Baicalin Improves Skeletal Muscle Atrophy by Attenuating DRP-1-Mediated Mitochondrial Fission in Aged Mice
Abstract
1. Introduction
2. Results
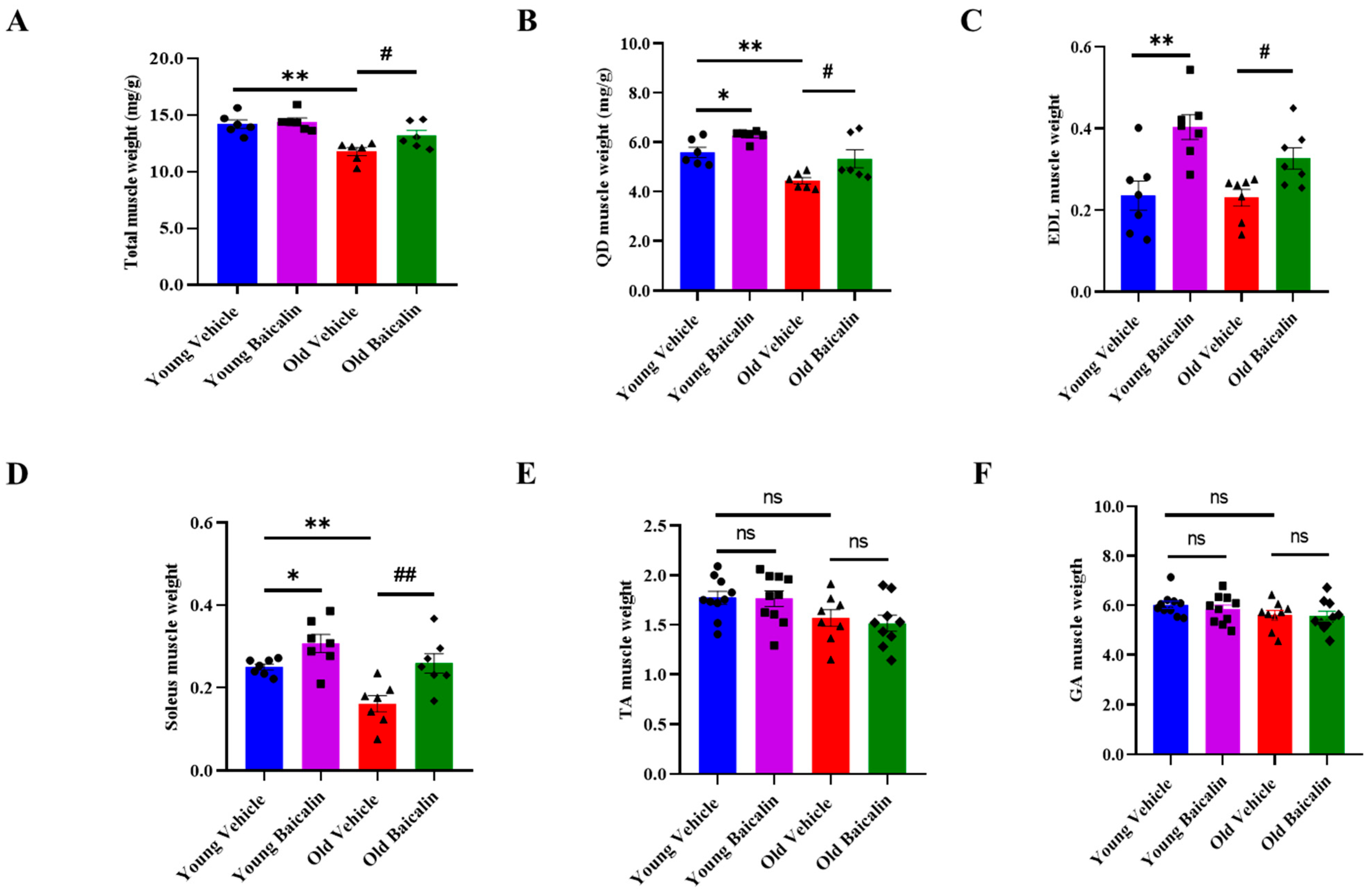
2.1. Baicalin-Treated Aged Mice Exhibit Higher Muscle Weight
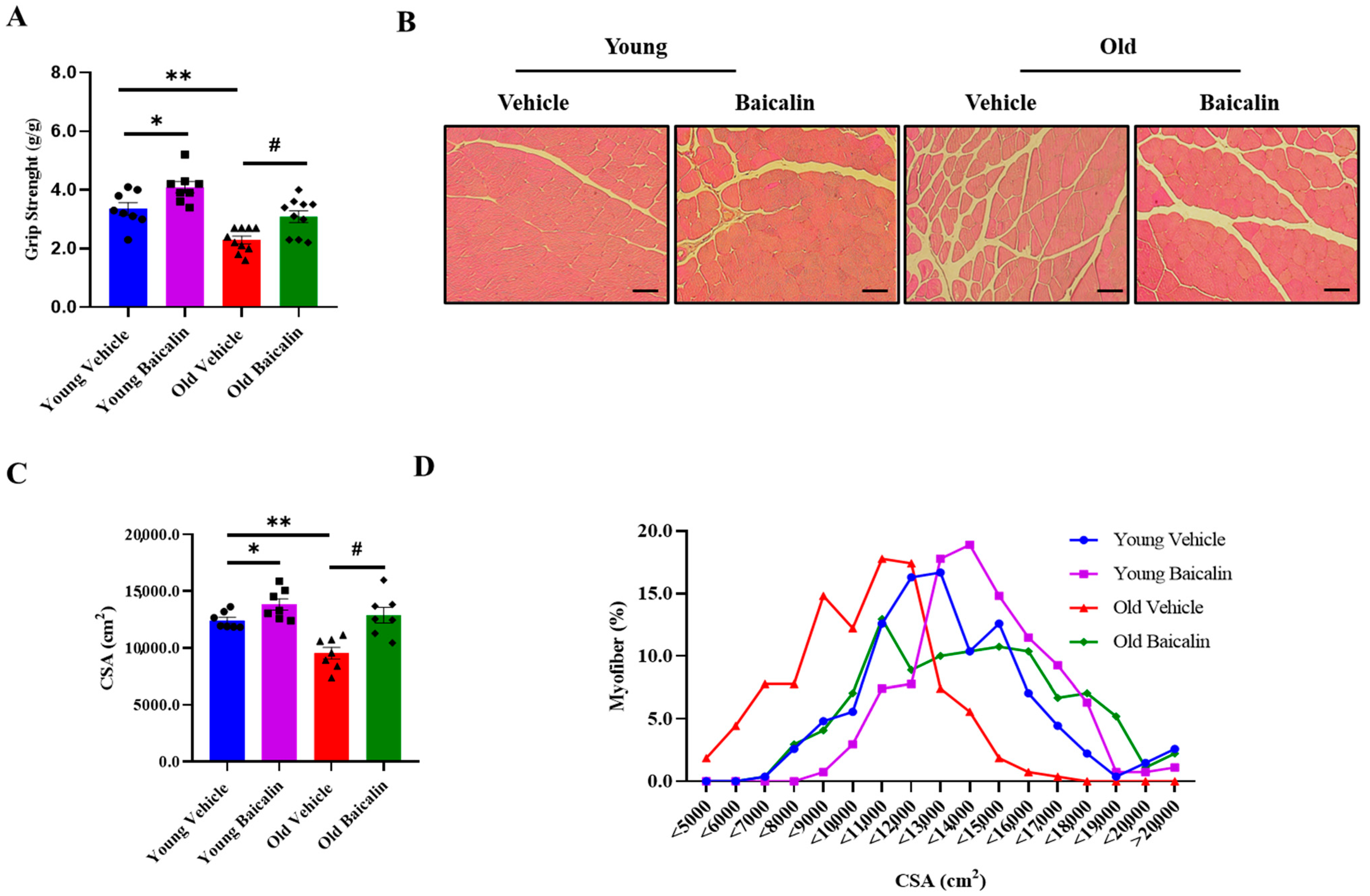
2.2. Baicalin-Treated Aged Mice Exhibit Higher Muscle Strength
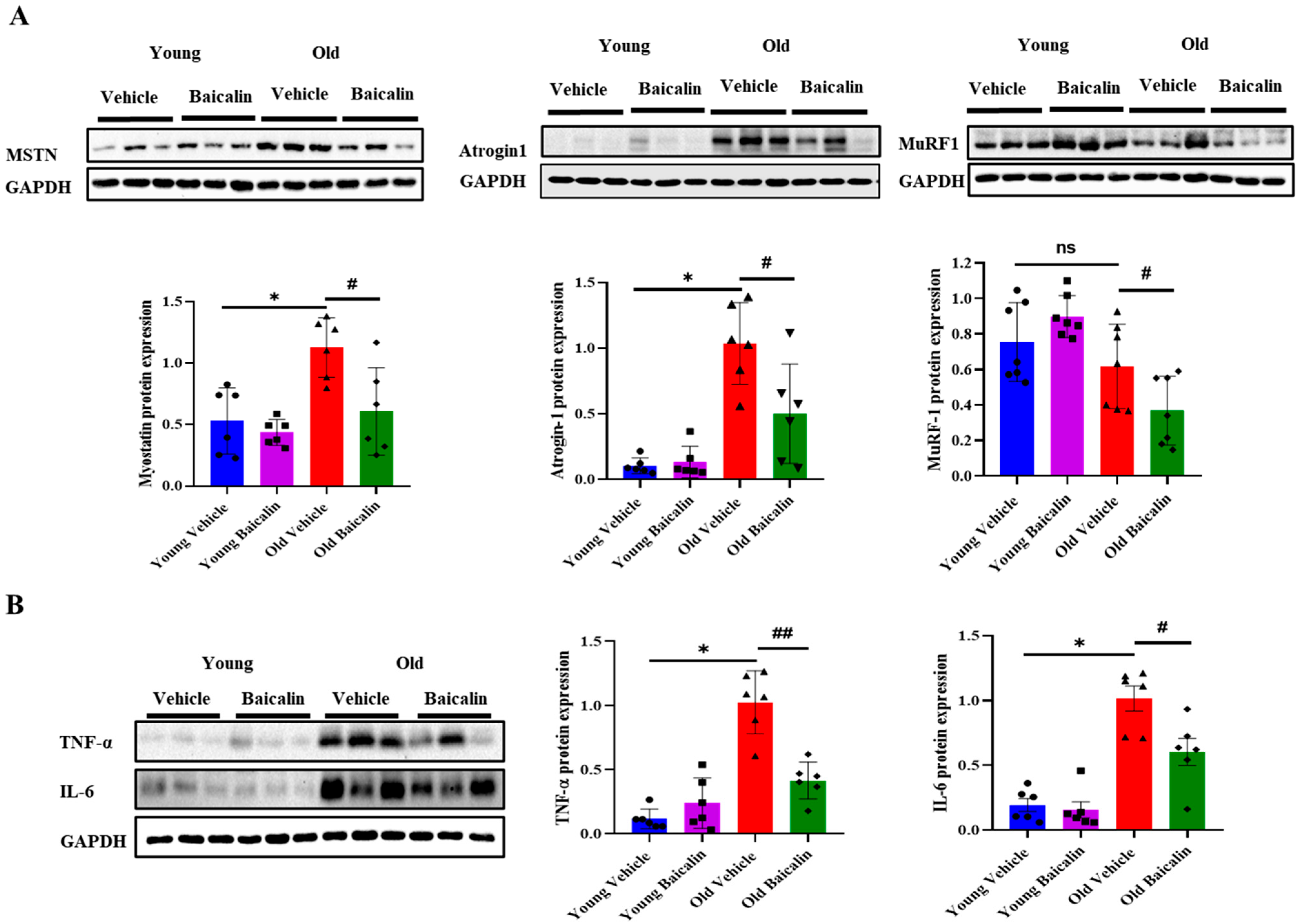
2.3. Baicalin-Treated Aged Mice Exhibit Lower Expression Levels of Muscle Atrophic Factors and Inflammatory Cytokines
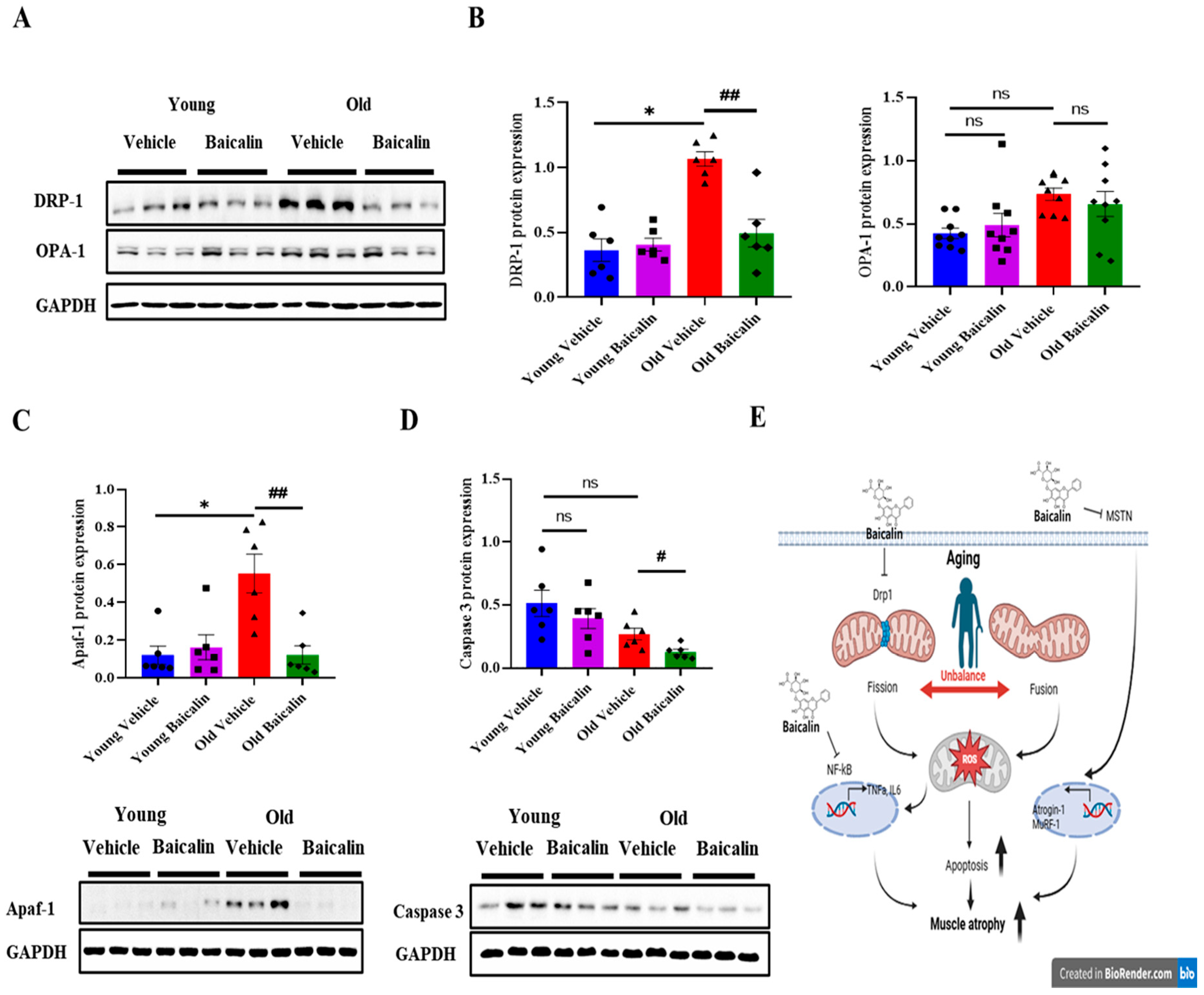
2.4. Baicalin-Treated Aged Mice Exhibit Lower Levels of Mitochondrial Dysfunction and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Animal Experiment
4.2. Grip Strength Test
4.3. Collection of Biological Samples
4.4. Histochemical Staining
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| QD | Quadriceps |
| MSTN | Myostatin |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin-6 |
| DRP-1 | Dynamin-related protein 1 |
| Apaf-1 | Apoptotic protease activating factor 1 |
| MuRF-1 | Muscle RING-finger protein-1 |
| Myo D | Myoblast determination protein 1 |
| FoxO | Forkhead box O |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OPA-1 | Optic atrophy 1 |
| ROS | Reactive oxygen species |
| BW | Body weight |
| EDL | Extensor digitorum longus |
| TA | Tibialis anterior |
| GA | Gastrocnemius |
| CSA | Cross-sectional area |
| Mfn | Mitofusin |
