Challenges Associated with Cleaning Plastic Food Packaging for Reuse
Abstract
:1. Introduction
2. Literature Review
3. Materials and Methods
3.1. Selection of Pack Form
3.2. Fouling Types and Fouling Deposition Method
3.3. Simulated Reuse
3.4. Cleaning Parameters
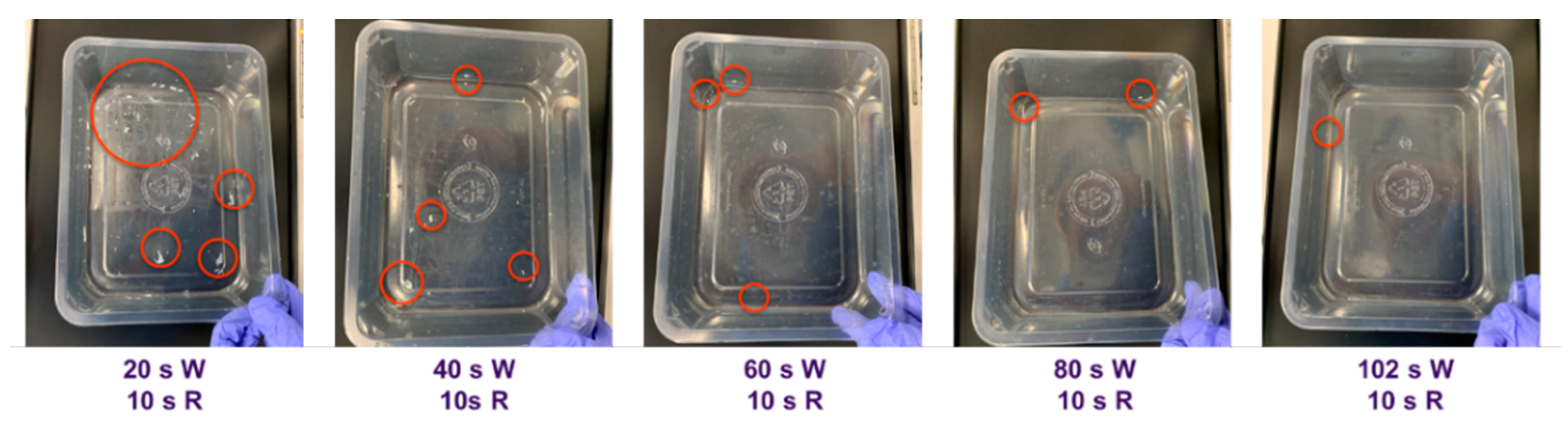
- Wash duration: Containers fouled with 2.00 g (±0.05) of food sample were cleaned for various wash durations with the standard 10 s rinse in the warewasher. For this test, the detergent (Jantex Pro Glass Wash Detergent 0.25% caustic soda, Bristol, UK) dose was set to 3 mL/L on the warewasher (i.e., 0.075% actual caustic soda concentration).
- Detergent dose: To investigate the influence of the detergent dose, containers were fouled and washed for various durations with 0 mL/L, 3 mL/L, and 6 mL/L of detergent. Detergent was only dispensed during the wash stage. The machine was drained before changing the detergent dose and run multiple times before use to ensure the correct dose of detergent was delivered.
- Fouling quantity: For orange juice, the quantity of fouling was determined by fouling a container before inverting it. It was found that when fouled with 2.00 g (±0.05) of orange juice, there was little to no dripping, therefore this quantity represented a realistic quantity of food residue. The same quantity was considered as an appropriate maximum amount of fouling for cream cheese. As the initial investigation with mayonnaise represented 0 RLU for the ATP reading, this fouling type was not studied further unless stated otherwise.
3.5. Cleanliness Assessment Method
3.6. Deformation and Material Properties
3.7. Analysis of Physio-Chemical Properties
- Tensile Testing: Before testing, the rPET packs were cut into small dumbbell shaped samples and equilibrated at 25 °C and 50% relative humidity. Tensile strength at yield and Young’s Modulus of the pack samples were measured using an Instron® 3366 universal testing system according to the ISO 527-2 standard testing parameters at a tensile rate of 80 mm/min. Measurements were performed on 6 samples across the area of the packs and the average results were recorded.
