Biopsychological Effects of Ashwagandha (Withania somnifera) in Athletes and Healthy Individuals: A Systematic Review
Abstract
1. Introduction
2. Results
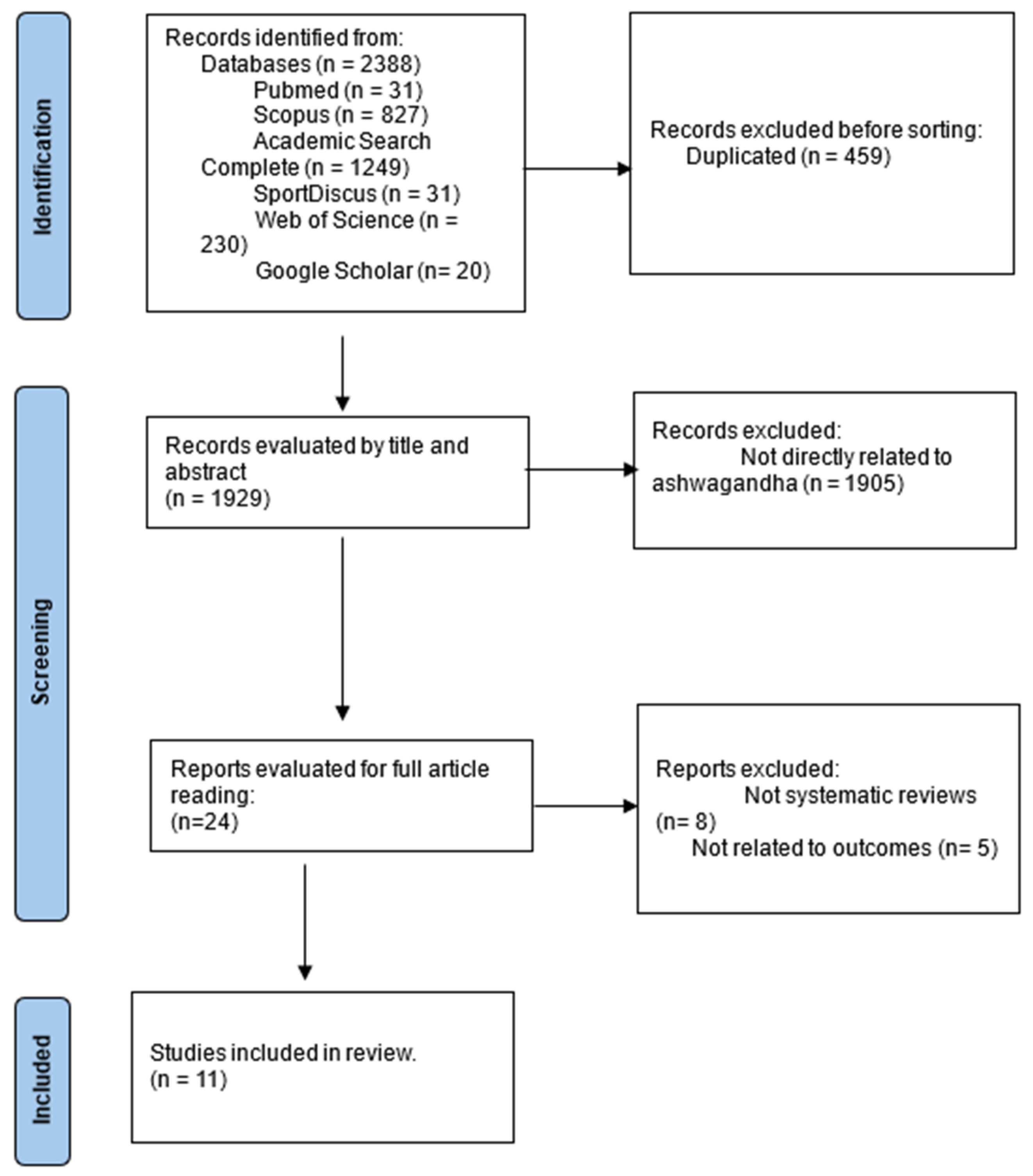
2.1. Study Selection
2.2. Methodological Quality
2.3. Study Characteristics
3. Materials and Methods
3.1. Search Strategy
3.2. Study Selection Process
3.3. Data Extraction and Synthesis
3.4. Outcomes
3.5. Quality Assessment
4. Discussion
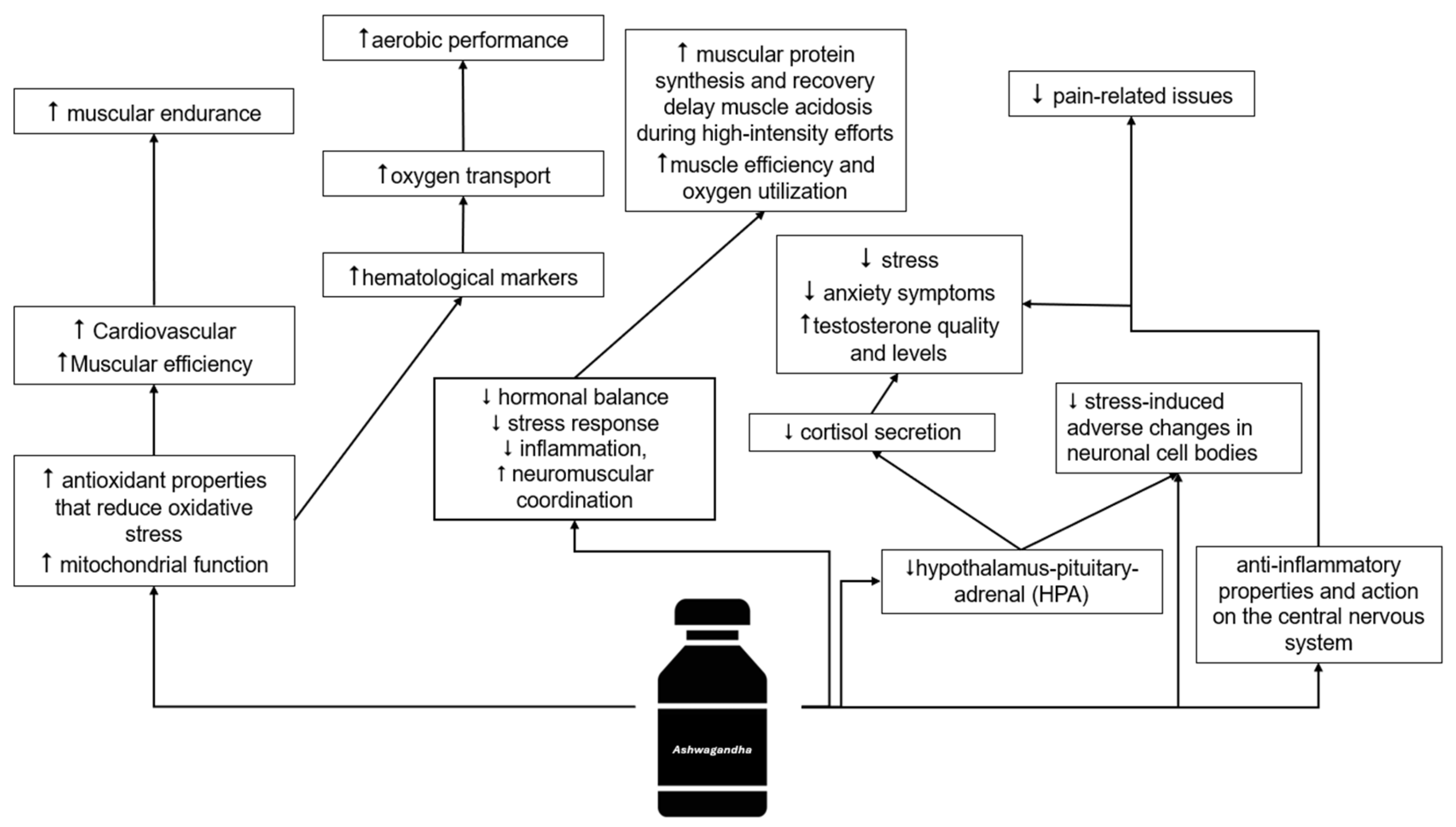
4.1. Sports Performance
4.2. Health
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Côté, J.; Macdonald, D.J.; Baker, J.; Abernethy, B. When “where” is more important than “when”: Birthplace and birthdate effects on the achievement of sporting expertise. J. Sports Sci. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. S2), 139–147. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.; Rodrigues, A.; Sabino, B.; Alves, R.; Correia, A.L.; Lopes, H. The Effect of Motivation on Physical Activity among Middle and High School Students. Sports 2024, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Bongard, V.; McDermott, A.Y.; Dallal, G.E.; Schaefer, E.J. Effects of age and gender on physical performance. Age 2007, 29, 77–85. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Cui, Y.; Chen, S.; Wu, Z. Relationship between sleep and muscle strength among Chinese university students: A cross-sectional study. J. Musculoskelet. Neuronal Interact. 2017, 17, 327. [Google Scholar]
- Dai, B.; Layer, J.S. Strength Assessments: Neuromuscular and Biomechanical Considerations. In Nutrition and Enhanced Sports Performance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 471–481. [Google Scholar]
- Sabir, Z.; Dierkes, J.; Hjartåker, A.; Rosendahl-Riise, H. The association of dietary patterns with muscle mass and strength in old age: The Hordaland Health Study. Eur. J. Nutr. 2023, 62, 2739–2750. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Stewart Jr, R.W.; Moyen, N.E.; Kavouras, S.A.; Dibrezzo, R.O.; Stone, M.S. Effects of 28-day beta-alanine supplementation on isokinetic exercise performance and body composition in female masters athletes. J. Strength Cond. Res. 2016, 30, 200–207. [Google Scholar] [CrossRef]
- Hackett, D.A. Training, supplementation, and pharmacological practices of competitive male bodybuilders across training phases. J. Strength Cond. Res. 2022, 36, 963–970. [Google Scholar] [CrossRef]
- Hall, M.; Manetta, E.; Tupper, K. Creatine supplementation: An update. Curr. Sports Med. Rep. 2021, 20, 338–344. [Google Scholar] [CrossRef]
- Jones, L.; Johnstone, I.; Day, C.; Le Marquer, S.; Hulton, A.T. The dose-effects of caffeine on lower body maximal strength, muscular endurance, and rating of perceived exertion in strength-trained females. Nutrients 2021, 13, 3342. [Google Scholar] [CrossRef]
- Juhn, M.S. Popular sports supplements and ergogenic aids. Sports Med. 2003, 33, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Gunes-Bayir, A.; Çemberci, İ.M. A review of ergogenic nutritional supplements for athletes. Arch. Sports Med. Physiother. 2023, 8, 003–010. [Google Scholar] [CrossRef]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D.; Langade, D.G. Efficacy and safety of Ashwagandha (Withania somnifera) root extract in insomnia and anxiety: A double-blind, randomized, placebo-controlled study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- Połumackanycz, M.; Forencewicz, A.; Wesołowski, M.; Viapiana, A. Ashwagandha (Withania somnifera L.)–roślina o udokumentowanych właściwościach prozdrowotnych. Farm Pol. 2020, 76, 442–447. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Moreno, Y.; Gho, C.; Petro, J.L.; Odriozola-Martínez, A.; Kreider, R.B. Effects of Ashwagandha (Withania somnifera) on physical performance: Systematic review and bayesian meta-analysis. J. Funct. Morphol. Kinesiol. 