Mouth Breathing and Obstructive Sleep Apnea in Children: An Umbrella Review
Abstract
1. Introduction
2. Objectives
Specific Objectives
- To synthesize the results of different systematic reviews on the relationship between mouth breathing and OSA in children.
- To assess the methodological quality of the included systematic reviews.
- To analyze different perspectives on the topic.
- To identify inconsistencies and knowledge gaps regarding the subject, guiding future research.
- To provide theoretical and practical implications for healthcare professionals, educators, and policymakers regarding the importance of assessing and managing mouth breathing in children.
3. Methodology
3.1. Study Eligibility Criteria
3.2. Information Sources
3.3. Search Strategy
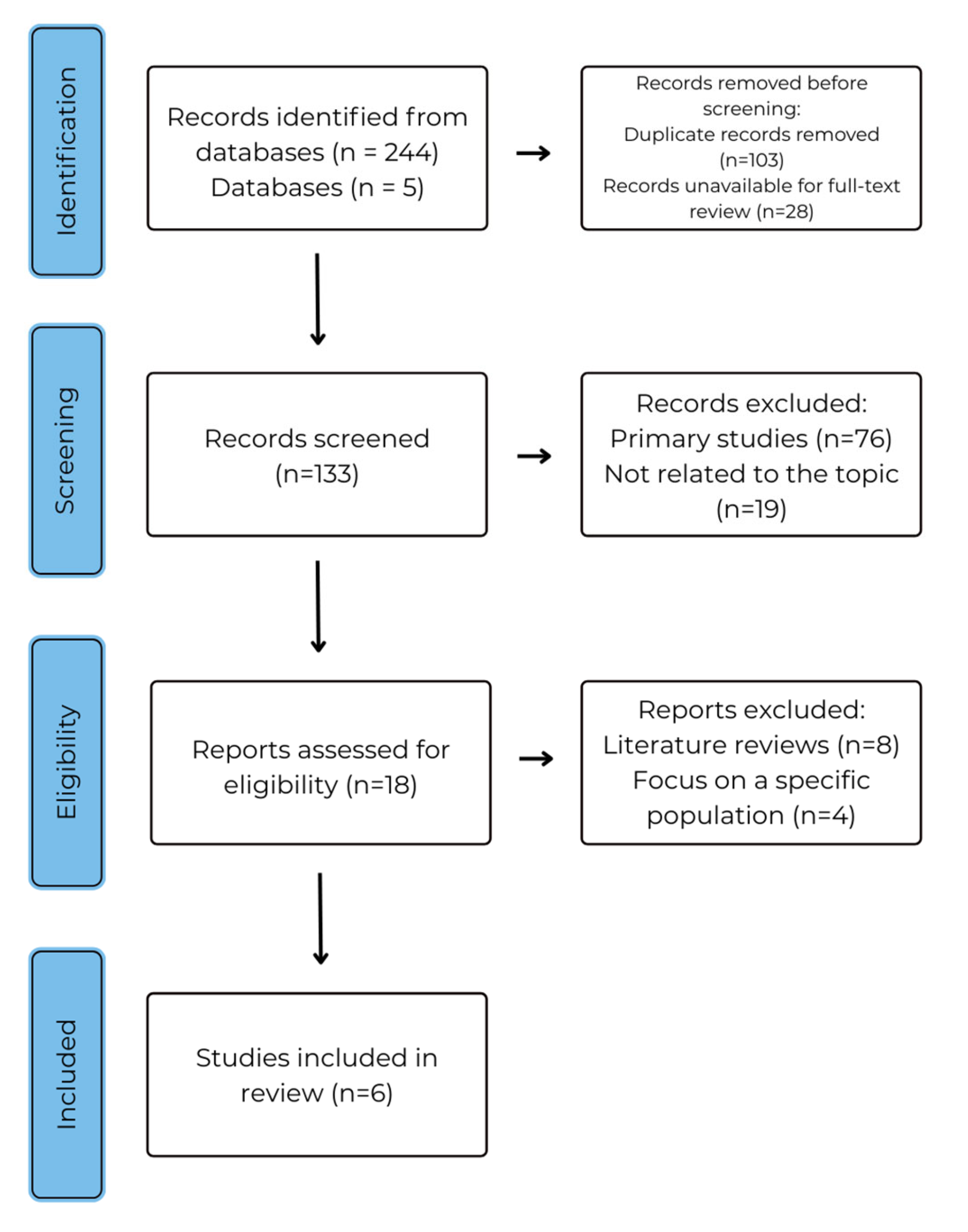
4. Results
4.1. Methodological Quality Assessment
4.2. Findings from Individual Studies
4.2.1. Epidemiology and Diagnosis of OSA
4.2.2. Impacts of OSA on Child Health and Development
- Postural Disorders
- b.
- Craniofacial Development
- c.
- Speech Development
4.2.3. Therapeutic Interventions for OSA
- Rapid Maxillary Expansion (RME)
- b.
