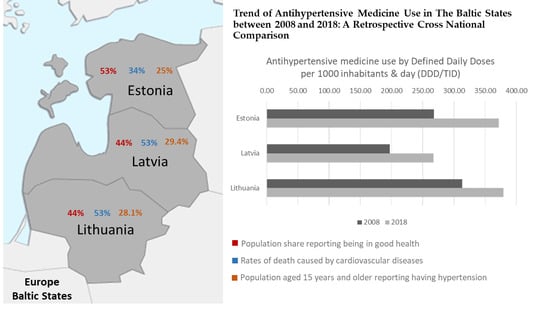
Trend of Antihypertensive Medicine Use in the Baltic States between 2008 and 2018: A Retrospective Cross-National Comparison
Abstract
:1. Introduction
2. Results
2.1. Utilization of Antihypertensives
2.2. Utilization by Pharmacological Group
3. Discussion
4. Materials and Methods
4.1. Data
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E.; et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [CrossRef]
- Arumugham, V.B.; Shahin, M.H. Therapeutic Uses of Diuretic Agents; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Stankūnienė, V.; Jasilionis, D. The Baltic Countries: Population, Family and Family Policy; Institute for Social Research: Vilnius, Lithuania, 2009; ISBN 978-9955-531-29-6. [Google Scholar]
- OECD Estonia: Country Health Profile 2019, State of Health in the EU; State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2019; ISBN 9789264289208.
- OECD Latvia: Country Health Profile 2019, State of Health in the EU; State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2019; ISBN 9789264647657.
- OECD Lithuania: Country Health Profile 2019, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2019; ISBN 9789264340787.
- OECD Estonia: Country Health Profile 2017, State of Health in the EU; State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017; ISBN 9789264283350.
- OECD Lithuania: Country Health Profile 2017, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017; ISBN 9789264283473.
- Causes of Death—Diseases of the Circulatory System, Residents, 2016 Health2019.png—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Causes_of_death_—_diseases_of_the_circulatory_system,_residents,_2016_Health2019.png (accessed on 15 May 2020).
- Cardiovascular Diseases Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Cardiovascular_diseases_statistics#Deaths_from_cardiovascular_diseases (accessed on 22 October 2020).
- Mägi, K.; Lepaste, M.; Szkultecka-Dębek, M. Drug Policy in Estonia. Value Health Reg. Issues 2018, 16, 1–4. [Google Scholar] [CrossRef]
- OECD Reviews of Health Systems: Lithuania 2018, OECD Reviews of Health Systems; OECD Publishing: Paris, France, 2018; ISBN 9789264300866.
- Lace, T. ESPN Thematic Report on Inequalities in Access to Healthcare: Latvia; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Vogler, S.; Österle, A.; Mayer, S. Inequalities in medicine use in Central Eastern Europe: An empirical investigation of socioeconomic determinants in eight countries. Int. J. Equity Health 2015, 14, 124. [Google Scholar] [CrossRef] [Green Version]
- Narayan, S.W.; Nishtala, P.S. Antihypertensive medicines utilization: A decade-long nationwide study of octogenarians, nonagenarians and centenarians. Geriatr. Gerontol. Int. 2017, 17, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, Y.; Song, J.; Tian, Y.; Wang, M.; Li, M.; Wang, X.; Huang, Z.; Li, L.; Zhao, Y.; et al. Antihypertensive drugs use over a 5-year period among children and adolescents in Beijing, China. Medicine 2019, 98, e17411. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Paulose-Ram, R.; Dillon, C.; Burt, V. Antihypertensive medication use among US adults with hypertension. Circulation 2006, 113, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundbøll, J.; Adelborg, K.; Mansfield, K.E.; Tomlinson, L.A.; Schmidt, M. Seventeen-Year Nationwide Trends in Antihypertensive Drug Use in Denmark. Am. J. Cardiol. 2017, 120, 2193–2200. [Google Scholar] [CrossRef]
- Kucan, M.; Mrsic-Pelcic, J.; Vitezic, D. Antihypertensive Drugs in Croatia: What Changes the Drug Usage Patterns? Clin. Ther. 2018, 40, 1159–1169. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Trends in age-specific coronary heart disease mortality in the European Union over three decades: 1980–2009. Eur. Heart J. 2013, 34, 3017–3027. [Google Scholar] [CrossRef]
- Björck, L.; Capewell, S.; O’Flaherty, M.; Lappas, G.; Bennett, K.; Rosengren, A. Decline in coronary mortality in Sweden between 1986 and 2002: Comparing contributions from primary and secondary prevention. PLoS ONE 2015, 10, e0124769. [Google Scholar] [CrossRef]
- Lisauskiene, I.; Garuoliene, K.; Gulbinovic, J. Utilization of cardiovascular medicines and cardiovascular mortality in Lithuania, Sweden and Norway in 2003–2012. Medicina 2017, 53, 259–267. [Google Scholar] [CrossRef]
- Lisauskiene, I. Changes in Cardiovascular Medicines Utilization and Morbidity and Mortality from Cardiovascular Disease in Lithuania. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2017. [Google Scholar]
- Baltic Statistics on Medicines|State Agency of Medicines of the Republic of Latvia. Available online: https://www.zva.gov.lv/en/news-and-publications/publications/baltic-statistics-medicines (accessed on 13 July 2020).