2021, 6, 20. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szelag, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current research on the health-promoting activities: A narrative review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Agnihotri, A.P.; Sontakke, S.D.; Thawani, V.R.; Saoji, A.; Goswami, V.S.S. Effects of Withania somnifera in patients of schizophrenia: A randomized, double blind, placebo controlled pilot trial study. Indian J. Pharmacol. 2013, 45, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef]
- Khalil, M.I.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. BioMed Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.R.; Arya, D.S.; Gupta, S.K. Withania somnifera provides cardioprotection and attenuates ischemia–reperfusion induced apoptosis. Clin. Nutr. 2008, 27, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D.; Langade, D.G. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: A double-blind, randomized, placebo-controlled clinical study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Kedia, A.W.; Sandrock, J.E.; Raub, B.J.; Kerksick, C.M.; Lopez, H.L. Effects of an aqueous extract of Withania somnifera on strength training adaptations and recovery: The STAR trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 43. [Google Scholar] [CrossRef]
- Pingali, U.; Pilli, R.; Fatima, N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacogn. Res. 2014, 6, 12. [Google Scholar] [CrossRef]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Asoudeh, F.; Bagheri, A.; Kalantar, Z.; Vahabi, Z.; Shab-bidar, S.; Rezvani, H.; Djafarian, K. Does Ashwagandha supplementation have a beneficial effect on the management of anxiety and stress? A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2022, 36, 4115–4124. [Google Scholar] [CrossRef]
- Arumugam, V.; Vijayakumar, V.; Balakrishnan, A.; Bhandari, R.; Boopalan, D.; Ponnurangam, R.; Thirupathy, V.; Kuppusamy, M. Effects of Ashwagandha (Withania Somnifera) on stress and anxiety: A systematic review and meta-analysis. Explore 2024, 20, 103062. [Google Scholar] [CrossRef]
- Cheah, K.L.; Norhayati, M.N.; Yaacob, L.H.; Rahman, R.A. Effect of Ashwagandha (Withania somnifera) extract on sleep: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0257843. [Google Scholar] [CrossRef]
- Della Porta, M.; Maier, J.A.; Cazzola, R. Effects of Withania somnifera on cortisol levels in stressed human subjects: A systematic review. Nutrients 2023, 15, 5015. [Google Scholar] [CrossRef] [PubMed]
- Didio, F.P.; Duarte, A.R.; Stefani, G.P. Effects of the Withania somnifera supplementation on sports performance: A systematic review and meta-analysis. N. Afr. J. Food Nutr. Res. 2022, 6, 20. [Google Scholar] [CrossRef]
- Gómez Afonso, A.; Fernandez-Lazaro, D.; Adams, D.P.; Monserda-Vilaro, A.; Fernandez-Lazaro, C.I. Effects of Withania somnifera (Ashwagandha) on hematological and biochemical markers, hormonal behavior, and oxidant response in healthy adults: A systematic review. Curr. Nutr. Rep. 2023, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J. Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: A systematic review of human trials. J. Herb. Med. 2021, 28, 100434. [Google Scholar] [CrossRef]
- Pratte, M.A.; Nanavati, K.B.; Young, V.; Morley, C.P. An alternative treatment for anxiety: A systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J. Altern. Complement. Med. 2014, 20, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, J.; Villafaina, S.; Adsuar, J.C.; Merellano-Navarro, E.; Collado-Mateo, D. Effects of Ashwagandha (Withania somnifera) on VO2Max: A systematic review and meta-analysis. Nutrients 2020, 12, 1119. [Google Scholar] [CrossRef]
