1. Introduction
The Chesapeake Bay, the largest estuary in North America, is about 200 miles long extending from the Susquehanna River in northeast Maryland to the Atlantic Ocean in Virginia. It is an important ecological and economical resource to the region due to its fishery and tourism industries. For many years, the health of the bay decreased dramatically from pollution and runoff of nutrients, sediments and toxic compounds. Once the Bay’s most viable commercial fishery, the oyster population has decreased significantly in the last 50 years. Native oyster populations in the Chesapeake Bay are at less than 1 percent of historic levels due to two protozoan diseases (MSX caused by
Haplosporidium nelsoni and Dermo caused by
Perkinsus marinus), overharvesting, and pollution [
1]. This reduction in the oyster reef area from approximately 810 km
2 (200,000 Ac) to 145 km
2 (36,000 Ac) has also affected the bay’s water quality. Individual oysters filter 4–34 L of water per hour, removing phytoplankton, sediments, pollutants, and microorganisms from the water column [
2]. Historic oyster populations of Chesapeake Bay could filter excess nutrients from the estuary’s entire water volume every three to four days. Today that would take nearly a year.
Loss of reef habitat within the Chesapeake Bay has been linked to this historic decline in the oyster populations. Restored reefs can enhance habitat function and oyster populations [
3,
4]. Oyster population restoration in Maryland has encouraged aquaculture practices that will help increase the number of oysters in the bay and thus enhance the filtering function of the oysters. These practices seed beds on the bay bottom with baby oysters, allow them to grow and then harvest the adults. The most economically friendly and common method of oyster restoration is spat-on-shell where oyster larvae are grown on shells in tanks until they metamorphose into juvenile oysters called spat. The spat-on-shell is then placed on the bay bottom where the oysters mature. The bottom needs to be built-up with a hard material that will support the shell called a reef. Taller reefs have been shown to provide better growth rates and survivorship than shorter reefs.
Reef construction begins with putting a hard material on the bottom surface of the bay that will support the oyster substrate preventing it from sinking into the soft mucky bottom (bottom conditioning). Old oyster shell was used for this hard material until the oyster shell became scarce [
5]. The historically low levels of native oyster populations have directly affected the amount of available shell for bed restoration [
6]. In lieu of oyster shell, many different materials have been used for oyster bed substrates [
7]. These include clam shell [
8,
9], gypsum [
10], coal ash [
9,
11,
12,
13], slate [
14], shale and tires [
1], recycled concrete, and most commonly limestone [
15,
16,
17]. Research has shown that restored reefs enhance habitat function and oyster populations [
3,
18]. The use of alternative substrates can also purvey advantages to benthic organisms by providing refuge from predation and increased settlement surface. Cost, expected life-span and availability of these alternative materials will determine their suitability for reef restoration. An economic analysis based on preliminary performance and the relative cost of alternative substrates indicated that, though crushed concrete was the most expensive choice, the number of oysters produced per unit substrate made it the most economical choice. The expected life-span of oyster shell reef material has been criticized on the basis of natural shell decomposition [
1]. Many alternative materials such as granite and concrete have persisted in marine and estuarine environments for decades [
19].
In the U.S., only 5% of the approximately two billion tons of aggregate used each year in concrete comes from recycled materials [
20]. Because of the increasing cost related to access, production, and transport of aggregate, the U.S. Environmental Protection Agency (EPA) and the Federal Highway Administration (FHWA) has promoted the beneficial use of recycled materials, including materials originating from pavements. Most of the recycled material is used in road materials, asphalt hot mixes, and new concrete mixes [
20]. Recently, this recycled aggregate has been used to recreate reefs and oyster beds. Alabama “Roads to Reefs” program has built a number of reefs in Mobile Bay to enhance habitats, with a portion of the reefs set aside for oyster nurseries. Virginia has constructed a reef system from a mixture of oyster shell reefs, recycled concrete from a deconstructed bridge, and fabricated concrete forms [
21]. The Maryland Department Natural Resource (MD DNR) Fisheries Service’s Artificial Reef Program has identified 21 reefs in the Chesapeake Bay, nine that have been built completely or partially with bridge slabs, bridge sections or concrete. Monitoring of these reefs has identified several populations of finfish, which have increased angler activities near the reefs. In possibly the largest single project, 60,000 tons of material from the replacement of the Woodrow Wilson Bridge was used to build four to five reefs in which over 1300 acres of new reefs were created from 2006 to 2008. In another notable project, Maryland has built a reef from the deconstruction of Memorial Stadium, the former home of the Baltimore Orioles professional baseball team.
Recycled concrete aggregate (RCA) is crushed concrete material created from the removal and milling of old concrete pavement/road infrastructure. It consists of sand, and gravel of many sizes and shapes. However, RCA may contain 10–30% subbase soil and asphalt pavement that accumulates during excavation. Thus, the RCA is a mixture of soil, concrete, and small amounts of bituminous concrete [
22]. The material is processed and sorted for reuse as base, sub-base, fill material for embankments, and in new concrete mix.
Crushed concrete was superior to crushed limestone and crushed oyster shell when evaluated as clutch material for oyster beds [
23]. However, some of these recycled materials may contain toxic substances, such as heavy metals and hydrocarbons that could leach when inundated with water, potentially affecting neighboring aquifers or streams and impairing aquatic health and function. It is important to assess RCA’s leaching characteristics before they are used [
11,
12]. Factors such as solubility, diffusion, desorption/adsorption, and advection influence the fate and transport of contaminants [
12]. For RCA to be used within the aquatic setting of the Chesapeake Bay, its chemical behavior under saturated conditions must be understood to avoid potential adverse impacts to the bay’s aquatic ecosystem. Understanding this behavior will ultimately help determine the suitability of RCA for supporting oyster aquaculture.
