Evaluation of Screening Tool of Older People’s Prescriptions (STOPP) Criteria in an Urban Cohort of Older People with HIV
Abstract
1. Introduction
2. Methods
2.1. Participant Recruitment and Data Collection
2.2. Measuring Potentially Inappropriate Prescriptions
2.3. Measuring Symptom Burden
2.4. Measuring Quality of Life
2.5. Measuring Social Determinants of Health
2.6. Statistical Analysis
2.7. Mediation Analysis
3. Results
3.1. Characteristics Associated with Potentially Inappropriate Prescribing
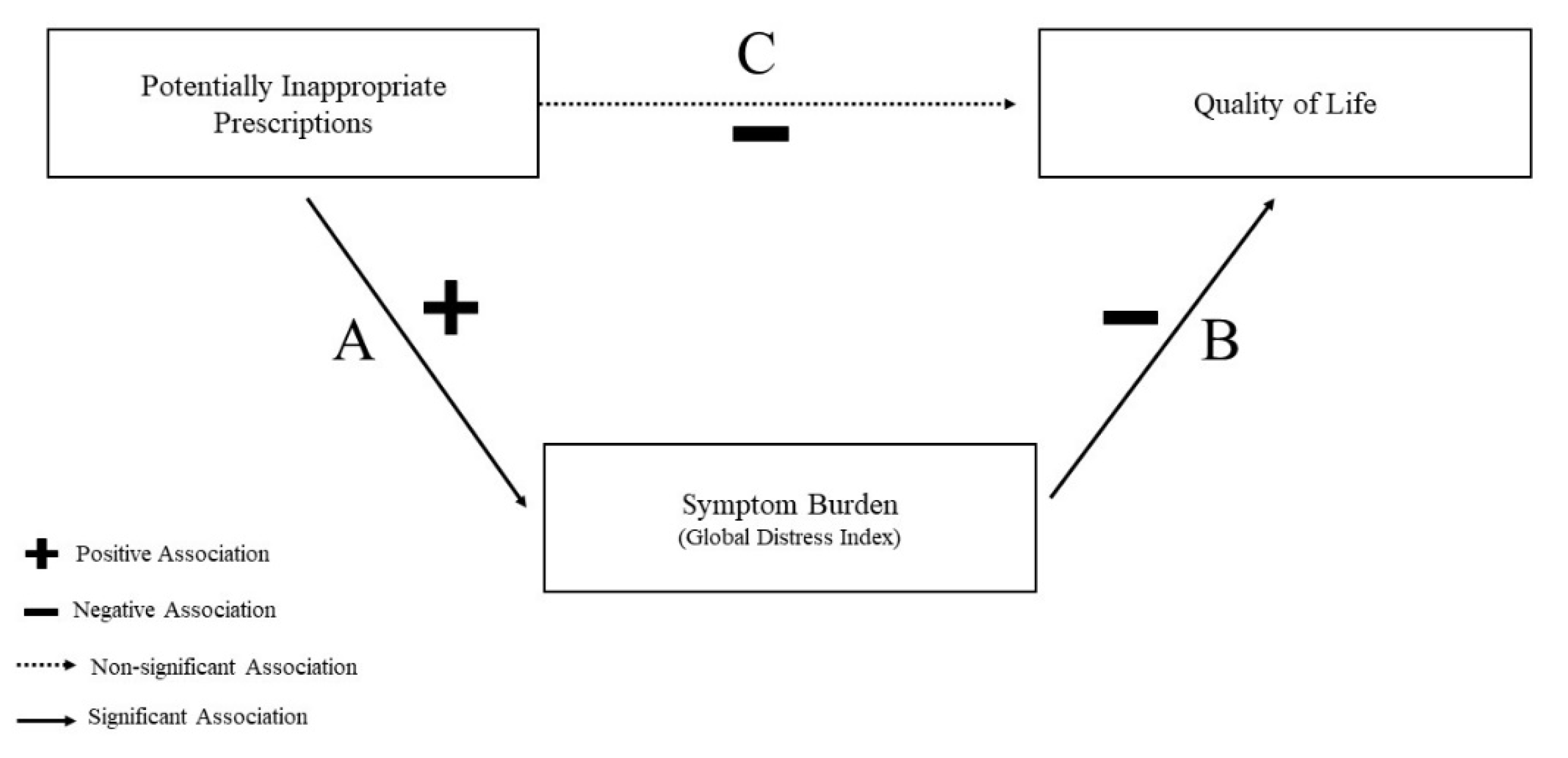
3.2. Mediation Analysis Results
4. Discussion
4.1. Clinical Relevance and Potential Interventions
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuzin, L.; Katlama, C.; Cotte, L.; Pugliese, P.; Cheret, A.; Bernaud, C.; Rey, D.; Poizot-Martin, I.; Chirouze, C.; Bani-Sadr, F.; et al. Ageing with HIV: Do comorbidities and polymedication drive treatment optimization? HIV Med. 2017, 18, 395–401. [Google Scholar] [CrossRef]
- Mata-Marín, J.A.; Martínez-Osio, M.H.; Arroyo-Anduiza, C.I.; Berrospe-Silva, M.d.l.Á.; Chaparro-Sánchez, A.; Cruz-Grajales, I.; Cruz-Herrera, J.E.; Uribe-Noguez, L.A.; Gaytán-Martínez, J.E.; Jerónimo-Morales, M. Comorbidities and polypharmacy among HIV-positive patients aged 50 years and over: A case–control study. BMC Res. Notes 2019, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.E.; Greenberg, A.E.; Hart, R.; Powers Happ, L.; Hadigan, C.; Castel, A. DC Cohort Executive Committee High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: Evolving health care needs of people aging with HIV in Washington, DC. HIV Med. 2017, 18, 724–735. [Google Scholar] [CrossRef]
- Livio, F.; Deutschmann, E.; Moffa, G.; Rrustemi, F.; Stader, F.; Elzi, L.; Braun, D.L.; Calmy, A.; Hachfeld, A.; Cavassini, M.; et al. Analysis of inappropriate prescribing in elderly patients of the Swiss HIV Cohort Study reveals gender inequity. J. Antimicrob. Chemother. 2021, 76, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Verdugo, R.; Blanco Ramos, J.R.; Abdel-Kader Martín, L.; Álvarez de Sotomayor, M. The challenge of aging and pharmacoterapeutic complexity in the HIV + patient. Farm Hosp. 2018, 42, 120–127. [Google Scholar] [CrossRef]
- Back, D.; Marzolini, C. The challenge of HIV treatment in an era of polypharmacy. J. Int. AIDS Soc. 2020, 23, e25449. [Google Scholar] [CrossRef] [PubMed]
- Dimitrow, M.S.; Airaksinen, M.S.A.; Kivelä, S.-L.; Lyles, A.; Leikola, S.N.S. Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: A systematic review. J. Am. Geriatr. Soc. 2011, 59, 1521–1530. [Google Scholar] [CrossRef]
