Are There Wheat Cultivars Allowing Enhanced Carbon Allocation to Soils?
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Plant Material
2.2. Field Experiment for Grain Yield and Biomass Production
2.3. Greenhouse Experiment for Grain Yield and Biomass Production
2.4. Selection of the Subset of 10 Genotypes
2.5. Assessment of C Transfer to the Soil
2.6. Descriptive Variables
2.6.1. Primary Variables
2.6.2. Secondary Variables
2.6.3. Data Analysis
3. Results
3.1. Variations in Grain Yield and Plant Biomass Amongst the 100 Genotypes
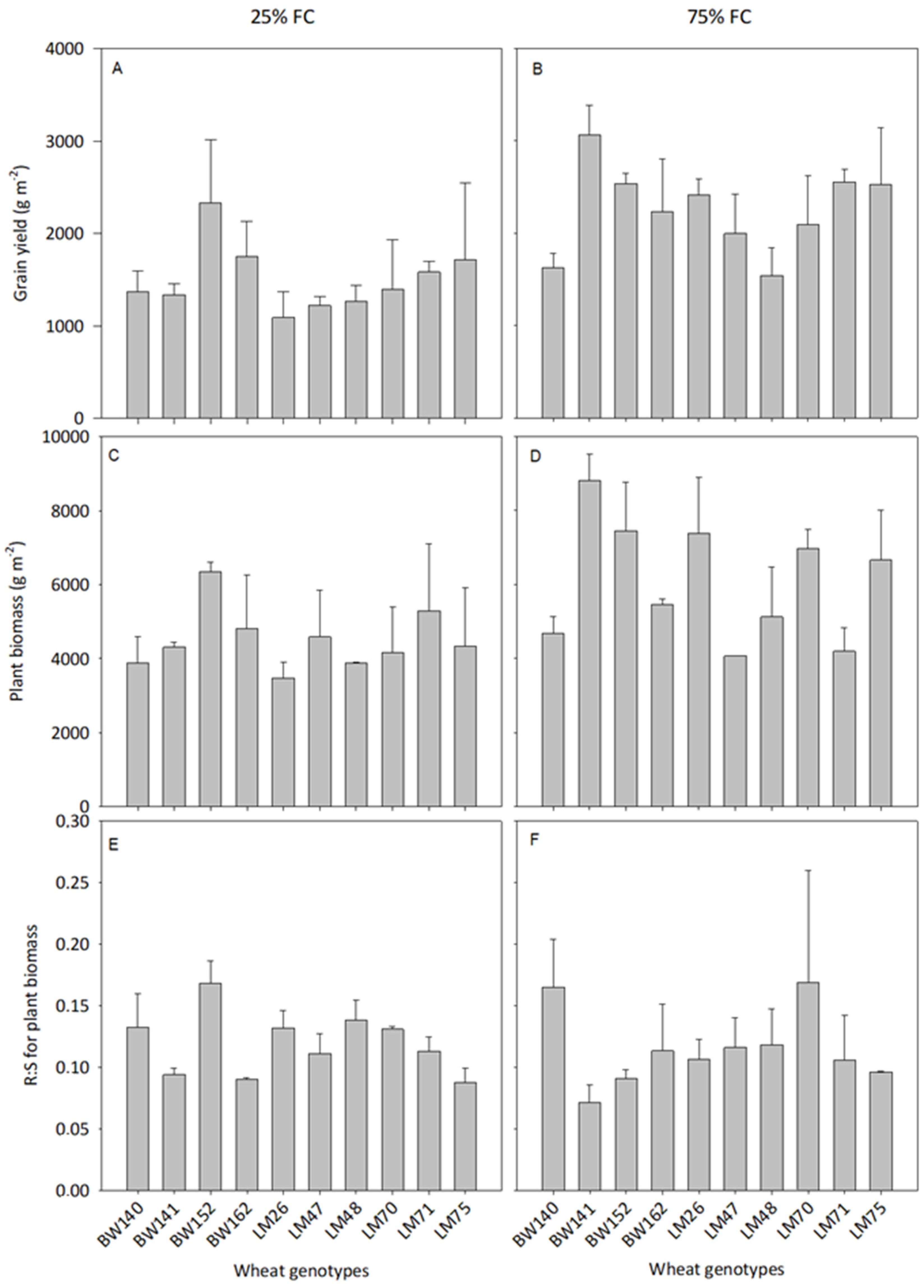
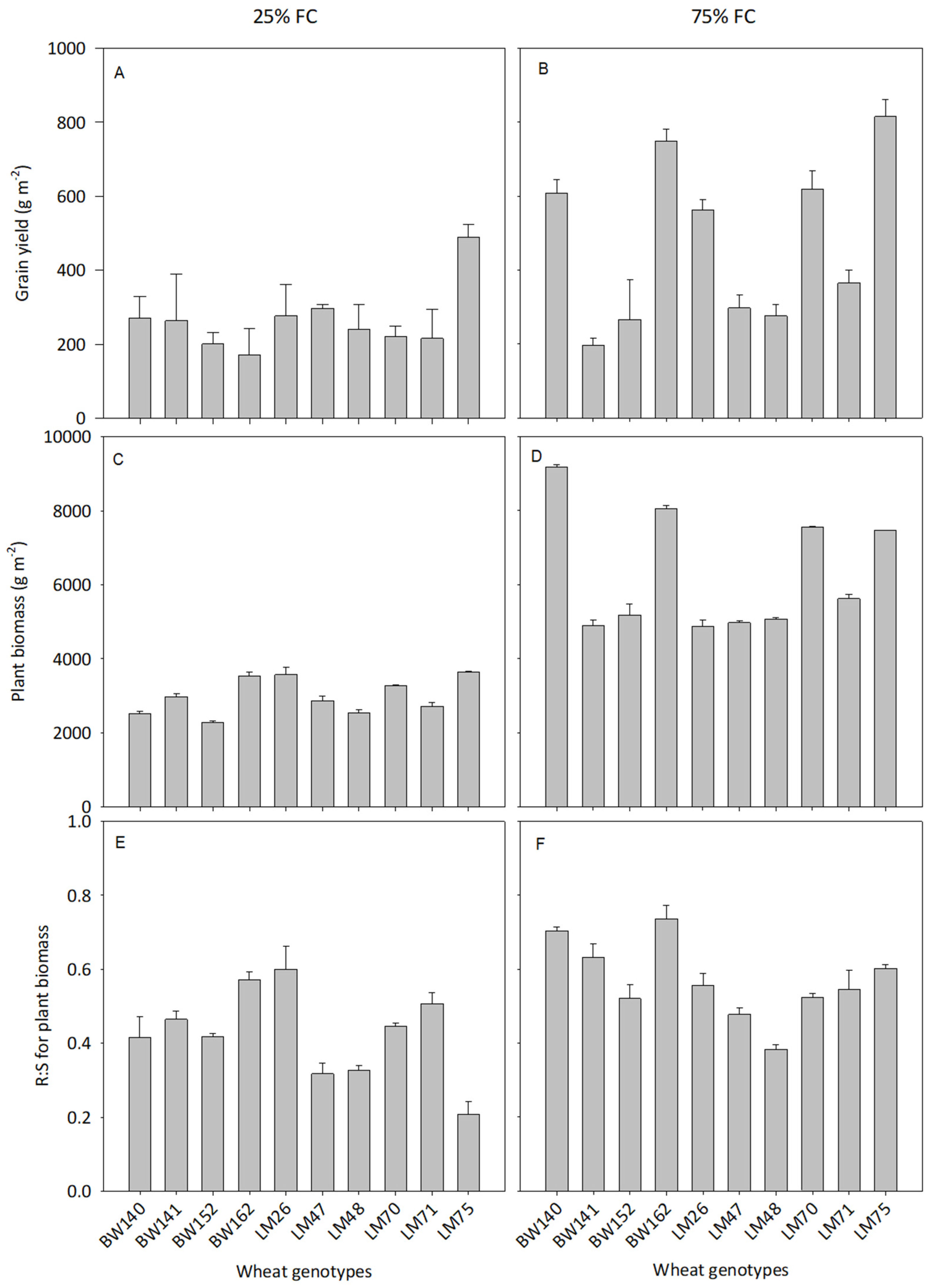
3.2. Variations Amongst the 10 Best Performing Genotypes
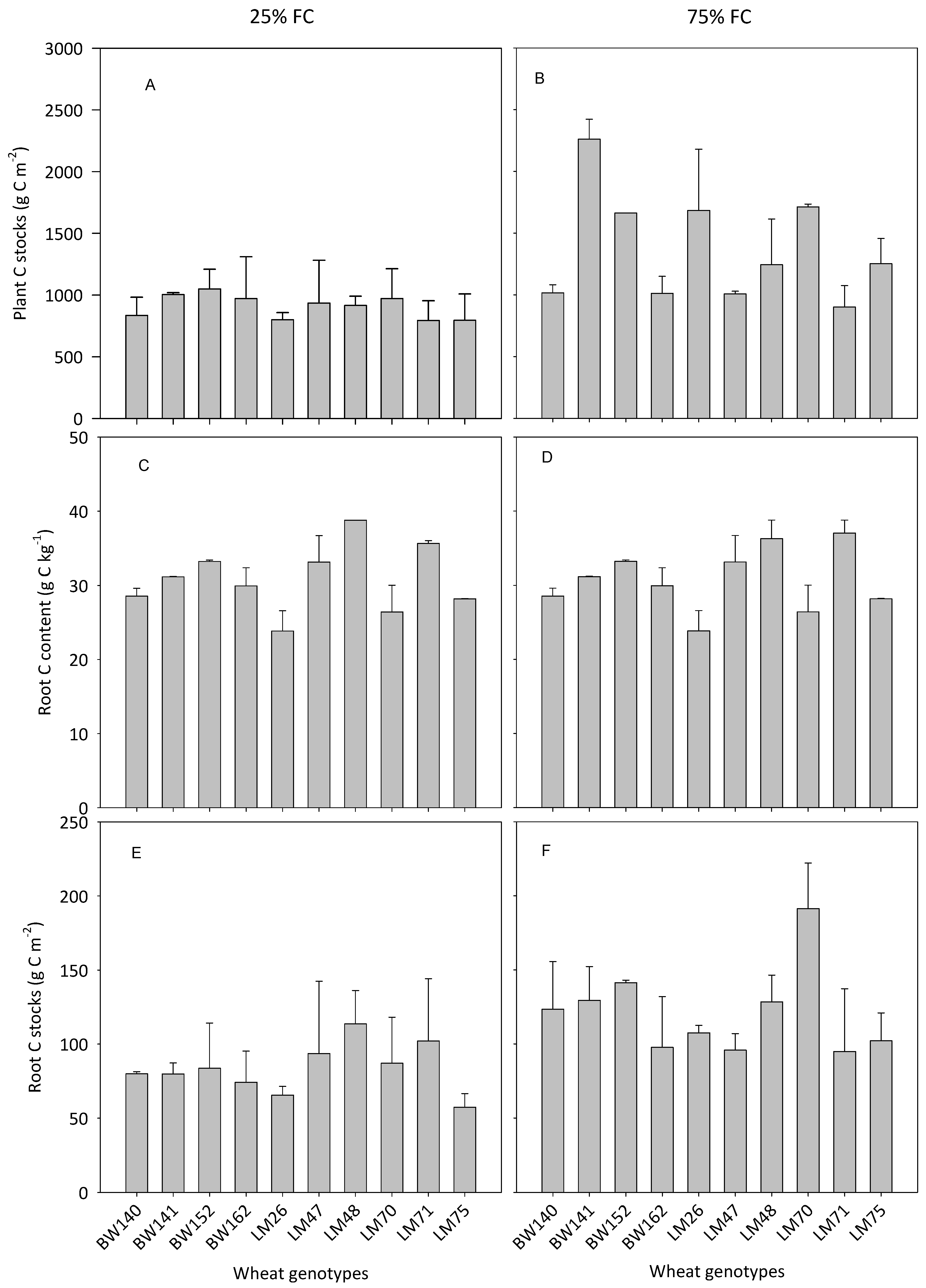
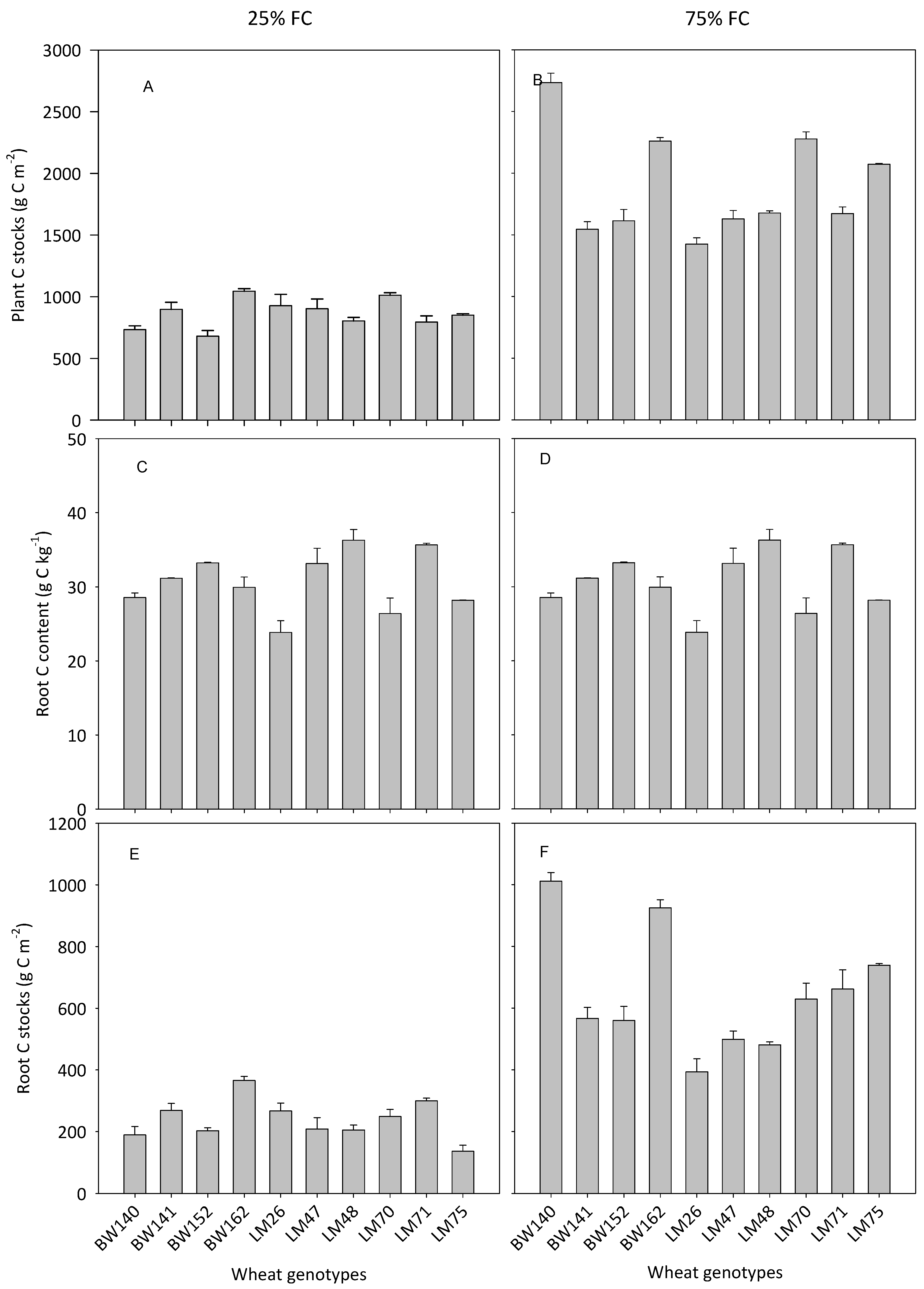
3.3. Variations in Below- and Aboveground C Allocation among the Selected Genotypes
4. Discussion
4.1. Large Variations in Below- and Aboveground C Allocation among the Selected Genotypes
4.2. Variations in the Ability of Wheat Genotypes to Transfer Atmospheric C into the Soil
4.3. Impact of Soil Water Availability on Plant C Storage and C Transfer to the Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Pandey, Y.; Vyas, R.P.; Kumar, J.; Singh, L.; Singh, H.C.; Yadav, P.C. Heritability, Correlation and Path Coefficient Analysis for Determining Interrelationships among Grain Yield and Related characters in Maize (Zea mays L.). Int. J. Pure App. Biosci. 2017, 5, 595–603. [Google Scholar] [CrossRef]
- Ray, J.D.; Gesch, R.W.; Sinclair, T.R.; Allen, L.H. The effect of vapor pressure deficit on maize transpiration response to drying soil. Plant Soil 2002, 239, 113–121. [Google Scholar] [CrossRef]