RCA has a rougher surface texture, lower specific gravity, and higher water absorption than natural aggregates. As the particle size decreases, the higher proportion of mortar causes the specific gravity to decrease and absorption to increase. Specific gravity of RCA ranges from 2.0 to 2.5 [
24]. RCA is generally more permeable than natural aggregate. Before RCA is used in the environment, the possibility of contaminant leaching and pH changes must be considered. Most of the analysis performed on RCA has focused on physical traits and the presence of chemicals that may affect the stability of concrete made with the RCA [
25]. When RCA is used for construction, dimensional instability and loss of strength in the concrete is related to the soluble sulfate content of the material. Therefore, research has assessed the leaching of sulfates from recycled aggregates. The amount of leachable sulfate was related to the amount of gypsum present in the construction waste materials [
26]. High soluble sulfate concentrations were found in mixed recycled aggregate that contained more than 30% of ceramic particles or more that 2% gypsum. RCA has a lower density and specific gravity compared to natural aggregates. RCA tends to have higher porosity and water absorption rates than natural aggregates. The major components include calcium (Ca), silicon (Si), aluminum (Al), iron (Fe), and other trace elements. RCA is typically alkaline due to the presence of residual cement paste and free lime. The primary oxides present in recycled concrete are calcium oxide (CaO), silicon dioxide (SiO
2), aluminum oxide (Al
2O
3), iron oxide (Fe
2O
3), and magnesium oxide (MgO).
Cementitious materials tend to leach heavy metals. Portland cement concrete subjected to the Toxicity Characteristic Leaching Procedure (TCLP) using acetic acid contained arsenic (As), beryllium (Be), cadmium (Cd), chromium (Cr), mercury (Hg), lead (Pb), and selenium (Se) in the leachate [
27]. As (19.9 mg/kg), Be (1.4 mg/kg), Cr (72.7 mg/kg), Pb (75.3 mg/kg), nickel (Ni; 72.0 mg/kg) and vanadium (V; 44.1 mg/kg) after a long-term leaching test [
28]. Assessed the leaching of heavy metals from RCA; mercury, lead and cadmium levels were below detection [
26]. The leachable levels of Cr, Ni, Cu, Zn, As, Se, Mo, Sb, and BA measured were all below the acceptable criteria designated by the EU Landfill Directive [
26]. In areas where deicing salts are used the RCA may contain high levels of chloride. Calcium-aluminum-silicate compounds found in cement paste can cause the pH of RCA-water solutions to often be greater than 11, which could have adverse effects on the surrounding environment.
The primary objective of this study was to determine the suitability of RCA from Maryland road projects as conditioning material for on-bottom oyster aquaculture in the Chesapeake Bay. The objective of the study evaluated how the introduction of RCA affects water chemistry and evaluated the effect of RCA on the survivorship and growth of oyster spat. The research was also to investigate the leaching performance of recycled concrete materials potentially contaminated with toxic organic substances, especially petroleum residues and PAH.
2. Material and Methods
Sample Collection: Recycled concrete aggregate was collected from three different stockpile sites for concrete recycling operations within Maryland (
Table 1). Approximately 3.82 m
3 of 5.08 cm and 10.16 cm crushed RCA were collected from the sites for the study. The study was conducted at the Morgan State University Estuarine Research Center. The center is located in St. Leonard, Maryland, on the Patuxent River, a sub-estuary of the Chesapeake Bay. The center’s facilities include an oyster hatchery, setting tanks, and 40,500 m
2 oyster lease. Recycled concrete pile and collected oyster shell washing are shown in
Figure 1.
RC (Recycled concrete)-Surge recycled concrete (7.62 cm to 20.32 cm) was used for oyster bottom conditioning materials; however, smaller material sizes were collected from each plant for extraction convenience. Bigger sizes would require additional processing for extraction such as crushing or grinding, which may generate inaccurate results. Therefore RC-6 (<3.81 cm) was collected from Machado Construction Co., Inc., (Baltimore, MD, USA) and The Recycling Center, and RC-2 (3.81 cm to 6.35 cm) was collected from Flanigan & Sons, Inc., (Baltimore, MD, USA) where RC-2 was the smallest size. Samples were collected from the crushed RCA stockpiles into 250 mL wide-mouth glass jars with Teflon-lined screw caps. The collected samples were cooled to 4 ± 2 °C immediately after collection. Collected RCA samples were extracted within 48 h of collection and analyzed with one week of extraction. Two separate samples were collected at the same time, placed under identical circumstances, and managed the same throughout field and laboratory procedures. Analyses of field duplicates give a measure of the precision associated with sample collection, preservation and storage, as well as laboratory procedures. Moisture content of the “as-tested” material was determined by standard gravimetric procedure at 105 ± 2 °C. The moisture contents of collected recycled concrete samples from Flanigan & Sons, Inc., Machado Construction Co., Inc., and The Recycling Center are 5.63 ± 1.56%, 6.59 ± 0.65%, 8.51 ± 1.35%, respectively. The oyster shells used as controls in the study were collected from a stockpile at the Morgan State University Estuarine Research Center. Prior to testing, the shells were washed to remove surface debris.
Four separate extraction methods were used to evaluate the leaching potential of chemicals from the RCA. Saturated RCA was subjected to the following methods: a sequential extraction to indirectly assess the potential mobility and bioavailability of heavy metals; a toxicity characteristic leaching procedure (TCLP) to determine the mobility of analytes present in liquid, solid and multiphasic wastes; a tank test (EPA method 1315) to evaluate mass transfer rates (release rates) and estimate the diffusivity of the RCA; and a flow-through leaching test to simulate the bottom conditions in the Patuxent River and also evaluate hydrocarbon leachability.