- Differential Scanning Calorimetry (DSC): The thermal characterisation of the packs was performed using TA instruments® Q200 DSC, New Castle, DE, USA. Approximately 5 mg of sample was heated from room temperature to 300 °C at a rate of 10 °C/min. Thermal transition values were determined using TA® Universal Analysis software, New Castle, DE, USA. Measurements were performed on 3 samples across the area of the packs and the average results were recorded.
- Fourier Transform Infrared (FTIR) Spectroscopy: FTIR spectroscopy was performed directly on small cut sections on the packs using a Shimadzu® IRTracer-100, Milton Keynes, UK, equipped with an attenuated total reflectance (ATR) attachment. Data was collected in the region of 400 to 4000 cm−1.
4. Results and Discussion
4.1. Cleaning Assurance Assessment Using ATP Swab Test
4.1.1. Orange Juice
4.1.2. Mayonnaise
4.1.3. Cream Cheese
4.2. Impact of Washing Procedure on the Pack Form
4.2.1. Wash Duration
4.2.2. Detergent Dose
4.2.3. Fouling Quantity
4.3. Pack Form Appearance under Simulated Reuse
4.3.1. Physical Deformation
4.3.2. Mechanical Deformation
4.3.3. Thermal (DSC) and Chemical (IR) Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Summary of Tensile Testing Data | ||||
|---|---|---|---|---|
| Number of Washes | Tensile Strength (MPa) | Young’s Modulus (MPa) | Yield Stress (MPa) | Strain at Yield (%) |
| 0 | 44.8 ± 8.9 | 1717 ± 277 | 49.4 ± 5.4 | 3.67 ± 0.21 |
| 5 | 57.0 ± 10.6 | 1984 ± 266 | 51.9 ± 7.2 | 3.89 ± 0.09 |
| 15 | 56.7 ± 11.2 | 2035 ± 344 | 57.6 ± 1.0 | 3.97 ± 0.07 |
| 20 | 61.1 ± 9.6 | 2076 ± 268 | 61.2 ± 1.0 | 4.17 ± 0.07 |
| 25 | 61.0 ± 3.9 | 2181 ± 145 | 61.3 ± 0.6 | 3.97 ± 0.01 |
| 30 | 63.3 ± 10.0 | 2127 ± 228 | 60.5 ± 3.6 | 4.02 ± 0.08 |
Appendix B
| Cream Cheese—2 g of Fouling—3 mL/L of Detergent on Machine | |||
| Wash Time (s) | Rinse Time (s) | ATP Test Result (RLU) | Observations after Wash |
| 0 | 10 | 290 | Visible fouling |
| 211 | Visible fouling | ||
| 155 | Visible fouling | ||
| 10 | 10 | 167 | Visible fouling |
| 207 | Visible fouling | ||
| 189 | Visible fouling | ||
| 20 | 10 | 249 | Visible fouling |
| 73 | Visible fouling | ||
| 189 | Visible fouling | ||
| 30 | 10 | 72 | Visible fouling |
| 40 | 10 | 22 | Visible fouling |
| 50 | 10 | 14 | Visible fouling |
| 60 | 10 | 13 | Visible fouling |
| 70 | 10 | 9 | Visible fouling |
| 80 | 10 | 2 | Visible fouling |
| 90 | 10 | 5 | Visible fouling |
| 102 (Standard wash) | 10 | 4 | Visible fouling |
| Cream Cheese—2 g of Fouling—0 mL/L of Detergent on Machine | |||
| Wash Time (s) | Rinse Time (s) | ATP Test Result (RLU) | Observations after Wash |
| 20 | 10 | 66 | |
| 30 | 10 | 83 | Visible fouling |
| 40 | 10 | 57 | Visible fouling |
| 50 | 10 | 26 | Visible fouling |
| 60 | 10 | 15 | Visible fouling |
| 70 | 10 | 12 | Visible fouling |
| 80 | 10 | 14 | Visible fouling |
| 90 | 10 | 20 | Visible fouling |
| 102 (Standard wash) | 10 | 13 | Visible fouling |
| Cream Cheese—2 g of Fouling—6 mL/L of Detergent on Machine | |||
| Wash Time (s) | Rinse Time (s) | ATP Test Result (RLU) | Observations after Wash |
| 20 | 10 | 42 | Visible fouling |
| 30 | 10 | 83 | Visible fouling |
