The J-Band of J-Aggregates as the Egorov Nano-Resonance †
Abstract
1. Introduction
2. Result for the Shape of the Optical Absorption Band from Quantum–Classical Mechanics of Elementary Electron Transfers and Egorov Nano-Resonance
3. Adaptation of Quantum–Classical Mechanics of Elementary Electron Transfers to Quantum–Classical Transitions in Extended Polymethine Chromophores
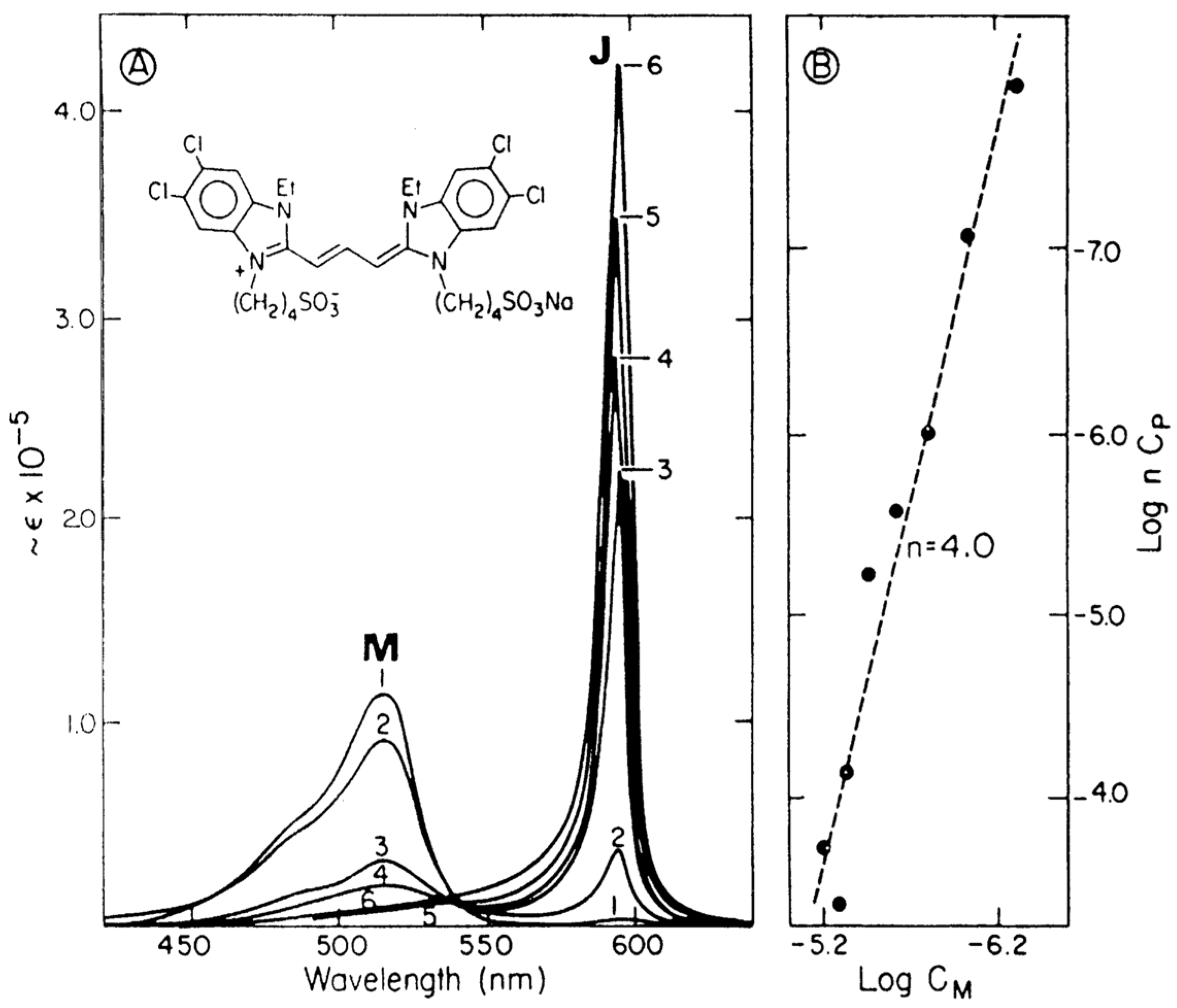
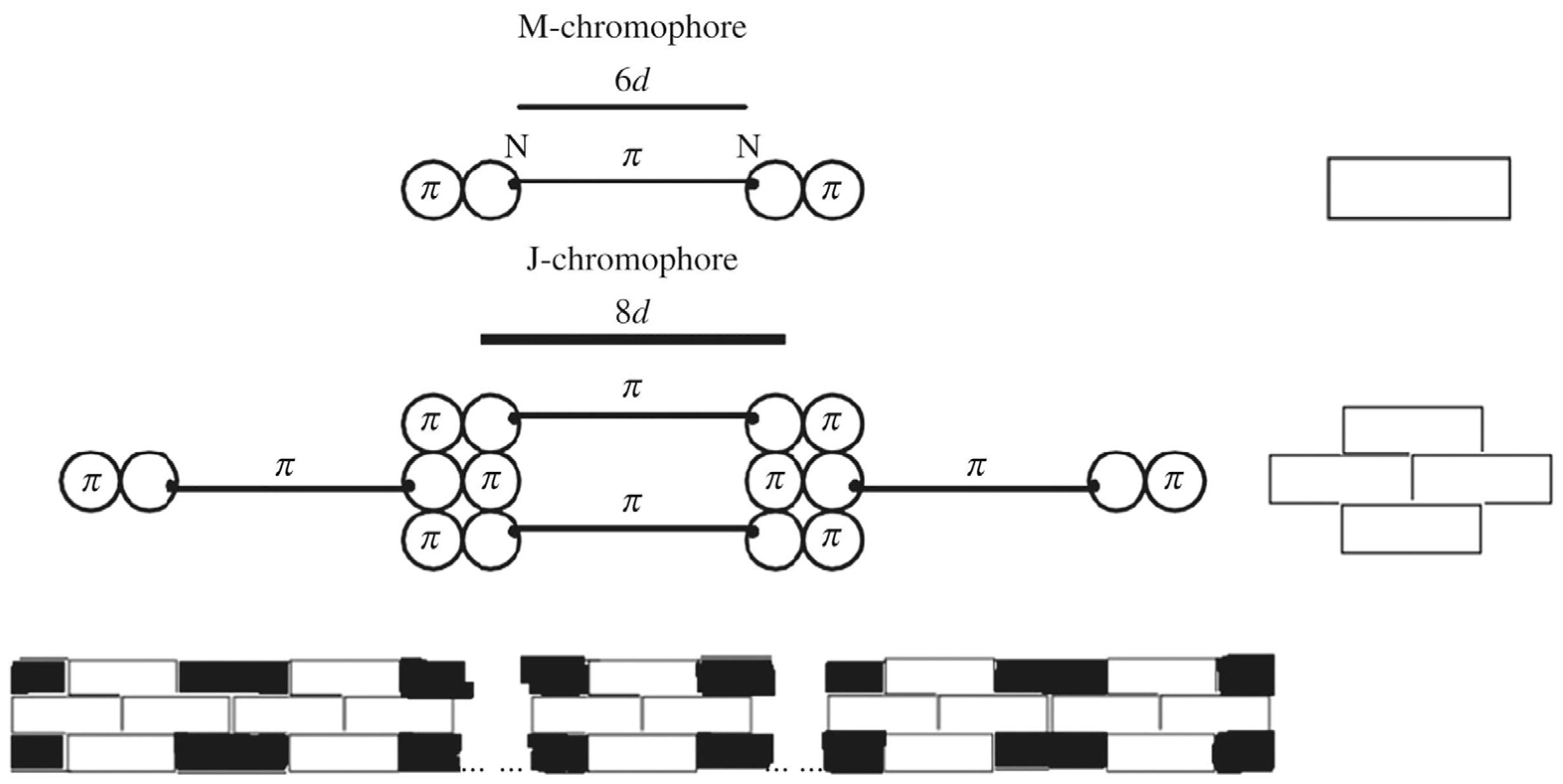
4. Explanation of the J-Band of J-Aggregates by Egorov Nano-Resonance
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jelley, E.E. Spectral absorption and fluorescence of dyes in the molecular state. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Scheibe, G. Variability of the absorption spectra of some sensitizing dyes and its cause. Angew. Chem. 1936, 49, 563. [Google Scholar]
- Franck, J.; Teller, E. Migration and photochemical action of excitation energy in crystals. J. Chem. Phys. 1938, 6, 861–872. [Google Scholar] [CrossRef]
- Knapp, E.W. Lineshapes of molecular aggregates, exchange narrowing and intersite correlation. Chem. Phys. 1984, 85, 73–82. [Google Scholar] [CrossRef]
- Makhov, D.V.; Egorov, V.V.; Bagatur’yants, A.A.; Alfimov, M.V. Efficient approach to the numerical calculation of optical line shapes for molecular aggregates. J. Chem. Phys. 1999, 110, 3196–3199. [Google Scholar] [CrossRef]
- Egorov, V.V.; Alfimov, M.V. Theory of the J-band: From the Frenkel exciton to charge transfer. Phys. Uspekhi 2007, 50, 985–1029. [Google Scholar] [CrossRef]
- Egorov, V.V. Theory of the J-band: From the Frenkel exciton to charge transfer. Phys. Procedia 2009, 2, 223–326. [Google Scholar] [CrossRef]
- Egorov, V.V. Quantum–classical mechanics: Nano-resonance in polymethine dyes. Mathematics 2022, 10, 1443. [Google Scholar] [CrossRef]
- Egorov, V.V. Quantum-classical mechanics as an alternative to quantum mechanics in molecular and chemical physics. Heliyon Phys. 2019, 5, e02579. [Google Scholar] [CrossRef]