- Vélez-Díaz-Pallarés, M.; Silveira, E.D.; Fradejas, J.F.; Llorente, B.M.; Fernández, C.P.; Errasquín, B.M.; Cruz-Jentoft, A.J.; Álvarez Díaz, A.M. Potentially inappropriate prescribing in older people living with HIV: A scoping review. JAIDS J. Acquir. Immune Defic. Syndr. 2023, 94, 445–460. [Google Scholar] [CrossRef]
- Mahony, D.O.; Sullivan, D.O.; Byrne, S.; Connor, M.N.O.; Ryan, C.; Gallagher, P. Corrigendum: STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2018, 47, 489. [Google Scholar] [CrossRef]
- Mekonnen, A.B.; Redley, B.; Courten, B.; Manias, E. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 4150–4172. [Google Scholar] [CrossRef]
- Vinuesa-Hernando, J.M.; Gimeno-Gracia, M.; Malo, S.; Sanjoaquin-Conde, I.; Crusells-Canales, M.J.; Letona-Carbajo, S.; Gracia-Piquer, R. Potentially inappropriate prescriptions and therapeutic complexity in older HIV patients with comorbidities. Int. J. Clin. Pharm. 2021, 43, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Loste, C.; Moltó, J.; Pérez-Álvarez, N.; Puig, J.; Echeverría, P.; Bonjoch, A.; Fumaz, C.R.; Lemos, B.; Estany, C.; Clotet, B.; et al. Potential prescribing issues among older HIV-infected subjects in a Mediterranean cohort: Does the current prevalence give cause for concern? Br. J. Clin. Pharmacol. 2021, 87, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Jing, B.; Growdon, M.E.; Yaffe, K.; Boscardin, W.J.; Boockvar, K.S.; Steinman, M.A. Medication misuse and overuse in community-dwelling persons with dementia. J. Am. Geriatr. Soc. 2023, 71, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, L.; Søgaard, J.; Hallas, J.; Kragstrup, J. Polypharmacy: Correlations with sex, age and drug regimenA prescription database study. Eur. J. Clin. Pharmacol. 1998, 54, 197–202. [Google Scholar] [CrossRef]
- Golchin, N.; Frank, S.H.; Vince, A.; Isham, L.; Meropol, S.B. Polypharmacy in the elderly. J. Res. Pharm. Pract. 2015, 4, 85–88. [Google Scholar] [CrossRef]
- Gokce Kutsal, Y.; Barak, A.; Atalay, A.; Baydar, T.; Kucukoglu, S.; Tuncer, T.; Hizmetli, S.; Dursun, N.; Eyigor, S.; Sarıdogan, M.; et al. Polypharmacy in the Elderly: A Multicenter Study. J. Am. Med. Dir. Assoc. 2009, 10, 486–490. [Google Scholar] [CrossRef]
- Greene, M.; Steinman, M.A.; McNicholl, I.R.; Valcour, V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J. Am. Geriatr. Soc. 2014, 62, 447–453. [Google Scholar] [CrossRef]
- A Pharmacist-Led Program to Evaluate and Reduce Polypharmacy and Potentially Inappropriate Prescribing in Older HIV-Positive Patients—McNicholl—2017—Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy—Wiley Online Library. Available online: https://accpjournals.onlinelibrary.wiley.com/doi/10.1002/phar.2043 (accessed on 12 May 2023).
