Mechanisms Underlying Muscle-Related Diseases and Aging: Insights into Pathophysiology and Therapeutic Strategies
Abstract
1. Introduction
1.1. Evolving Definitions, Burden, and Heterogeneity
1.2. Hallmarks of Muscle Aging: Beyond Atrophy
1.3. Interconnected Pathophysiology: A Network Model
1.4. Therapeutic Landscape: Converging and Diverging Pathways
1.5. Scope and Structure of This Review
2. Mitochondrial Mechanisms in Muscle Aging and Disease
2.1. Mitochondrial Quality Control (MQC) System
2.1.1. Mitochondrial Biogenesis
2.1.2. Mitochondrial Dynamics
2.1.3. Mitophagy
2.1.4. Exophers
2.2. Mitochondrial Alterations Induced by Aging
2.2.1. Reduced Mitochondrial Biogenesis and Content
2.2.2. Disrupted Mitochondrial Dynamics
2.2.3. Increased Oxidative Stress
2.2.4. Altered Mitochondrial Unfolded Protein Response (UPRmt)
3. Non-Mitochondrial Mechanisms in Muscle Aging and Disease
3.1. Muscle Stem Cell Dysfunction
3.2. Neuromuscular Junction Dysfunction
3.3. Inflammation and Immune Cell Infiltration
4. Therapeutic Strategies Targeting Muscle Aging and Muscle-Related Diseases
4.1. Lifestyle Interventions: Exercise and Dietary Restriction
4.2. Pharmacological Strategies Targeting Metabolic and Proteostatic Pathways
4.3. Biological Interventions Targeting Stem Cell Function and Neuromuscular Integrity
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Park, S.; Le, T.P.; Byun, H.J.; Lee, S.; Lee, M.; Huh, K.M.; Lee, J.Y. Synergistic effects of conductive hydrogels and electrical stimulation in volumetric muscle loss. Chem. Eng. J. 2025, 512, 162362. [Google Scholar] [CrossRef]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human skeletal muscle aging atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Green, A.E.; Kim, Y.A.; Bae, S.J.; Ha, K.T.; Gariani, K.; Lee, M.R.; Menzies, K.J.; Ryu, D. Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol Metab. 2020, 35, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Bossola, M.; Allocca, E.; Menghi, A.; Pesce, V.; Lezza, A.M.S.; Bernabei, R.; Landi, F.; Marzetti, E. Update on mitochondria and muscle aging: All wrong roads lead to sarcopenia. Biol. Chem. 2018, 399, 421–436. [Google Scholar] [CrossRef]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Musci, R.V.; Hinkley, J.M.; Miller, B.F. Mitochondria as a Target for Mitigating Sarcopenia. Front. Physiol. 2019, 9, 1883. [Google Scholar] [CrossRef]
- Alway, S.E.; Mohamed, J.S.; Myers, M.J. Mitochondria Initiate and Regulate Sarcopenia. Exerc. Sport Sci. Rev. 2017, 45, 58–69. [Google Scholar] [CrossRef]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; De Vito, G.; Narici, M.; Boreham, C. Neuromuscular Junction Aging: A Role for Biomarkers and Exercise. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 576–585. [Google Scholar] [CrossRef]
- Antuna, E.; Cachan-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Chatre, L.; Verdonk, F.; Rocheteau, P.; Crochemore, C.; Chretien, F.; Ricchetti, M. A novel paradigm links mitochondrial dysfunction with muscle stem cell impairment in sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt B, 2546–2553. [Google Scholar] [CrossRef]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramirez-Pardo, I.; Segales, J.; Hernansanz-Agustin, P.; Curtabbi, A.; Deryagin, O.; Pollan, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1506–1508. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Shah, V.; Oresajo, O.; Scime, A. p107 mediated mitochondrial function controls muscle stem cell proliferative fates. Nat. Commun. 2021, 12, 5977. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Scime, A. Mitochondrial Function in Muscle Stem Cell Fates. Front. Cell Dev. Biol. 2020, 8, 480. [Google Scholar] [CrossRef]
- Miao, Y.M.; Xie, L.Y.; Song, J.M.; Cai, X.; Yang, J.H.; Ma, X.L.; Chen, S.L.; Xie, P. Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction. Physiol. Rep. 2024, 12, e15917. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Ranjit, R.; Richardson, A.; Van Remmen, H. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J. Cachexia Sarcopenia Muscle 2021, 12, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Zhang, J.M.; Shu, X.Q.; Bai, L.; Xu, W.T.; Wang, A.L.; Chen, A.Z.; Tu, W.Y.; Wang, J.W.; Zhang, K.J.; et al. Loss of mitochondrial protein CHCHD10 in skeletal muscle causes neuromuscular junction impairment. Hum. Mol. Genet. 2020, 29, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, M.E.; Hepple, R.T. Mitochondrial Mechanisms of Neuromuscular Junction Degeneration with Aging. Cells 2020, 9, 197. [Google Scholar] [CrossRef]
- Liu, W.X.; Klose, A.; Forman, S.; Paris, N.D.; Pierre, L.W.L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Verschuuren, J.J.; Palace, J.; Murai, H.; Tannemaat, M.R.; Kaminski, H.J.; Bril, V. Advances and ongoing research in the treatment of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 189–202. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015, 25, 1082–1083. [Google Scholar] [CrossRef]
- Ennis, W.J.; Sui, A.; Bartholomew, A. Stem Cells and Healing: Impact on Inflammation. Adv. Wound Care 2013, 2, 369–378. [Google Scholar] [CrossRef]
- Palacios, D.; Mozzetta, C.; Consalvi, S.; Caretti, G.; Saccone, V.; Proserpio, V.; Marquez, V.E.; Valente, S.; Mai, A.; Forcales, S.V.; et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 2010, 7, 455–469. [Google Scholar] [CrossRef]
- Van Dyke, J.M.; Smit-Oistad, I.M.; Macrander, C.; Krakora, D.; Meyer, M.G.; Suzuki, M. Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS). Exp. Neurol. 2016, 277, 275–282. [Google Scholar] [CrossRef]
- Wang, J.; Leung, K.S.; Chow, S.K.; Cheung, W.H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop. Transl. 2017, 10, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.; Chung, T.; Lovett, J.; Ward, C.; Joca, H.; Yang, H.; Khadeer, M.; Tian, J.; Xue, Q.L.; Le, A.; et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight 2020, 5, e136091. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Niculescu, A.G.; Grumezescu, A.M.; Beuran, M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. Int. J. Mol. Sci. 2024, 25, 4300. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Yang, S.; McCarthy, C.; Brown, J.B.; Martin, C.K.; Redman, L.M.; Ravussin, E.; Shen, W.; Muller, M.J.; Bosy-Westphal, A. Proportion of caloric restriction-induced weight loss as skeletal muscle. Obesity 2024, 32, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Strasser, B.; Burtscher, M. A mito-centric view on muscle aging and function. Front. Public Health 2023, 11, 1330131. [Google Scholar] [CrossRef]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef]
- Zhou, L.S.; Yang, Y.; Mou, L.; Xia, X.; Liu, M.; Xu, L.J.; Liu, R.; Liu, J.P.; Zhang, H.Y.; Ao, X.J.; et al. Melatonin Ameliorates Age-Related Sarcopenia via the Gut-Muscle Axis Mediated by Serum Lipopolysaccharide and Metabolites. J. Cachexia Sarcopenia Muscle 2025, 16, e13722. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Demontis, R.; Santangelo, C.; Pini, N.; Bonelli, M.; Rosato, E.; Roberti, P.; Locatelli, M.; Tartaglia, A.; Marramiero, L.; et al. New Perspectives for Postmortem Human Satellite Cells of Different Embryological Origin. Front. Physiol. 2022, 13, 886149. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Feng, Q.; Cui, Y.; Huard, J.; Robbins, P.D.; Wang, Z.; Mu, X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging 2022, 14, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- Jadiya, P.; Tomar, D. Mitochondrial Protein Quality Control Mechanisms. Genes 2020, 11, 563. [Google Scholar] [CrossRef]