- Smith, S.J.; Lopresti, A.L.; Teo, S.Y.; Fairchild, T.J. Examining the Effects of Herbs on Testosterone Concentrations in Men: A Systematic Review. Adv. Nutr. 2021, 12, 744–765. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef]
- Ambiye, V.R.; Langade, D.; Dongre, S.; Aptikar, P.; Kulkarni, M.; Dongre, A. Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: A pilot study. Evid.-Based Complement. Altern. Med. 2013, 2013, 571420. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.M.; Sulaiman, S.A.; Ismail, H.C.; Zakaria, H.; Musa, K.I. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr. Cancer Ther. 2013, 12, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, K.R.; Bowie, C.R.; Schlicht, P.J.; Fleet, D.; Brar, J.S.; Jindal, R. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry 2013, 74, 16816. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
- Khyati, S.; Anup, B. A randomized double blind placebo controlled study of ashwagandha on generalized anxiety disorder. Int. Ayurvedic Med. J. 2013, 1, 1–7. [Google Scholar]
- Malik, A.; Mehta, V.; Dahiya, V. Effect of Ashwagandha (Withania somnifera) root powder supplementation on the VO2Max and hemoglobin in hockey players. Int. J. Behav. Soc. Mov. Sci. 2013, 2, 91–99. [Google Scholar]
- Pingali, U.; Pilli, R.; Fatima, N. Effect of Withania somnifera extract on mental stress induced changes in hemodynamic properties and arterial wave reflections in healthy subjects. Curr. Top. Nutraceuticals Res. 2013, 11, 151. [Google Scholar]
- Usharani, P.; Kumar, C.U.; Pokuri, V.K. Evaluation of the analgesic activity of standardized aqueous extract of Withania somnifera in healthy human volunteers using Hot Air Pain Model. Res. J. Life Sci. 2013, 1, 1–6. [Google Scholar]
- Gannon, J.M.; Forrest, P.E.; Chengappa, K.R. Subtle changes in thyroid indices during a placebo-controlled study of an extract of Withania somnifera in persons with bipolar disorder. J. Ayurveda Integr. Med. 2014, 5, 241. [Google Scholar] [CrossRef]
- Kuchewar, V.V.; Borkar, M.A.; Nisargandha, M.A. Evaluation of antioxidant potential of Rasayana drugs in healthy human volunteers. Int. Q. J. Res. Ayurveda 2014, 35, 46–49. [Google Scholar] [CrossRef]
- Sufiyan Ahmad Ghawte, S.N.; Ahmad, J.; Mulla, G. Withania somnifera L. Dunal a potential herb for the treatment of rheumatoid arthritis. Ann. Phytomed. 2014, 3, 98–102. [Google Scholar]
- Usharani, P.; Fatima, N.; Kumar, C.U.; Kishan, P.V. Evaluation of a highly standardized Withania somnifera extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double blind, placebo controlled study. Int. J. Ayurveda Pharma Res. 2014, 2, 22–32. [Google Scholar]
- Usharani, P.; Kishan, P.V.; Nishat Fatima, N.F.; Kumar, C.U. A comparative study to evaluate the effect of highly standardised aqueous extracts of Phyllanthus emblica, Withania somnifera and their combination on endothelial dysfunction and biomarkers in patients with type II Diabetes Mellitus. Int. J. Pharm. Sci. Res. 2014, 5, 2687–2697. [Google Scholar]
- Murthy, M.N.K.; Gundagani, S.; Nutalapati, C.; Pingali, U. Evaluation of Analgesic Activity of Standardised Aqueous Extract of Withania somnifera in Healthy Human Volunteers using Mechanical Pain Model. J. Clin. Diagn. Res. 2019, 13, FC1–FC4. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D.; Smith, S.J. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of ashwagandha (Withania somnifera) in aging, overweight males. Am. J. Men’s Health 2019, 13, 1557988319835985. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J.M.; Brar, J.; Rai, A.; Chengappa, K.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiatry 2019, 31, 123–129. [Google Scholar]
- Andallu, B.; Radhika, B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J. Exp. Biol. 2000, 38, 607–609. [Google Scholar]
