The Comparative Safety and Efficacy of Resmetirom and Semaglutide in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review
Abstract
1. Introduction
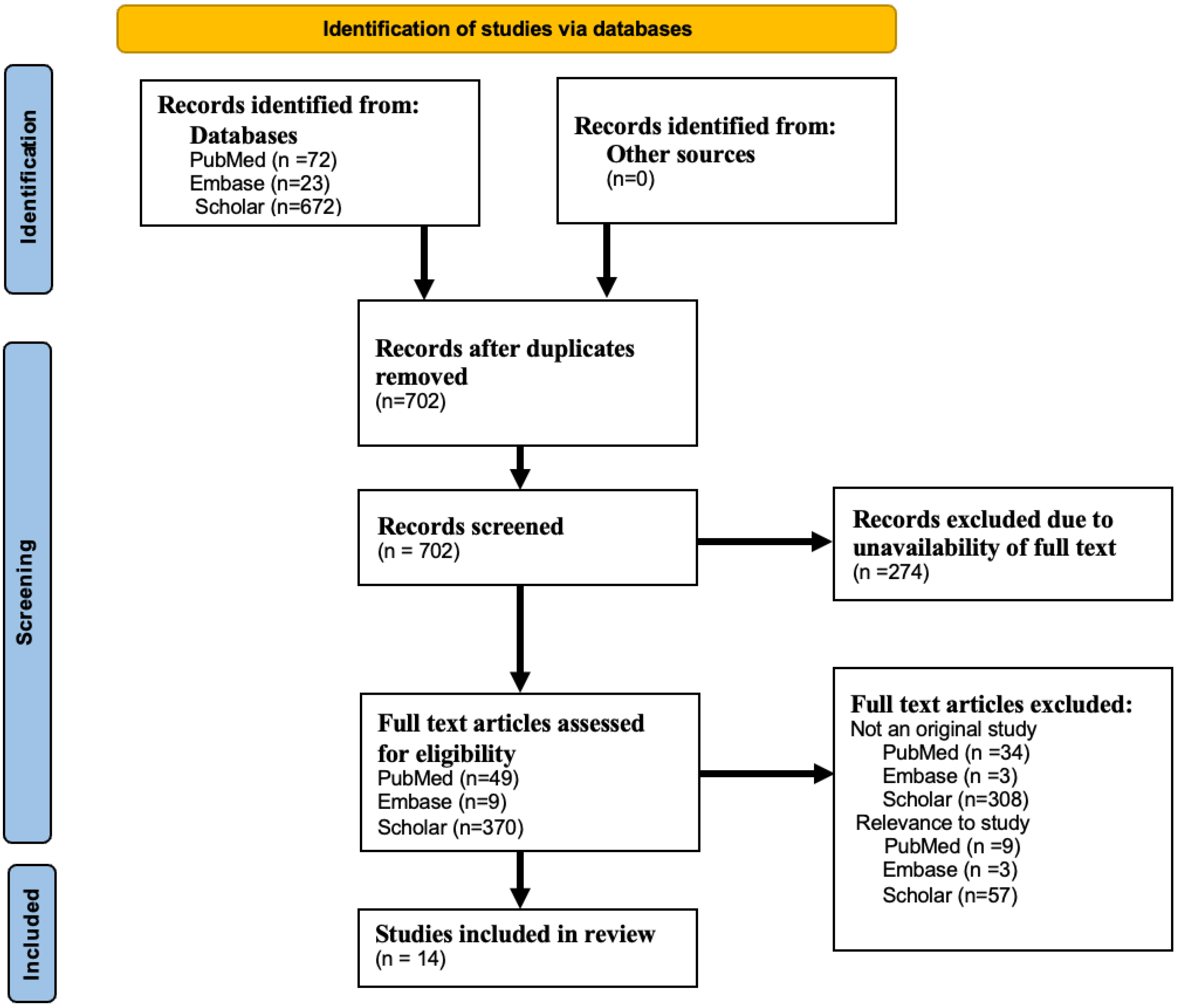
2. Methods
2.1. Eligibility Criteria, Study Selection, and Data Extraction
2.2. Statistical Analysis
3. Results
Comparative Insights
4. Discussion
4.1. Comparative Analysis of Efficacy: Resmetirom vs. Semaglutide in MASLD/NASH
4.2. Safety and Efficacy
4.3. Regulatory Considerations and Role of Surrogate Endpoints
4.4. Strengths
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2023, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur. J. Clin. Nutr. 2009, 64, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Wong, V.W.; Farrell, G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J. Gastroenterol. Hepatol. 2011, 26, 163–172. [Google Scholar] [CrossRef]
- Dajani, A.; AbuHammour, A. Treatment of nonalcoholic fatty liver disease: Where do we stand? an overview. Saudi J. Gastroenterol. 2016, 22, 91. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar] [CrossRef]
- Edmison, J.; McCullough, A.J. Pathogenesis of non-alcoholic steatohepatitis: Human data. Clin. Liver Dis. 2007, 11, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2017, 67, 123–133. [Google Scholar] [CrossRef]
- Nseir, W.; Hellou, E.; Assy, N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. WJG 2014, 20, 9338–9344. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic fatty liver disease and hypercholesterolemia: Roles of thyroid hormones, metabolites, and agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E. The Glucagon-Like peptide-1 receptor agonist, semaglutide, for the treatment of nonalcoholic steatohepatitis. Hepatology 2021, 74, 2290–2292. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Management of endocrine disease: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur. J. Endocrinol. 2019, 181, R211–R234. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sanyal, A.J.; Engebretsen, K.A.; Kliers, I.; Østergaard, L.; Vanni, D.; Bugianesi, E.; Rinella, M.E.; Roden, M.; Ratziu, V. Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment. Pharmacol. Ther. 2024, 60, 1525–1533. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; McCarty, K.; Frias, J.P.; Taub, R.; Alkhouri, N. Effects of resmetirom on noninvasive endpoints in a 36-Week phase 2 active treatment extension study in patients with NASH. Hepatol. Commun. 2021, 5, 573–588. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Abdelmalek, M.F.; Armstrong, M.J.; Jara, M.; Kjær, M.S.; Krarup, N.; Lawitz, E.; Ratziu, V.; Sanyal, A.J.; Schattenberg, J.M.; et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 511–522. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Taub, R.A.; Barbone, J.M.; Harrison, S.A. Hepatic fat reduction due to resmetirom in patients with nonalcoholic steatohepatitis is associated with improvement of quality of life. Clin. Gastroenterol. Hepatol. 2022, 20, 1354–1361.e7. [Google Scholar] [CrossRef]