Sequential extraction: The sequential extractions procedure gives a general assessment of the potential risk of desorption and remobilization of compounds from environmental samples. Despite limitations of the procedure, insight into the transport of trace elements and their subsequent biological and physico-chemical availability can be ascertained. The five chemical fractions are defined by an extraction sequence that follows the order of decreasing solubility [
29]: exchangeable elements > element bounded to carbonates > element bounded to iron > elements bounded to organic matter > residual. Four replicates of 1 g of ground, air-dried RCA were used for the extractions. For each test, the solution and solid phases were separated by centrifugation at 10,000 rpm for 10 min. The solution was filtered through a 0.45 μm filter and the solid residues were preserved for the subsequent extractions. Collected filtrate was acidified (pH < 2) with nitric acid and analyzed for metals using a PerkinElmer ELAN 6100, (PerkinElmer, Inc., Walthanm, MA, USA) inductively coupled plasma mass spectrometry (ICP-MS). Exchangeable elements were extracted at room temperature for 1 h with 8 mL of 1 M magnesium chloride solution (MgCl
2, pH 7.0) with continuous agitation for 1 h. The elements bounded to carbonates were extracted using the residue from fraction 1. Magnesium chloride targets exchangeable elements, which are loosely bound to particles through electrostatic interactions. These elements are easily exchangeable with cations in the solution. This step targets elements like calcium, magnesium, potassium, and sodium that are weakly adsorbed on the surface of soil particles. The residue was leached at room temperature with 8 mL of 1 M sodium acetate (CH
3COONa) adjusted to pH 5.0 with acetic acid (CH
3COOH). Sodium acetate solution, acidified to pH 5.0, is used to dissolve carbonate minerals, releasing elements bound to carbonates. Elements like calcium, magnesium, and heavy metals that precipitate as carbonates are targeted in this step. Continuous agitation was maintained. Lead carbonate tests prior to the experimental trials determined that 3 h were necessary for complete extraction. The elements bounded to iron were extracted using the residue from fraction 2. The residue from fraction 2 was extracted with 20 mL of 0.04 M hydroxylamine (NH
2OH)• hydrogen chloride (HCl) in 25% (
v/
v) acetic acid (CH
3COOH). This fraction experiment was performed at 96 ± 3 °C with occasional agitation for 6 h. This solution targets elements bound to amorphous and crystalline iron and manganese oxides by reducing these oxides, which releases the bound elements. This step releases elements like iron, manganese, and other metals associated with oxides. The next extraction consisted of the elements bounded to organic matter. 20 mL of 7 M Sodium hypochlorite (NaOCl) adjusted to pH 8.5 with hydrogen chloride (HCl) were added to the residue from fraction 3, and the mixture was heated to 90 ± 2 °C for 2 h with occasional agitation. Sodium hypochlorite (NaOCl) which is a strong oxidizing agent at alkaline pH oxidizes organic matter, releasing elements bound to organic compounds. Elements complexed with or bound to organic matter are targeted in this step. After centrifugation, a second 20 mL aliquot of sodium hypochlorite (NaOCl, adjusted to pH 8.5 with hydrogen chloride, HCl) was then added and the sample was heated again to 90 ± 2 °C for 2 h with intermittent agitation. The final step was extraction of residual elements. The residue from fraction 4 was digested by the aqua regia method with a mixture of concentrated nitric acid (HNO
3) and hydrogen chloride (HCl) in a 1 to 3 ratio. Aqua regia is used to dissolve residual fractions, which include elements tightly bound within mineral lattices. This step targets elements that remain in the residue after the previous extractions, typically those that are part of the crystal structure of minerals.
Toxicity characteristic leaching procedure (TCLP): EPA’s TCLP [
18] was used to determine the mobility of inorganic analytes present in the RCA. The procedure is used to simulate the leaching of organic and inorganic compounds from materials placed in landfills. The results of the test determine if a particular waste is considered hazardous or non-hazardous for future disposal regulations. The RCA was crushed to smaller than 9.5 mm and added to an acid extractant (acetate buffer) at a solid to liquid ratio of 1:20 by mass in 250 mL Polytetrafluoroethylene (PTFE) bottles. Extractions were carried out in triplicate. An agitation apparatus rotated the extraction vessels continuously (at 30 rpm) for 18 h. Analysis of the samples was performed in triplicate. After mixing, the suspension was filtered with a borosilicate glass fiber filter (0.45 μm) and the filtrate was collected in 50 mL centrifuge tubes and stored at 4 °C until chemical analysis. The laboratory followed a strict quality assurance and quality control protocol. Heavy metal concentrations in the leachate were measured using ICP-MS.
Tank test: EPA preliminary version of method 1315-semi-dynamic tank leaching procedure [
30] was used to evaluate mass transfer rates (release rates) and estimate the diffusivity of the RCA. The method consisted of tank leaching of recycled concrete with periodic renewal of the three different salinity solutions: deionized water, 10–15% salinity and 25–35% salinity. Salinity levels were achieved with Instant Ocean Sea salt. Leachate was withdrawn and analyzed. RCA was subjected to leaching in a closed tank using an eluent to RCA volume ratio (L/V) of 5. Three bed treatments were evaluated. Approximately 500 g of recycled concrete, 400 g of recycle concrete with 100 g of oyster shell, and 500 g of oyster shell were placed into 1 L PVC cylinders. A 1000 mL of eluents were added to PVC cylinders and changed at six intervals. Both RCA and oyster shell were used together to simulate the field conditions. Pure oyster shells were used as a control. The eluent was renewed after 2, 27, 42, 121, 360, and 504 h. The tank leaching test assessed the potential and speed of leaching of the recycled concrete over the long term. Triplicates of each treatment were run. Half of the collected water samples were measured for pH, alkalinity, conductivity, chloride, oxidation-reduction potential (ORP), nitrate, salinity, and total dissolved solids (TDS). The other half of the collected water samples were filtered using borosilicate glass fiber filters, acidified (pH < 2) with 0.2 mL of nitric acid, and then stored at 4 °C until analysis with ICP-MS.
Flow-through leaching test: Column experiments were used to evaluate the effects of material size and water flow rate on the concentration of chemical leaching from the RCA. Two flow regimes based on the Patuxent River’s seasonal minimum (13 cm
3/s in August; slow flow rate) and maximum (30 cm
3/s in March; fast flow rate) river bottom velocities were applied to the column experiments. The column effluent was measured for pH, alkalinity, conductivity, chloride, salinity, TDS, and organic and inorganic constituents. Ambient Patuxent River water was used for the column experiments to infer recycled concrete performance under specific environmental conditions. Approximately 47 kg of RCA, 47 kg of RCA with 3 kg of oyster shell, and pure oyster shell were placed in each PVC column (15.2 cm diameter, 86.4 cm height). The column design consisted of two sections. The upper section was filled with the materials. The top of each column had an outlet (5 cm diameter). The lower section had a screen that prevented sidewall flow when brackish water was added. At each column’s base was an inlet (2.5 cm diameter) connected to a brackish water supply system that was directly connected to the Patuxent River. In total, twelve columns were used in this study: two RCA with fast flow, two RCA with slow flow, two RCA and oyster shell with fast flow, two RCA and oyster shell with slow flow, one oyster shell with fast flow, one oyster shell with slow flow, and two controls. The brackish water flow was maintained with a flow control water pump, and water samples were collected at 0, 1, 6, 21, 28, 139, 288, 508, and 1016 h. The flow through leaching test are shown in
Figure 2.