| FIS1 | Fission |
| AIF | Apoptosis-inducing factor |
| NBF | Natural buffer formalin |
| DMSO | Dimethyl sulfoxide |
| RIPA | Radioimmunoprecipitation |
| SEM | Standard error mean |
References
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—A narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Lo, J.H.; U, K.P.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Lei, Y.; Gan, M.; Qiu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: From molecular mechanisms to therapeutic insights. Cell. Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Glass, Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Li, Y.P.; Schwartz, R.J.; Waddell, I.D.; Holloway, B.R.; Reid, M.B. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998, 12, 871–880. [Google Scholar] [CrossRef]
- Ding, J.; Yang, G.; Sun, W.; Li, Y.; Wang, N.; Wang, J.; Zhao, Y. Association of interleukin-6 with sarcopenia and its components in older adults: A systematic review and meta-analysis of cross-sectional studies. Ann. Med. 2024, 56, 2384664. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.M.; Kim, H.H.; Ha, K.T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Cipolat, S.; de Brito, O.M.; Zilio, B.D.; Scorrano, L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Li, L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int. J. Mol. Sci. 2017, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.H. Mitochondrial Dysfunction in Aging and Diseases of Aging. Biology 2019, 8, 48. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Implications of mitochondrial fusion and fission in skeletal muscle mass and health. Semin. Cell Dev. Biol. 2023, 143, 46–53. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007, 14, 56–65. [Google Scholar] [CrossRef]
- Yu, T.; Sheu, S.S.; Robotham, J.L.; Yoon, Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef]
- Dai, C.Q.; Guo, Y.; Chu, X.Y. Neuropathic Pain: The Dysfunction of Drp1, Mitochondria, and ROS Homeostasis. Neurotox. Res. 2020, 38, 553–563. [Google Scholar] [CrossRef]
- Sharp, W.W.; Beiser, D.G.; Fang, Y.H.; Han, M.; Piao, L.; Varughese, J.; Archer, S.L. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit. Care Med. 2015, 43, e38–e47. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Wei, J.; Fan, R.; Zuo, Y.; Shi, M.; Wu, H.; Zhou, M.; Lin, J.; Wu, M.; et al. Inhibition of Drp1 by Mdivi-1 attenuates cerebral ischemic injury via inhibition of the mitochondria-dependent apoptotic pathway after cardiac arrest. Neuroscience 2015, 311, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, Y.; Sui, X.; Shen, L.; Han, J.; Wang, H.; Xi, Q.; Zhuang, Q.; Meng, Q.; Wu, G. Phosphorylation of Dynamin-Related Protein 1 (DRP1) Regulates Mitochondrial Dynamics and Skeletal Muscle Wasting in Cancer Cachexia. Front. Cell Dev. Biol. 2021, 9, 673618. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, Z.; Xiao, D.; Wang, Y.; Zhao, L.; An, Y.; Gao, Y. Baicalein inhibits mitochondrial apoptosis induced by oxidative stress in cardiomyocytes by stabilizing MARCH5 expression. J. Cell. Mol. Med. 2020, 24, 2040–2051. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Qin, J.; Chen, N.; Lu, S.; Jin, J.; Li, Y. Baicalin Improves Cardiac Outcome and Survival by Suppressing Drp1-Mediated Mitochondrial Fission After Cardiac Arrest-Induced Myocardial Damage. Oxid. Med. Cell. Longev. 2021, 2021, 8865762. [Google Scholar] [CrossRef]
- Pan, Y.; Song, D.; Zhou, W.; Lu, X.; Wang, H.; Li, Z. Baicalin inhibits C2C12 myoblast apoptosis and prevents against skeletal muscle injury. Mol. Med. Rep. 2019, 20, 709–718. [Google Scholar] [CrossRef]
- Khin, P.P.; Hong, Y.; Yeon, M.; Lee, D.H.; Lee, J.H.; Jun, H.S. Dulaglutide improves muscle function by attenuating inflammation through OPA-1-TLR-9 signaling in aged mice. Aging 2021, 13, 21962–21974. [Google Scholar] [CrossRef]
- Lim, J.Y.; Frontera, W.R. Skeletal muscle aging and sarcopenia: Perspectives from mechanical studies of single permeabilized muscle fibers. J. Biomech. 2023, 152, 111559. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, S.; Scapaticci, R.; Masiello, E.; Messina, C.; Aliprandi, A.; Salerno, V.M.; Russo, A.; Pegreffi, F. Advancements in sarcopenia diagnosis: From imaging techniques to non-radiation assessments. Front. Med. Technol. 