A Beneficial Role of Rooibos in Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
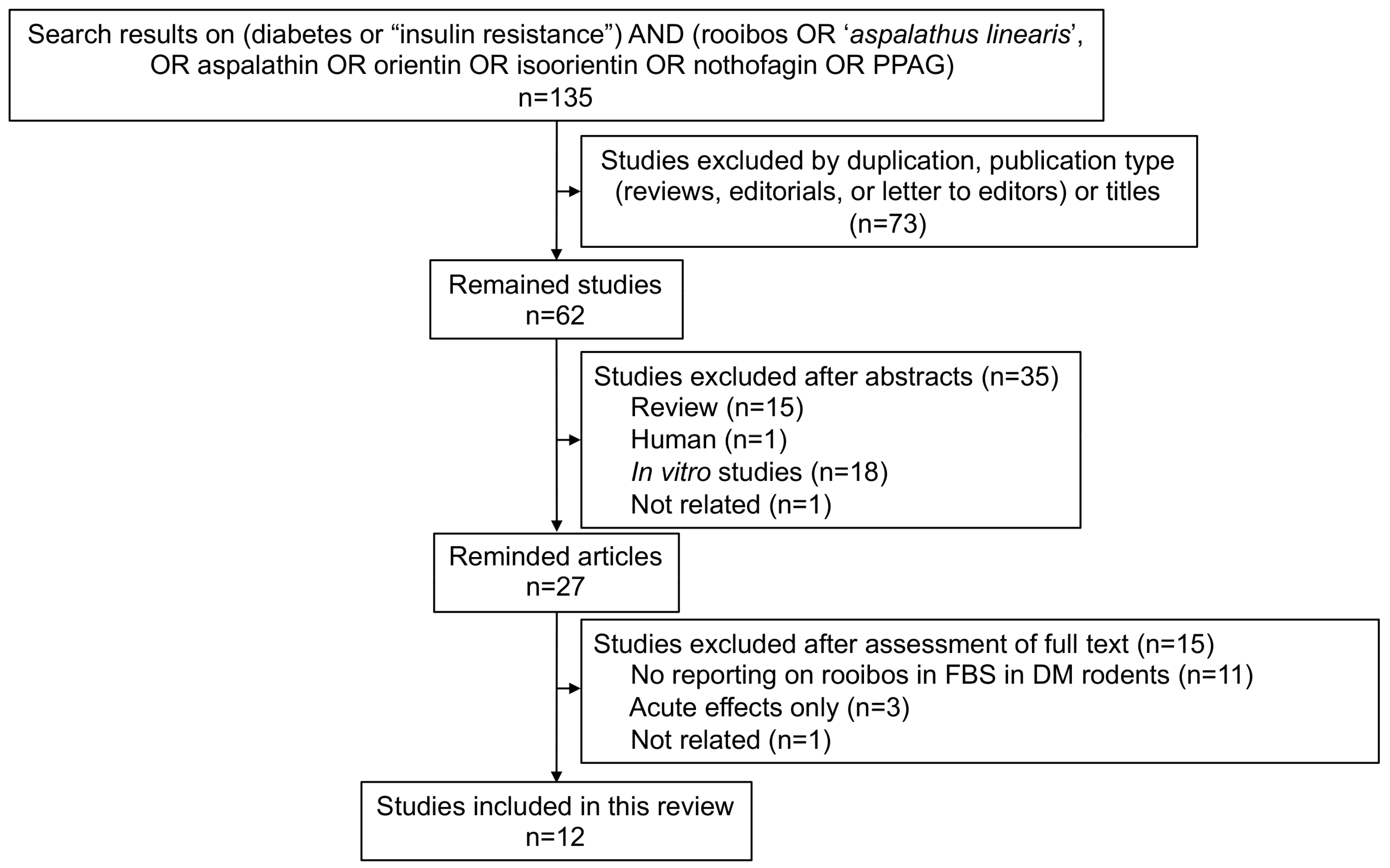
2.1. Search Results
2.2. Study Characteristics and Quality Assessment
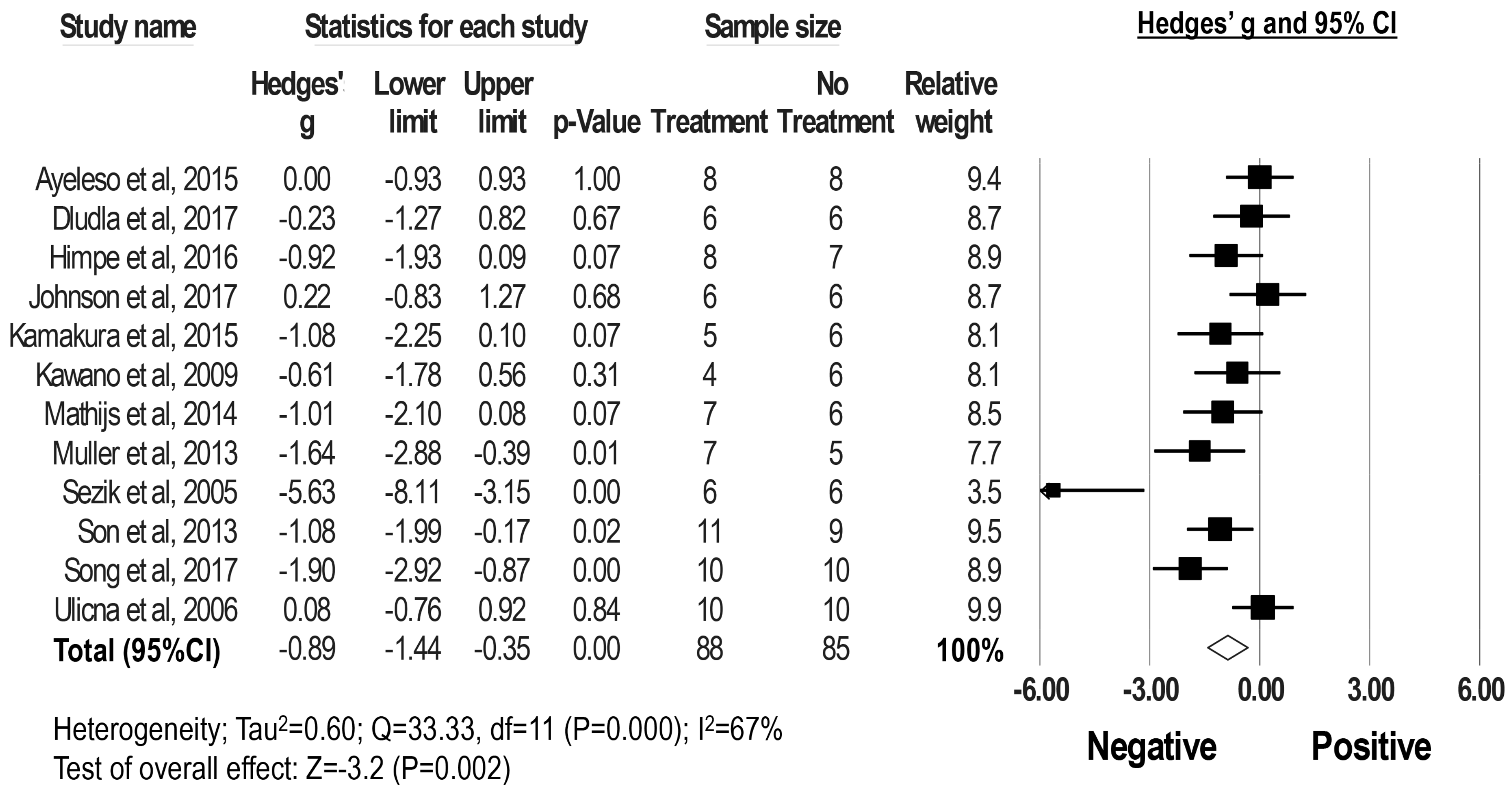