The brackish water used in the experiments was drawn 200 m offshore at the Morgan State University Patuxent Environmental & Aquatic Research Laboratory (PEARL), St. Leonard, Maryland, on the Patuxent River, a sub-estuary of the Chesapeake Bay. The waters surrounding the center contain state managed and private oyster bars. Collected water samples were measured for inorganic constituents, pH, alkalinity, conductivity, chloride, ORP, salinity, TDS, and metals. For metal analysis, collected water samples were filtered through 0.45 μm borosilicate glass fiber filters, acidified (pH < 2) with 0.2 mL of nitric acid, and then stored at 4 °C. Inorganic chemicals in the acidified samples were analyzed using a PerkinElmer ELAN 6100 inductively coupled plasma mass spectrometry (ICP-MS). The pH and total alkalinity were measured with an Orion 9107BN pH meter (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Standard solutions used in this project were obtained from Forston Labs (Forston Labs, Inc., Fort Collins, CO, USA). Conductivity, chloride, ORP, salinity, and TDS were measured with a Forston Labs LabNavigator (Forston Labs, Inc., Fort Collins, CO, USA), a multi-parameter instrument.
Oyster Growth and Survivorship: The growth and survival experiments were conducted in four flow-through fiberglass tanks that measured 178 × 91 × 25 cm3. Standpipes, cut to 19 cm, were placed in each tank to keep the water level consistent at 19 cm throughout the experiment. Patuxent River ambient seawater was used. Flow was maintained at minimum flow rates for the Patuxent River. Two of the tanks contained shell and two contained shell and RCA. The material was stacked in 60 × 60 × 16 cm3 piles in the center of each tank. Each tank contained 75,708 cm3 of seawater. In the shell and RCA tanks, the RCA was covered with a layer of shell at a volume ratio of 3:1. Ambient Patuxent River water was allowed to flow over the materials for two days prior to the spat planting. Oyster spat was acquired from the State of Maryland Hatchery at Piney Point. Larvae were spawned from locally collected oysters and set on oyster shell. To simulate the 1 million spat per acre required by Maryland Department of Natural Resources for oyster aquaculture, 140 spat were placed in each tank. Each shell was numbered, and the number and length of each spat was measured and recorded. Shells were arranged into two lines on top of the materials already in the tank. One line had shells facing up and the other had shells facing down. Length and survivorship were recorded. The pH, salinity, dissolved oxygen, and temperature were checked regularly using a Yellow Springs Instrument Professional Plus multi-parameter meter (YSI, Inc., Yellow Springs, OH, USA). The experiment was run twice, once in the beginning of the spawning season (July–August) and again later in the season (September–October). Growth was analyzed as average length with a two-way ANOVA: treatment (RCA, shell) and season (summer, fall). Survivorship was analyzed with a three-way ANOVA on percent survivorship.
Hydrocarbon Leaching: The long-term leaching potential of the RCA was assessed using a tank leaching test. The RCA samples were collected from three concrete dumping sites: Flanigan & Sons, Inc.; Machado Construction Co., Inc.; and The Recycling Center, MD. Typically, RC-Surge recycled concrete is used for oyster conditioning materials, but smaller sizes (<1mm) were collected from each plant for extraction convenience. Volatile organic compounds (VOCs) are preferentially volatilized [