- Smith, M.R.; Myers, S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Chang. 2018, 8, 834. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Bhabani, S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ji, C.; Ma, W.; Mohammat, A.; Wang, S.; Smith, P. Widespread decreases in topsoil inorganic carbon stocks across China’s grasslands during 1980s–2000s. Glob. Chang. Biol. 2012, 18, 3672–3680. [Google Scholar] [CrossRef]
- Hirte, J.; Leifeld, J.; Abiven, S.; Oberholzer, H.-R.; Mayer, J. Below ground carbon inputs to soil via root biomass and rhizodeposition of field-grown maize and wheat at harvest are independent of net primary productivity. Agric. Ecosyst. Environ. 2018, 265, 556–566. [Google Scholar] [CrossRef]
- Lynch, J.P.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mutema, M.; Chaplot, V. What crop type for atmospheric carbon sequestration: Results from a global data analysis. Agric. Ecosyst. Environ. 2017, 243, 34–46. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mutema, M.; Minasny, B.; Chaplot, V. Crops for increasing soil organic carbon stocks—A global meta analysis. Geoderma 2020, 367, 114230. [Google Scholar] [CrossRef]
- Amanullah, Jr.; Stewart, B.A. Dry matter partitioning, growth analysis and water use efficiency response of oats (Avena sativa L.) to excessive nitrogen and phosphorus application. J. Agr. Sci. Tech. 2013, 15, 479–489. [Google Scholar]
- Yang, C.H.; Chai, Q.; Huang, G.B. Root distribution and yield responses of wheat/maize intercropping to alternate irrigation in the arid areas of northwest China. Plant Soil Environ. 2010, 56, 253–262. [Google Scholar] [CrossRef]
- Bolinder, M.A.; Angers, D.A.; Dubuc, J.P. Estimating shoot to root ratios and annual carbon inputs in soils for cereal crops. Agric. Ecosyst. Environ. 1997, 63, 61–66. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.H.M.; Belford, R.K.; Tennant, D. Root: Shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 1990, 121, 89–98. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Kaštovská, E.; Šantrůčková, H. Fate and dynamics of recently fixed C in pasture plant-soil system under field conditions. Plant Soil 2007, 300, 61–69. [Google Scholar] [CrossRef]