- Egorov, V.V. On electrodynamics of extended multiphonon transitions and nature of the J-band. Chem. Phys. 2001, 269, 251–283. [Google Scholar] [CrossRef]
- Egorov, V.V. Nature of the optical transition in polymethine dyes and J-aggregates. J. Chem. Phys. 2002, 116, 3090–3103. [Google Scholar] [CrossRef]
- Egorov, V.V. Nature of the optical band shapes in polymethine dyes and H-aggregates: Dozy chaos and excitons. Comparison with dimers, H*- and J-aggregates. R. Soc. Open Sci. 2017, 4, 160550. [Google Scholar] [CrossRef] [PubMed]
- Egorov, V.V. Quantum-classical mechanics: Luminescence spectra in polymethine dyes and J-aggregates. Nature of the small Stokes shift. Results Phys. 2019, 13, 102252. [Google Scholar] [CrossRef]
- Perlin, Y.E. Modern methods in the theory of many-phonon processes. Sov. Phys. Uspekhi 1964, 6, 542–565. [Google Scholar] [CrossRef]
- Dähne, S. Color and constitution: One hundred years of research. Science 1978, 199, 1163–1167. [Google Scholar] [CrossRef]
- Kachkovskii, A.D. The nature of electronic transitions in linear conjugated systems. Russ. Chem. Rev. 1997, 66, 647–664. [Google Scholar] [CrossRef]
- James, T.H. (Ed.) The Theory of the Photographic Process; Macmillan: New York, NY, USA, 1977. [Google Scholar]
- Brooker, L.G.S.; Sprague, R.H.; Smith, C.P.; Lewis, G.L. Color and constitution. I. Halochromism of anhydronium bases related to the cyanine dyes. J. Am. Chem. Soc. 1940, 62, 1116–1125. [Google Scholar] [CrossRef]
- Egorov, V.V. Dryad Digital Repository. Data R. Soc. Open Sci. 2017, 4, 160550. [Google Scholar] [CrossRef] [PubMed]
- Herz, A.H. Aggregation of sensitizing dyes in solution and their adsorption onto silver halides. Adv. Colloid Interface Sci. 1977, 8, 237–298. [Google Scholar] [CrossRef]
- Kuhn, H.; Kuhn, C. Chromophore coupling effects. In J-Aggregates; Kobayashi, T., Ed.; World Scientific: Singapore, 1996; pp. 1–40. [Google Scholar]