31] and tend to evaporate quickly when concrete is milled on the demolition sites and during the stockpiling period. VOCs in the leachate from recycled asphalt pavement were found below detection limit (BDL), with various extraction tests, including the TCLP, Synthetic Precipitation Leaching Procedure (SPLP), Deionized Water Leaching Procedure, and Column Leaching Procedure. Therefore, VOCs were not tested in this study. EPA Method 3570 for extracting organic compounds, especially semivolatile organic compounds (SVOCs), was used to determine the mobility of petroleum analytes present in the RCA. Collected RCA samples were shaken for 4 h with methylene chloride (CH
2Cl
2) in sealed extraction tubes. Sample extracts were collected, dried by sodium sulfate (Na
2SO
4), and concentrated using a modification of the Kuderna-Danish concentration method. The filtered extracts were analyzed for SVOCs by EPA method 8270D using a gas chromatography mass spectrometry (GC-MS) with a narrow-bore fused-silica capillary column, 30 m length, 0.25 mm inside diameter, and 0.25 μm film thickness (PerkinElmer, Inc., Waltham, MA, USA).
3. Results and Discussion
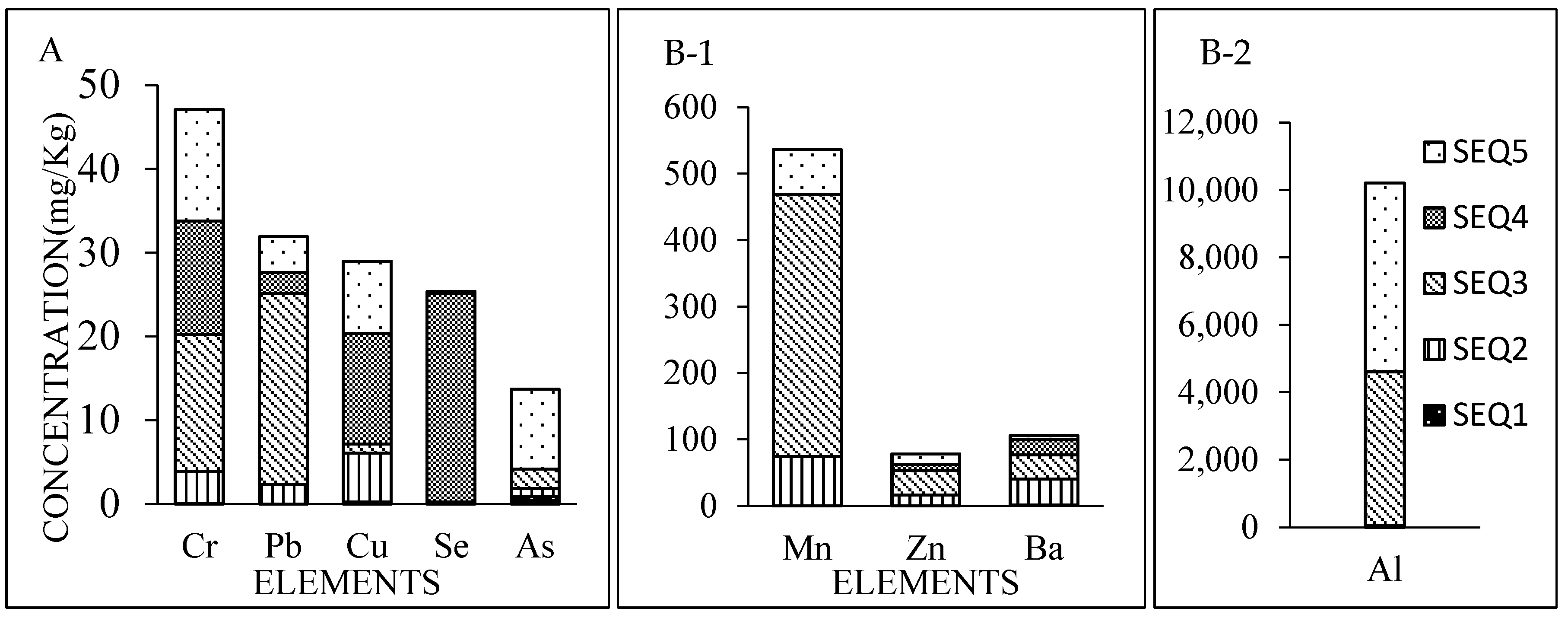
Sequential Extractions Leaching Test: The concentration of mercury (Hg) in the leachate was below detection for all extractions (
Table 2). The lowest total concentrations leached from the RCA were 0.63, 0.10, and 0.07 mg/kg for beryllium (Be), cadmium (Cd), and thallium (Tl), respectively (
Figure 3). The total amount leached from the RCA for chromium (Cr), lead (Pb), copper (Cu), selenium (Se) and arsenic (As) were 47.1, 31.9, 29.0, 25.4, and 13.7 mg/kg, respectively (
Figure 4). The Cr concentrations of each of the sequential fractions were below the Maryland criteria (1100 μg/L). However, the total amount leached exceeded the limit with the largest fraction occurring during sequential extraction (SEQ) 3 representing the fraction of metal that is bound to the iron-manganese oxides. This fraction will be mobilized as reducing conditions increase. No Pb or Se leached with the exchangeable fraction (SEQ1). The greatest amount of leached Pb occurred during SEQ3 but concentrations exceeded the Maryland criteria for SEQ 2 through 5. Ninety-eight percent of the Se leached with SEQ4 representing the fraction bound to organic matter, which could be mobilized under increasing oxidizing conditions. For each SEQ extraction, leached copper (Cu) and arsenic (As) concentrations were greater than the MWQS.
The highest total metal concentrations that leached from the RCA were 10,203, 536, 78.2, and 106 mg/kg of aluminum (Al), manganese (Mn), zinc (Zn) and barium (Ba), respectively (
Figure 4). Less than 1% of the total metal concentrations leached from SEQ1, which represents the exchangeable fraction. For Zn, each sequential extraction contained approximately equal percentages of the total amount leached that exceeded the MWQS (90 μg/L).
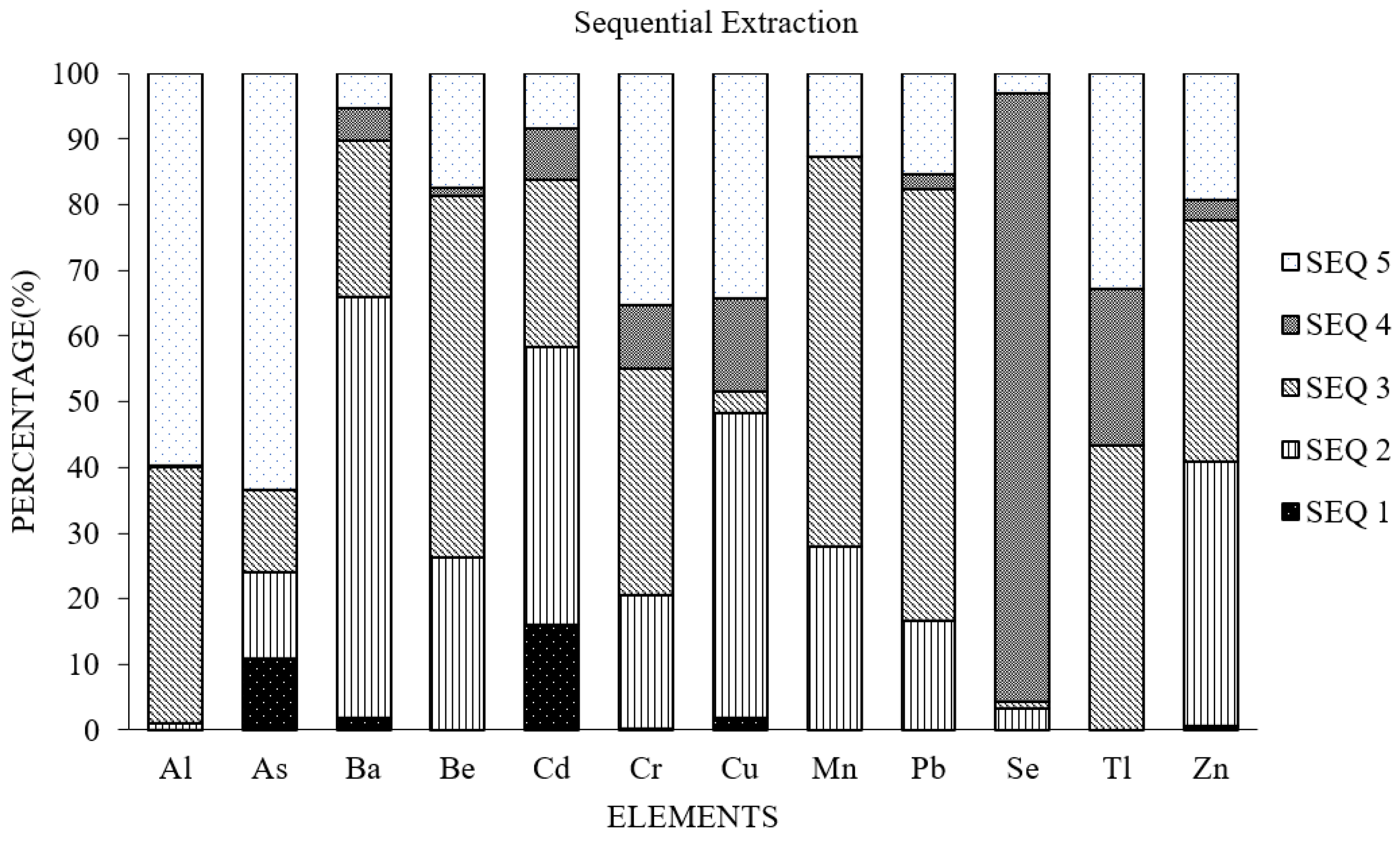
Figure 5 shows the amount of metal leached for each fraction as a percentage of the total metal leached.