- Baker, M.J.; Palmer, C.S.; Stojanovski, D. Mitochondrial protein quality control in health and disease. Br. J. Pharmacol. 2014, 171, 1870–1889. [Google Scholar] [CrossRef]
- Dillon, L.M.; Rebelo, A.P.; Moraes, C.T. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 2012, 64, 231–241. [Google Scholar] [CrossRef]
- Halling, J.F.; Jessen, H.; Nohr-Meldgaard, J.; Buch, B.T.; Christensen, N.M.; Gudiksen, A.; Ringholm, S.; Neufer, P.D.; Prats, C.; Pilegaard, H. PGC-1alpha regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E513–E525. [Google Scholar] [CrossRef]
- Baraldo, M.; Geremia, A.; Pirazzini, M.; Nogara, L.; Solagna, F.; Türk, C.; Nolte, H.; Romanello, V.; Megighian, A.; Boncompagni, S.; et al. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J. Cachexia Sarcopenia Muscle 2020, 11, 208–225. [Google Scholar] [CrossRef]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, H.; Niu, Y.; Niu, W.; Fu, L. The role of AMPK/mTOR/S6K1 signaling axis in mediating the physiological process of exercise-induced insulin sensitization in skeletal muscle of C57BL/6 mice. Biochim. Biophys. Acta 2012, 1822, 1716–1726. [Google Scholar] [CrossRef]
- Parkington, J.D.; LeBrasseur, N.K.; Siebert, A.P.; Fielding, R.A. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J. Appl. Physiol. 2004, 97, 243–248. [Google Scholar] [CrossRef]
- Chen, Z.P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Shepherd, D.L.; Durr, A.J.; Stanton, D.S.; Mohamed, J.S.; Hollander, J.M.; Alway, S.E. The role of SIRT1 in skeletal muscle function and repair of older mice. J. Cachexia Sarcopenia Muscle 2019, 10, 929–949. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Zhao, T.; Fan, J.; Abu-Zaid, A.; Burley, S.K.; Zheng, X.F.S. Nuclear mTOR Signaling Orchestrates Transcriptional Programs Underlying Cellular Growth and Metabolism. Cells 2024, 13, 781. [Google Scholar] [CrossRef]
- Fan, J.; Yuan, Z.; Burley, S.K.; Libutti, S.K.; Zheng, X.F.S. Amino acids control blood glucose levels through mTOR signaling. Eur. J. Cell Biol. 2022, 101, 151240. [Google Scholar] [CrossRef]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef]
- Raven, K.D.; Kapetanovic, R. Mitochondrial dynamics: Regulating cell metabolism, homoeostasis, health and disease. Semin. Cell Dev. Biol. 2024, 161–162, 20–21. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Marinkovic, M.; Novak, I. A brief overview of BNIP3L/NIX receptor-mediated mitophagy. FEBS Open Bio 2021, 11, 3230–3236. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Q.; Sun, H.; Liu, L.; Wang, C.; Li, Z.; Xu, Y.; Wang, L.; Zhang, L.; Zhang, H.; et al. BNIP3 (BCL2 interacting protein 3) regulates pluripotency by modulating mitochondrial homeostasis via mitophagy. Cell Death Dis. 2022, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Xu, Z.; Liu, L.; Guo, Q.; Wu, H.; Liang, X.; Zhou, D.; Xiao, L.; Liu, L.; Liu, Y.; et al. Mitophagy Directs Muscle-Adipose Crosstalk to Alleviate Dietary Obesity. Cell Rep. 2018, 23, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.N.; Kim, Y.; Erlich, A.T.; Zarrin-Khat, D.; Hood, D.A. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 2018, 596, 3567–3584. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nouri, K.; Feng, Y.; Schimmer, A.D. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020, 11, 841. [Google Scholar] [CrossRef]
- Pharaoh, G.; Sataranatarajan, K.; Street, K.; Hill, S.; Gregston, J.; Ahn, B.; Kinter, C.; Kinter, M.; Van Remmen, H. Metabolic and Stress Response Changes Precede Disease Onset in the Spinal Cord of Mutant SOD1 ALS Mice. Front. Neurosci. 2019, 13, 487. [Google Scholar] [CrossRef]
- Bota, D.A.; Van Remmen, H.; Davies, K.J. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002, 532, 103–106. [Google Scholar] [CrossRef]
- Bota, D.A.; Davies, K.J. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002, 4, 674–680. [Google Scholar] [CrossRef]
- Guo, C.; Xiao, Y.; Gu, J.; Zhao, P.; Hu, Z.; Zheng, J.; Hua, R.; Hai, Z.; Su, J.; Zhang, J.V.; et al. ClpP/ClpX deficiency impairs mitochondrial functions and mTORC1 signaling during spermatogenesis. Commun. Biol. 2023, 6, 1012. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho-Junior, H.J.; Bucci, C.; Leeuwenburgh, C.; Marzetti, E. Mitochondrial-derived vesicles in skeletal muscle remodeling and adaptation. Semin. Cell Dev. Biol. 2023, 143, 37–45. [Google Scholar] [CrossRef]
- Konig, T.; McBride, H.M. Mitochondrial-derived vesicles in metabolism, disease, and aging. Cell Metab. 2024, 36, 21–35. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Roberts, R.F.; Tang, M.Y.; Fon, E.A.; Durcan, T.M. Defending the mitochondria: The pathways of mitophagy and mitochondrial-derived vesicles. Int. J. Biochem. Cell Biol. 2016, 79, 427–436. [Google Scholar] [CrossRef]
- Konig, T.; Nolte, H.; Aaltonen, M.J.; Tatsuta, T.; Krols, M.; Stroh, T.; Langer, T.; McBride, H.M. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat. Cell Biol. 2021, 23, 1271–1286. [Google Scholar] [CrossRef]
- Li, A.; Qin, Y.; Gong, G. The Changes of Mitochondria during Aging and Regeneration. Adv. Biol. 2024, 8, e2300445. [Google Scholar] [CrossRef]
- Frankowska, N.; Lisowska, K.; Witkowski, J.M. Proteolysis dysfunction in the process of aging and age-related diseases. Front. Aging 2022, 3, 927630. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef]
- Liang, W.; Sagar, S.; Ravindran, R.; Najor, R.H.; Quiles, J.M.; Chi, L.; Diao, R.Y.; Woodall, B.P.; Leon, L.J.; Zumaya, E.; et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 2023, 14, 5031. [Google Scholar] [CrossRef]
- Turek, M.; Banasiak, K.; Piechota, M.; Shanmugam, N.; Macias, M.; Sliwinska, M.A.; Niklewicz, M.; Kowalski, K.; Nowak, N.; Chacinska, A.; et al. Muscle-derived exophers promote reproductive fitness. EMBO Rep. 2021, 22, e52071. [Google Scholar] [CrossRef]
- Nicolas-Avila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martinez, L.; Sanchez-Diaz, M.; Diaz-Garcia, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e123. [Google Scholar] [CrossRef]
- Sligar, J.; DeBruin, D.A.; Saner, N.J.; Philp, A.M.; Philp, A. The importance of mitochondrial quality control for maintaining skeletal muscle function across health span. Am. J. Physiol. Cell Physiol. 2022, 322, C461–C467. [Google Scholar] [CrossRef]
- Marzetti, E. Musculoskeletal Aging and Sarcopenia in the Elderly. Int. J. Mol. Sci. 2022, 23, 2808. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Sladowska, M.; Turek, M.; Kim, M.J.; Drabikowski, K.; Mussulini, B.H.M.; Mohanraj, K.; Serwa, R.A.; Topf, U.; Chacinska, A. Proteasome activity contributes to pro-survival response upon mild mitochondrial stress in Caenorhabditis elegans. PLoS Biol. 2021, 19, e3001302. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, Y.; Zhang, X.; Tomaszewski, A.; McNamara, J.T.; Li, R. Mitochondria-Associated Proteostasis. Annu. Rev. Biophys. 2020, 49, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Hood, D.A. Manifestations of Age on Autophagy, Mitophagy and Lysosomes in Skeletal Muscle. Cells 2021, 10, 1054. [Google Scholar] [CrossRef]
- Xiao, Y.; Karam, C.; Yi, J.; Zhang, L.; Li, X.; Yoon, D.; Wang, H.; Dhakal, K.; Ramlow, P.; Yu, T.; et al. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 2018, 138, 25–36. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, Y.; Zhang, Z. Effects of Exercise-Induced ROS on the Pathophysiological Functions of Skeletal Muscle. Oxid. Med. Cell. Longev. 2021, 2021, 3846122. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Hollander, J.; Fiebig, R.; Gore, M.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression in skeletal muscle: Fiber-specific adaptation to endurance training. Am. J. Physiol. 1999, 277, R856–R862. [Google Scholar] [CrossRef]