- Ratziu, V.; Francque, S.; Behling, C.A.; Cejvanovic, V.; Cortez-Pinto, H.; Iyer, J.S.; Krarup, N.; Le, Q.; Sejling, A.-S.; Tiniakos, D.; et al. Artificial intelligence scoring of liver biopsies in a phase ii trial of semaglutide in non-alcoholic steatohepatitis. Hepatology 2024, 80, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Calanna, S.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.; Sejling, A.-S.; Newsome, P.N. Semaglutide for the treatment of non-alcoholic steatohepatitis: Trial design and comparison of non-invasive biomarkers. Contemp. Clin. Trials 2020, 97, 106174. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Palle, M.S.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef]
- Dusilová, T.; Kovář, J.; Laňková, I.; Thieme, L.; Hubáčková, M.; Šedivý, P.; Pajuelo, D.; Burian, M.; Dezortová, M.; Miklánková, D.; et al. Semaglutide Treatment Effects on Liver Fat Content in Obese Subjects with Metabolic-Associated Steatotic Liver Disease (MASLD). J. Clin. Med. 2024, 13, 6100. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okanoue, T.; Palle, M.S.; Sejling, A.; Tawfik, M.; Roden, M. Similar weight loss with semaglutide regardless of diabetes and cardiometabolic risk parameters in individuals with metabolic dysfunction-associated steatotic liver disease: Post hoc analysis of three randomised controlled trials. Diabetes Obes. Metab. 2025, 27, 710–718. [Google Scholar] [CrossRef]
- Kitsunai, H.; Shinozaki, Y.; Furusawa, S.; Kitao, N.; Ito, M.; Kurihara, H.; Oba-Yamamoto, C.; Takeuchi, J.; Nakamura, A.; Takiyama, Y.; et al. The Effects of Oral Semaglutide on Hepatic Fibrosis in Subjects with Type 2 Diabetes in Real-World Clinical Practice: A Post Hoc Analysis of the Sapporo-Oral SEMA Study. Pharmaceuticals 2025, 18, 129. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ratziu, V.; Anstee, Q.M.; Noureddin, M.; Sanyal, A.J.; Schattenberg, J.M.; Bedossa, P.; Bashir, M.R.; Schneider, D.; Taub, R.; et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2023, 59, 51–63. [Google Scholar] [CrossRef]
- Ravela, N.; Shackelford, P.; Blessing, N.; Yoder, L.; Chalasani, N.; Samala, N. Early experience with resmetirom to treat metabolic dysfunction–associated steatohepatitis with fibrosis in a real-world setting. Hepatol. Commun. 2025, 9, e0670. [Google Scholar] [CrossRef]
- Alkhouri, N.; Herring, R.; Kabler, H.; Kayali, Z.; Hassanein, T.; Kohli, A.; Huss, R.S.; Zhu, Y.; Billin, A.N.; Damgaard, L.H.; et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J. Hepatol. 2022, 77, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Kakkar, R.; Chahal, D.; Yoshida, E.M.; Hussaini, T. Efficacy and safety of semaglutide in non-alcoholic fatty liver disease. World J. Gastroenterol. 2023, 29, 5327–5338. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef] [PubMed]