The greatest amount of leached chromium, lead and selenium occurred during sequence 3 representing the iron-bound fraction, while the greatest amount of released arsenic came from the residual fraction. The percentage of residual chromium in the total chromium was 28.3 percent, which was similar to that of copper (29.7 percent). Arsenic had the greatest total percentage in the residual fraction (69.8 percent). This indicates that arsenic will have a lower tendency to leach. However, the exchangeable percentages of arsenic and cadmium were higher (6 to 8.2 percent) than the other elements (0 to 0.8). The order of total metal concentrations leached from recycled concrete was as follows: aluminum > manganese > barium > zinc > chromium > lead > copper > selenium > arsenic > beryllium > cadmium > thallium > mercury (
Table 2). In the sequential leaching study, the only detectable metals found after the first leach test (SEQ 1), were barium, arsenic, and aluminum. However, the sequential strength of the leaching solutions indicated that most of the arsenic present in the RCA was in the residual fraction. Thus, arsenic in our samples had the least potential to leach from the RCA. The Landfill Directive sets limits of the metals for three classifications: inert, non-hazardous, and hazardous. Based on these limit concentrations, the RCA evaluated in their work was classified as non-hazardous based on the concentrations of nickel (Ni) and antimony (Sb). They found that the composition of the RCA had an influence on the leached concentrations. RCA samples from asphalt and concrete (did not contain gypsum or bitumen) were classified as inert.
Direct comparisons of the sequential extraction concentrations to the Maryland salt water criteria are difficult to make because of the influence of general water quality chemistry (hardness, pH) on bound metal toxicity. Also, the increasing strength of the extraction solutions provides an assessment of metal leaching that may occur under changing environmental conditions such as moisture content and re-dox status where the leaching may occur, that is, in the soil environment, typically. In the marine environment, the concentration of leached metal would be diluted by the surrounding waters. Therefore, the tank leaching test should be a more accurate indicator of the risks of metal leaching from the RCA. However, the sequential leaching procedure provides insight into metal availability and mobility from the RCA that may occur in the various phases found in the soil and sediment environments.
Toxicity Characteristic Leaching Procedure (TCLP): Based on the TCLP results, none of the leached elements were at concentrations higher than the regulatory maximum concentration of contaminants for toxicity characteristics (
Table 2). Manganese and barium leached at higher concentrations (1.35 mg/L and 0.55 mg/L, respectively) than the other observed elements. There were no detectable levels of beryllium, mercury, and thallium in the extractant.
Tank Leaching Test: The resulting cumulative concentrations of the leached metals from the tank test were totaled from the six eluents collected over the test period (
Table 3). No detectable levels of beryllium, cadmium, mercury, and thallium were found in any of the leachate samples. The RCA treatment resulted in the greatest cumulative concentrations of aluminum for all salinity levels. The aluminum concentration (0.79 mg/kg) from the RCA was higher in the low salinity than in the high salinity (0.49 mg/kg) and the deionized water (0.57 mg/kg) leachate. Aluminum concentrations were lower in the high salinity for all bed treatments than in the low salinity. The cumulative concentration of leached aluminum in the oyster shell treatment decreased with increasing salinity. Salinity can slow down the rate of leaching. Leaching is the process of moving salts by percolating water through it. Samples with higher initial salinity have slower infiltration rates, which in turn slows down the rate of leaching, however, the lowest concentration of aluminum in the shell occurred in the deionized water (0.050 mg/kg) leachate. The cumulative concentration of leached aluminum in the RCA-oyster shell treatment decreased with increasing salinity resulting in 0.072 mg/kg in 20–25% salinity leachates. In general, the RCA and RCA-Shell treatments released a higher aluminum concentration than the oyster shell alone (
Table 3).
RCA in the 10–15% salinity had the highest cumulative concentration of chromium (Cr) (8.8 μg/kg). As shown in
Table 3, the salt-water treatment had a higher cumulative chromium (Cr) concentration (5.6 to 8.8 μg/kg) than the deionized water application (1.8 to 4.5 μg/kg) for all treatments. The cumulative concentrations of heavy metals did not exceed 1 mg/kg in any treatment except for aluminum in RCA with saltwater. The highest concentrations of arsenic occurred in RCA-oyster shell (23.3 μg/kg) and oyster shell (33.9 μg/kg) in saltwater (
Table 3). In a tank test using non-saline water, the cumulative release of the tested heavy metals remained relatively constant, and concentrations were below the EU Landfill Directive limits. Only arsenic leaching increased over time. The researchers concluded that concentrations detected for Ni (nickel), Cr (chromium), Sb (antimony), Zn (zinc), and Cu (copper) merited that these metals be identified as potential pollutants to consider when using RCA in environmental applications. Although leached metal concentration for all metals tested in our work remained below 1 mg/kg, the concentrations of Zn (zinc), Cr (chromium), Mn (manganese), and As (arsenic) did increase over time in the non-saline water tank test. For the salt-water tank test, there was an increase overtime for As (arsenic), Cu (copper), Mn (manganese), Se (selenium), and Zn (zinc). Yet, concentrations remained below 1 mg/kg for all metals tested.
Metal concentrations found in the salt-water tank test were below the Maryland water quality criteria except for copper and selenium. The highest Cu (copper) concentration (9.9 μg/L) was found in the Shell treatment and decreased to 6.2 μg/L and 3.3 μg/L for the RCA + Shell and RCA treatments, respectively. The RCA Cu (copper) concentration was below the acute criteria of 4.0 μg/L but was higher than the chronic criteria level of 2.6 μg/L.