| 40 | 10 | 57 | Visible fouling |
| 50 | 10 | 26 | Visible fouling |
| 60 | 10 | 15 | Visible fouling |
| 70 | 10 | 12 | Visible fouling |
| 80 | 10 | 14 | Visible fouling |
| 90 | 10 | 20 | Visible fouling |
| 102 (Standard wash) | 10 | 13 | No noticeable fouling |
| Cream Cheese—1 g of Fouling—3 mL/L of Detergent on Machine | |||
| Wash Time (s) | Rinse Time (s) | ATP Test Result (RLU) | Observations after Wash |
| 20 | 10 | 63 | Visible fouling |
| 30 | 10 | 32 | Visible fouling |
| 40 | 10 | 20 | Visible fouling |
| 50 | 10 | 8 | Visible fouling |
| 60 | 10 | 4 | Visible fouling |
| 70 | 10 | 4 | No noticeable fouling |
| 80 | 10 | 5 | No noticeable fouling |
| 90 | 10 | 3 | Visible fouling |
| 102 (Standard wash) | 10 | 7 | No noticeable fouling |
Appendix C
| DSC Data | 0 | 5 | 15 | 20 | 25 | 30 |
| Tg (°C) (±standard deviation) | 66.4 (±0.5) | 66.3 (±0.8) | 70.4 (±0.9) | 70.0 (±0.2) | 70.2 (±0.1) | 69.8 (±0.1) |
| Tc (°C) (±standard deviation) | 125.9 (±0.5) | 124.5 (±2.9) | 124.3 (±2.0) | 124.5 (±0.5) | 124.1 (±0.4) | 123.4 (±0.3) |
| Tm (±standard deviation) (°C) | 247.7 (±0.2) | 249.3 (±2.2) | 248.1 (±0.8) | 247.7 (±0.2) | 247.8 (±0.2) | 247.7 (±0.1) |
References
- Ellen MacArthur Foundation. The New Plastics Economy, Rethinking The Future of Plastics. Available online: https://www.mckinsey.com/~/media/McKinsey/dotcom/client_service/Sustainability/PDFs/The%20New%20Plastics%20Economy.ashx (accessed on 6 September 2021).
- Gogliettino, M.; Balestrieri, M.; Ambrosio, R.L.; Anastasio, A.; Smaldone, G.; Proroga, Y.T.R.; Moretta, R.; Rea, I.; De Stefano, L.; Agrillo, B.; et al. Extending the Shelf-Life of Meat and Dairy Products via PET-Modified Packaging Activated with the Antimicrobial Peptide MTP1. Front. Microbiol. 2020, 10, 2963. [Google Scholar] [CrossRef] [PubMed]
- Smith, L. A House of Commons Library Briefing Paper on plastic waste in the UK. Briefing Paper Number 08515. Available online: https://researchbriefings.files.parliament.uk/documents/CBP-8515/CBP-8515.pdf (accessed on 17 May 2022).
- Smithers, R. ‘Lunch on The Go’ Habit Generates 11bn Items of Packaging Waste a Year. The Guardian, London. Available online: https://www.theguardian.com/environment/2019/may/14/lunch-on-go-habit-generates-11bn-items-packaging-waste-year-uk (accessed on 11 November 2021).
- Clark, N.; Trimingham, R.L.; Wilson, G.T. Incorporating consumer insights into the UK food packaging supply chain in the transition to a Circular Economy. Sustainability 2020, 12, 6106. [Google Scholar] [CrossRef]
- The Grocer Vision. Whitepapers on ‘Grocery Packaging in A Sustainable Future’. Available online: https://drlkaenwvxlk9.cloudfront.net/Uploads/l/z/m/grocervisionpwcbeyondplastics_869862.pdf (accessed on 5 July 2022).
- Ross, S.; Evans, D. The environmental effect of reusing and recycling plastic-based packaging system. J. Clean. Prod. 2003, 11, 561–571. [Google Scholar] [CrossRef]
- Zero Waste Europe. Press Release ‘Reusable vs. Single-Use packaging: A Review of Environmental Impact’. Available online: https://zerowasteeurope.eu/library/reusable-vs-single-use-packaging-a-review-of-environmental-impact/ (accessed on 28 September 2021).