2024, 6, 1467155. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Woods, P.C.; Paluch, A.E.; Miller, M.S. Effects of age on human skeletal muscle: A systematic review and meta-analysis of myosin heavy chain isoform protein expression, fiber size, and distribution. Am. J. Physiol. Cell Physiol. 2024, 327, C1400–C1415. [Google Scholar] [CrossRef] [PubMed]
- Antuna, E.; Cachan-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.E9–526.E17. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Beavers, K.M.; Hsu, F.C.; Isom, S.; Kritchevsky, S.B.; Church, T.; Goodpaster, B.; Pahor, M.; Nicklas, B.J. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. Sports Exerc. 2010, 42, 2189–2196. [Google Scholar] [CrossRef]
- Chang, K.V.; Wu, W.T.; Chen, Y.H.; Chen, L.R.; Hsu, W.H.; Lin, Y.L.; Han, D.S. Enhanced serum levels of tumor necrosis factor-alpha, interleukin-1beta, and -6 in sarcopenia: Alleviation through exercise and nutrition intervention. Aging 2023, 15, 13471–13485. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 2003, 23, 5409–5420. [Google Scholar] [CrossRef]
- Favaro, G.; Romanello, V.; Varanita, T.; Andrea Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 2019, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- She, R.; Tian, H.; Sun, F.; Ge, J.; Mei, Z. Naotaifang formula regulates Drp1-induced remodeling of mitochondrial dynamics following cerebral