- Liu, G.G.; Christensen, D.B. The Continuing Challenge of Inappropriate Prescribing in the Elderly: An Update of the Evidence. J. Am. Pharm. Assoc. 2002, 42, 847–857. [Google Scholar] [CrossRef]
- Fulone, I.; Lopes, L.C. Potentially inappropriate prescriptions for elderly people taking antidepressant: Comparative tools. BMC Geriatr. 2017, 17, 278. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M. Race/Ethnicity, Socioeconomic Status, and Polypharmacy among Older Americans. Pharmacy 2019, 7, 41. [Google Scholar] [CrossRef]
- Cashion, W.; McClellan, W.; Howard, G.; Goyal, A.; Kleinbaum, D.; Goodman, M.; Prince, V.; Muntner, P.; McClure, L.A.; McClellan, A.; et al. Geographic region and racial variations in polypharmacy in the United States. Ann. Epidemiol. 2015, 25, 433–438.e1. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Shirai, K.; Tang, C.; Hu, Y.; Wang, Y.; Hao, Y.; Dong, J.-Y. Prevalence and trends of polypharmacy in U.S. adults, 1999–2018. Glob. Health Res. Policy 2023, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.E.; Hays, H.; Castel, A.D.; Subramanian, T.; Happ, L.P.; Jaurretche, M.; Binkley, J.; Kalmin, M.M.; Wood, K.; Hart, R.; et al. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: Technical challenges and innovative solutions. J. Am. Med. Inform. Assoc. 2016, 23, 635–643. [Google Scholar] [CrossRef]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.W.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.L.; Gough, K.; Pascoe, M.C.; Drosdowsky, A.; Chang, V.T.; Schofield, P. The modified Memorial Symptom Assessment Scale Short Form: A modified response format and rational scoring rules. Qual. Life Res. 2018, 27, 1903–1910. [Google Scholar] [CrossRef]
- Earnshaw, V.A.; Smith, L.R.; Chaudoir, S.R.; Amico, K.R.; Copenhaver, M.M. HIV stigma mechanisms and well-being among PLWH: A test of the HIV stigma framework. AIDS Behav. 2013, 17, 1785–1795. [Google Scholar] [CrossRef]
- Christopoulos, K.A.; Neilands, T.B.; Hartogensis, W.; Geng, E.H.; Sauceda, J.; Mugavero, M.J.; Crane, H.M.; Fredericksen, R.J.; Moore, R.D.; Mathews, W.C.; et al. Internalized HIV Stigma Is Associated with Concurrent Viremia and Poor Retention in a Cohort of US Patients in HIV Care. J. Acquir. Immune Defic. Syndr. 2019, 82, 116–123. [Google Scholar] [CrossRef]
- EuroQol Group EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool—National Academy of Medicine. Available online: https://nam.edu/standardized-screening-for-health-related-social-needs-in-clinical-settings-the-accountable-health-communities-screening-tool/ (accessed on 14 September 2022).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- STOPP-START—CGA Toolkit Plus. Available online: https://www.cgakit.com/m-2-stopp-start (accessed on 22 May 2023).