- Sillassen, C.D.B.; Kamp, C.B.; Petersen, J.J.; Faltermeier, P.; Siddiqui, F.; Grand, J.; Dominguez, H.; Frølich, A.; Gæde, P.H.; Gluud, C.; et al. Adverse effects with semaglutide: A protocol for a systematic review with meta-analysis and trial sequential analysis. BMJ Open 2024, 14, e084190. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Intervention | Control | Study Design | N (Total) | Age (Mean ± SD) | % Female | % Diabetes | BMI (kg/m2) (Mean ± SD) | Waist Circumference | Fibrosis Stage Inclusion | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harrison et al. (2024, NEJM MAESTRO-NASH)—[12] | Resmetirom 80/100 mg | Placebo n = 321 | Phase 3, RCT, 52 weeks (MAESTRO-NASH) | 966 | 56.6 ± 10.9 | 56% | 67% | 35.7 ± 6.8 | N/A | F1B-F3 | NASH resolution w/o fibrosis worsening; fibrosis improvement ≥1 stage |
| Newsome et al. (2024, ESSENCE)—[16] | Semaglutide 2.4 mg | Placebo | Phase 3, RCT (ongoing, baseline data) | 800 | 56 ± 11.6 | 57% | 55.5% | 34.6 ± 7.2 | N/A | F2–F3 | Resolution of NASH w/o fibrosis worsening; fibrosis improvement w/o NASH worsening |
| Harrison et al. (2021)—[17] | Resmetirom 80/100 mg | Placebo | Phase 2, RCT extension, 36 weeks | 31 | 48.2 ± 12.3 | 48% | 45.2% | 35.3 ± 5.2 | N/A | F1–F3 | MRI-PDFF ↓, PRO-C3 ↓, liver stiffness ↓ (Fibroscan) |
| Newsome et al. (2021)—[18] | Semaglutide (0.1/0.2/0.4 mg) | Placebo n = 80 | Phase 2, RCT | 320 | 55 ± 11 | Not stated | 62% | 36 ± 6 | N/A | F2–F3 | NASH resolution; fibrosis improvement |
| Loomba et al. (2023)—[19] | Semaglutide 2.4 mg | Placebo n = 24 | Phase 2, RCT, 48 weeks | 71 | 59.5 ± 8 | 69% | 75% | 34.9 ± 5.9 | N/A | Cirrhosis | Fibrosis improvement ≥1 stage w/o NASH worsening |
| Younossi et al. (2022)—[20] | Resmetirom 80 mg | Placebo n = 41 | Phase 2, RCT, 36 weeks | 125 | 50 ± 11 | 50% | 39% | 35 ± 6 | N/A | Non-cirrhotic NASH | HRQL improvement; PDFF reduction ≥30% |
| Ratziu et al. (2024, AI subset)—[21] | Semaglutide 0.4 mg | N/A | Post hoc analysis of Phase 2 trial (72 weeks) | 251 | 54.5 | 61% | 64.1% | 36.0 | N/A | F1–F3 | AI-assessed NASH resolution and fibrosis response |
| Harrison et al. (2020)—[22] | Semaglutide (0.1/0.2/0.4 mg) | Placebo | Phase 2, RCT | 320 | 55 ± 11 | Not stated | 62% | 36 ± 6 | N/A | F1–F3 | NASH resolution w/o fibrosis worsening; fibrosis improvement |
| Harrison et al. (2023)—[23] | Resmetirom 80/100 mg | Placebo n = 320 | Phase 3, RCT, 52 weeks | 972 | 56 ± NA | 57% | 49% | 35 | N/A | Presumed NASH | Safety, LDL-C, hepatic fat reduction, liver stiffness |
| Flint et al. (2021)—[24] | Semaglutide 0.4 mg | Placebo n = 33 | Phase 2, RCT, 48 weeks | 67 | Not specified | Not stated | Not stated | Not stated | N/A | NA | Change in liver steatosis (MRI-PDFF) and stiffness (MRE) |