- Greciano, V.; Macías Saint-Gerons, D.; González-Bermejo, D.; Montero, D.; Catalá-López, F.; de la Fuente Honrubia, C. Use of Antihypertensive Drugs in Spain: National Trends from 2000 to 2012. Rev. Esp. Cardiol. 2015, 68, 899–903. [Google Scholar] [CrossRef]
- Drug Consumption in Norway 2012–2016—NIPH. Available online: https://www.fhi.no/en/publ/2017/drug-consumption-2012-2016/ (accessed on 17 January 2020).
- Saadeh, R.; Qato, D.; Khader, A.; Shahin, Y.; Seita, A. Trends in the utilization of antihypertensive medications among Palestine refugees in Jordan, 2008–2012. J. Pharm. Policy Pract. 2015, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Sarganas, G.; Knopf, H.; Grams, D.; Neuhauser, H.K. Trends in Antihypertensive Medication Use and Blood Pressure Control among Adults with Hypertension in Germany. Am. J. Hypertens. 2016, 29, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zanchetti, A. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laucevičius, A.; Rinkūnienė, E.; Ryliškytė, L.; Kasiulevičius, V.; Jatužis, D.; Petrulionienė, Ž.; Badarienė, J.; Navickas, R.; Jucevičienė, A.; Kutkienė, S.; et al. Primary Prevention Strategy for Cardiovascular Disease in Lithuania. Semin. Cardiovasc. Med. 2019, 25, 14–39. Available online: https://content.sciendo.com/view/journals/semcard/25/1/article-p14.xml?language=en (accessed on 20 November 2020). [CrossRef] [Green Version]
- Sica, D.A. Centrally Acting Antihypertensive Agents: An Update. J. Clin. Hypertens. 2007, 9, 399–405. [Google Scholar] [CrossRef]
- Carnagarin, R.; Matthews, V.; Gregory, C.; Schlaich, M.P. Pharmacotherapeutic strategies for treating hypertension in patients with obesity. Expert Opin. Pharmacother. 2018, 19, 643–651. [Google Scholar] [CrossRef]
- Montgomery, B.D.; Mansfield, P.R.; Spurling, G.K.; Ward, A.M. Do advertisements for antihypertensive drugs in Australia promote quality prescribing? A cross-sectional study. BMC Public Health 2008, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Gore, R.; Parker, R. Analysing power and politics in health policies and systems. Glob. Public Health 2019, 14, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Fickweiler, F.; Fickweiler, W.; Urbach, E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: A systematic review. BMJ Open 2017, 7, e016408. [Google Scholar] [CrossRef] [Green Version]
- Brakoulias, V.; Starcevic, V.; Belloch, A.; Dell’Osso, L.; Ferrão, Y.A.; Fontenelle, L.F.; Lochner, C.; Marazziti, D.; Martin, A.; Matsunaga, H.; et al. International prescribing practices in obsessive-compulsive disorder (OCD). Hum. Psychopharmacol. Clin. Exp. 2016, 31, 319–324. [Google Scholar] [CrossRef]
- Parati, G.; Esler, M. The human sympathetic nervous system: Its relevance in hypertension and heart failure. Eur. Heart J. 2012, 33, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Garuoliene, K.; Godman, B.; Gulbinovic, J.; Schiffers, K.; Wettermark, B. Differences in utilization rates between commercial and administrative databases: Implications for future health-economic and cross-national studies. Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ (accessed on 28 January 2021).