The eluents from each leaching interval were also analyzed for pH, alkalinity, conductivity, ORP, salinity, TDS, and heavy metals (
Table 3). The pH of the eluents from the tank tests of the RCA, RCA-oyster shell, and oyster shell was evaluated at different salinities. In general, recycled concrete had higher pH values than the oyster shell with deionized water applications. RCA in deionized water achieved the highest pH of 10.1. Of the control treatments, deionized water had a pH 5.3 and salt water had a pH 8.1. Eluent pH trended as follows: RCA > RCA-oyster shell > oyster shell > control for both treatments. In saline applications, the RCA and RCA-oyster shell treatments increased the leachate pH above the control to 8.6 and 8.5, respectively. The eluents collected from the tank test of RCA may contain dissolved solids that cause high pH, including calcium oxide (free lime content), aluminum and iron as oxides, oxyhydroxides, and hydroxides from cement. Aydilek (2015) found a positive correlation between the calcium oxide concentration in RCA and the resulting leachate pH [
32]. RCA with 17% CaO (calcium oxide) had a leachate pH of 11.8 while RCA containing 13% CaO (calcium oxide) produced leachate with a pH of 10.4. Reagent water was the liquid used in the test. The pH of the RCA was alkaline, ranging from 10.5 to 12.3. There was no significant drop in pH at the higher L/S although there was a decreasing trend in pH for some of the samples tested. Other researchers have found that over time the pH of RCA decreases to 8.0 [
33,
34]. These values are similar to the leachate pH of the tested samples in this research for the salinity treatment.
ORP, a measure of a substance’s ability to oxidize or reduce another chemical substance, decreased over time for the RCA treatments more than the controls did. The reduction of ORP for all of the treatments was related to the release of reductants such as iron and manganese. The salinity treatment exhibited higher ORP values due to the oxidants contained in sea salts. Conductivity, the ability to conduct an electrical current, evaluates the amount of inorganic dissolved solids. The general conductivity trend was as follows: RCA > RCA-oyster shell > oyster shell > control in deionized application. Conductivity for all treatments was the same at both salinity levels. Salinity is directly proportional to the amount of chloride ions; therefore, salinity correlated to increased conductivity. The range of conductivity was 4268 to 4313 μS/m for saltwater and 66 to 126 μS/m for deionized water treatments, respectively. TDS that measures the concentrations of common ions in water is similar to conductivity measurements. TDS concentrations generally followed the trend: RCA-oyster shell > RCA > oyster shell > control at deionized application. TDS was 32 to 41 mg/L in the salt water and 41 to 68 mg/L in the deionized water treatments. Alkalinity indicates the buffering capacity of a solution by measuring its ability to maintain a stable pH. Alkalinity generally trended as follows: RCA-oyster shell > RCA > oyster shell > control in deionized application. Due to the chloride in sea salt, alkalinity increased with the salinity. RCA-oyster shell had a higher alkalinity (112.2 mg/L) than the other treatments. Alkalinity ranged from 103 to 112 mg/L in saltwater and 22 to 36 mg/L in deionized water treatments.
Flow-Through-Leaching Test: This study evaluated the potential leachability of chemicals from recycled concrete with brackish water supplied from the Patuxent River, near St. Leonard, Maryland. The leachability tests were run in fast (30 cm
3/s) and slow (13 cm
3/s) flow conditions. Based on the detected concentrations, the analyzed chemicals from the recycled concrete were divided in three groups: high, medium, and low. The high group consisted of selenium, barium, and zinc (
Table 4). However, high concentrations of these chemicals have also been detected in the brackish water. Therefore, these chemicals naturally exist in the Patuxent River (36.6 to 56.6 μg/L for selenium, 28.6 to 36.1 μg/L for barium, and 7.8 to 12.4 μg/L for zinc).
As shown in
Table 4, the highest barium concentrations were seen in RCA in fast-flow conditions (91.5 μg/L) and RCA-oyster shell in low-flow conditions (67.4 μg/L). The highest zinc concentrations were 46.6 μg/L (RCA in fast flow) and 48.7 μg/L (oyster shell in low flow). However, selenium, barium, and zinc concentrations gradually decreased with time. The medium concentration group included aluminum, copper, chromium, and arsenic. The highest aluminum concentrations were seen in oyster shell under fast flow (5.7 μg/L) and oyster shell under low flow (10.1 μg/L). The highest copper concentrations were in RCA under fast flow (6.6 μg/L) and oyster shell under low flow (5.2 μg/L). The highest arsenic concentrations were in RCA under fast flow (14.7 μg/L) and oyster shell under low flow (8.3 μg/L). The highest concentration in the low leachability group did not exceed 2 μg/L. The highest concentrations of cadmium and mercury were less than 0.1 μg/L, which was detected only at the beginning of the column experiment. The highest manganese concentrations (1.8 μg/L and 1.2 μg/L) occurred with RCA in fast-flow and low flow conditions. Manganese concentration also decreased with time. The highest chromium concentrations were 2.8 μg/L (RCA with fast flow) and 2.76 μg/L (control with low flow). Chromium’s leaching concentration did not decrease with time. Beryllium and thallium were not detected from the collected samples. The collected samples from the column experiment were also analyzed for pH, conductivity, TDS, ORP, alkalinity, and salinity (
Table 4). At the beginning of column experiment, RCA and RCA-oyster shell had slightly higher pH values than the oyster shell and control, but the pH for all treatments stabilized to 7.9 to 8.0 after seven days even though recycled concrete contains aluminum and iron as oxides, oxyhydroxides, and hydroxides that may raise pH. Initial values of conductivity, TDS, ORP and alkalinity were higher than values detected over time except for ORP. The values for the RCA, RCA-oyster shell, and oyster shell with brackish water were not significantly different, and the values stabilized over time. The salinity of brackish water changed depending on the surrounding area’s weather conditions with a range of 5 to 14‰. Additional graphs for the leaching and flow through test results are available in
Supplementary Materials.