| Dusilová et al. (2024)—[25] | Semaglutide (SC) | Basic dietary interventions | Cross-over, non-diabetic males | 16 | Not specified | 0% | 0% | Not specified | 120 ± 10 cm | NA | Change in liver fat and VLDL-TG fatty acid composition |
| Harrison et al. (2019)—[26] | Resmetirom 80 mg | Placebo n = 41 | Phase 2, RCT, 36 weeks | 125 | Not specified | Not stated | Not stated | Not stated | N/A | F1–F3 | Reduction in hepatic fat (MRI-PDFF) |
| Armstrong et al. (2025)—[27] | Semaglutide (0.4 mg daily/2.4 mg weekly) | N/A | Post hoc analysis of pooled RCTs (48–72 weeks) | 300 | 56.3 ± 10.2 | 53% | 69.7% | 35.2 ± 5.8 | N/A | F1–F4 (subset) | Weight change with semaglutide by T2D status |
| Kitsunai et al. (2025)—[28] | Oral Semaglutide | N/A | Real-world, post hoc analysis | 169 | Not stated | Not stated | Type 2 DM | Not stated | N/A | NA | Change in FIB-4, hepatic steatosis index |
| Feature | Resmetirom | Semaglutide |
|---|---|---|
| Mechanism | THR-β agonist [hepato-specific] | GLP-1 receptor agonist |
| Main Outcome | Fat reduction, LDL lowering, NAS improvement | NASH resolution, weight loss |
| Effect on Liver fibrosis | Reduced markers of fibrosis, including liver stiffness when assessed by transient elastography | Not significant statistically |
| Weight loss | No significant weight loss observed | 13%in 0.4 mg group vs. 1% in placebo |
| Trial Duration | 52 weeks | ongoing |
| Histological Benefit | 27% NASH resolution vs. 6%in placebo | 36% in 0.2 mg group NASH resolution vs. 59% NASH resolution in 0.4 mg group vs. 17% placebo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udaikumar, J.; Nimmagadda, R.; Lella, V.V.; Achuta, K.M.; Kuppili, S.; Avula, S.R.; Sarwar, R. The Comparative Safety and Efficacy of Resmetirom and Semaglutide in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review. Pharmacoepidemiology 2025, 4, 14. https://doi.org/10.3390/pharma4030014
Udaikumar J, Nimmagadda R, Lella VV, Achuta KM, Kuppili S, Avula SR, Sarwar R. The Comparative Safety and Efficacy of Resmetirom and Semaglutide in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review. Pharmacoepidemiology. 2025; 4(3):14. https://doi.org/10.3390/pharma4030014
Chicago/Turabian StyleUdaikumar, Jahnavi, Rithish Nimmagadda, Vindhya Vasini Lella, Kesava Manikanta Achuta, Satwik Kuppili, Suraj Reddy Avula, and Raiya Sarwar. 2025. "The Comparative Safety and Efficacy of Resmetirom and Semaglutide in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review" Pharmacoepidemiology 4, no. 3: 14. https://doi.org/10.3390/pharma4030014
APA StyleUdaikumar, J., Nimmagadda, R., Lella, V. V., Achuta, K. M., Kuppili, S., Avula, S. R., & Sarwar, R. (2025). The Comparative Safety and Efficacy of Resmetirom and Semaglutide in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review. Pharmacoepidemiology, 4(3), 14. https://doi.org/10.3390/pharma4030014