- Rangel-Castro, J.I.; Killham, K.; Ostle, N.; Nicol, G.W.; Anderson, I.C.; Scrimgeour, C.M. Stable isotope probing analysis of the influence of liming on root exudates utilization by soil microorganisms. Environ. Microbiol. 2005, 7, 828–838. [Google Scholar] [CrossRef] [PubMed]
- De Neergaard, A.; Gorissen, A. Carbon allocation to roots, rhizodeposits and soil after pulse labeling: A comparison of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.). Biol. Fertil. Soils 2004, 39, 228–234. [Google Scholar] [CrossRef]
- Brüggemann, E.N.; Gessler, A.; Kayler, Z.; Keel, S.G.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J.; et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Soil Classification Working Group. Soil Classification—A Taxonomic System for South Africa; Department of Agricultural Development: Pretoria, South Africa, 1991. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. A Framework for International Classification, Correlation and Communication; World Soil Resources Report No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- DAFF. Wheat Production Guideline; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2010. [Google Scholar]
- Liu, X.; Bonhomme, J.; Merbach, I.; Kümmel, S.; Richnow, H.H. Uptake of a-HCH by wheat from the gas phase and translocation to soil analyzed by a stable carbon isotope labeling experiment. Chemosphere 2021, 264, 128489. [Google Scholar] [CrossRef] [PubMed]