- Ahmad, M.K.; Mahdi, A.A.; Shukla, K.K.; Islam, N.; Rajender, S.; Madhukar, D.; Shankhwar, S.N.; Ahmad, S. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil. Steril. 2010, 94, 989–996. [Google Scholar] [CrossRef]
- Nasimi Doost Azgomi, R.; Nazemiyeh, H.; Bazargani, H.; Fazljou, S.M.B.; Nejatbaksh, F.; Jazani, A.M.; Asrbadr, Y.A.; Zomorrodi, A. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: A triple-blind randomised clinical trial. Andrologia 2018, 50, e13041. [Google Scholar] [CrossRef]
- Chengappa, K.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive use of a standardized extract of Withania somnifera (Ashwagandha) to treat symptom exacerbation in schizophrenia: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2018, 79, 22496. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and safety of ashwagandha root extract in subclinical hypothyroid patients: A double-blind, randomized placebo-controlled trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, R.M.; Tricco, A.C.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny, C.; et al. Reporting guideline for overviews of reviews of healthcare interventions: Development of the PRIOR statement. BMJ 2022, 378, e070849. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Shetty, A.; Langade, D.G. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in improving cardiorespiratory endurance in healthy athletic adults. Int. Q. J. Res. Ayurveda 2015, 36, 63–68. [Google Scholar]
- Tiwari, S.; Gupta, S.K.; Pathak, A.K. A double-blind, randomized, placebo-controlled trial on the effect of Ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults. J. Ethnopharmacol. 2021, 272, 113929. [Google Scholar] [CrossRef] [PubMed]
- Jayawanth Manjunath, M. Effect of Withania somnifera supplementation on rotenone-induced oxidative damage in cerebellum and striatum of the male mice brain. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 43–56. [Google Scholar] [CrossRef]
- Begum, V.H.; Sadique, J. Effect of Withania somnifera on glycosaminoglycan synthesis in carrageenin-induced air pouch granuloma. Biochem. Med. Metab. Biol. 1987, 38, 272–277. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Pérez-Idárraga, A.; Odriozola-Martínez, A.; Kreider, R.B. The 4r’s framework of nutritional strategies for post-exercise recovery: A review with emphasis on new generation of carbohydrates. Int. J. Environ. Res. Public Health 2021, 18, 103. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Reid, M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. 2008, 104, 853–860. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- McKenna, M.J.; Medved, I.; Goodman, C.A.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X. N-acetylcysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. J. Physiol. 2006, 576, 279–288. [Google Scholar] [CrossRef]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; McKenna, M.J. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J. Appl. Physiol. 2004, 96, 211–217. [Google Scholar] [CrossRef]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Sostaric, P.S.; Gong, X.; McKenna, M.J. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 2004, 97, 1477–1485. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Shah, B.; Shenoy, S.; Chauhan, S.; Lavekar, G.S.; Padhi, M.M. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of acute alkalosis and acidosis on performance: A meta-analysis. Sports Med. 2011, 41, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance—An umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef]
- Abedon, B.; Auddy, B.; Hazra, J.; Mitra, A.; Ghosal, S. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: A double-blind, randomized, placebo-controlled study. JANA 2008, 11, 50–56. [Google Scholar]