- McDowell, D.A. Food Hygiene Challenges in Replacing Single Use Food Service Ware with Reusable Food Service Items. Available online: https://www.eppa-eu.org/uploads/Bestanden/FINAL%20EPPA%20Report%20-%20Professor%20David%20McDowell.pdf (accessed on 22 July 2021).
- Pilz, H.; Brandt, B.; Fehringer, R. The Impact of Plastic packaging on Life Cycle Energy Consumption and Greenhouse Gas Emissions in Europe, Plastics Europe. Available online: http://matchpack2015.web2016.netivity.nl/data/filling/pdf/105/The-impact-of-plastics-on-life-cycle-energy-consumption-and-greenhouse-gas-emissions-in-Europe.pdf (accessed on 11 November 2021).
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-related food losses and waste: An overview of drivers and issues. Sustainability 2019, 11, 264. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Purnell, P.; Iacovidou, E.; Velis, C.A.; Atseyinku, M. Post-consumer plastic packaging waste in England: Assessing the yield of multiple collection-recycling schemes. Waste Manag. 2018, 75, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Megale Coelho, P.; Corona, B.; Klooster, R.; Worrell, E. Sustainability of reusableackaging–Current situation and trends. Resour. Conserv. Recycl. X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Harnoto, M.F. A Comparative Life Cycle Assessment of Compostable and Reusable Takeout Clamshells at the University of California, Berkeley. Available online: https://nature.berkeley.edu/classes/es196/projects/2013final/HarnotoM_2013.pdf (accessed on 22 July 2021).
- Reloop, Zero Waste Europe. Reusable vs. Single-Use packaging—A Review of Environmental Impacts. Available online: https://zerowasteeurope.eu/wp-content/uploads/2020/12/zwe_reloop_report_reusable-vs-single-use-packaging-a-review-of-environmental-impact_en.pdf.pdf_v2.pdf (accessed on 22 July 2021).
- Loop. Cleaning Systems. Available online: https://loopstore.co.uk/cleaning (accessed on 11 August 2021).
- Food Standards Australia New Zealand. A Guide to the Food Safety Standards Appendix 6- Cleaning and Sanitising Surfaces and Utensils, 3rd ed. Available online: https://www.foodstandards.gov.au/publications/Pages/safefoodaustralia3rd16.aspx (accessed on 13 August 2021).
- NSF. Dishwashers. Available online: https://www.nsf.org/knowledge-library/dishwashers (accessed on 15 July 2021).
- Guh, A.; Carling, P. Options for Evaluating Environmental Cleaning, Centres for Disease Control and Prevention. Available online: https://www.cdc.gov/hai/pdfs/toolkits/Environ-Cleaning-Eval-Toolkit12-2-2010.pdf (accessed on 11 August 2021).
- Thermo Fischer Scientific. Hygiene and Sanitation Monitoring Using ATP Bioluminescence. Available online: https://www.thermofisher.co.nz/Uploads/file/Scientific/Microbiology-Products/1542342447_ATP-Hygiene-monitoring_White-Paper_FDB_NZ.PDF (accessed on 11 August 2021).
- Hygiena. ATP Testing: A Proven Method to Measure Cleanliness. Available online: https://www.hygiena.com/wp-content/uploads/2020/09/ATP-Testing-A-Proven-Method-to-Measure-Cleanliness.pdf (accessed on 11 August 2021).
- Kupski, B.; Ceylan, E.; Stewart, C. Performance Evaluation of Various ATP Detecting Units; Silliker, Inc.: Chicago, IL, USA, 2009. [Google Scholar]
- Shama, G.; Malik, D.J. The uses and abuses of rapid bioluminescence-based ATP assays. Int. J. Hyg. Environ. Health 2013, 216, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Agency, Cleaning. Available online: https://www.food.gov.uk/print/pdf/node/194 (accessed on 21 September 2021).