- Nevo, E.; Chen, G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010, 33, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Waines, J.G. Determination of Optimum Root and Shoot Size in Bread Wheat for Increased Water and Nutrient Use Efficiency and Grain Yield; Report to California Wheat Commission: GH 2011–2012 Experiments; California Wheat Commission: Woodland, CA, USA, 2012. [Google Scholar]
- Akman, H.; Akgün, N.; Tamkoç, A. Comparison of root and shoot traits of different wheat species and wild wheat relatives: Does feature of shoot biomass have positive and significant relationships with grain yield and root traits? Rev. Fac. Agron. 2017, 34, 428–447. [Google Scholar]
- Corneo, P.E.; Suenaga, H.; Kertesz, M.A.; Dijkstra, F.A. Effect of twenty four wheat genotypes on soil biochemical 734 and microbial properties. Plant Soil. 2016, 404, 141–155. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef]
- Canarini, A.; Merchant, A.; Dijkstra, F.A. Drought effects on Helianthus annuus and Glycine max metabolites: From phloem to root exudates. Rhizosphere 2016, 2, 85–97. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Magalhães, P.C.; Ávila, R.G.; Diniz, M.M.; De Souza, T.C. Partitioning between primary and secondary metabolism of carbon allocated to roots in four maize genotypes under water deficit and its effects on productivity. Crop J. 2015, 3, 379–386. [Google Scholar] [CrossRef]
- Aljazairi, S.; Arias, C.; Nogues, S. Carbon and nitrogen allocation and partitioning in traditional and modern wheat genotypes under pre-industrial and future CO2 conditions. Plant Biol. 2015, 17, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Gavito, M.E.; Curtis, P.S.; Mikkelsen, T.N.; Jakobsen, I. Interactive effects of soil temperature, atmospheric carbon dioxide and soil N on root development, biomass and nutrient uptake of winter wheat during vegetative growth. J. Exp. Bot. 2001, 52, 1913–1923. [Google Scholar] [CrossRef]