- Hollander, J.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression in skeletal muscle: Fiber-specific effect of age. Mech. Ageing Dev. 2000, 116, 33–45. [Google Scholar] [CrossRef]
- Hood, D.A.; Tryon, L.D.; Carter, H.N.; Kim, Y.; Chen, C.C. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem. J. 2016, 473, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fano-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic. Biol. Med. 2019, 130, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Nissanka, N.; Mareco, E.A.; Rossi, S.; Peralta, S.; Diaz, F.; Rotundo, R.L.; Carvalho, R.F.; Moraes, C.T. Overexpression of PGC-1alpha in aging muscle enhances a subset of young-like molecular patterns. Aging Cell 2018, 17, e12707. [Google Scholar] [CrossRef] [PubMed]
- Bujak, A.L.; Crane, J.D.; Lally, J.S.; Ford, R.J.; Kang, S.J.; Rebalka, I.A.; Green, A.E.; Kemp, B.E.; Hawke, T.J.; Schertzer, J.D.; et al. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metab. 2015, 21, 883–890. [Google Scholar] [CrossRef]
- Thompson, A.M.; Wagner, R.; Rzucidlo, E.M. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H533–H541. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Szabo, Z.; Atalay, M.; Boldogh, I.; Naito, H.; Goto, S.; Nyakas, C.; Radak, Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 2010, 131, 21–28. [Google Scholar] [CrossRef]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Vernier, M.; Giguere, V. Aging, senescence and mitochondria: The PGC-1/ERR axis. J. Mol. Endocrinol. 2021, 66, R1–R14. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, M.; Chen, X.; Xiao, X. Biological function of EPHB4 in the aging process of vascular endothelial cells: mtDNA molecular mechanism and MAPK/PGC-1/TFAM signaling pathway. Int. J. Biol. Macromol. 2025, 293, 138536. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.A.; Kumar, R.; Mulligan, J.D.; Davis, A.J.; Saupe, K.W. Effects of aging on cardiac and skeletal muscle AMPK activity: Basal activity, allosteric activation, and response to in vivo hypoxemia in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1270–R1275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liu, C.; Chen, Y.; Xing, X.; Zheng, D. Whole body vibration activates AMPK/CPT1 signaling pathway of skeletal muscle in young and aging mice based on metabolomics study. Endocr. J. 2022, 69, 585–596. [Google Scholar] [CrossRef]
- Seo, A.Y.; Joseph, A.M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Implications of mitochondrial fusion and fission in skeletal muscle mass and health. Semin. Cell Dev. Biol. 2023, 143, 46–53. [Google Scholar] [CrossRef]
- Iqbal, S.; Ostojic, O.; Singh, K.; Joseph, A.M.; Hood, D.A. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 2013, 48, 963–970. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Shaw, P.X.; Werstuck, G.; Chen, Y. Oxidative stress and aging diseases. Oxid. Med. Cell. Longev. 2014, 2014, 569146. [Google Scholar] [CrossRef]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef] [PubMed]
- Puengpan, S.; Phetrungnapha, A.; Sattayakawee, S.; Tunsophon, S. Phycocyanin attenuates skeletal muscle damage and fatigue via modulation of Nrf2 and IRS-1/AKT/mTOR pathway in exercise-induced oxidative stress in rats. PLoS ONE 2024, 19, e0310138. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Nargund, A.M.; Haynes, C.M. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta 2013, 1833, 410–416. [Google Scholar] [CrossRef]
- Jovaisaite, V.; Mouchiroud, L.; Auwerx, J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 2014, 217 Pt 1, 137–143. [Google Scholar] [CrossRef]
- Mesbah Moosavi, Z.S.; Hood, D.A. The unfolded protein response in relation to mitochondrial biogenesis in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2017, 312, C583–C594. [Google Scholar] [CrossRef] [PubMed]
- Naresh, N.U.; Haynes, C.M. Signaling and Regulation of the Mitochondrial Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2019, 11, a033944. [Google Scholar] [CrossRef]
- Shin, C.S.; Meng, S.; Garbis, S.D.; Moradian, A.; Taylor, R.W.; Sweredoski, M.J.; Lomenick, B.; Chan, D.C. LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding. Nat. Commun. 2021, 12, 265. [Google Scholar] [CrossRef]
- Seli, E.; Wang, T.; Horvath, T.L. Mitochondrial unfolded protein response: A stress response with implications for fertility and reproductive aging. Fertil. Steril. 2019, 111, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Carvajal, F.; Sanhueza, M. The Mitochondrial Unfolded Protein Response: A Hinge Between Healthy and Pathological Aging. Front. Aging Neurosci. 2020, 12, 581849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Zhang, Z.; Wang, R.; Bo, H.; Zhang, Y. Exercise Improves the Coordination of the Mitochondrial Unfolded Protein Response and Mitophagy in Aging Skeletal Muscle. Life 2023, 13, 1006. [Google Scholar] [CrossRef]
- Gopinath, S.D.; Rando, T.A. Stem cell review series: Aging of the skeletal muscle stem cell niche. Aging Cell 2008, 7, 590–598. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Garcia-Prat, L.; Serrano, A.L.; Perdiguero, E.; Munoz-Canoves, P. Muscle stem cell aging: Regulation and rejuvenation. Trends Endocrinol. Metab. 2015, 26, 287–296. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Karaz, S.; Stuelsatz, P.; Gurriaran-Rodriguez, U.; Michaud, J.; Dammone, G.; Sizzano, F.; Mashinchian, O.; Ancel, S.; Migliavacca, E.; et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 2019, 24, 433–446.e437. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Neves, J.; Munoz-Canoves, P. Muscle stem cell aging: Identifying ways to induce tissue rejuvenation. Mech. Ageing Dev. 2020, 188, 111246. [Google Scholar] [CrossRef]
- Memczak, S.; Belmonte, J.C. Overcoming muscle stem cell aging. Curr. Opin. Genet. Dev. 2023, 83, 102127. [Google Scholar] [CrossRef] [PubMed]
- Fulzele, S.; Mendhe, B.; Khayrullin, A.; Johnson, M.; Kaiser, H.; Liu, Y.; Isales, C.M.; Hamrick, M.W. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging 2019, 11, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, Z.; Song, M.; Zhou, Y.; Shen, Y. A microRNA switch controls dietary restriction-induced longevity through Wnt signaling. EMBO Rep. 2019, 20, e46888. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, Y.; Song, M.; Dai, Y.; Antebi, A.; Shen, Y. BLMP-1 is a critical temporal regulator of dietary-restriction-induced response in Caenorhabditis elegans. Cell Rep. 2024, 43, 113959. [Google Scholar] [CrossRef]
- Pan, C.; Xiong, Y.; Lv, X.; Xia, Y.; Zhang, S.; Chen, H.; Fan, J.; Wu, W.; Liu, F.; Wu, H.; et al. UbcD1 regulates Hedgehog signaling by directly modulating Ci ubiquitination and processing. EMBO Rep. 2017, 18, 1922–1934. [Google Scholar] [CrossRef]
- Fan, J.; Gao, Y.; Lu, Y.; Wu, W.; Yuan, S.; Wu, H.; Chen, D.; Zhao, Y. PKAc-directed interaction and phosphorylation of Ptc is required for Hh signaling inhibition in Drosophila. Cell Discov. 2019, 5, 44. [Google Scholar] [CrossRef]
- Jang, Y.C.; Van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Gallego, E. Mechanisms controlling neuromuscular junction stability. Cell. Mol. Life Sci. 2015, 72, 1029–1043. [Google Scholar] [CrossRef]