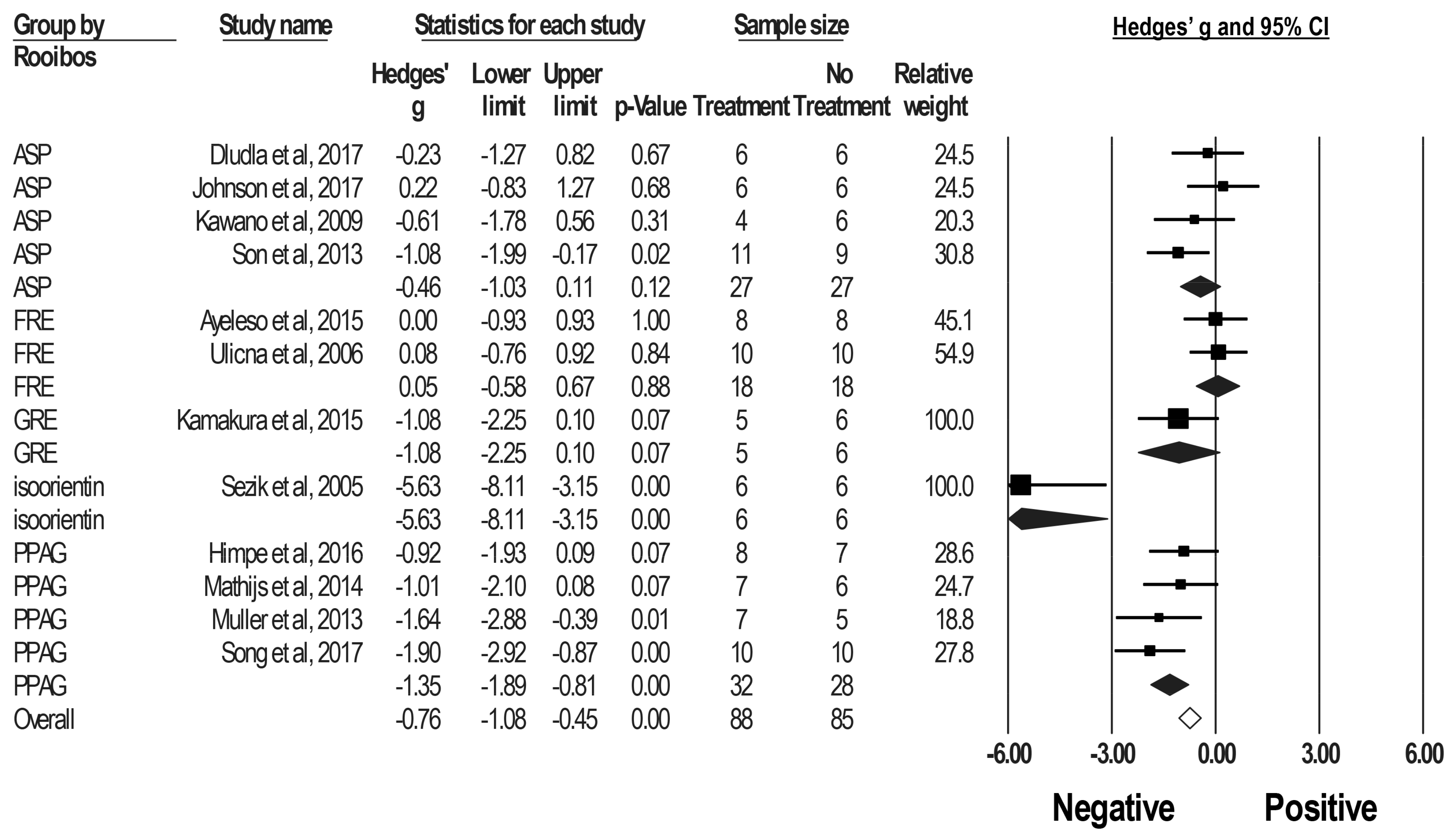
2.3. Effect of Rooibos Tea Extracts and Associated Polyphenols on Blood Glucose Levels in DM Rodent Models
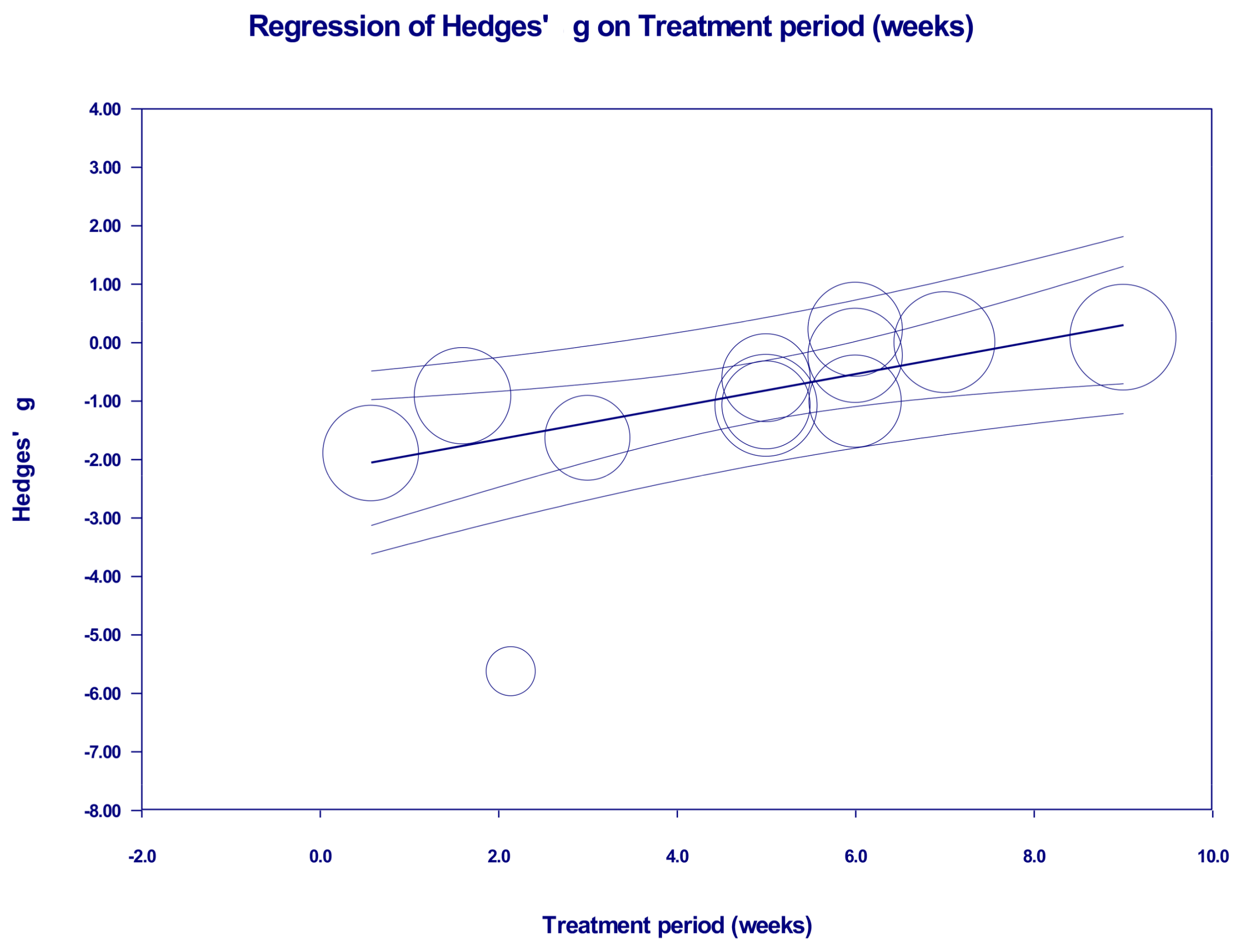
2.4. Meta-Regression Analyses