- Jain, S.; Shukla, S.D.; Sharma, K.; Bhatnagar, M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother. Res. 2001, 15, 544–548. [Google Scholar] [CrossRef]
- Mehta, A.K.; Binkley, P.; Gandhi, S.S.; Ticku, M.K. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J. Med. Res. 1991, 94, 312–315. [Google Scholar]
- Jie, F.; Yin, G.; Yang, W.; Yang, M.; Gao, S.; Lv, J.; Li, B. Stress in regulation of GABA amygdala system and relevance to neuropsychiatric diseases. Front. Neurosci. 2018, 12, 562. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol. Bull. 2003, 37, 133–146. [Google Scholar] [PubMed]
- Kumar, V.; Dey, A.; Hadimani, M.B.; Marcovic, T.; Emerald, M. Chemistry and pharmacology of Withania somnifera: An update. CellMed 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Sharma, D.; Salvi, M. Neuroprotective effects of Withania somnifera dunal.: A possible mechanism. Neurochem. Res. 2009, 34, 1975–1983. [Google Scholar] [CrossRef]
- Kumar, A.; Chanana, P. Role of nitric oxide in stress-induced anxiety: From pathophysiology to therapeutic target. Vitam. Horm. 2017, 103, 147–167. [Google Scholar] [PubMed]
- Gautam, M.; Agrawal, M.; Gautam, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatry 2012, 54, 244–247. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghoray, M.; Saha, S.C.; Patil, M.; Kandimalla, R.; Prockow, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Kelgane, S.B.; Salve, J.; Sampara, P.; Debnath, K. Efficacy and tolerability of ashwagandha root extract in the elderly for improvement of general well-being and sleep: A prospective, randomized, double-blind, placebo-controlled study. Cureus 2020, 12, e7083. [Google Scholar] [CrossRef]
- Ramakanth, G.S.H.; Kumar, C.U.; Kishan, P.V.; Usharani, P. A ran domized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J. Ayurveda Integr. Med. 2016, 7, 151–157. [Google Scholar] [CrossRef]
- Dongre, S.; Langade, D.; Bhattacharyya, S. Efficacy and safety of Ashwagandha (Withania somnifera) root extract in improving sexual function in women: A pilot study. BioMed Res. Int. 2015, 2015, 284154. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Rajender, S.; Shankhwar, S.N.; Singh, V.; Dalela, D. Withania somnifera improves semen quality in stress-related male fertility. Evid. Based Complement. Altern. Med. 2011, 2011, 576962. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Wong, S.K.; Hasan, W.N.W.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Noshahr, Z.S.; Shahraki, M.R.; Ahmadvand, H.; Nourabadi, D.; Nakhaei, A. Protective effects of Withania somnifera root on inflammatory markers and insulin resistance in fructose-fed rats. Rep. Biochem. Mol. Biol. 2015, 3, 62. [Google Scholar]
- Shahraki, M.R.; Noshahr, Z.S.; Ahmadvand, H.; Nakhaie, A. Anti-nociceptive and anti-inflammatory effects of Withania somnifera root in fructose fed male rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Fakjiel-Madajczyk, A.; Kurant, Z.; Kurant, D.; Gryczka, K.; Falkowski, M.; Wisniewska, M.; Slupski, M.; Ohla, J.; Zabrzynski, J. Can Ashwagandha Benefit the Endocrine System?—A Review. Int. J. Mol. Sci. 2023, 24, 16513. [Google Scholar] [CrossRef]



| AMSTAR-2 Items | AMSTAR-2 Score | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (A to Z) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Akhgarjand, C., et al., 2022 [31] | Yes | No | Yes | Partially Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | No | No | Yes | High |
| Arumugam, V., et al., 2024 [32] | Yes | No | Yes | Partially Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | High |
| Bonilla, D.A., et al., 2021 [18] | No | Yes | Yes | Partially Yes | No | No | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | High |
| Cheah, K.L., et al., 2021 [33] | No | Yes | Yes | Partially Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | High |