- Barlow, A.; Prusak, E.S.; Barlow, B.; Nightingale, G. Interventions to reduce polypharmacy and optimize medication use in older adults with cancer. J. Geriatr. Oncol. 2021, 12, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Schenker, Y.; Park, S.Y.; Jeong, K.; Pruskowski, J.; Kavalieratos, D.; Resick, J.; Abernethy, A.; Kutner, J.S. Associations Between Polypharmacy, Symptom Burden, and Quality of Life in Patients with Advanced, Life-Limiting Illness. J. Gen. Intern. Med. 2019, 34, 559–566. [Google Scholar] [CrossRef]
- Muthén, B.O.; Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Griffin, M.R.; Ray, W.A.; Schaffner, W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann. Intern. Med. 1988, 109, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant Acute Kidney Injury Due to Non-steroidal Anti-inflammatory Drugs: Inpatient Setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, M.; Juurlink, D.N.; Lee, D.S.; Rochon, P.A.; Kopp, A.; Naglie, G.; Austin, P.C.; Laupacis, A.; Stukel, T.A. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: A population-based cohort study. Lancet 2004, 363, 1751–1756. [Google Scholar] [CrossRef]
- Meng, L.-C.; Kojima, T.; Suzuki, Y.; Weng, S.-E.; Chen, H.-M.; Huang, S.-T.; Akishita, M.; Chen, L.-K.; Hsiao, F.-Y. Medication overload: A closer look at polypharmacy and potentially inappropriate medications among older people in Taiwan and Japan. Arch. Gerontol. Geriatr. 2023, 115, 105100. [Google Scholar] [CrossRef]
- Nguyen, T.N.M.; Laetsch, D.C.; Chen, L.-J.; Holleczek, B.; Meid, A.D.; Brenner, H.; Schöttker, B. Comparison of Five Lists to Identify Potentially Inappropriate Use of Non-Steroidal Anti-Inflammatory Drugs in Older Adults. Pain Med. 2021, 22, 1962–1969. [Google Scholar] [CrossRef]
- Schmidt-Mende, K.; Andersen, M.; Wettermark, B.; Hasselström, J. Drug–disease interactions in Swedish senior primary care patients were dominated by non-steroid anti-inflammatory drugs and hypertension—A population-based registry study. Scand. J. Prim. Health Care 2020, 38, 330–339. [Google Scholar] [CrossRef]
- Hudhra, K.; García-Caballos, M.; Casado-Fernandez, E.; Jucja, B.; Shabani, D.; Bueno-Cavanillas, A. Polypharmacy and potentially inappropriate prescriptions identified by Beers and STOPP criteria in co-morbid older patients at hospital discharge. J. Eval. Clin. Pract. 2016, 22, 189–193. [Google Scholar] [CrossRef]
- Cahir, C.; Fahey, T.; Teeling, M.; Teljeur, C.; Feely, J.; Bennett, K. Potentially inappropriate prescribing and cost outcomes for older people: A national population study. Br. J. Clin. Pharmacol. 2010, 69, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, A.J.; Miller, G.E.; Sarpong, E.M.; Yang, E.; Brandt, N.; Fick, D.M. Prevalence of Potentially Inappropriate Medication Use in Older Adults Using the 2012 Beers Criteria. J. Am. Geriatr. Soc. 2015, 63, 486–500. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Lizaraso, F.; Carvajal, A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R.; DeBerry, J.J.; Aggarwal, S. Chronic P—Ain in HIV. Mol. Pain. 2020, 16, 1744806920927276. [Google Scholar] [CrossRef]
- Gallagher, P.; O’Mahony, D. Constipation in old age. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 875–887. [Google Scholar] [CrossRef]
- Chau, D.L.; Walker, V.; Pai, L.; Cho, L.M. Opiates and elderly: Use and side effects. Clin. Interv. Aging 2008, 3, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Edelman, E.J.; Gordon, K.; Becker, W.C.; Goulet, J.L.; Skanderson, M.; Gaither, J.R.; Brennan Braden, J.; Gordon, A.J.; Kerns, R.D.; Justice, A.C.; et al. Receipt of Opioid Analgesics by HIV-Infected and Uninfected Patients. J. Gen. Intern. Med. 2013, 28, 82–90. [Google Scholar] [CrossRef]
- Prescription Opioid Trends in the United States. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/prescription-opioid-trends-in-the-united-states (accessed on 4 April 2025).