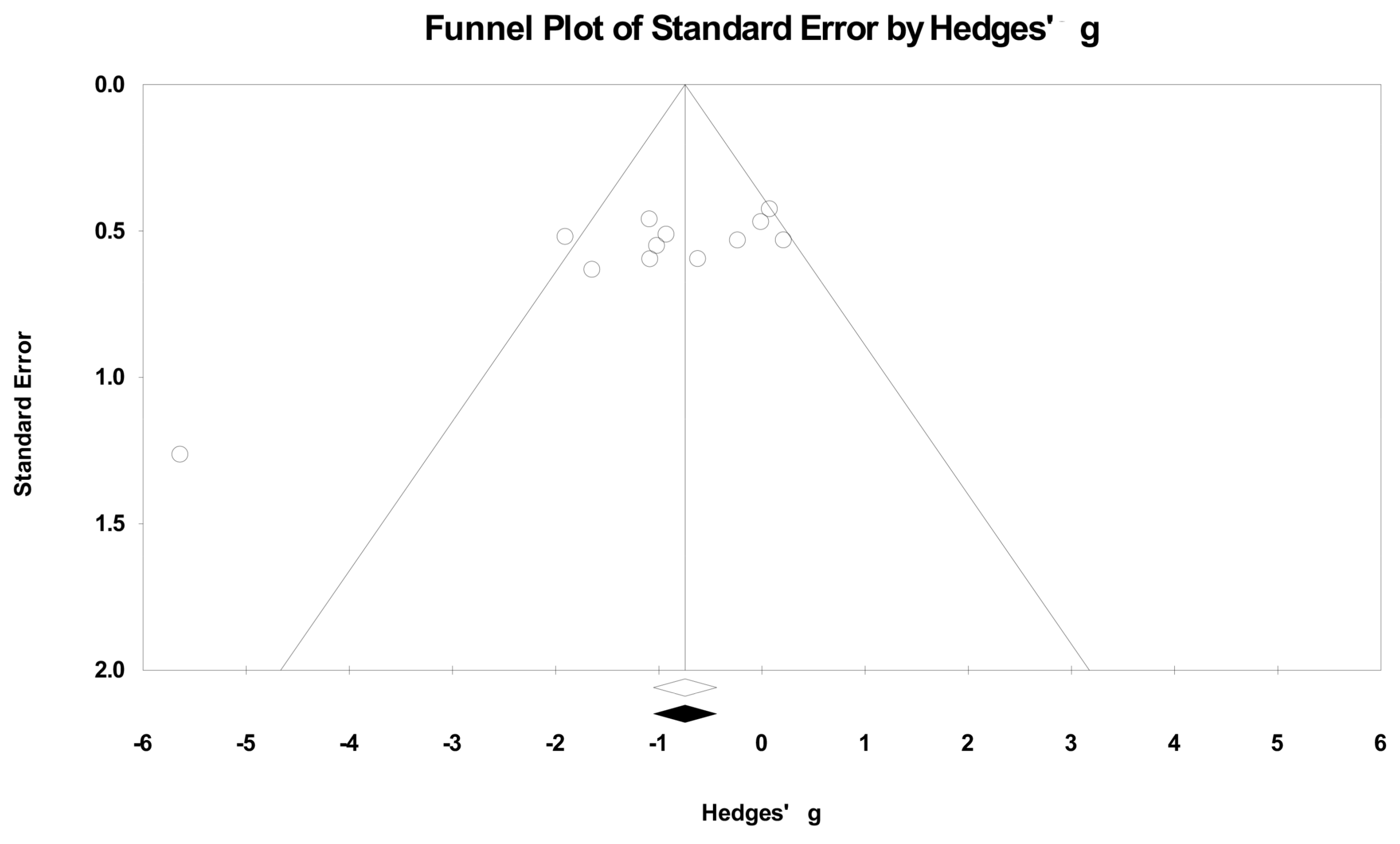
2.5. Publication Bias
3. Discussion
3.1. Main Findings
3.2. Interpretation
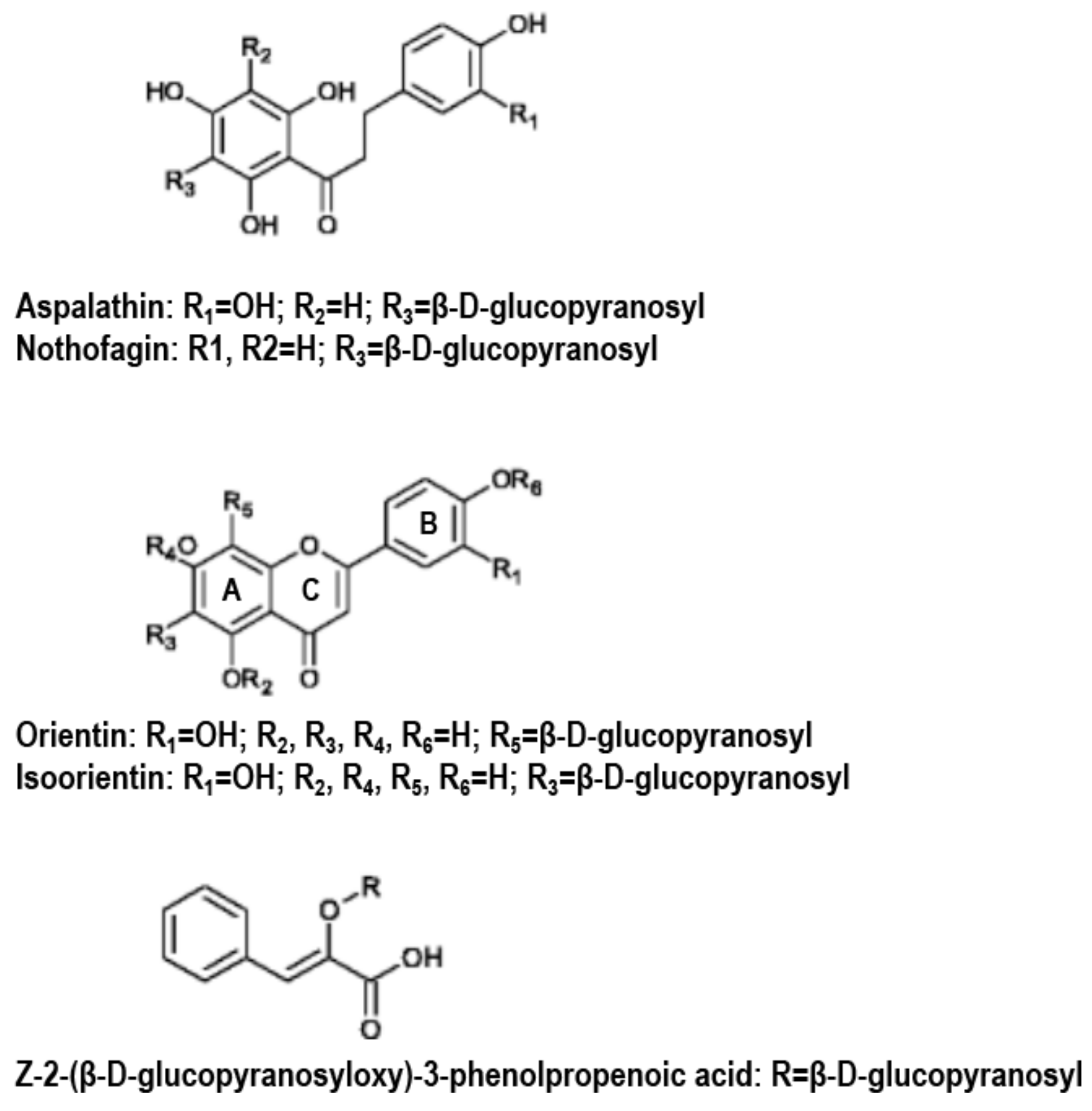
3.2.1. Structures and Pharmacological Properties of Phenolic Compounds Rich in Rooibos Extracts
3.2.2. FREs, GREs and Major Phenolic Compounds in Rooibos in DM Rodent Models
3.2.3. Strength and Limitations
4. Materials and Methods
4.1. Data Sources and Search Strategies
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction and Quality Assessment
4.4. Data Synthesis and Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; IDF: Brussels, Belgium, 2017; Available online: http://www.diabetesatlas.org (accessed on 7 March 2018).