| Della Porta, M., et al., 2023 [34] | Yes | Yes | Yes | Partially Yes | Yes | No | No | Yes | No | No | No meta-analysis was conducted | No meta-analysis was conducted | No | No | No meta-analysis was conducted | Yes | Moderate |
| Didio, F.P., et al., 2022 [35] | Yes | Yes | Yes | Partially Yes | No | No | No | Yes | Yes | No | Yes | No | No | No | No | Yes | Moderate |
| Gómez Afonso, A., et al., 2023 [36] | No | No | Yes | Partially Yes | Yes | Yes | No | Yes | Yes | No | No meta-analysis was conducted | No meta-analysis was conducted | No | Yes | No meta-analysis was conducted | Yes | Moderate |
| Lopresti, A.L., and S.J. Smith, 2021 [37] | No | No | Yes | Partially Yes | No | Yes | No | Yes | Yes | No | No meta-analysis was conducted | No meta-analysis was conducted | No | Yes | No meta-analysis was conducted | Yes | Moderate |
| Pratte, M.A., et al., 2014 [38] | No | No | No | Partially Yes | Yes | Yes | No | Yes | Yes | No | No meta-analysis was conducted | No meta-analysis was conducted | Yes | No | No meta-analysis was conducted | Yes | Moderate |
| Pérez-Gómez, J., et al., 2020 [39] | Yes | No | No | Partially Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | High |
| Smith, S.J. et al., 2021 [40] | Yes | Yes | Yes | Partially Yes | Yes | Yes | No | Yes | Yes | No | No meta-analysis was conducted | No meta-analysis was conducted | Yes | No | No meta-analysis was conducted | No | High |
| Studies (A to Z) | Objective | No. of Studies (Characteristics) | Outcomes | Results and Conclusions |
|---|---|---|---|---|
| Akhgarjand, C., et al., 2022 [31] | To determine the effects of ashwagandha on stress and anxiety in adults. | Studies: 12 Sample: 783 Population: healthy adults and adults with stress or anxiety-related illnesses Age: 18–50 Sex: 50% undeclared Dosage: 250–1000 mg/day | Stress and anxiety. | Ashwagandha supplementation significantly reduced anxiety (SMD: −1.55, 95% CI: [−2.37, 0.74]; p = 0.005; I2 = 93.8%) and stress level (SMD: −1.75; 95% CI: −2.29, −1.22; p = 0.005; I2 = 83.1%) compared to the placebo. Additionally, the non-linear dose–response analysis indicated a favorable effect of Ashwagandha supplementation on anxiety until 12,000 mg/d and stress at a dose of 300–600 mg/d. A dose–response meta-analysis of RCTs demonstrated a positive impact on stress and anxiety levels because of Ashwagandha supplementation. However, the certainty of the evidence was low for both outcomes. |
| Arumugam, V., et al., 2024 [32] | To evaluate the effects and safety of ashwagandha on psychosomatic functions related to stress and anxiety in patients. | Studies: 13 Sample: 802 Population: undeclared Age: 16–80 Sex: 45% undeclared Dosage: 240–4000 mg/day | Stress and anxiety. | The findings of the meta-analysis showed a significant effect of Ashwagandha formulations on the PSS (MD = −4.72, 95% CI = [−8.45 to −0.99]), Anxiety (MD = −2.19, 95% CI = [−3.83 to −0.55]), and serum cortisol levels (MD = −2.58, 95% CI = [−4.99 to −0.16]) compared to the placebo group. Ashwagandha provides benefits like those of conventional treatments, as evidenced by the effect sizes reported in this meta-analysis. |
| Bonilla, D.A., et al., 2021 [18] | Evaluation of the effect of ashwagandha supplementation on physical performance. | Studies: 13 Sample: 615 Population: healthy individuals|Tier: 1 Age: 16–45 Sex: 55% male Dosage: 120–1250 mg/day | Muscle strength, VO2Max, muscle fatigue, tiredness and physical recovery. | Ashwagandha supplementation was more effective than placebo in improving variables related to strength/power, cardiorespiratory fitness and fatigue/recovery in healthy men and women. In fact, the probability of at least a small effect size on physical performance favoring the subjects