- Rengel, Z. The role of crop residues in improving soil fertility. In Nutrient Cycling in Terrestrial Ecosystems; Springer: Berlin/Heidelberg, Germany, 2007; pp. 183–214. [Google Scholar]
- Luquet, D.; Perrier, L.; Clément-Vidal, A.; Jaffuel, S.; Verdeil, J.L.; Roques, S.; Soutiras, A.; Baptiste, C.; Fabre, D.; Bastianelli, D. Genotypic covariation of traits underlying sorghum stem biomass production and quality and its regulation by water availability: Insight from studies at organ and tissue levels. GCB Bioenergy 2018, 11, 444–462. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Whipps, J.M.; Budge, S.P. Screening for sclerotial mycoparasites of Sclerotinia sclerotiorum. Mycol. Res. 1990, 94, 607–612. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Cheng, W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol. Biochem. 2001, 33, 1915–1925. [Google Scholar] [CrossRef]
- Hutsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Warembourg, F.R.; Estelrich, D.H. Towards a better understanding of carbon flow in the rhizosphere: A time-dependent approach using carbon-14. Biol. Fertil. Soils 2000, 30, 528–534. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Perrier, L.; Rouan, L.; Jaffuel, S.; Clement-Vidal, A.; Roques, S.; Soutiras, A.; Luquet, D. Plasticity of sorghum stem biomass accumulation in response to water deficit: A multiscale analysis from internode tissue to plant level. Front. Plant Sci. 2017, 8, 1516. [Google Scholar] [CrossRef] [PubMed]
- Lacube, S.; Fournier, C.; Palaffre, C.; Millet, E.J.; Tardieu, F.; Parent, B. Distinct controls of leaf widening and elongation by light and evaporative demand in maize. Plant Cell Environ. 2017, 40, 2017–2028. [Google Scholar] [CrossRef]
- Barthel, M.; Hammerle, A.; Sturm, P.; Baur, T.; Gentsch, L.; Knohl, A. The diel imprint of leaf metabolism on the δ13C signal of soil respiration under control and drought conditions. New Phytol. 2011, 192, 925–938. [Google Scholar] [CrossRef]