- Suksomboon, N.; Poolsup, N.; Boonkaew, S.; Suthisisang, C.C. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. J. Ethnopharmacol. 2011, 137, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Kam, A.; Wong, K.H.; Zhou, X.; Omar, E.A.; Alqahtani, A.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Herbal medicines for the management of diabetes. Adv. Exp. Med. Biol. 2012, 771, 396–413. [Google Scholar] [PubMed]
- Chang, C.L.; Lin, Y.; Bartolome, A.P.; Chen, Y.C.; Chiu, S.C.; Yang, W.C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Altern. Med. 2013, 2013, 378657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.T.; Li, W.L.; De Kimpe, N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [PubMed]
- Kibiti, C.M.; Afolayan, A.J. Herbal therapy: A review of emerging pharmacological tools in the management of diabetes mellitus in Africa. Pharmacogn. Mag. 2015, 11, S258–S274. [Google Scholar] [CrossRef] [PubMed]
- Harlev, E.; Nevo, E.; Mirsky, N.; Ofir, R. Antidiabetic attributes of desert and steppic plants: A review. Planta Med. 2013, 79, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of South African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. 2007, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, S.E.; Muller, C.J.; Joubert, E.; de Beer, D.; Johnson, R.; Opoku, A.R.; Louw, J. Amelioration of palmitate-induced insulin resistance in C(2)C(1)(2) muscle cells by rooibos (Aspalathus linearis). Phytomedicine 2013, 20, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-A(y) mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-beta-d-glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.; Persson, K.; Hagg, S.; Andersson, R.G. Effects of green tea, black tea and Rooibos tea on angiotensin-converting enzyme and nitric oxide in healthy volunteers. Public Health Nutr. 2010, 13, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Villano, D.; Pecorari, M.; Testa, M.F.; Raguzzini, A.; Stalmach, A.; Crozier, A.; Tubili, C.; Serafini, M. Unfermented and fermented rooibos teas (Aspalathus linearis) increase plasma total antioxidant capacity in healthy humans. Food Chem. 2010, 123, 679–683. [Google Scholar] [CrossRef]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, A.O.; Oguntibeju, O.O.; Brooks, N.L. Impact of Co-administration of Red Palm Oil (Elaeis guineensis Arecaceae) and Rooibos (Aspalathus linearis Fabaceae) on Glycaemic Parameters, Liver Function and Key Glycolytic Enzymes in Diabetic Rats. Trop. J. Pharm. Res. 2015, 14, 1613–1619. [Google Scholar] [CrossRef]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Essop, M.F.; Gabuza, K.B.; Ghoor, S.; Huisamen, B.; Johnson, R. Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression. Molecules 2017, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Himpe, E.; Cunha, D.A.; Song, I.; Bugliani, M.; Marchetti, P.; Cnop, M.; Bouwens, L. Phenylpropenoic Acid Glucoside from Rooibos Protects Pancreatic Beta Cells against Cell Death Induced by Acute Injury. PLoS ONE 2016, 11, e0157604. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.V.; Muller, C.J.; Huisamen, B.; Essop, M.F.; Louw, J. The Transcription Profile Unveils the Cardioprotective Effect of Aspalathin against Lipid Toxicity in an In Vitro H9c2 Model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, I.; Da Cunha, D.A.; Himpe, E.; Ladriere, L.; Chellan, N.; Roux, C.R.; Joubert, E.; Muller, C.; Cnop, M.; Louw, J.; et al. Phenylpropenoic acid glucoside augments pancreatic beta cell mass in high-fat diet-fed mice and protects beta cells from ER stress-induced apoptosis. Mol. Nutr. Food Res. 2014, 58, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Joubert, E.; Pheiffer, C.; Ghoor, S.; Sanderson, M.; Chellan, N.; Fey, S.J.; Louw, J. Z-2-(beta-D-glucopyranosyloxy)-3-phenylpropenoic acid, an alpha-hydroxy acid from rooibos (Aspalathus linearis) with hypoglycemic activity. Mol. Nutr. Food Res. 2013, 57, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Sezik, E.; Aslan, M.; Yesilada, E.; Ito, S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005, 76, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Roels, S.; Martens, G.A.; Bouwens, L. Circulating microRNA-375 as biomarker of pancreatic beta cell death and protection of beta cell mass by cytoprotective compounds. PLoS ONE 2017, 12, e0186480. [Google Scholar] [CrossRef] [PubMed]