- Liu, W.; Wei-LaPierre, L.; Klose, A.; Dirksen, R.T.; Chakkalakal, J.V. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife 2015, 4, e09221. [Google Scholar] [CrossRef][Green Version]
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. New mechanisms driving muscle stem cell regenerative decline with aging. Int. J. Dev. Biol. 2018, 62, 583–590. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musarò, A. Neuromuscular Junction as an Entity of Nerve-Muscle Communication. Cells 2019, 8, 906. [Google Scholar] [CrossRef]
- Personius, K.E.; Parker, S.D. TrkB expression at the neuromuscular junction is reduced during aging. Muscle Nerve 2013, 47, 532–538. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The neuromuscular junction: Aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Stowe, J.M.; Sieck, D.C.; Ermilov, L.G.; Greising, S.M.; Zhang, C.; Shokat, K.M.; Sieck, G.C. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J. Appl. Physiol. 2014, 117, 910–920. [Google Scholar] [CrossRef]
- Greising, S.M.; Stowe, J.M.; Sieck, G.C.; Mantilla, C.B. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp. Gerontol. 2015, 72, 184–191. [Google Scholar] [CrossRef]
- Simó, A.; Just-Borràs, L.; Cilleros-Mañé, V.; Hurtado, E.; Nadal, L.; Tomàs, M.; Garcia, N.; Lanuza, M.A.; Tomàs, J. BDNF-TrkB Signaling Coupled to nPKCε and cPKCβI Modulate the Phosphorylation of the Exocytotic Protein Munc18-1 During Synaptic Activity at the Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 207. [Google Scholar] [CrossRef]
- Snyder-Warwick, A.K.; Satoh, A.; Santosa, K.B.; Imai, S.; Jablonka-Shariff, A. Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell 2018, 17, e12776. [Google Scholar] [CrossRef]
- Ang, S.T.J.; Crombie, E.M.; Dong, H.; Tan, K.T.; Hernando, A.; Yu, D.J.; Adamson, S.; Kim, S.; Withers, D.J.; Huang, H.; et al. Muscle 4EBP1 activation modifies the structure and function of the neuromuscular junction in mice. Nat. Commun. 2022, 13, 7792. [Google Scholar] [CrossRef]
- Genin, E.C.; Madji Hounoum, B.; Bannwarth, S.; Fragaki, K.; Lacas-Gervais, S.; Mauri-Crouzet, A.; Lespinasse, F.; Neveu, J.; Ropert, B.; Auge, G.; et al. Mitochondrial defect in muscle precedes neuromuscular junction degeneration and motor neuron death in CHCHD10(S59L/+) mouse. Acta Neuropathol. 2019, 138, 123–145. [Google Scholar] [CrossRef]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495. [Google Scholar] [CrossRef]
- Dagdeviren, S.; Jung, D.Y.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X.; et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-kappaB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef] [PubMed]
- Belizario, J.E.; Fontes-Oliveira, C.C.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 2016, 5, 619. [Google Scholar] [CrossRef]
- Ma, J.F.; Sanchez, B.J.; Hall, D.T.; Tremblay, A.K.; Di Marco, S.; Gallouzi, I.E. STAT3 promotes IFNgamma/TNFalpha-induced muscle wasting in an NF-kappaB-dependent and IL-6-independent manner. EMBO Mol. Med. 2017, 9, 622–637. [Google Scholar] [CrossRef]
- Chen, F.; Fu, J.; Feng, H. IL-6 Promotes Muscle Atrophy by Increasing Ubiquitin-Proteasome Degradation of Muscle Regeneration Factors After Cerebral Infarction in Rats. Neuromolecular Med. 2025, 27, 3. [Google Scholar] [CrossRef]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Mourkioti, F.; Rosenthal, N. NF-kappaB signaling in skeletal muscle: Prospects for intervention in muscle diseases. J. Mol. Med. 2008, 86, 747–759. [Google Scholar] [CrossRef]
- Choudhary, S.; Sinha, S.; Zhao, Y.; Banerjee, S.; Sathyanarayana, P.; Shahani, S.; Sherman, V.; Tilton, R.G.; Bajaj, M. NF-kappaB-inducing kinase (NIK) mediates skeletal muscle insulin resistance: Blockade by adiponectin. Endocrinology 2011, 152, 3622–3627. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.; Gosker, H.R.; Verhees, K.J.; Langen, R.C.; Schols, A.M. TNF-alpha-induced NF-kappaB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1alpha. Endocrinology 2015, 156, 1770–1781. [Google Scholar] [CrossRef]
- Biggar, Y.; Kamath, A.A.; Breedon, S.A.; Storey, K.B. NF-kappaB signaling and its anti-apoptotic effects in liver & skeletal muscle of dehydrated Xenopus laevis. Exp. Cell Res. 2025, 449, 114579. [Google Scholar] [CrossRef]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of Transforming Growth Factor-beta in Skeletal Muscle Fibrosis: A Review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef]
- Song, Y.; Shen, H.; Schenten, D.; Shan, P.; Lee, P.J.; Goldstein, D.R. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 103–109. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuv. Res. 2018, 21, 350–359. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2008, 2, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Renault, V.M.; Rando, T.A. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 2005, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Wang, Y.; Xiong, Y.; Chen, X.C.; Ma, M.L.; Cai, R.; Gao, Y.; Sun, Y.M.; Yang, G.S.; Pang, W.J. Sirt1 AS lncRNA interacts with its mRNA to inhibit muscle formation by attenuating function of miR-34a. Sci. Rep. 2016, 6, 21865. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Zhang, W.; Tong, H.; Li, S.; Yan, Y. WISP1 promotes bovine MDSC differentiation via recruitment of ANXA1 for the regulation of the TGF-beta signalling pathway. Mol. Cell. Biochem. 2020, 470, 215–227. [Google Scholar] [CrossRef]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Muinos Lopez, E.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004. [Google Scholar] [CrossRef]
- Irfan, M.; Marzban, H.; Chung, S. C5L2 CRISPR KO enhances dental pulp stem cell-mediated dentinogenesis via TrkB under TNFalpha-induced inflammation. Front. Cell Dev. Biol. 2024, 12, 1338419. [Google Scholar] [CrossRef]
- Zuo, B.; Fan, X.; Xu, D.; Zhao, L.; Zhang, B.; Li, X. Deciphering the mitochondria-inflammation axis: Insights and therapeutic strategies for heart failure. Int. Immunopharmacol. 2024, 139, 112697. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.S.; Roda, V.M.P.; Andrieux, P.; Kalil, J.; Chevillard, C.; Cunha-Neto, E. Inflammation and mitochondria in the pathogenesis of chronic Chagas disease cardiomyopathy. Exp. Biol. Med. 2023, 248, 2062–2071. [Google Scholar] [CrossRef]
- Malnoe, D.; Bories, M.; Pierre-Jean, M.; Marchand, T.; Le Corre, P. Inflammation Decreases Ciclosporin Metabolism in Allogeneic Hematopoietic Stem Cell Transplantation Recipients. J. Clin. Pharmacol. 2025, 65, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Giffard, R.G. Inflammation, mitochondria, and the inhibition of adult neurogenesis. J. Neurosci. Res. 2011, 89, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Z.; Han, Z.; He, Z. Mesenchymal stem cell-conditioned media suppresses inflammation-associated overproliferation of pulmonary artery smooth muscle cells in a rat model of pulmonary hypertension. Exp. Ther. Med. 2016, 11, 467–475. [Google Scholar] [CrossRef]
- Medeiros, C.; Frederico, M.J.; da Luz, G.; Pauli, J.R.; Silva, A.S.; Pinho, R.A.; Velloso, L.A.; Ropelle, E.R.; De Souza, C.T. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J. Cell. Physiol. 2011, 226, 666–674. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Fealy, C.E.; Mulya, A.; Kirwan, J.P. Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype. Acta Physiol. 2019, 225, e13216. [Google Scholar] [CrossRef]
- Kim, Y.; Triolo, M.; Hood, D.A. Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxid. Med. Cell. Longev. 2017, 2017, 3165396. [Google Scholar] [CrossRef]