- Costa, P.D.; Nélio, J.A.; Sebastião, C.C.B.; Frederico, J.V.P.; Nilda, F.F.S. ATP-bioluminescence assay as an alternative for hygiene-monitoring procedures of stainless-steel milk contact surfaces. Braz. J. Microbiol. 2006, 37, 345–349. [Google Scholar] [CrossRef]
- Griffith, C. Improving surface sampling andetection of contamination. In Handbook of Hygiene Control in Theood Industry; Lelieveld, H.L.M., Mostert, M.A., Holah, J., Eds.; Woodhead Publishing: Sawston, UK, 2005; pp. 588–618. [Google Scholar]
- Hygiena. Hygiena SystemSURE Plus™: Instruction Manual. Available online: https://www.sanitationtools.com/media/luminometer-operators-manual-v4.0.pdf (accessed on 12 August 2021).
- Hygiena (noate—A). A Comparative Study of Commercial ATP Hygiene Monitoring Systems. Available online: https://d163axztg8am2h.cloudfront.net/static/doc/9a/72/6a2bdc2f5e07097e41972ba05d66.pdf (accessed on 12 August 2021).
- Hygiena (noate—B). Lower and Upper RLU Limitsor ATP Monitoring Programs. Available online: https://d163axztg8am2h.cloudfront.net/static/doc/c1/b1/d472875da397448c7747d185c9e2.pdf (accessed on 6 September 2021).
- Corbitt, A.J.; Bennion, N.; Forsythe, S.J. Adenylate kinase amplification of ATP bioluminescence for hygiene monitoring in the food and beverage industry. Lett. Appl. Microbiol. 2000, 30, 443–447. [Google Scholar] [CrossRef]
- Hygiena. Hygiena UltraSnap ™ Surface ATP Test: Instruction Manual. Available online: https://cdn.brandfolder.io/VZSMQ4LE/at/mj3w6kh453z8mk28xx98n/ATP-UltraSnap-Kit-Insert.pdf (accessed on 3 September 2021).
- Wypych, G. Handbook of Polymers, 2nd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2016; pp. 398–403. ISBN 978-1-895198-92-8. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]













| Wash Time (s) | Rinse Time (s) | ATP Test Result (RLU) | Average (RLU) |
|---|---|---|---|
| 20 | 10 | 4 | 4 |
| 10 | 10 | 6 | 6 |
| 0 | 10 | 4 | 2.67 |
| 2 | |||
| 2 | |||
| 0 | 7 | 7 | 16.33 |
| 16 | |||
| 26 | |||
| 0 | 5 | 322 | 398.67 |
| 155 | |||
| 719 |
| Number of Standard Washes | Total Wash and Rinse Time (m:s) | Images | ||
|---|---|---|---|---|
| Top View | Bottom View | Side View | ||
| 0 | - |  |  |  |
| 5 | 8:30 wash 0:50 rinse |  |  |  |
| 10 | 17:00 wash 1:40 rinse |  |  |  |
| 15 | 25:30 wash 2:30 rinse |  |  |  |
| 20 | 34:00 wash 3:20 rinse |  |  |  |
| 25 | 42:30 wash 4:10 rinse |  |  |  |
| 30 | 51:00 wash 5:00 rinse |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, S.; Sian, M.; Larder, R.; Hatton, F.L.; Woolley, E. Challenges Associated with Cleaning Plastic Food Packaging for Reuse. Waste 2023, 1, 21-39. https://doi.org/10.3390/waste1010003
Nahar S, Sian M, Larder R, Hatton FL, Woolley E. Challenges Associated with Cleaning Plastic Food Packaging for Reuse. Waste. 2023; 1(1):21-39. https://doi.org/10.3390/waste1010003
Chicago/Turabian StyleNahar, Samsun, Mala Sian, Ryan Larder, Fiona L. Hatton, and Elliot Woolley. 2023. "Challenges Associated with Cleaning Plastic Food Packaging for Reuse" Waste 1, no. 1: 21-39. https://doi.org/10.3390/waste1010003
APA StyleNahar, S., Sian, M., Larder, R., Hatton, F. L., & Woolley, E. (2023). Challenges Associated with Cleaning Plastic Food Packaging for Reuse. Waste, 1(1), 21-39. https://doi.org/10.3390/waste1010003