- Ulicna, O.; Vancova, O.; Bozek, P.; Carsky, J.; Sebekova, K.; Boor, P.; Nakano, M.; Greksak, M. Rooibos tea (Aspalathus linearis) partially prevents oxidative stress in streptozotocin-induced diabetic rats. Physiol. Res. 2006, 55, 157–164. [Google Scholar] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A nonparametric ‘trim and fill’ method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and structural characterization of alpha-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS(n) and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Kinne, R.K.; Castaneda, F. SGLT inhibitors as new therapeutic tools in the treatment of diabetes. In Diabetes-Perspectives in Drug Therapy; Springer: Berlin/Heidelberg, Germany, 2011; pp. 105–126. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Grasset, E.; Heyman, M.; Dumontier, A.M.; Beau, J.P.; Desjeux, J.F. Congenital selective malabsorption of glucose and galactose. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Lee, W.S.; You, G.; Brown, D.; Hediger, M.A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J. Clin. Investig. 1994, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Idris, I.; Donnelly, R. Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug. Diabetes Obes. Metab. 2009, 11, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Smith, D.; Shulman, G.I.; Papachristou, D.; DeFronzo, R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Investig. 1987, 79, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.R.; Vila-Vicosa, D.; Machuqueiro, M.; Marques, A.P.; Dore, T.M.; Rauter, A.P. Targeting Type 2 Diabetes with C-Glucosyl Dihydrochalcones as Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: Synthesis and Biological Evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.; Wang, H.; Meng, F. In silico modeling of aspalathin and nothofagin against SGLT2. J. Ther. Comput. Chem. 2015, 14, 15550056. [Google Scholar]
- Ayeleso, A.; Brooks, N.; Oguntibeju, O. Modulation of antioxidant status in streptozotocin-induced diabetic male Wistar rats following intake of red palm oil and/or rooibos. Asian Pac. J. Trop. Med. 2014, 7, 536–544. [Google Scholar] [CrossRef]
- Ayeleso, A.; Oguntibeju, O.O.; Brooks, N.L. Assessment of Lipid Profiles, Antioxidant Status and Liver Histopathology in Male Wistar Rats Following Dietary Intake of Rooibos (Aspalathus linearis). Int. J. Pharmacol. 2013, 9, 348–357. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Joubert, E.; Johnson, R.; Louw, J.; Opoku, A.R.; Muller, C.J. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015, 59, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Gelderblom, W.C.; Louw, A.; de Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.A.; Novelli, E.L.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimaraes, J.F.; Fernandes Junior, A. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kamalakkannan, N.; Prince, P.S. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Arumugam, T.V.; Brown, L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jang, I.S.; Yang, K.E.; Yoon, S.J.; Baek, S.; Lee, J.Y.; Suzuki, T.; Chung, K.Y.; Woo, S.H.; Choi, J.S. Effect of rutin from tartary buckwheat sprout on serum glucose-lowering in animal model of type 2 diabetes. Acta Pharm. 2016, 66, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wei, G.; You, Y.; Huang, Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.; Zhang, C.; et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017, 31, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Choo, C.Y.; Sulong, N.Y.; Man, F.; Wong, T.W. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo alpha-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Syimal’ain Azmi, N.; Noor Mohamad Zin, N.S.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement. Altern. Med. 2017, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; de Beer, D.; Malherbe, C.J.; Muller, N.; Bonnet, S.L.; van der Westhuizen, J.H.; Ferreira, D. Occurrence and sensory perception of Z-2-(β-d-glucopyranosyloxy)-3-phenylpropenoic acid in rooibos (Aspalathus linearis). Food Chem. 2013, 136, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Wiedenfeld, H. Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J. Ethnopharmacol. 2001, 78, 145–149. [Google Scholar] [CrossRef]
- Perez, R.M.; Ocegueda, A.; Munoz, J.L.; Avila, J.G.; Morrow, W.W. A study of the hypoglycemic effect of some Mexican plants. J. Ethnopharmacol. 1984, 12, 253–262. [Google Scholar] [CrossRef]
- Roman-Ramos, R.; Flores-Saenz, J.L.; Partida-Hernandez, G.; Lara-Lemus, A.; Alarcon-Aguilar, F. Experimental study of the hypoglycemic effect of some antidiabetic plants. Arch. Investig. Med. 1991, 22, 87–93. [Google Scholar]
- Baser, K.H.C.; Honda, G.; Miki, W. Herb Drugs and Herbalists in Turkey; ILCAA: Tokyo, Japan, 1986; p. 27. [Google Scholar]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0; The Cochrane Collaboration: London, UK, 2017; Available online: https://training.cochrane.org/handbook (accessed on 7 March 2018).