- Zou, K.; De Lisio, M.; Huntsman, H.D.; Pincu, Y.; Mahmassani, Z.; Miller, M.; Olatunbosun, D.; Jensen, T.; Boppart, M.D. Laminin-111 improves skeletal muscle stem cell quantity and function following eccentric exercise. Stem Cells Transl. Med. 2014, 3, 1013–1022. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Lee, T.X.Y.; Wu, J.; Jean, W.H.; Condello, G.; Alkhatib, A.; Hsieh, C.C.; Hsieh, Y.W.; Huang, C.Y.; Kuo, C.H. Reduced stem cell aging in exercised human skeletal muscle is enhanced by ginsenoside Rg1. Aging 2021, 13, 16567–16576. [Google Scholar] [CrossRef]
- Moradi, N.; Sanfrancesco, V.C.; Champsi, S.; Hood, D.A. Regulation of lysosomes in skeletal muscle during exercise, disuse and aging. Free Radic. Biol. Med. 2024, 225, 323–332. [Google Scholar] [CrossRef]
- Moore, D.R.; Atherton, P.J.; Rennie, M.J.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol. 2011, 201, 365–372. [Google Scholar] [CrossRef]
- Nishimune, H.; Stanford, J.A.; Mori, Y. Role of Exercise in Maintaining the Integrity of the Neuromuscular Junction. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Russell, A.P.; Foletta, V.C.; Snow, R.J.; Wadley, G.D. Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochim. Biophys. Acta 2014, 1840, 1276–1284. [Google Scholar] [CrossRef]
- Lambert, C.P.; Wright, N.R.; Finck, B.N.; Villareal, D.T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 2008, 105, 473–478. [Google Scholar] [CrossRef]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef]
- Keith, N.; Bronson, R.T.; Lipman, R.D.; Ding, W.; Lamont, L.; Cosmas, A.C.; Manfredi, T.G. Diet restriction and age alters skeletal muscle capillarity in B6C3F1 mice. J. Am. Aging Assoc. 2000, 23, 141–145. [Google Scholar] [CrossRef]
- Nam, S.Y.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Low-dose growth hormone treatment combined with diet restriction decreases insulin resistance by reducing visceral fat and increasing muscle mass in obese type 2 diabetic patients. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1101–1107. [Google Scholar] [CrossRef]
- Magwere, T.; Goodall, S.; Skepper, J.; Mair, W.; Brand, M.D.; Partridge, L. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 36–47. [Google Scholar] [CrossRef]
- Colom, B.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc. Res. 2007, 74, 456–465. [Google Scholar] [CrossRef]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.J.; Cheng, H.L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef]
- Hepple, R.T.; Qin, M.; Nakamoto, H.; Goto, S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: Implications for sarcopenia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1231–R1237. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef]
- Tauriainen, E.; Storvik, M.; Finckenberg, P.; Merasto, S.; Martonen, E.; Pilvi, T.K.; Korpela, R.; Mervaala, E.M. Skeletal muscle gene expression profile is modified by dietary protein source and calcium during energy restriction. J. Nutr. Nutr. 2011, 4, 49–62. [Google Scholar] [CrossRef]
- Cerletti, M.; Jang, Y.C.; Finley, L.W.; Haigis, M.C.; Wagers, A.J. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 2012, 10, 515–519. [Google Scholar] [CrossRef]
- Jang, Y.C.; Liu, Y.; Hayworth, C.R.; Bhattacharya, A.; Lustgarten, M.S.; Muller, F.L.; Chaudhuri, A.; Qi, W.; Li, Y.; Huang, J.Y.; et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell 2012, 11, 770–782. [Google Scholar] [CrossRef]
- Mercken, E.M.; Majounie, E.; Ding, J.; Guo, R.; Kim, J.; Bernier, M.; Mattison, J.; Cookson, M.R.; Gorospe, M.; de Cabo, R.; et al. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging 2013, 5, 692–703. [Google Scholar] [CrossRef]
- Wang, C.C.; Adochio, R.L.; Leitner, J.W.; Abeyta, I.M.; Draznin, B.; Cornier, M.A. Acute effects of different diet compositions on skeletal muscle insulin signalling in obese individuals during caloric restriction. Metabolism 2013, 62, 595–603. [Google Scholar] [CrossRef]
- Suga, T.; Kinugawa, S.; Takada, S.; Kadoguchi, T.; Fukushima, A.; Homma, T.; Masaki, Y.; Furihata, T.; Takahashi, M.; Sobirin, M.A.; et al. Combination of exercise training and diet restriction normalizes limited exercise capacity and impaired skeletal muscle function in diet-induced diabetic mice. Endocrinology 2014, 155, 68–80. [Google Scholar] [CrossRef]
- Wong, K.E.; Mikus, C.R.; Slentz, D.H.; Seiler, S.E.; DeBalsi, K.L.; Ilkayeva, O.R.; Crain, K.I.; Kinter, M.T.; Kien, C.L.; Stevens, R.D.; et al. Muscle-Specific Overexpression of PGC-1alpha Does Not Augment Metabolic Improvements in Response to Exercise and Caloric Restriction. Diabetes 2015, 64, 1532–1543. [Google Scholar] [CrossRef]
- Keogh, K.; Kenny, D.A.; Cormican, P.; McCabe, M.S.; Kelly, A.K.; Waters, S.M. Effect of Dietary Restriction and Subsequent Re-Alimentation on the Transcriptional Profile of Bovine Skeletal Muscle. PLoS ONE 2016, 11, e0149373. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Wu, L.; Wei, H.; Liu, Y.; Li, T.; Tan, B.; Kong, X.; Yao, K.; Chen, S.; et al. Effects of dietary protein restriction on muscle fiber characteristics and mTORC1 pathway in the skeletal muscle of growing-finishing pigs. J. Anim. Sci. Biotechnol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Margolis, L.M.; Rivas, D.A.; Berrone, M.; Ezzyat, Y.; Young, A.J.; McClung, J.P.; Fielding, R.A.; Pasiakos, S.M. Prolonged Calorie Restriction Downregulates Skeletal Muscle mTORC1 Signaling Independent of Dietary Protein Intake and Associated microRNA Expression. Front. Physiol. 2016, 7, 445. [Google Scholar] [CrossRef]
- Oku, Y.; Tanabe, R.; Nakaoka, K.; Yamada, A.; Noda, S.; Hoshino, A.; Haraikawa, M.; Goseki-Sone, M. Influences of dietary vitamin D restriction on bone strength, body composition and muscle in rats fed a high-fat diet: Involvement of mRNA expression of MyoD in skeletal muscle. J. Nutr. Biochem. 2016, 32, 85–90. [Google Scholar] [CrossRef]
- Elashry, M.I.; Matsakas, A.; Wenisch, S.; Arnhold, S.; Patel, K. The effect of caloric restriction on the forelimb skeletal muscle fibers of the hypertrophic myostatin null mice. Acta Histochem. 2017, 119, 582–591. [Google Scholar] [CrossRef]
- Stockinger, J.; Maxwell, N.; Shapiro, D.; deCabo, R.; Valdez, G. Caloric Restriction Mimetics Slow Aging of Neuromuscular Synapses and Muscle Fibers. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 21–28. [Google Scholar] [CrossRef]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell. Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef]
- Walters, R.O.; Arias, E.; Diaz, A.; Burgos, E.S.; Guan, F.X.; Tiano, S.; Mao, K.; Green, C.L.; Qiu, Y.P.; Shah, H.; et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. 2018, 25, 663–676.e6. [Google Scholar] [CrossRef]
- Yoshida, S.; Yamahara, K.; Kume, S.; Koya, D.; Yasuda-Yamahara, M.; Takeda, N.; Osawa, N.; Chin-Kanasaki, M.; Adachi, Y.; Nagao, K.; et al. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell 2018, 17, e12796. [Google Scholar] [CrossRef]
- Serna, J.D.C.; Caldeira da Silva, C.C.; Kowaltowski, A.J. Functional changes induced by caloric restriction in cardiac and skeletal muscle mitochondria. J. Bioenerg. Biomembr. 2020, 52, 269–277. [Google Scholar] [CrossRef]
- Bareja, A.; Draper, J.A.; Katz, L.H.; Lee, D.E.; Grimsrud, P.A.; White, J.P. Chronic caloric restriction maintains a youthful phosphoproteome in aged skeletal muscle. Mech. Ageing Dev. 2021, 195, 111443. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Saavedra, D.; Moody, L.; Tang, X.; Goldberg, Z.J.; Wang, A.P.; Chen, H.; Pan, Y.X. Caloric restriction following early-life high fat-diet feeding represses skeletal muscle TNF in male rats. J. Nutr. Biochem. 2021, 91, 108598. [Google Scholar] [CrossRef]