- Campbell, N.; Boustani, M.; Limbil, T.; Ott, C.; Fox, C.; Maidment, I.; Schubert, C.C.; Munger, S.; Fick, D.; Miller, D.; et al. The cognitive impact of anticholinergics: A clinical review. CIA 2009, 4, 225–233. [Google Scholar] [CrossRef]
- Dagli, R.J.; Sharma, A. Polypharmacy: A Global Risk Factor for Elderly People. J. Int. Oral Health 2014, 6, i–ii. [Google Scholar]
- Tseng, A.; Szadkowski, L.; Walmsley, S.; Salit, I.; Raboud, J. Association of Age With Polypharmacy and Risk of Drug Interactions With Antiretroviral Medications in HIV-Positive Patients. Ann. Pharmacother. 2013, 47, 1429–1439. [Google Scholar] [CrossRef]
- Wastesson, J.W.; Morin, L.; Laroche, M.-L.; Johnell, K. How Chronic Is Polypharmacy in Old Age? A Longitudinal Nationwide Cohort Study. J. Am. Geriatr. Soc. 2019, 67, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W.; Liao, K.-F.; Liao, C.-C.; Muo, C.-H.; Liu, C.-S.; Sung, F.-C. Polypharmacy Correlates With Increased Risk for Hip Fracture in the Elderly: A Population-Based Study. Medicine 2010, 89, 295. [Google Scholar] [CrossRef] [PubMed]
- Rochon, P.A.; Gurwitz, J.H. Optimising drug treatment for elderly people: The prescribing cascade. BMJ 1997, 315, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Ziere, G.; Dieleman, J.P.; Hofman, A.; Pols, H.A.P.; Van Der Cammen, T.J.M.; Stricker, B.H.C. Polypharmacy and falls in the middle age and elderly population. Br. J. Clin. Pharmacol. 2006, 61, 218–223. [Google Scholar] [CrossRef]
- Lesko, C.R.; Moore, R.D.; Tong, W.; Lau, B. Association of injection drug use with incidence of HIV-associated non-AIDS-related morbidity by age, 1995–2014. AIDS 2016, 30, 1447–1455. [Google Scholar] [CrossRef]
- Tsao, J.C.I.; Plankey, M.W.; Young, M.A. Pain, psychological symptoms and prescription drug misuse in HIV: A literature review. J. Pain. Manag. 2012, 5, 111–118. [Google Scholar]
- Holtzman, C.; Armon, C.; Tedaldi, E.; Chmiel, J.S.; Buchacz, K.; Wood, K.; Brooks, J.T. Polypharmacy and Risk of Antiretroviral Drug Interactions Among the Aging HIV-Infected Population. J. Gen. Intern. Med. 2013, 28, 1302–1310. [Google Scholar] [CrossRef]
- Assari, S.; Wisseh, C.; Bazargan, M. Obesity and Polypharmacy among African American Older Adults: Gender as the Moderator and Multimorbidity as the Mediator. Int. J. Environ. Res. Public Health 2019, 16, 2181. [Google Scholar] [CrossRef]
- Bazargan, M.; Smith, J.; Saqib, M.; Helmi, H.; Assari, S. Associations between Polypharmacy, Self-Rated Health, and Depression in African American Older Adults; Mediators and Moderators. Int. J. Environ. Res. Public Health 2019, 16, 1574. [Google Scholar] [CrossRef]
- Trevisan, C.; Limongi, F.; Siviero, P.; Noale, M.; Cignarella, A.; Manzato, E.; Sergi, G.; Maggi, S. Mild polypharmacy and MCI progression in older adults: The mediation effect of drug–drug interactions. Aging Clin. Exp. Res. 2021, 33, 49–56. [Google Scholar] [CrossRef]
- Bose, S.; Kelly, L.; Shahn, Z.; Novack, L.; Banner-Goodspeed, V.; Subramaniam, B. Sedative polypharmacy mediates the effect of mechanical ventilation on delirium in critically ill COVID-19 patients: A retrospective cohort study. Acta Anaesthesiol. Scand. 2022, 66, 1099–1106. [Google Scholar] [CrossRef]
- Weng, Y.-A.; Deng, C.-Y.; Pu, C. Targeting continuity of care and polypharmacy to reduce drug–drug interaction. Sci. Rep. 2020, 10, 21279. [Google Scholar] [CrossRef]