who took the ashwagandha supplement is very high (>95%). A low-to-moderate overall risk of bias was identified in the trials analyzed in this study. Meta-analytic findings indicated that Ashwagandha supplementation was more effective than placebo in enhancing physical performance variables in healthy men and women. In strength and power, compared to placebo, the combined treatment effect of ashwagandha supplementation was moderate (95% CI: [0.40 to 0.95]). In cardiorespiratory fitness, compared to placebo, the aggregated treatment effect of Ashwagandha supplementation on cardiorespiratory fitness was exceptionally high (95% CI: [1.40 to 2.31]). In fatigue and recovery, the combined treatment effect of Ashwagandha supplementation, when compared to placebo, was notably substantial (95% CI: [−3.01 to −1.049]), with moderate to high variability across studies even after excluding outliers (diamond ratio = 1.89). |
| Cheah, K.L., et al., 2021 [33] | Determining the effect of ashwagandha on sleep. | Studies: 5 Sample: 80 Population: healthy adults, adults with insomnia Age: 18–75 Sex: 100% undeclared Dosage: 120–600 mg/day | Sleep (quantity and quality) | Ashwagandha extract seems to have a beneficial effect on improving sleep, both subjectively and objectively, in adults. Ashwagandha extract with a dosage ≥ 600 mg/day and a treatment duration ≥ 8 weeks appears to be the most effective. Ashwagandha extract demonstrated a modest yet significant improvement in overall sleep quality (SMD: −0.59; 95% CI: [−0.75 to −0.42]; I2 = 62%). |
| Della Porta, M., et al., 2023 [34] | To provide a systematic that focuses on the efficacy of ashwagandha in reducing cortisol levels in stressed human beings.” | Studies: 9 Sample: 620 Population: healthy adults Age: over 18 Sex: 55% undeclared Dosage: 125–5000 mg/day | Stress and anxiety. | Taking an ashwagandha supplement for a period of 30 to 112 days seems to have a stress-reducing effect, reducing cortisol levels in stressed individuals. |
| Didio, F.P., et al., 2022 [35] | To evaluate the effects of ashwagandha supplementation on sports performance in physical exercisers. | Studies: 6 Sample: 245 Population: healthy active adults, inactive adults, elite athletes|Tier: 0, 1 and 4 Age: 18–50 Sex: 62% male Dosage: 500–1250 mg/day | VO2Max and maximum strength | Chronic use, for more than 30 days, of oral supplementation with Ashwagandha, at doses of between 500 and 1250 mg, before or after exercise, can improve physical performance in exercisers, especially regarding cardiorespiratory fitness. About muscle strength, there was no positive effect of Ashwagandha supplementation in adults. In the maximum oxygen uptake, Ashwagandha supplementation seems to result in a slight improvement in VO2Max among adults (+3.45 mL/kg/min; 95% CI: [0.30 to 6.60]; I2 = 74%; p = 0.03) when compared to the control group. In the one repetition maximum test, Ashwagandha supplementation appears to have no significant effect on increasing maximum strength in the lower limbs (+4.95 kg; 95% CI: [0.75 to 9.15]; I2 = 0%; p = 0.66) or upper limbs (+13.77 kg; 95% CI: [−0.44 to 27.98]; I2 = 50%; p = 0.16) among adults compared to the control group. |
| Gómez Afonso, A., et al., 2023 [36] | To evaluate the health benefits of ashwagandha supplementation in healthy adults. | Studies: 10 Sample: 542 Population: healthy adults, active adults, adults with low levels of anxiety and adults with some excess weight Age: 18–55 Sex: 63% male Dosage: 240–1000 mg/day | Testosterone and other hormones | Results showed that supplemented individuals displayed reduced levels of oxidative stress and inflammation, and counterbalanced hormone levels. No beneficial effects were found on hematological markers. |