- 2019 ACTS Annual Report: Statistical Quality Assurance Applied to IQVIA’s Information Offerings. IQVIA. Available online: https://www.iqvia.com/library/publications/acts-2019-33rd-edition-quality-assurance-report-of-iqvia (accessed on 18 November 2020).
- WHO Collaborating Centre for Drug Statistics Methodology—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 17 January 2020).
- Statistics Estonia. RV0212: Population at the Beginning of the Year and Mean Annual Population by Sex and Age. Available online: http://andmebaas.stat.ee/Index.aspx?DataSetCode=RV0212 (accessed on 25 November 2019).
- Lithuanian Official Statistics Portal. Indicators Database. Resident Population at the Beginning of the Year. Available online: https://osp.stat.gov.lt/statistiniu-rodikliu-analize?indicator=S3R167#/ (accessed on 25 November 2019).
- Statistics|Central Statistical Bureau of Latvia. Available online: https://www.csb.gov.lv/en/statistics (accessed on 25 November 2019).



| Country | DDD/TID in 2008 | DDD/TID in 2018 | Mean Annual Increase in DDD/TID | 95% CI of Mean Annual Increase | p-Value, Time Series Analysis * | R2 | ||
|---|---|---|---|---|---|---|---|---|
| Antiadrenergic agents centrally acting C02AC | Estonia | 1.25 | 3.01 | 0.17 | 0.12–0.23 | <0.0001 | 0.839 | |
| Latvia | 7.1 | 16.17 | 1.04 | 0.34–1.75 | 0.0086 | 0.554 | ||
| Lithuania | 21.85 | 30.9 | 0.89 | 0.59–1.19 | <0.0001 | 0.832 | ||
| Diuretics C03 | Estonia | 22.76 | 27.17 | 0.56 | 0.33; 0.77 | 0.0003 | 0.772 | |
| Latvia | 17.01 | 17.54 | 0.2 | 0.02; 0.4 | 0.034 | 0.407 | ||
| Lithuania | 18.97 | 22.71 | 0.39 | 0.22; 0.55 | 0.0005 | 0.755 | ||
| Beta blockers C07 | Plain | Estonia | 29.5 | 57.4 | 2.98 | 2.55; 3.42 | <0.0001 | 0.964 |
| Latvia | 29.1 | 50.7 | 2.63 | 1.97; 3.30 | <0.0001 | 0.899 | ||
| Lithuania | 42.4 | 69.4 | 2.77 | 2.27; 3.27 | <0.0001 | 0.946 | ||
| Combinations with diuretics | Estonia | 0.1 a | 0 | - | - | - | - | |
| Latvia | 1.3 | 3.4 | 0.26 | 0.21; 0.30 | <0.0001 | 0.9489 | ||
| Lithuania | 0.1 | 5.1 | 0.63 | 0.48; 0.79 | <0.0001 | 0.901 | ||
| Other combinations | Estonia | 0.0 | 0.0 | - | - | - | - | |
| Latvia | 0.0 | 0.6 d | - | - | - | - | ||
| Lithuania | 0.0 | 0.5 e | - | - | - | - | ||
| Calcium channel blockers C08 | Plain | Estonia | 66.6 | 56.0 | −1.00 | −2.16; 0.17 | 0.0856 | 0.293 |
| Latvia | 34.7 | 29.6 | −0.67 | −1.45; 0.06 | 0.0662 | 0.327 | ||
| Lithuania | 39.7 | 24.1 | −1.72 | −2.25; −1.2 | <0.0001 | 0.859 | ||
| Combinations with diuretics | Estonia | 0.0 | 0.0 | - | - | - | - | |
| Latvia | 0.0 | 0.3 d | - | - | - | - | ||
| Lithuania | 0.0 | 1.1 d | - | - | - | - | ||
| Other | Estonia | 0.0 | 0.0 | - | - | - | - | |