- López-Centeno, B.; Badenes-Olmedo, C.; Mataix-Sanjuan, A.; Bellón, J.; Pérez-Latorre, L.; López, J.; Benedí, J.; Khoo, S.; Marzolini, C.; Calvo-Alcántara, M.; et al. Potentially inappropriate medications in older adults living with HIV. HIV Med. 2020, 21, 541–546. [Google Scholar] [CrossRef]
- Parodi López, N.; Svensson, S.A.; Wallerstedt, S.M. Clinical relevance of potentially inappropriate medications and potential prescribing omissions according to explicit criteria—A validation study. Eur. J. Clin. Pharmacol. 2022, 78, 1331–1339. [Google Scholar] [CrossRef]
- Lönnbro, J.; Wallerstedt, S.M. Clinical relevance of the STOPP/START criteria in hip fracture patients. Eur. J. Clin. Pharmacol. 2017, 73, 499–505. [Google Scholar] [CrossRef]
- Sergi, G.; Rui, M.D.; Sarti, S.; Manzato, E. Polypharmacy in the Elderly. Drugs Aging 2011, 28, 509–518. [Google Scholar] [CrossRef]
- Kaur, S.; Mitchell, G.; Vitetta, L.; Roberts, M.S. Interventions that can Reduce Inappropriate Prescribing in the Elderly. Drugs Aging 2009, 26, 1013–1028. [Google Scholar] [CrossRef]
- Rognstad, S.; Brekke, M.; Fetveit, A.; Dalen, I.; Straand, J. Prescription peer academic detailing to reduce inappropriate prescribing for older patients: A cluster randomised controlled trial. Br. J. Gen. Pract. 2013, 63, e554–e562. [Google Scholar] [CrossRef]
- Clyne, B.; Fitzgerald, C.; Quinlan, A.; Hardy, C.; Galvin, R.; Fahey, T.; Smith, S.M. Interventions to Address Potentially Inappropriate Prescribing in Community-Dwelling Older Adults: A Systematic Review of Randomized Controlled Trials. J. Am. Geriatr. Soc. 2016, 64, 1210–1222. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Peterson, K.; Winthrop, K.; Cantor, A.; Lazur, B.H.; Buckley, D.I. Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: Summary and update of a systematic review. J. Int. Med. Res. 2018, 46, 3337–3357. [Google Scholar] [CrossRef]
- Tamblyn, R.; Huang, A.; Perreault, R.; Jacques, A.; Roy, D.; Hanley, J.; McLeod, P.; Laprise, R. The medical office of the 21st century (MOXXI): Effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ 2003, 169, 549–556. [Google Scholar]
- Clyne, B.; Bradley, M.C.; Hughes, C.M.; Clear, D.; McDonnell, R.; Williams, D.; Fahey, T.; Smith, S.M. Addressing potentially inappropriate prescribing in older patients: Development and pilot study of an intervention in primary care (the OPTI-SCRIPT study). BMC Health Serv. Res. 2013, 13, 307. [Google Scholar] [CrossRef]
| Demographic | Total (N = 1048) | Had a PIP (N = 486) | Did Not Have a PIP (N = 562) | p-Value |
|---|---|---|---|---|
| Age [years] (Mean [St.Dev.]) | 61.26 (7.00) | 62.03 (6.98) | 60.60 (6.95) | 0.0006 |
| Race/Ethnicity | <0.0001 | |||
| Black, non-Hispanic | 830 (79.20) | 418 (86.01) | 412 (73.31) | |
| White, non-Hispanic | 142 (13.55) | 37 (7.61) | 105 (18.68) | |
| Hispanic | 44 (4.20) | 16 (3.29) | 28 (4.98) | |
| Other | 13 (1.24) | 2 (0.41) | 11 (1.96) | |
| Unknown | 19 (1.81) | 13 (2.67) | 6 (1.07) | |
| Gender | <0.0001 | |||
| Male | 750 (71.56) | 317 (65.23) | 433 (77.05) | |
| Female | 281 (26.81) | 154 (31.69) | 127 (22.60) | |
| Transgender: male-to-female | 16 (1.53) | 14 (2.88) | 2 (0.36) | |
| Transgender: female-to-male | 1 (0.10) | 1 (0.21) | 0 (0.00) | |
| HIV Transmission Factor | <0.0001 | |||
| Men who have sex with Men (MSM) | 383 (36.55) | 142 (29.22) | 241 (42.88) | |
| Intravenous Drug Use (IDU) | 87 (8.30) | 59 (12.14) | 28 (4.98) | |
| MSM and IDU | 11 (1.05) | 3 (0.62) | 8 (1.42) | |
| High Risk Heterosexual (HRH) | 358 (34.16) | 184 (37.86) | 174 (30.96) | |
| Perinatal | 1 (0.10) | 1 (0.21) | 0 (0.00) | |
| Other | 207 (19.75) | 96 (19.75) | 111 (19.75) | |