| Lopresti, A.L., and S.J. Smith, 2021 [37] | Assess the effects of ashwagandha on mental and/or physical conditions, and/or human performance | Studies: 41 Sample: 2248 Population: healthy and active adults, unhealthy adults Age: 16–75 Sex: 56% male Dosage: 125–5000 mg/day | Stress and anxiety, sexual functions, athletic performance, pain, fatigue, sleep, well-being | Overall, the strongest evidence for therapeutic efficacy of Ashwagandha is the alleviation of stress and anxiety symptoms. As an intervention to enhance sexual function and fertility, performance, fatigue, pain, and other disorders research is promising, particularly as a natural treatment for male infertility. However, with the limited number of studies, high heterogeneity and overall quality, the strength of these findings is diminished. |
| Pratte, M.A., et al., 2014 [38] | To identify and evaluate the effects of ashwagandha on anxiety and stress. | Studies: 5 Sample: 400 Population: individuals with some stress or anxiety problems Age: undeclared Sex: 18–55 Dosage: 125–4000 mg/day | Stress and anxiety | Most studies have concluded that there is a significant improvement in symptoms for the ashwagandha group when compared to a variety of controls, including placebo and psychotherapy. |
| Pérez-Gómez, J., et al., 2020 [39] | To determine the effects of ashwagandha supplementation on VO2Max. | Studies: 5 Sample: 182 Population: healthy adults and athletes|Tier: 1 and 2 Age: 16–45 Sex: 52% male Dosage: 300–500 mg/day | VO2Max | Results showed a significant enhancement in VO2Max in healthy adults and athletes (p = 0.04). The mean difference was 3.00 (95% CI from [0.18 to 5.82]) with high heterogeneity. |
| Smith, S.J. et al., 2021 [40] | To assess the efficacy of individual herbal ingredients on testosterone concentrations, in addition to their fractions or binding proteins, in men. | Studies: 32 Sample: 2488 Population: adult men Age: 18–72 Sex: 98% male | Saliva, serum or plasma testosterone concentrations | The results demonstrated positive effects of Ashwagandha supplementation on testosterone concentrations in men. |
| Databases | Search Strategy |
|---|---|
| Pubmed | (Withania somnifera [Title/Abstract]) OR (ashwagandha [Title/Abstract]) |
| Scopus | (TITLE-ABS-KEY (ashwagandha) OR TITLE-ABS-KEY (Withania AND somnifera)) AND (LIMIT-TO (DOCTYPE, “re”)) |
| Academic Search Complete & SportDiscus | TI (Withania somnifera OR ashwagandha) OR AB (Withania somnifera OR ashwagandha) |
| Web of Science | (TI= (ashwagandha OR Withania somnifera)) OR AB= (ashwagandha OR Withania somnifera) |
| Google Scholar | allintitle: ashwagandha; systematic review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.F.; Ferreira, R.M.; Maia, F.; Fernandes, L.G.; Leão, C.; Pimenta, N. Biopsychological Effects of Ashwagandha (Withania somnifera) in Athletes and Healthy Individuals: A Systematic Review. Muscles 2025, 4, 24. https://doi.org/10.3390/muscles4030024
Ferreira JF, Ferreira RM, Maia F, Fernandes LG, Leão C, Pimenta N. Biopsychological Effects of Ashwagandha (Withania somnifera) in Athletes and Healthy Individuals: A Systematic Review. Muscles. 2025; 4(3):24. https://doi.org/10.3390/muscles4030024
Chicago/Turabian StyleFerreira, João Francisco, Ricardo Maia Ferreira, Filipe Maia, Luís Gonçalves Fernandes, César Leão, and Nuno Pimenta. 2025. "Biopsychological Effects of Ashwagandha (Withania somnifera) in Athletes and Healthy Individuals: A Systematic Review" Muscles 4, no. 3: 24. https://doi.org/10.3390/muscles4030024
APA StyleFerreira, J. F., Ferreira, R. M., Maia, F., Fernandes, L. G., Leão, C., & Pimenta, N. (2025). Biopsychological Effects of Ashwagandha (Withania somnifera) in Athletes and Healthy Individuals: A Systematic Review. Muscles, 4(3), 24. https://doi.org/10.3390/muscles4030024