- Born, M.; Oppenheimer, J.R. Quantum theory of the molecules. Ann. Phys. 1927, 84, 457–484. [Google Scholar] [CrossRef]
- Franck, J.; Dymond, E.G. Elementary processes of photochemical reactions. Trans. Faraday Soc. 1925, 21, 536–542. [Google Scholar] [CrossRef]
- Condon, E.U. A theory of intensity distribution in band systems. Phys. Rev. 1926, 28, 1182–1201. [Google Scholar] [CrossRef]
- Condon, E.U. Nuclear motions associated with electron transitions in diatomic molecules. Phys. Rev. 1928, 32, 858–872. [Google Scholar] [CrossRef]
- Condon, E.U. The Franck-Condon principle and related topics. Am. J. Phys. 1947, 15, 365–374. [Google Scholar] [CrossRef]
- Petrenko, A.; Stein, M. Toward a molecular reorganization energy-based analysis of third-order nonlinear optical properties of polymethine dyes and J-aggregates. J. Phys. Chem. A 2019, 123, 9321–9327. [Google Scholar] [CrossRef]
- Egorov, V.V.; Thomas, S. Quantum-classical mechanics: On the problem of a two-photon resonance band shape in polymethine dyes. Nano-Struct. Nano-Objects 2021, 25, 100650. [Google Scholar] [CrossRef]
- Petrenko, A.; Stein, M. Molecular reorganization energy as a key determinant of J-band formation in J-aggregates of polymethine dyes. J. Phys. Chem. A 2015, 119, 6773–6780. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, V.V. The J-Band of J-Aggregates as the Egorov Nano-Resonance. Comput. Sci. Math. Forum 2023, 7, 31. https://doi.org/10.3390/IOCMA2023-14414
Egorov VV. The J-Band of J-Aggregates as the Egorov Nano-Resonance. Computer Sciences & Mathematics Forum. 2023; 7(1):31. https://doi.org/10.3390/IOCMA2023-14414
Chicago/Turabian StyleEgorov, Vladimir V. 2023. "The J-Band of J-Aggregates as the Egorov Nano-Resonance" Computer Sciences & Mathematics Forum 7, no. 1: 31. https://doi.org/10.3390/IOCMA2023-14414
APA StyleEgorov, V. V. (2023). The J-Band of J-Aggregates as the Egorov Nano-Resonance. Computer Sciences & Mathematics Forum, 7(1), 31. https://doi.org/10.3390/IOCMA2023-14414