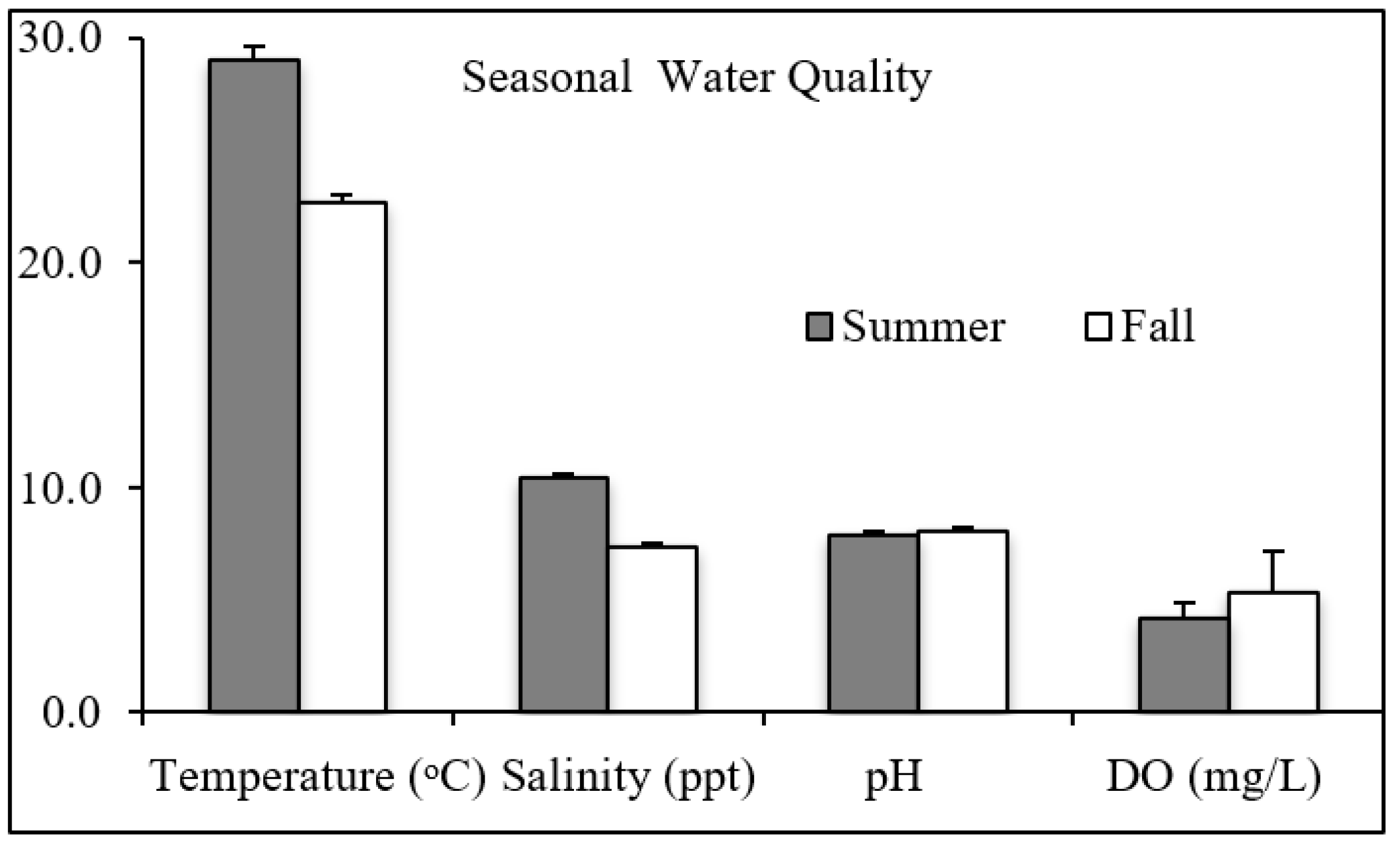
Oyster Growth and Survivorship: The oyster growth and survivorship experiments were run twice (July and October). The first time-period experienced high rainfall, which kept salinities below the 9‰ required for oyster spawning until midsummer. Fortunately, water temperatures remained within the optimum range for spat growth into October. Temperature, salinity, and DO differ by seasons (
t-test,
p < 0.05) (
Figure 4). Summer’s temperatures (average 29.1 °C) were higher than fall’s (average 22.8 °C). Summer temperatures changed little during the experiment, ranging from 27.8 to 29.5 °C. Fall temperatures had a slightly larger range, 20.1 to 24.9 °C, and decreased during the experiment. Salinity was also lower during the fall, averaging 7.4‰ relative to the summer average salinity of 10.4‰. The fall average values for DO, 5.3 mg/L, were higher than the summer average of 4.2 mg/L. The fall variance in DO values, ±0.71 mg/L, was lower than the summer variance, ±1.8 mg/L. pH was not different between seasons. For all treatments and time-periods, pH ranged from 7.7 to 8.3. Temperature, salinity, pH, and DO were monitored during the experiments (
Figure 6). The recorded values were within historical characteristics of the Patuxent River. As shown in
Figure 6, there was no statistical difference in any of the water quality parameters between treatments (
t-test,
p < 0.05).
There was no difference (Tukey HSD,
p = 0.98, df = 2) in initial length between shell (5.5 ± 2.9 mm) and RCA + shell (5.54 ± 3.2 mm) treatments. After two weeks, there was no significant difference (ANOVA,
p = 0.99, df = 1) between the mean length of oysters in the RCA + shell (9.32 mm ± 4.87 mm) and shell (9.36 mm ± 4.62 mm) treatments (
Figure 7). In the fall, the experiment was run for six weeks, and an interim measurement was taken at week two. Two hundred and thirty-two shells with a total of 583 spat were planted. The mean length of oysters at the beginning was 8.73 mm. There was no difference (Tukey HSD,
p = 0.98, df = 2) in initial oyster length between shell (8.73 ± 3.67 mm) and RCA + shell (8.73 ± 3.44 mm). Over the six-week period, the spat grew an average of 12.1 mm. Treatment had no effect on the initial, two-week, or six-week length. Survivorship was high and did not differ between treatments. In the summer, spat were mapped on the shell. When the shells were recovered, the map was used to identify spat that survived the experiment. Thirteen were missing from the 280 spat identified from each treatment, resulting in a survivorship of 95 percent for each treatment. The shells were not mapped in the fall treatment; therefore, the number of dead oysters identified on each shell was used as a measure of survivorship. Of the 578 counted, 15 were dead. The 99.99 percent survivorship was not significantly different in either treatment (ANOVA on arcsine square root transformed data (
p = 0.4, f = 0.59, df = 1).
Hydrocarbon leaching: The leaching of hydrocarbon compounds from RCA material was the focus of this study. These hydrocarbon compounds could possibly have been spread onto concrete pavement by vehicles through fluid spills, accidents, and general vehicle wear and tear. The leaching evaluation was performed by determining the concentration of a pollutant and comparing that concentration to an applicable pollutant concentration guidance standard. Since there is no current policy for hydrocarbon compound leaching of RCA, the State of Maryland Department of the Environment cleanup standard for soil and groundwater was adopted. RCA samples from three sites were used in this study. All results of organic chemical concentration were Below Detection Limit (BDL).