- Engels, M.; Wang, C.; Matoso, A.; Maidan, E.; Wands, J. Tea not Tincture: Hepatotoxicity Associated with Rooibos Herbal Tea. ACG Case Rep. J. 2013, 1, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Sinisalo, M.; Enkovaara, A.L.; Kivisto, K.T. Possible hepatotoxic effect of rooibos tea: a case report. Eur. J. Clin. Pharmacol. 2010, 66, 427–428. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) | Rooibos or Poly-phenols | Dose, Route | Duration | Animal Models | Total n (T/no-T) | Age or Weight at a Baseline | Diet | Fasting or ad Lib. | Blood Sample |
|---|---|---|---|---|---|---|---|---|---|
| Ayeleso A et al., (2015) [18] | FRE | 2 g/100 mL boiling water. As drinking water | 7 w | STZ-induced DM rats (50 mg/kg i.m.) | 16 (8/8) | 176–255 g | Control | Overnight fasting | Plasma |
| Dludla PV et al., (2017) [19] | ASP (98%) | 13 or 130 mg/kg BW via daily oral gavage | 6 w | db/db mice | 12 (6/6) | 9 w | Control | 16-h fasting | Plasma |
| Himpe E et al., (2016) [20] | PPAG (99%) | 10 mg/kg BW via daily oral gavage. | 11 d | STZ-induced DM mice (200 mg/kg i.p.) | 15 (8/7) | 9–11 w, approx. 25 g | Control | Ad lib. | Whole blood |
| Johnson R et al., (2017) [21] | ASP (98%) | 13 or 130 mg/kg/day via daily oral gavage | 6 w | db/db mice | 12 (6/6) | 9 w | Control | 4-h fasting | Plasma |
| Kamakura R et al., (2015) [13] | GRE (6.62% ASP) | Add to diet at 0.3% and then 0.6%. | 5 w | KK-Ay mice | 11 (5/6) | 4 w | Control | 3-h fasting | Whole blood |
| Kawano A et al., (2009) [22] | ASP (98.5%) | Added to diet at 0.2% | 5 w | db/db mice | 10 (4/6) | 6 w | Control | 4-h fasting | Whole blood |
| Mathijs I et al., (2014) [23] | PPAG (99%) | 10 mg/kg BW via daily oral gavage | 6 w | OBIR mice | 13 (7/6) | 15 w | High fat and fructose | Fasting | Whole blood |
| Muller CJ et al., (2013) [24] | PPAG (99%) | 0.3–3 mg/kg BW via daily oral gavage | 3 w | OBIR rats | 12 (7/5) | 24 w | High fat and sucrose | 4-h fasting | Plasma |
| Sezik E et al., (2005) [25] | isoorientin | 15 or 30 mg/kg BW/d via daily oral gavage | 15 d | STZ-induced DM rats (55mg/kg i.p.) | 12 (6/6) | 200–250 g | Control | 18–20 h fasting | Whole blood |
| Son MJ et al., (2013) [26] | ASP | 0.1% dietary supplement | 5 w | ob/ob mice | 20 (11/9) | 6 w | Control | 3-h fasting | Serum |
| Song I et al., (2017) [27] | PPAG | A dose of 10 mg/kg BW via daily oral gavage | 4 d | STZ-induced DM mice (200 mg/kg i.p.) | 20 (10/10) | 9–11 w, approx. 25 g | Control | Ad lib. | Whole blood |
| Ulicna O et al., (2006) [28] | FRE | 2.5 g/1L of boiling water, 5 mL/kg BW/d via gavage | 9 w | STZ-induced DM rats (45 mg/kg i.v.) | 20 (10/10) | 290–340 g | Control | Ad lib. | Plasma |
| Subgroups | Effect Size | Heterogeneity (I2) | ||||
|---|---|---|---|---|---|---|
| No. of Studies | g | 95% CI | P-value | |||
| Rooibos and Polyphenols | ||||||
| FRE | 2 | 0.05 | −0.58 | 0.67 | 0.88 | <0.001 |
| GRE | 1 | −1.08 | −2.25 | 0.10 | 0.07 | <0.001 |
| ASP | 4 | −0.46 | −1.03 | 0.11 | 0.12 | 18.12 |
| PPAG | 4 | −1.35 | −1.89 | −0.81 | <0.001 | <0.001 |
| Isoorientin | 1 | −5.63 | −8.11 | −3.15 | <0.001 | <0.001 |
| DM rodent models | ||||||
| db/db | 3 | −0.18 | −0.80 | 0.45 | 0.02 | <0.001 |
| ob/ob | 1 | −1.08 | −1.99 | −0.17 | 0.58 | <0.001 |
| KK-Ay | 1 | −1.08 | −2.25 | 0.10 | 0.07 | <0.001 |
| OBIR | 2 | −1.28 | −2.10 | −0.46 | 0.002 | <0.001 |
| STZ | 5 | −1.29 | −2.54 | −0.05 | 0.04 | 84.65 |
| Rodent | ||||||
| Mice | 8 | −0.84 | −1.28 | −0.39 | <0.001 | 30.09 |
| Rats | 4 | −1.41 | −3.03 | 0.22 | 0.09 | 86.70 |
| Blood sample | ||||||
| Plasma | 6 | −0.54 | −1.26 | 0.19 | 0.15 | 67.42 |
| Serum | 1 | −1.08 | −1.99 | −0.17 | 0.02 | <0.001 |
| Whole blood | 5 | −1.43 | −2.47 | −0.39 | 0.01 | 70.58 |
| Sampling time point | ||||||
| Non-fasting | 3 | −0.88 | −2.03 | 0.27 | 0.134 | 77.02 |
| Fasting (>3h) | 9 | −0.91 | −1.58 | −0.24 | 0.007 | 67.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, M.; Nishida, N.; Shimada, M. A Beneficial Role of Rooibos in Diabetes Mellitus: A Systematic Review and Meta-Analysis. Molecules 2018, 23, 839. https://doi.org/10.3390/molecules23040839
Sasaki M, Nishida N, Shimada M. A Beneficial Role of Rooibos in Diabetes Mellitus: A Systematic Review and Meta-Analysis. Molecules. 2018; 23(4):839. https://doi.org/10.3390/molecules23040839
Chicago/Turabian StyleSasaki, Moe, Nami Nishida, and Masako Shimada. 2018. "A Beneficial Role of Rooibos in Diabetes Mellitus: A Systematic Review and Meta-Analysis" Molecules 23, no. 4: 839. https://doi.org/10.3390/molecules23040839
APA StyleSasaki, M., Nishida, N., & Shimada, M. (2018). A Beneficial Role of Rooibos in Diabetes Mellitus: A Systematic Review and Meta-Analysis. Molecules, 23(4), 839. https://doi.org/10.3390/molecules23040839