| Latvia | 0.0 | 1.3 c | - | - | - | - | ||
| Lithuania | 0.0 | 3.8 f | - | - | - | - | ||
| Angiotensin-converting enzyme inhibitors C09A, C09B | Plain | Estonia | 99.8 | 85.8 | −1.32 | −2.36; −0.30 | 0.0172 | 0.485 |
| Latvia | 66.3 | 52.4 | −1.06 | −2.02; −0.09 | 0.0355 | 0.404 | ||
| Lithuania | 133.1 | 86.7 | −4.18 | −4.84; −3.51 | <0.0001 | 0.957 | ||
| Combinations with diuretics | Estonia | 23.4 | 33.0 | 1.00 | 0.69; 1.32 | <0.0001 | 0.851 | |
| Latvia | 23.6 | 31.6 | 0.50 | −0.09; 1.09 | 0.088 | 0.289 | ||
| Lithuania | 28.6 | 31.2 | 0.77 | 0.57; 0.97 | <0.0001 | 0.893 | ||
| Other | Estonia | 0.2 | 25.2 | 2.6 | 2.14; 3.05 | <0.0001 | 0.949 | |
| Latvia | 0.2 | 32.4 | 2.89 | 2.27; 3.51 | <0.0001 | 0.925 | ||
| Lithuania | 1.5 | 39.7 | 3.54 | 2.86; 4.22 | <0.0001 | 0.940 | ||
| Angiotensin II receptor blockers C09C, C09D | Plain | Estonia | 16.1 | 46.0 | 2.70 | 1.61; 3.77 | 0.0003 | 0.780 |
| Latvia | 6.7 | 11.4 | 0.51 | 0.25; 0.76 | 0.0014 | 0.697 | ||
| Lithuania | 21.1 | 32.3 | 0.31 | −1.40; 2.02 | 0.6917 | 0.018 | ||
| Combinations with diuretics | Estonia | 6.6 | 18.5 | 1.15 | 0.61; 1.68 | 0.0009 | 0.724 | |
| Latvia | 3.6 | 10.9 | 0.83 | 0.61; 1.05 | <0.0001 | 0.891 | ||
| Lithuania | 3.1 | 14.6 | 1.20 | 0.75; 1.64 | 0.0002 | 0.807 | ||
| Other | Estonia | 0.0 | 18.1 b | - | - | - | - | |
| Latvia | 0.0 | 6.8 | 0.81 | 0.66; 0.95 | <0.0001 | 0.947 | ||
| Lithuania | 0.0 | 10.1 b | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treciokiene, I.; Bratcikoviene, N.; Gulbinovic, J.; Wettermark, B.; Taxis, K. Trend of Antihypertensive Medicine Use in the Baltic States between 2008 and 2018: A Retrospective Cross-National Comparison. Pharmacoepidemiology 2022, 1, 1-11. https://doi.org/10.3390/pharma1010001
Treciokiene I, Bratcikoviene N, Gulbinovic J, Wettermark B, Taxis K. Trend of Antihypertensive Medicine Use in the Baltic States between 2008 and 2018: A Retrospective Cross-National Comparison. Pharmacoepidemiology. 2022; 1(1):1-11. https://doi.org/10.3390/pharma1010001
Chicago/Turabian StyleTreciokiene, Indre, Nomeda Bratcikoviene, Jolanta Gulbinovic, Bjorn Wettermark, and Katja Taxis. 2022. "Trend of Antihypertensive Medicine Use in the Baltic States between 2008 and 2018: A Retrospective Cross-National Comparison" Pharmacoepidemiology 1, no. 1: 1-11. https://doi.org/10.3390/pharma1010001
APA StyleTreciokiene, I., Bratcikoviene, N., Gulbinovic, J., Wettermark, B., & Taxis, K. (2022). Trend of Antihypertensive Medicine Use in the Baltic States between 2008 and 2018: A Retrospective Cross-National Comparison. Pharmacoepidemiology, 1(1), 1-11. https://doi.org/10.3390/pharma1010001