- Myers, M.J.; Shaik, F.; Shaik, F.; Alway, S.E.; Mohamed, J.S. Skeletal Muscle Gene Expression Profile in Response to Caloric Restriction and Aging: A Role for SirT1. Genes 2021, 12, 691. [Google Scholar] [CrossRef]
- Swaminathan, A.; Fokin, A.; Venckunas, T.; Degens, H. Methionine restriction plus overload improves skeletal muscle and metabolic health in old mice on a high fat diet. Sci. Rep. 2021, 11, 1260. [Google Scholar] [CrossRef]
- Ham, D.J.; Borsch, A.; Chojnowska, K.; Lin, S.; Leuchtmann, A.B.; Ham, A.S.; Thurkauf, M.; Delezie, J.; Furrer, R.; Burri, D.; et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat. Commun. 2022, 13, 2025. [Google Scholar] [CrossRef]
- Lv, S.; Shen, Q.; Li, H.; Chen, Q.; Xie, W.; Li, Y.; Wang, X.; Ding, G. Caloric restriction delays age-related muscle atrophy by inhibiting 11beta-HSD1 to promote the differentiation of muscle stem cells. Front. Med. 2022, 9, 1027055. [Google Scholar] [CrossRef]
- Swaminathan, A.; Cesanelli, L.; Venckunas, T.; Degens, H. Impact of methionine restriction on muscle aerobic metabolism and hypertrophy in young and old mice on an obesogenic diet. Growth Factors 2022, 40, 108–118. [Google Scholar] [CrossRef]
- Ersoy, U.; Altinpinar, A.E.; Kanakis, I.; Alameddine, M.; Gioran, A.; Chondrogianni, N.; Ozanne, S.E.; Peffers, M.J.; Jackson, M.J.; Goljanek-Whysall, K.; et al. Lifelong dietary protein restriction induces denervation and skeletal muscle atrophy in mice. Free Radic. Biol. Med. 2024, 224, 457–469. [Google Scholar] [CrossRef]
- Li, S.; Zhong, H.; Wang, Z.; Chen, J.; Huang, Z.; Zou, T.; You, J. Dietary protein restriction regulates skeletal muscle fiber metabolic characteristics associated with the FGF21-ERK1/2 pathway. iScience 2024, 27, 109249. [Google Scholar] [CrossRef]
- Vermeij, W.P.; Alyodawi, K.; van Galen, I.; von der Heide, J.L.; Birkisdottir, M.B.; Van’t Sant, L.J.; Ozinga, R.A.; Komninos, D.S.J.; Smit, K.; Rijksen, Y.M.A.; et al. Improved health by combining dietary restriction and promoting muscle growth in DNA repair-deficient progeroid mice. J. Cachexia Sarcopenia Muscle 2024, 15, 2361–2374. [Google Scholar] [CrossRef]
- Zador, E. Paradoxical Effect of Caloric Restriction on Overload-Induced Muscle Growth in Diastolic Heart Failure. JACC Basic Transl. Sci. 2024, 9, 241–243. [Google Scholar] [CrossRef]
- Zhang, T.R.; Chiang, C.H.; Hsu, T.C.; Wang, C.Y.; Chen, C.Y. Age and dietary restriction modulate mitochondrial quality in quadriceps femoris muscle of male mice. Biogerontology 2024, 25, 447–459. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Cheng, X.F.; Cui, Y.Y.; Xia, Q.; Yan, X.Y.; Zhang, M.Y.; Lan, G.Q.; Liu, J.Q.; Shan, T.Z.; Huang, Y.N. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef]
- Ringholm, S.; Olesen, J.; Pedersen, J.T.; Brandt, C.T.; Halling, J.F.; Hellsten, Y.; Prats, C.; Pilegaard, H. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1α. Exp. Gerontol. 2013, 48, 1311–1318. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Sin, T.K.; Yung, B.Y.; Siu, P.M. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: Implications in skeletal muscle aging and insulin resistance. Cell Physiol. Biochem. 2015, 35, 541–552. [Google Scholar] [CrossRef]
- Wang, D.T.; Yin, Y.; Yang, Y.J.; Lv, P.J.; Shi, Y.; Lu, L.; Wei, L.B. Resveratrol prevents TNF-alpha-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Dirks Naylor, A.J. Cellular effects of resveratrol in skeletal muscle. Life Sci. 2009, 84, 637–640. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado Diaz, D.C.; Kostek, M.C. Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of Duchenne muscular dystrophy. Clin. Nutr. 2013, 32, 104–111. [Google Scholar] [CrossRef]
- Su, L.Y.; Huang, W.C.; Kan, N.W.; Tung, T.H.; Huynh, L.B.P.; Huang, S.Y. Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model. Nutrients 2023, 15, 249. [Google Scholar] [CrossRef]
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Attenuating Oxidative Stress and JNK/NF-kappaB Pathway in a SIRT1-Independent Mechanism. J. Cell. Biochem. 2017, 118, 2654–2663. [Google Scholar] [CrossRef]
- Tang, H.; Shrager, J.B.; Goldman, D. Rapamycin protects aging muscle. Aging 2019, 11, 5868–5870. [Google Scholar] [CrossRef]
- Ramos, F.J.; Chen, S.C.; Garelick, M.G.; Dai, D.F.; Liao, C.Y.; Schreiber, K.H.; MacKay, V.L.; An, E.H.; Strong, R.; Ladiges, W.C.; et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012, 4, 144ra103. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Fry, C.S.; Drummond, M.J.; Gundermann, D.M.; Walker, D.K.; Glynn, E.L.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Mammalian Target of Rapamycin Complex 1 Activation Is Required for the Stimulation of Human Skeletal Muscle Protein Synthesis by Essential Amino Acids. J. Nutr. 2011, 141, 856–862. [Google Scholar] [CrossRef]
- Bolster, D.R.; Kubica, N.; Crozier, S.J.; Williamson, D.L.; Farrell, P.A.; Kimball, S.R.; Jefferson, L.S. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J. Physiol. 2003, 553 Pt 1, 213–220. [Google Scholar] [CrossRef]
- Ghaffari, M.H.; Schuh, K.; Dusel, G.; Frieten, D.; Koch, C.; Prehn, C.; Adamski, J.; Sauerwein, H.; Sadri, H. Mammalian target of rapamycin signaling and ubiquitin-proteasome-related gene expression in skeletal muscle of dairy cows with high or normal body condition score around calving. J. Dairy Sci. 2019, 102, 11544–11560. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hambright, W.S.; Takayama, K.; Mu, X.; Lu, A.; Cummins, J.H.; Matsumoto, T.; Yurube, T.; Kuroda, R.; Kurosaka, M.; et al. Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice. Mol. Ther. Methods Clin. Dev. 2019, 14, 64–76. [Google Scholar] [CrossRef]
- Kido, K.; Sase, K.; Yokokawa, T.; Fujita, S. Enhanced skeletal muscle insulin sensitivity after acute resistance-type exercise is upregulated by rapamycin-sensitive mTOR complex 1 inhibition. Sci. Rep. 2020, 10, 8509. [Google Scholar] [CrossRef]
- Yang, Y.; Sadri, H.; Prehn, C.; Adamski, J.; Rehage, J.; Danicke, S.; von Soosten, D.; Metges, C.C.; Ghaffari, M.H.; Sauerwein, H. Proteasome activity and expression of mammalian target of rapamycin signaling factors in skeletal muscle of dairy cows supplemented with conjugated linoleic acids during early lactation. J. Dairy Sci. 2020, 103, 2829–2846. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, H.N.; Li, S.; Jr, A.D.; Chellappa, K.; Davis, J.G.; Guan, Y.; Frederick, D.W.; Chu, W.; Zhao, H.; et al. Rapamycin maintains NAD(+)/NADH redox homeostasis in muscle cells. Aging 2020, 12, 17786–17799. [Google Scholar] [CrossRef]
- Bhat, O.M.; Yuan, X.; Kukreja, R.C.; Li, P.L. Regulatory role of mammalian target of rapamycin signaling in exosome secretion and osteogenic changes in smooth muscle cells lacking acid ceramidase gene. FASEB J. 2021, 35, e21732. [Google Scholar] [CrossRef]
- Elliehausen, C.J.; Minton, D.M.; Nichol, A.D.; Konopka, A.R. Skeletal muscle mitochondrial respiration in a model of age-related osteoarthritis is impaired after dietary rapamycin. Exp. Gerontol. 2021, 155, 111579. [Google Scholar] [CrossRef]
- Orenduff, M.C.; Coleman, M.F.; Glenny, E.M.; Huffman, K.M.; Rezeli, E.T.; Bareja, A.; Pieper, C.F.; Kraus, V.B.; Hursting, S.D. Differential effects of calorie restriction and rapamycin on age-related molecular and functional changes in skeletal muscle. Exp. Gerontol. 2022, 165, 111841. [Google Scholar] [CrossRef]