| Unknown | 1 (0.10) | 1 (0.21) | 0 (0.00) | |
| Viral Suppression (<200 copies/mL) a | <0.0001 | |||
| Virally Suppressed | 961 (91.70) | 455 (93.62) | 506 (90.04) | |
| Not Virally Suppressed | 50 (4.77) | 28 (5.76) | 22 (3.91) | |
| Unknown | 37 (3.53) | 3 (0.62) | 34 (6.05) | |
| Type of HIV Care Site | <0.0001 | |||
| Community | 483 (46.09) | 261 (53.70) | 222 (39.50) | |
| Hospital | 565 (53.91) | 225 (46.30) | 340 (60.50) | |
| Social Determinants of Health | ||||
| Housing Need | 291 (27.77) | 148 (30.45) | 143 (25.44) | 0.0710 |
| Transportation Need | 164 (15.65) | 93 (19.14) | 71 (12.63) | 0.0039 |
| Utility Need | 103 (9.83) | 49 (10.08) | 54 (9.61) | 0.7972 |
| Food Insecurity | 360 (34.35) | 191 (39.30) | 169 (30.07) | 0.0017 |
| Quality of Life | ||||
| Pain/Discomfort Problems | 469 (44.75) | 254 (52.26) | 215 (38.26) | <0.0001 |
| Mobility Problems | 265 (25.29) | 151 (31.07) | 114 (20.28) | <0.0001 |
| Self-Care Problems | 74 (7.06) | 46 (9.47) | 28 (4.98) | 0.0047 |
| Usual Activities Problems | 213 (20.32) | 127 (26.13) | 86 (15.30) | <0.0001 |
| Anxiety/Depression Problems | 356 (33.97) | 187 (38.48) | 169 (30.07) | 0.0042 |
| Symptom Burden Score (Mean [St. Dev.]) | ||||
| Psychological Symptoms | 0.79 (0.94) | 0.86 (0.95) | 0.73 (0.92) | 0.0094 |
| Physical Symptoms | 0.38 (0.48) | 0.48 (0.51) | 0.30 (0.43) | <0.0001 |
| General Distress Index | 0.66 (0.72) | 0.76 (0.75) | 0.57 (0.68) | <0.0001 |
| System | N (%) |
|---|---|
| Musculoskeletal System | 245 (23.38) |
| Analgesic Drugs | 172 (16.41) |
| Central Nervous System | 140 (13.36) |
| Coagulation System | 88 (8.40) |
| Antimuscarinic/Anticholinergic Drugs | 71 (6.77) |
| Cardiovascular System | 57 (5.44) |
| Endocrine System | 43 (4.10) |
| Fall Risk Increase | 35 (3.34) |
| Respiratory System | 11 (1.05) |
| Urogenital System | 6 (0.57) |
| Gastrointestinal System | 6 (0.57) |
| Renal System | 0 (0.00) |
| Any STOPP Criteria | 486 (46.37) |
| Demographic | Crude Model | Model I b | Model II c |
|---|---|---|---|
| Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) | Adjusted IRR (95% CI) | |
| Age | 1.03 (1.02, 1.04) | 1.03 (1.02, 1.04) | 1.03 (1.02, 1.05) |
| Race/Ethnicity | |||
| Black, non-Hispanic | REF | REF | REF |
| Hispanic | 0.67 (0.41, 1.09) | 0.77 (0.47, 1.25) | 0.70 (0.40, 1.21) |
| White, non-Hispanic | 0.61 (0.45, 0.81) | 0.67 (0.50, 0.92) | 0.62 (0.44, 0.88) |
| Other/Unknown | 0.78 (0.45, 1.35) | 0.78 (0.45, 1.33) | 0.79 (0.42, 1.48) |
| Gender Identity | |||
| Male | REF | REF | REF |
| Female | 1.20 (0.98, 1.48) | 1.05 (0.83, 1.33) | 1.07 (0.82, 1.41) |
| Transgender | 1.68 (0.86, 3.31) | 1.66 (0.86, 3.20) | 1.59 (0.68, 3.76) |
| HIV Transmission Factor | |||
| Men who have sex with Men (MSM) a | REF | REF | REF |
| High-Risk Heterosexual (HRH) | 1.39 (1.12 1.73) | 1.12 (0.86, 1.45) | 1.03 (0.77, 1.38) |
| Intravenous Drug Use (IDU) | 2.18 (1.58, 3.02) | 1.68 (1.20, 2.35) | 1.67 (1.15, 2.41) |
| Other/Unknown | 1.18 (0.91, 1.53) | 0.99 (0.76, 1.31) | 0.96 (0.71, 1.31) |
| Viral Suppression (<200 copies/mL) | |||
| Not Virally Suppressed | REF | REF | REF |
| Virally Suppressed | 0.94 (0.62, 1.43) | 0.96 (0.63, 1.44) | 1.16 (0.71, 1.88) |
| Unknown | 0.12 (0.04, 0.33) | 0.15 (0.05, 0.42) | 0.19 (0.07, 0.56) |
| Site Type | |||
| Community | REF | REF | REF |
| Hospital | 0.77 (0.64, 0.93) | 0.75 (0.62, 0.92) | 0.85 (0.68, 1.07) |
| Social Determinants of Health | |||
| Housing Need | 1.20 (0.97, 1.49) | - | 1.19 (0.95, 1.48) |