ischemia-reperfusion injury. Free Radic. Biol. Med. 2025, 229, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, C.; Bellemin, S.; Martin, E.; Carre-Pierrat, M.; Mollereau, B.; Gieseler, K.; Walter, L. DRP-1-mediated apoptosis induces muscle degeneration in dystrophin mutants. Sci. Rep. 2018, 8, 7354. [Google Scholar] [CrossRef] [PubMed]
- Wojtysiak, D.; Calik, J.; Krawczyk, J.; Wojciechowska-Puchalka, J. Postmortem Degradation of Desmin and Dystrophin in Breast Muscles from Capons and Cockerels. Ann. Anim. Sci. 2019, 19, 835–846. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, H.M.M.; Lee, J.H. Baicalin Improves Skeletal Muscle Atrophy by Attenuating DRP-1-Mediated Mitochondrial Fission in Aged Mice. Muscles 2025, 4, 35. https://doi.org/10.3390/muscles4030035
Mo HMM, Lee JH. Baicalin Improves Skeletal Muscle Atrophy by Attenuating DRP-1-Mediated Mitochondrial Fission in Aged Mice. Muscles. 2025; 4(3):35. https://doi.org/10.3390/muscles4030035
Chicago/Turabian StyleMo, Hla Myat Mo, and Jong Han Lee. 2025. "Baicalin Improves Skeletal Muscle Atrophy by Attenuating DRP-1-Mediated Mitochondrial Fission in Aged Mice" Muscles 4, no. 3: 35. https://doi.org/10.3390/muscles4030035
APA StyleMo, H. M. M., & Lee, J. H. (2025). Baicalin Improves Skeletal Muscle Atrophy by Attenuating DRP-1-Mediated Mitochondrial Fission in Aged Mice. Muscles, 4(3), 35. https://doi.org/10.3390/muscles4030035