| Bulk density | 1.04 |
| Phosphorus (g kg−1) | 39 |
| Potassium (g kg−1) | 241 |
| Calcium (g kg−1) | 1.4 |
| Magnesium (mg kg−1) | 369 |
| Electrical conductivity (cmol L−1) | 11 |
| pH KCl | 4.6 |
| Organic carbon (%) | 2.6 |
| Nitrogen (%) | 0.23 |
| Clay (%) | 28 |
| GY | SB | RB | PB | R:S | |
|---|---|---|---|---|---|
| ----------------------g m2 ----------------------- | |||||
| Mean | 1387 | 2498 | 305 | 4189 | 0.12 |
| Median | 1309 | 2332 | 263 | 3930 | 0.11 |
| Min | 75 | 1179 | 65 | 1976 | 0.03 |
| Max | 4696 | 8658 | 1219 | 13,529 | 0.38 |
| Q1 | 959 | 1827 | 189 | 3026 | 0.09 |
| Q3 | 1644 | 2908 | 365 | 4893 | 0.15 |
| CV% | 47 | 37 | 57 | 37 | 41 |
| SEM | 84 | 121 | 22 | 200 | 0.01 |
| Skew | 1.53 | 2.04 | 2.03 | 1.82 | 1.67 |
| Kurt | 4.35 | 7.15 | 5.76 | 5.66 | 4.39 |
| GY | SB | RB | PB | R:S | SCC | RCC | SCS | RCS | PCS | |
|---|---|---|---|---|---|---|---|---|---|---|
| -----------------------g m2 ----------------------- | ----------%-------- | --------------g m2 ---------------- | ||||||||
| Glasshouse | ||||||||||
| 25% Field capacity | ||||||||||
| Mean | 326 | 1873 | 792 | 2992 | 0.43 | 33 | 31 | 625 | 239 | 865 |
| Median | 266 | 1826 | 762 | 2918 | 0.44 | 34 | 31 | 621 | 225 | 854 |
| Min | 58 | 1349 | 368 | 2156 | 0.15 | 25 | 21 | 409 | 103 | 592 |
| Max | 869 | 2415 | 1263 | 3983 | 0.70 | 37 | 39 | 805 | 379 | 1109 |
| CV% | 68 | 24 | 36 | 24 | 34 | 19 | 23 | 25 | 34 | 23 |
| SEM | 28 | 57 | 37 | 94 | 0.02 | 1 | 1 | 20 | 10 | 26 |
| 75% Field capacity | ||||||||||
| Mean | 414 | 3726 | 2149 | 6289 | 0.57 | 33 | 31 | 1243 | 647 | 1890 |
| Median | 397 | 3440 | 1823 | 5508 | 0.57 | 34 | 31 | 1186 | 605 | 1716 |
| Min | 136 | 2779 | 1290 | 4531 | 0.37 | 25 | 21 | 851 | 333 | 1324 |