| Food Insecurity | 1.28 (1.04, 1.57) | - | 1.16 (0.91, 1.48) |
| Transportation Need | 1.26 (0.97, 1.63) | - | 1.19 (0.90, 1.57) |
| Utility Need | 0.97 (0.70, 1.33) | - | 0.84 (0.61, 1.16) |
| Mediation of the relationship between PIP and having problems with anxiety/depression. | ||
| Path | Model Variables | Regression Estimates (95% CI) |
| Path A | PIP → GDI | 0.08 (0.04, 0.11) A |
| Path B | GDI → Anxiety/Depression | 18.19 (12.16, 27.19) B,C |
| Path C | PIP → Anxiety/Depression | 1.16 (1.06, 1.28) B,D |
| Mediated Path | PIP → GDI → Anxiety/Depression | 0.22 (0.11, 0.33) E |
| Mediation of the relationship between PIP and having problems with usual activities. | ||
| Path | Model Variables | Regression Estimates (95% CI) |
| Path A | PIP → GDI | 0.08 (0.04, 0.11) A |
| Path B | GDI → Usual Activities | 4.64 (3.56, 6.05) B,C |
| Path C | PIP → Usual Activities | 1.28 (1.16, 1.42) B,D |
| Mediated Path | PIP → GDI → Usual Activities | 0.12 (0.06, 0.18) E |
| Mediation of the relationship between PIP and mobility problems. | ||
| Path | Model Variables | Regression Estimates (95% CI) |
| Path A | PIP → GDI | 0.08 (0.04, 0.11) A |
| Path B | GDI → Mobility | 3.45 (2.72, 4.37) B,C |
| Path C | PIP → Mobility | 3.31 (2.61, 4.20) B,D |
| Mediated Path | PIP → GDI → Mobility | 0.09 (0.05, 0.14) E |
| Mediation of the relationship between PIP and self-care problems. | ||
| Path | Model Variables | Regression Estimates (95% CI) |
| Path A | PIP → GDI | 0.08 (0.04, 0.11) A |
| Path B | GDI → Self-Care | 4.21 (3.00, 5.91) B,C |
| Path C | PIP → Self-Care | 1.44 (1.09, 1.45) B,D |
| Mediated Path | PIP → GDI → Self-Care | 0.11 (0.06, 0.18) E |
| Mediation of the relationship between PIP and pain and discomfort. | ||
| Path | Model Variables | Regression Estimates (95% CI) |
| Path A | PIP → GDI | 0.08 (0.04, 0.11) A |
| Path B | GDI → Pain/Discomfort | 5.15 (3.83, 6.92) B,C |
| Path C | PIP → Pain/Discomfort | 1.26 (1.13, 1.40) B,D |
| Mediated Path | PIP → GDI → Pain/Discomfort | 0.12 (0.06, 0.19) E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, L.F.; Resnik, J.B.; Simmens, S.; Bhandaru, V.; Benator, D.; Wingate, L.; Castel, A.D.; Monroe, A.K., on behalf of the DC Cohort Executive Committee. Evaluation of Screening Tool of Older People’s Prescriptions (STOPP) Criteria in an Urban Cohort of Older People with HIV. Pharmacoepidemiology 2025, 4, 10. https://doi.org/10.3390/pharma4020010
O’Connor LF, Resnik JB, Simmens S, Bhandaru V, Benator D, Wingate L, Castel AD, Monroe AK on behalf of the DC Cohort Executive Committee. Evaluation of Screening Tool of Older People’s Prescriptions (STOPP) Criteria in an Urban Cohort of Older People with HIV. Pharmacoepidemiology. 2025; 4(2):10. https://doi.org/10.3390/pharma4020010
Chicago/Turabian StyleO’Connor, Lauren F., Jenna B. Resnik, Sam Simmens, Vinay Bhandaru, Debra Benator, La’Marcus Wingate, Amanda D. Castel, and Anne K. Monroe on behalf of the DC Cohort Executive Committee. 2025. "Evaluation of Screening Tool of Older People’s Prescriptions (STOPP) Criteria in an Urban Cohort of Older People with HIV" Pharmacoepidemiology 4, no. 2: 10. https://doi.org/10.3390/pharma4020010
APA StyleO’Connor, L. F., Resnik, J. B., Simmens, S., Bhandaru, V., Benator, D., Wingate, L., Castel, A. D., & Monroe, A. K., on behalf of the DC Cohort Executive Committee. (2025). Evaluation of Screening Tool of Older People’s Prescriptions (STOPP) Criteria in an Urban Cohort of Older People with HIV. Pharmacoepidemiology, 4(2), 10. https://doi.org/10.3390/pharma4020010