- Sirago, G.; Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Mammalian Target of Rapamycin (mTOR) Signaling at the Crossroad of Muscle Fiber Fate in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 13823. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, P.; Li, C.; Zhang, Y.; Yang, C.; Zhou, X.; Wang, X.; Su, Z.; Ming, W.; Zeng, L.; et al. Sodium Butyrate Induces Mitophagy and Apoptosis of Bovine Skeletal Muscle Satellite Cells through the Mammalian Target of Rapamycin Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 13474. [Google Scholar] [CrossRef]
- Ato, S.; Oya, C.; Ogasawara, R. Rapamycin administration causes a decrease in muscle contractile function and systemic glucose intolerance concomitant with reduced skeletal muscle Rictor, the mTORC2 component, expression independent of energy intake in young rats. PLoS ONE 2024, 19, e0312859. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Teng, F.; Li, J.; Guan, Y.; Xu, J.; Lv, X.; Guan, F.; Zhang, M.; Chen, L. Berberine Improves Cognitive Deficiency and Muscular Dysfunction via Activation of the AMPK/SIRT1/PGC-1a Pathway in Skeletal Muscle from Naturally Aging Rats. J. Nutr. Health Aging 2018, 22, 710–717. [Google Scholar] [CrossRef]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta 2012, 1822, 185–195. [Google Scholar] [CrossRef]
- Jeong, H.W.; Hsu, K.C.; Lee, J.W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Potue, P.; Wunpathe, C.; Maneesai, P.; Kukongviriyapan, U.; Prachaney, P.; Pakdeechote, P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019, 10, 1880–1892. [Google Scholar] [CrossRef]
- Amarsanaa, K.; Kim, H.J.; Ko, E.A.; Jo, J.; Jung, S.C. Nobiletin Exhibits Neuroprotective Effects against Mitochondrial Complex I Inhibition via Regulating Apoptotic Signaling. Exp. Neurobiol. 2021, 30, 73–86. [Google Scholar] [CrossRef]
- Wang, H.H.; Sun, Y.N.; Qu, T.Q.; Sang, X.Q.; Zhou, L.M.; Li, Y.X.; Ren, F.Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef]
- Lee, S.H.; Li, X.H.; Lu, Q.Y.; Zhan, C.L.; Kim, J.D.; Lee, G.H.; Sim, J.M.; Cui, X.S. Nobiletin enhances mitochondrial function by regulating SIRT1/PGC-1alpha signaling in porcine oocytes during in vitro maturation. Biochem. Biophys. Res. Commun. 2024, 706, 149747. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, Y.; Zhang, H.; Meng, X.; Jin, L.; Yang, J.; Wang, W.; Ning, G.; Zhang, Y.; Zhang, Z. Berberine attenuates the abnormal ectopic lipid deposition in skeletal muscle. Free Radic. Biol. Med. 2020, 159, 66–75. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Zhang, Y.; Li, K.; Gao, X.J.; Guo, M.Y. Berberine Depresses Inflammation and Adjusts Smooth Muscle to Ameliorate Ulcerative Colitis of Cats by Regulating Gut Microbiota. Microbiol. Spectr. 2022, 10, e0320722. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Du, C.; Peng, X.; Fan, W.; Chang, B.; Shan, C. Berberine attenuates obesity-induced skeletal muscle atrophy via regulation of FUNDC1 in skeletal muscle of mice. Sci. Rep. 2025, 15, 4918. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef]
- Yang, G.; Lin, C.C.; Yang, Y.; Yuan, L.; Wang, P.; Wen, X.; Pan, M.H.; Zhao, H.; Ho, C.T.; Li, S. Nobiletin Prevents Trimethylamine Oxide-Induced Vascular Inflammation via Inhibition of the NF-kappaB/MAPK Pathways. J. Agric. Food Chem. 2019, 67, 6169–6176. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, S.G.; Yue, G.G.; Kwok, H.F.; Lee, J.K.; Zheng, T.; Shaw, P.C.; Simmonds, M.S.J.; Lau, C.B. Natural flavone tricin exerted anti-inflammatory activity in macrophage via NF-kappaB pathway and ameliorated acute colitis in mice. Phytomedicine 2021, 90, 153625. [Google Scholar] [CrossRef]
- Chang, B.Y.; Bae, J.H.; Lim, C.Y.; Kim, Y.H.; Kim, T.Y.; Kim, S.Y. Tricin-enriched Zizania latifolia ameliorates non-alcoholic fatty liver disease through AMPK-dependent pathways. Food Sci. Biotechnol. 2023, 32, 2117–2129. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhou, X.Y.; Lan, C.; Wen, Y.P.; Fu, H.J.; Li, Z.C.; Li, Y.P.; Li, S.Y.; Huang, F.H.; Wang, L.; et al. Tricin Delays Aging and Enhances Muscle Function via Activating AMPK-Mediated Autophagy in Diverse Model Organisms. J. Agric. Food Chem. 2025, 73, 10246–10264. [Google Scholar] [CrossRef]
- Gherardi, G.; Weiser, A.; Bermont, F.; Migliavacca, E.; Brinon, B.; Jacot, G.E.; Hermant, A.; Sturlese, M.; Nogara, L.; Vascon, F.; et al. Mitochondrial calcium uptake declines during aging and is directly activated by oleuropein to boost energy metabolism and skeletal muscle performance. Cell Metab. 2025, 37, 477–495.e411. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Ratajczak, J.; Doig, C.L.; Oakey, L.A.; Callingham, R.; Da Silva Xavier, G.; Garten, A.; Elhassan, Y.S.; Redpath, P.; Migaud, M.E.; et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 2017, 6, 819–832. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e1716. [Google Scholar] [CrossRef]
- Khan, N.A.; Auranen, M.; Paetau, I.; Pirinen, E.; Euro, L.; Forsstrom, S.; Pasila, L.; Velagapudi, V.; Carroll, C.J.; Auwerx, J.; et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med. 2014, 6, 721–731. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, M.; Xiao, X.; Lu, Y.; Zhao, W.; Zheng, K.; Yu, K.; He, Y.; Zhong, Q.; Zhou, L.; et al. Sarcosine decreases in sarcopenia and enhances muscle regeneration and adipose thermogenesis by activating anti-inflammatory macrophages. Nat. Aging 2025. [Google Scholar] [CrossRef]
- Cichello, S.A.; Weisinger, R.S.; Schuijers, J.; Jois, M. 1-Sarcosine-angiotensin II infusion effects on food intake, weight loss, energy expenditure, and skeletal muscle UCP3 gene expression in a rat model. J. Cachexia Sarcopenia Muscle 2014, 5, 239–246. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Felix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Rodriguez, J.; Pierre, N.; Naslain, D.; Bontemps, F.; Ferreira, D.; Priem, F.; Deldicque, L.; Francaux, M. Urolithin B, a newly identified regulator of skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2017, 8, 583–597. [Google Scholar] [CrossRef]
- Luan, P.; D’Amico, D.; Andreux, P.A.; Laurila, P.P.; Wohlwend, M.; Li, H.; Imamura de Lima, T.; Place, N.; Rinsch, C.; Zanou, N.; et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci. Transl. Med. 2021, 13, eabb0319. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Biswas, N.; Gnyawali, S.; Singh, K.; Gorain, M.; Polcyn, C.; Khanna, S.; Roy, S.; Sen, C.K. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci. Rep. 2020, 10, 20184. [Google Scholar] [CrossRef]
- Faitg, J.; D’Amico, D.; Rinsch, C.; Singh, A. Mitophagy Activation by Urolithin A to Target Muscle Aging. Calcif. Tissue Int. 2024, 114, 53–59. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, Z.; Jiang, J.; Xu, W.; Yuan, A.; Liao, F.; Ding, S.; Pu, J. Urolithin A Protects against Hypoxia-Induced Pulmonary Hypertension by Inhibiting Pulmonary Arterial Smooth Muscle Cell Pyroptosis via AMPK/NF-kappaB/NLRP3 Signaling. Int. J. Mol. Sci. 2024, 25, 8246. [Google Scholar] [CrossRef]
- Moradi, N.; Champsi, S.; Hood, D.A. Sulforaphane, Urolithin A, and ZLN005 induce time-dependent alterations in antioxidant capacity, mitophagy, and mitochondrial biogenesis in muscle cells. Sports Med. Health Sci. 2025, 7, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, A.; Karagounis, L.G.; Bennett, A.J.; D’Amico, D.; Fouassier, A.M.; Jones, S.W.; Tsintzas, K. The polyphenol metabolite urolithin A suppresses myostatin expression and augments glucose uptake in human skeletal muscle cells. Nutr. Metab. 2025, 22, 12. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J. Beta-Hydroxy-Beta-Methyl Butyrate (HMB): From Experimental Data to Clinical Evidence in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 668–672. [Google Scholar] [CrossRef]