| Max | 811 | 5192 | 3575 | 9305 | 0.78 | 37 | 39 | 1822 | 1059 | 2881 |
| CV% | 50 | 26 | 38 | 30 | 26 | 19 | 23 | 27 | 35 | 28 |
| SEM | 27 | 127 | 104 | 247 | 0.02 | 1 | 1 | 44 | 29 | 69 |
| Field | ||||||||||
| 25% Field capacity | ||||||||||
| Mean | 1700 | 2507 | 302 | 4508 | 0.12 | 33 | 31 | 832 | 95 | 927 |
| Median | 1401 | 2534 | 277 | 4042 | 0.12 | 34 | 31 | 827 | 80 | 968 |
| Min | 621 | 1598 | 152 | 2475 | 0.08 | 25 | 21 | 534 | 45 | 582 |
| Max | 4488 | 3775 | 642 | 8100 | 0.19 | 37 | 39 | 1215 | 214 | 1409 |
| CV% | 53 | 27 | 39 | 34 | 24 | 8 | 16 | 26 | 47 | 27 |
| SEM | 116 | 87 | 15 | 199 | 0.004 | 0.36 | 0.62 | 28.10 | 5.79 | 32.30 |
| 75% Field capacity | ||||||||||
| Mean | 2062 | 3714 | 403 | 6179 | 0.12 | 34 | 31 | 1254 | 121 | 1375 |
| Median | 1720 | 3154 | 382 | 5462 | 0.10 | 35 | 31 | 1052 | 112 | 1170 |
| Min | 1118 | 1954 | 135 | 3555 | 0.06 | 30 | 21 | 675 | 53 | 728 |
| Max | 4383 | 8658 | 1006 | 13,529 | 0.26 | 37 | 39 | 2869 | 302 | 3022 |
| CV% | 44 | 42 | 44 | 39 | 43 | 6 | 15 | 42 | 43 | 39 |
| SEM | 117 | 202 | 23 | 313 | 0.01 | 0.26 | 0.61 | 67.57 | 6.69 | 69.93 |
| DF | GY | SB | RB | R:S | |
|---|---|---|---|---|---|
| Rep | 1 | 1.54 | 3.2 | 13.9 | 0.07 |
| Block | 18 | 117.1 *** | 236.4 | 18.5 | 0.10 *** |
| Genotype | 99 | 101.7 *** | 556.5 | 52.1 * | 0.06 * |
| Water regime | 1 | 43.7 * | 43.1 * | 2.8 * | 1.12 * |
| Cluster | Treatment | GY | SB | RB | PB | R:S |
|---|---|---|---|---|---|---|
| 2A11 | Overall | 613.8 | 992.5 | 224.1 | 1830.4 | 0.44 |
| Drought | 480.9 | 777.6 | 187.1 | 1445.7 | 0.44 | |
| Well-watered | 746.7 | 1207.5 | 261.1 | 2215.2 | 0.45 | |
| 2A12 | Overall | 586.8 | 860.7 | 216.2 | 1663.6 | 0.44 |
| Drought | 385.5 | 678.1 | 166.0 | 1229.6 | 0.43 | |
| Well-watered | 771.3 | 1028.2 | 262.1 | 2061.5 | 0.44 | |