- Portal, S.; Eliakim, A.; Nemet, D.; Halevy, O.; Zadik, Z. Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J. Pediatr. Endocrinol. Metab. 2010, 23, 641–650. [Google Scholar] [CrossRef]
- Slater, G.J.; Jenkins, D. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med. 2000, 30, 105–116. [Google Scholar] [CrossRef]
- Alon, T.; Bagchi, D.; Preuss, H.G. Supplementing with beta-hydroxy-beta-methylbutyrate (HMB) to build and maintain muscle mass: A review. Res. Commun. Mol. Pathol. Pharmacol. 2002, 111, 139–151. [Google Scholar]
- Russ, D.W.; Acksel, C.; Boyd, I.M.; Maynard, J.; McCorkle, K.W.; Edens, N.K.; Garvey, S.M. Dietary HMB and beta-alanine co-supplementation does not improve in situ muscle function in sedentary, aged male rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1294–1301. [Google Scholar] [CrossRef]
- Shirvani, H.; Rahmati-Ahmadabad, S.; Kowsari, E.; Fry, H.; Kazemi, M.; Kaviani, M. Effects of 2-week HMB-FA supplementation with or without eccentric resistance exercise on expression of some genes related to muscle protein turnover and serum irisin and IGF-1 concentrations. Gene 2020, 760, 145018. [Google Scholar] [CrossRef]
- Cavallucci, V.; Pani, G. The Leucine Catabolite and Dietary Supplement beta-Hydroxy-beta-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. Metabolites 2021, 11, 512. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ueda, H.; Sugita, N.; Ochi, E. Low Dose of beta-Hydroxy-beta-Methylbutyrate (HMB) Alleviates Muscle Strength Loss and Limited Joint Flexibility following Eccentric Contractions. J. Am. Coll. Nutr. 2021, 40, 211–218. [Google Scholar] [CrossRef]
- Viana, M.V.; Becce, F.; Pantet, O.; Schmidt, S.; Bagnoud, G.; Thaden, J.J.; Ten Have, G.A.M.; Engelen, M.; Voidey, A.; Deutz, N.E.P.; et al. Impact of beta-hydroxy-beta-methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: A RCT. Clin. Nutr. 2021, 40, 4878–4887. [Google Scholar] [CrossRef]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of beta-hydroxy beta-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Souza, N.P.; Amado, L.R.N.; Melo, J.O.F.; Reis, I.A.; Anastacio, L.R. The effect of beta-hydroxy beta-methylbutyrate (HMB) with nutritional counselling on anthropometric muscle mass markers, strength, functionality, and quality of life in patients on the waiting list for liver transplantation: A double-blind study. Nutrition 2023, 110, 112021. [Google Scholar] [CrossRef]
- Manzano, M.; Giron, M.D.; Salto, R.; Burgio, C.; Reinoso, A.; Cabrera, E.; Rueda, R.; Lopez-Pedrosa, J.M. Arginine and Lysine Supplementation Potentiates the Beneficial beta-Hydroxy ss-Methyl Butyrate (HMB) Effects on Skeletal Muscle in a Rat Model of Diabetes. Nutrients 2023, 15, 4706. [Google Scholar] [CrossRef]
- Cohen-Or, M.; Chapnik, N.; Froy, O. beta-Hydroxy-beta-methylbutyrate (HMB) increases muscle mass and diminishes weight gain in high-fat-fed mice. J. Nutr. Biochem. 2025, 142, 109926. [Google Scholar] [CrossRef]
- Pham, T.; MacRae, C.L.; Broome, S.C.; D’Souza, R.F.; Narang, R.; Wang, H.W.; Mori, T.A.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 1657–1669. [Google Scholar] [CrossRef]
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281. [Google Scholar] [CrossRef]
- Rudolph, T.E.; Mayorga, E.J.; Roths, M.; Rhoads, R.P.; Baumgard, L.H.; Selsby, J.T. The effect of Mitoquinol (MitoQ) on heat stressed skeletal muscle from pigs, and a potential confounding effect of biological sex. J. Therm. Biol. 2021, 97, 102900. [Google Scholar] [CrossRef]
- Broome, S.C.; Pham, T.; Braakhuis, A.J.; Narang, R.; Wang, H.W.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. MitoQ supplementation augments acute exercise-induced increases in muscle PGC1alpha mRNA and improves training-induced increases in peak power independent of mitochondrial content and function in untrained middle-aged men. Redox Biol. 2022, 53, 102341. [Google Scholar] [CrossRef]
- Pin, F.; Huot, J.R.; Bonetto, A. The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef]
- Graham, Z.A.; DeBerry, J.J.; Cardozo, C.P.; Bamman, M.M. A 50 kdyne contusion spinal cord injury with or without the drug SS-31 was not associated with major changes in muscle mass or gene expression 14 d after injury in young male mice. Physiol. Rep. 2021, 9, e14751. [Google Scholar] [CrossRef]
- Graham, Z.A.; DeBerry, J.J.; Cardozo, C.P.; Bamman, M.M. SS-31 does not prevent or reduce muscle atrophy 7 days after a 65 kdyne contusion spinal cord injury in young male mice. Physiol. Rep. 2022, 10, e15266. [Google Scholar] [CrossRef] [PubMed]
- Ancel, S.; Michaud, J.; Migliavacca, E.; Jomard, C.; Fessard, A.; Garcia, P.; Karaz, S.; Raja, S.; Jacot, G.E.; Desgeorges, T.; et al. Nicotinamide and pyridoxine stimulate muscle stem cell expansion and enhance regenerative capacity during aging. J. Clin. Investig. 2024, 134, e163648. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth Differentiation Factor 11 Is a Circulating Factor that Reverses Age-Related Cardiac Hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Rebo, J.; Mehdipour, M.; Gathwala, R.; Causey, K.; Liu, Y.; Conboy, M.J.; Conboy, I.M. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat. Commun. 2016, 7, 13363. [Google Scholar] [CrossRef]
- Sahu, A.; Clemens, Z.J.; Shinde, S.N.; Sivakumar, S.; Pius, A.; Bhatia, A.; Picciolini, S.; Carlomagno, C.; Gualerzi, A.; Bedoni, M.; et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat. Aging 2021, 1, 1148–1161. [Google Scholar] [CrossRef]
- Fish, L.A.; Fallon, J.R. Multiple MuSK signaling pathways and the aging neuromuscular junction. Neurosci. Lett. 2020, 731, 135014. [Google Scholar] [CrossRef]
- Zhou, Z.; Dun, L.; Wei, B.; Gan, Y.; Liao, Z.; Lin, X.; Lu, J.; Liu, G.; Xu, H.; Lu, C.; et al. Musk Ketone Induces Neural Stem Cell Proliferation and Differentiation in Cerebral Ischemia via Activation of the PI3K/Akt Signaling Pathway. Neuroscience 2020, 435, 1–9. [Google Scholar] [CrossRef]
- Madison, R.D.; Robinson, G.A. Muscle-Derived Extracellular Vesicles Influence Motor Neuron Regeneration Accuracy. Neuroscience 2019, 419, 46–59. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Deng, C.; Qiu, J.; Wang, K.; Chang, M.; Zhou, S.; Gu, Y.; Shen, Y.; Wang, W.; et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants 2022, 12, 44. [Google Scholar] [CrossRef]
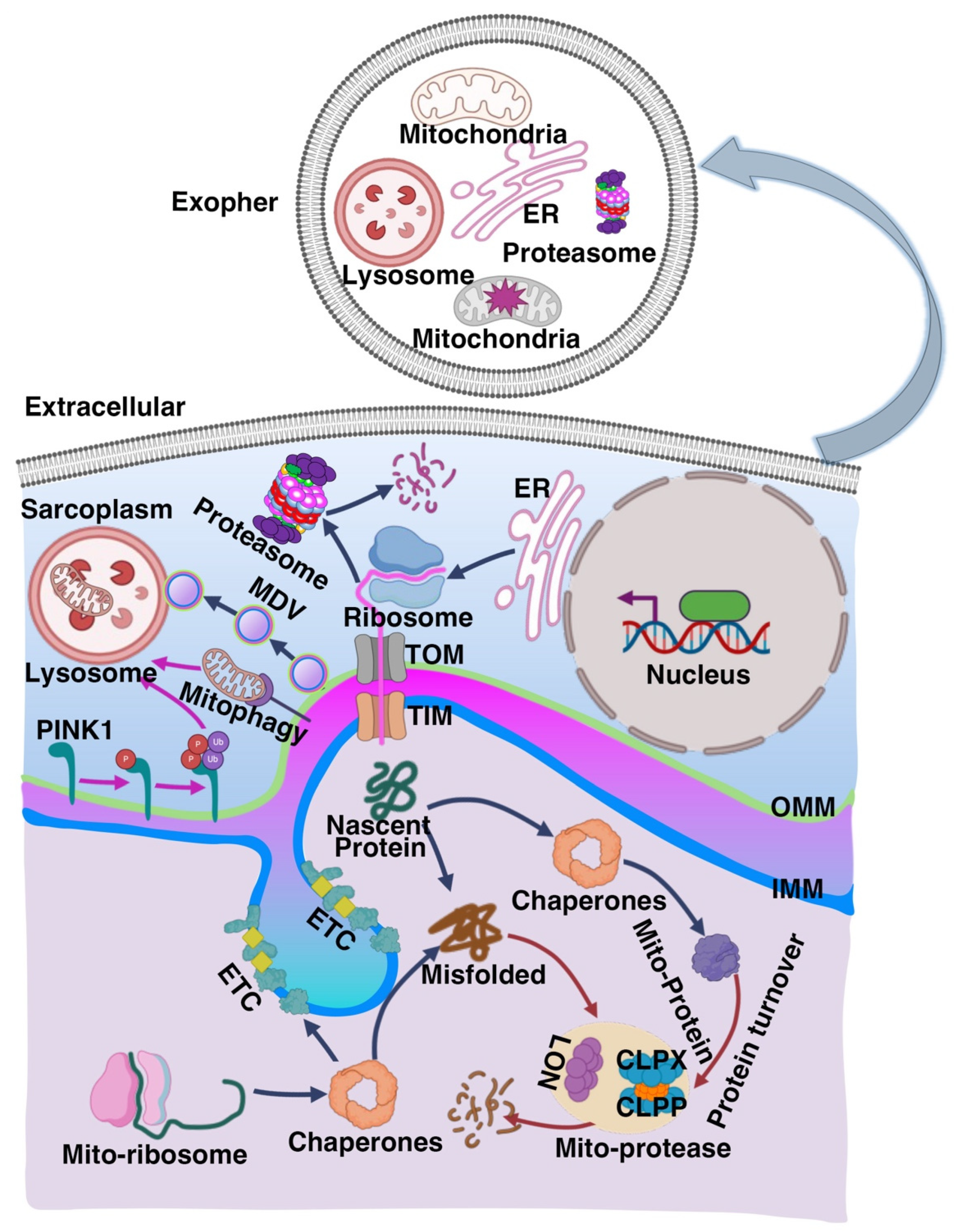
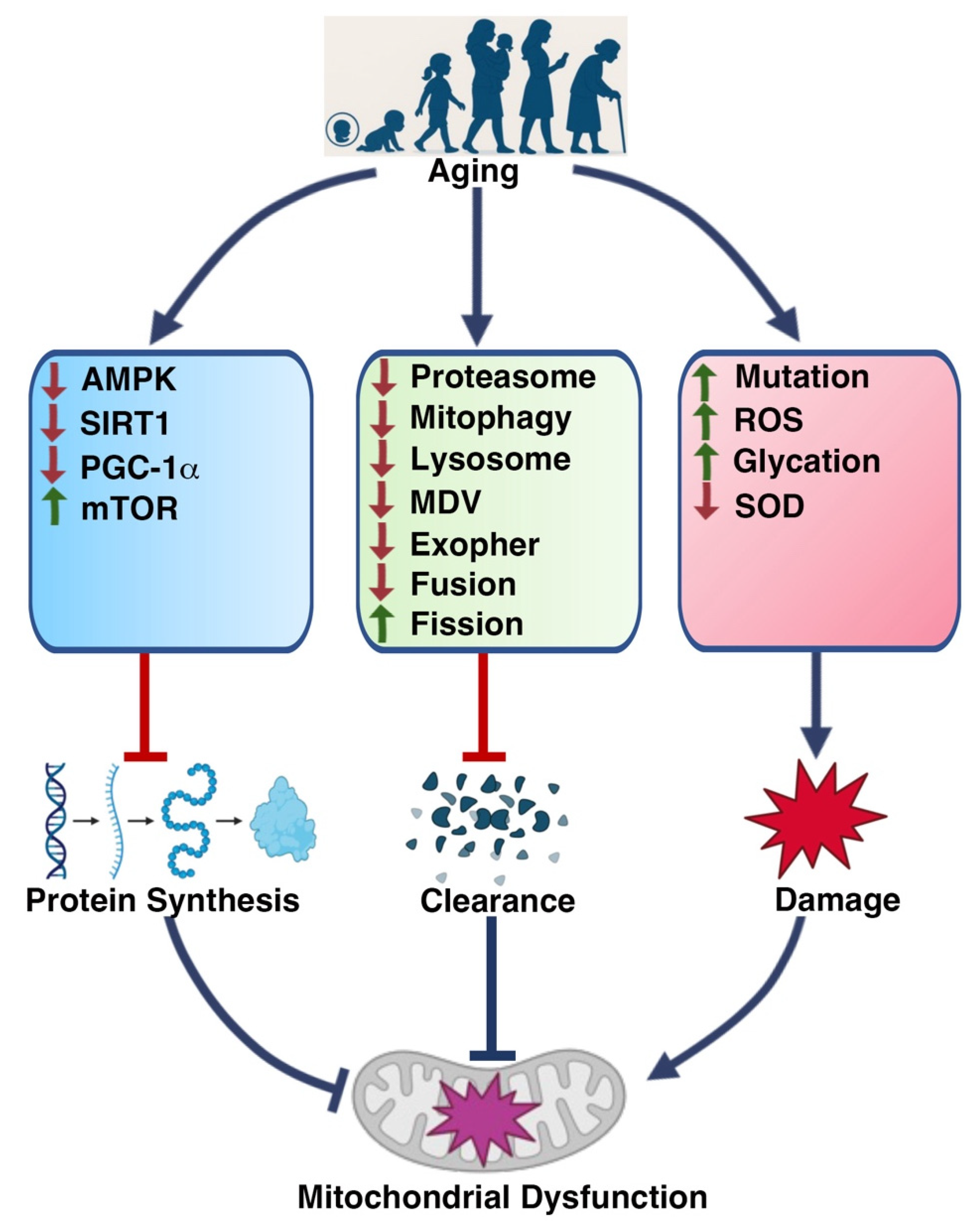

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Khanzada, Z.; Xu, Y. Mechanisms Underlying Muscle-Related Diseases and Aging: Insights into Pathophysiology and Therapeutic Strategies. Muscles 2025, 4, 26. https://doi.org/10.3390/muscles4030026
Fan J, Khanzada Z, Xu Y. Mechanisms Underlying Muscle-Related Diseases and Aging: Insights into Pathophysiology and Therapeutic Strategies. Muscles. 2025; 4(3):26. https://doi.org/10.3390/muscles4030026
Chicago/Turabian StyleFan, Jialin, Zara Khanzada, and Yunpeng Xu. 2025. "Mechanisms Underlying Muscle-Related Diseases and Aging: Insights into Pathophysiology and Therapeutic Strategies" Muscles 4, no. 3: 26. https://doi.org/10.3390/muscles4030026
APA StyleFan, J., Khanzada, Z., & Xu, Y. (2025). Mechanisms Underlying Muscle-Related Diseases and Aging: Insights into Pathophysiology and Therapeutic Strategies. Muscles, 4(3), 26. https://doi.org/10.3390/muscles4030026