| 2A1 | Overall | 481.5 | 983.3 | 227.3 | 1686.3 | 0.45 |
| Drought | 317.3 | 795.1 | 186.3 | 1295.0 | 0.40 | |
| Well-watered | 649.6 | 1176.1 | 269.2 | 2087.1 | 0.49 | |
| 2A2 | Overall | 576.4 | 986.1 | 238.4 | 1795.3 | 0.46 |
| Drought | 432.5 | 824.9 | 197.6 | 1447.5 | 0.42 | |
| Well-watered | 717.8 | 1147.3 | 279.5 | 2143.0 | 0.50 | |
| 2B1 | Overall | 706.6 | 1159.1 | 352.9 | 2218.6 | 0.50 |
| Drought | 610.3 | 968.5 | 243.9 | 1822.7 | 0.43 | |
| Well-watered | 802.9 | 1349.8 | 461.9 | 2614.5 | 0.57 | |
| 2B21 | Overall | 568.4 | 1182.4 | 270.7 | 2005.7 | 0.44 |
| Drought | 413.4 | 929.9 | 223.8 | 1555.4 | 0.42 | |
| Well-watered | 719.1 | 1427.9 | 316.4 | 2443.7 | 0.46 | |
| 2B22 | Overall | 695.3 | 1125.9 | 264.8 | 2067.0 | 0.48 |
| Drought | 502.7 | 873.8 | 210.8 | 1567.2 | 0.45 | |
| Well-watered | 885.6 | 1376.5 | 318.7 | 2566.8 | 0.50 | |
| Grand Mean | 603.0 | 1040.5 | 256.1 | 1893.4 | 0.46 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaplot, V.; Mathew, I.; Clulow, A.; Shimelis, H. Are There Wheat Cultivars Allowing Enhanced Carbon Allocation to Soils? Appl. Biosci. 2023, 2, 115-135. https://doi.org/10.3390/applbiosci2010010
Chaplot V, Mathew I, Clulow A, Shimelis H. Are There Wheat Cultivars Allowing Enhanced Carbon Allocation to Soils? Applied Biosciences. 2023; 2(1):115-135. https://doi.org/10.3390/applbiosci2010010
Chicago/Turabian StyleChaplot, Vincent, Isack Mathew, Alistair Clulow, and Hussein Shimelis. 2023. "Are There Wheat Cultivars Allowing Enhanced Carbon Allocation to Soils?" Applied Biosciences 2, no. 1: 115-135. https://doi.org/10.3390/applbiosci2010010
APA StyleChaplot, V., Mathew, I., Clulow, A., & Shimelis, H. (2023). Are There Wheat Cultivars Allowing Enhanced Carbon Allocation to Soils? Applied Biosciences, 2(1), 115-135. https://doi.org/10.3390/applbiosci2010010