- Montelukast
4.3. Results of Syntheses
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palomo, J.M.; Dias, V.; Macedo De Menezes, L. Obstructive sleep apnea: A review for the orthodontist. Online Dent. Press J. Orthod. 2023, 28, 23–24. [Google Scholar] [CrossRef]
- Izu, S.C.; Itamoto, C.H.; Pradella-Hallinan, M.; Pizarro, G.U.; Tufik, S.; Pignatari, S.; Fujita, R.R. Ocorrência da Síndrome da Apneia Obstrutiva do sono (SAOS) em Crianças Respiradoras Orais. Braz. J. Otorhinolaryngol. 2010, 76, 552–556. Available online: https://www.scielo.br/j/bjorl/a/MdgJ9RSG93n9QkQPjtQcL6f/?lang=pt&format=html (accessed on 8 January 2024). [CrossRef]
- Silva, C.F.F.S.d.; Gomes, V.C.A.; Boas, L.S.e.S.V.; Pezzin, A.C. Avaliação das Alterações do sono em Crianças com Síndrome do Respirador Oral. Rev. Eletrônica Acervo Saúde 2019, (Suppl. 24), e637. Available online: https://acervomais.com.br/index.php/saude/article/view/637 (accessed on 8 January 2024). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of pediatric obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Park, V.; Bogaardt, H.; Docking, K. The impact of childhood obstructive sleep apnea on speech and oral language development: A systematic review. Sleep Med. 2021, 81, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Neiva, P.D.; Kirkwood, R.N.; Mendes, P.L.; Zabjek, K.; Becker, H.G.; Mathur, S. Postural disorders in mouth breathing children: A systematic review. Braz. J. Phys. Ther. 2018, 22, 7–19. [Google Scholar] [CrossRef]
- Habumugisha, J.; Cheng, B.; Ma, S.Y.; Zhao, M.Y.; Bu, W.Q.; Wang, G.L.; Liu, Q.; Zou, R.; Wang, F. A non-randomized concurrent controlled trial of myofunctional treatment in the mixed dentition children with functional mouth breathing assessed by cephalometric radiographs and study models. BMC Pediatr. 2022, 22, 506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes Barbosa, D.; Fernandes Bana, L.; Cristina, M.; Michel, B.; Meira ECruz, M.; Zancanella, E.; Machado Júnior, A.J. Rapid maxillary expansion in pediatric patients with obstructive sleep apnea: An umbrella review. Braz. J. Otorhinolaryngol. 2023, 89, 494–502. Available online: https://www.bjorl.org/ (accessed on 8 January 2024). [CrossRef]
- Ji, T.; Lu, T.; Qiu, Y.; Li, X.; Liu, Y.; Tai, J.; Guo, Y.; Zhang, J.; Wang, S.; Zhao, J.; et al. The efficacy and safety of montelukast in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 2021, 78, 193–201. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews that Include Randomised or Non-Randomised Studies of Healthcare Interventions, or both. BMJ 2017, 358, 4008. Available online: https://www.bmj.com/content/358/bmj.j4008 (accessed on 23 January 2024). [CrossRef]
- Guilleminault, C.; Stoohs, R.; Clerk, A.; Cetel, M.; Maistros, P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 1993, 104, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, R.C.; Passerot, G.; Paulucci, B.; Miniti, A. Mouth breathing in children: Different repercussions according to the diagnosis. Rev. Bras. Otorrinolaringol. 2004, 70, 665–670. [Google Scholar] [CrossRef]
- Felcar, J.M.; Bueno, I.R.; Massan, A.C.S.; Torezan, R.P.; Cardoso, J.R. Prevalência de Respiradores Orais em Crianças de Idade Escolar. Ciênc Saúde Coletiva 2010, 15, 437–444. Available online: https://www.scielo.br/j/csc/a/n85ytvNm6tDdsW3xvZGJP4h/?format=html&lang=pt (accessed on 26 January 2025). [CrossRef]
- Pagel, J.M.L.; Mattos, J.L. Allergic Rhinitis and Its Effect on Sleep. Otolaryngol. Clin. N. Am. 2024, 57, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L. New approaches to the diagnosis of sleep-disordered breathing in children. Sleep Med. 2010, 11, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Harari, D.; Redlich, M.; Miri, S.; Hamud, T.; Gross, M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope 2010, 120, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Juliano, M.L.; Machado, M.A.; de Carvalho, L.B.; Zancanella, E.; Santos, G.M.; do Prado, L.B.; do Prado, G.F. Polysomnographic findings are associated with cephalometric measurements in mouth-breathing children. J. Clin. Sleep Med. 2009, 5, 554–561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, E.J.; Choi, J.H.; Kim, K.W.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Shin, C.; Lee, K.Y.; Lee, S.H. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur. Arch. Otorhinolaryngol. 2011, 268, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Metgud, D.; Angadi, P.V.; Panthee, A. Association of orofacial dysfunction and sleep disordered breathing among Indian primary school children. J. Oral Biol. Craniofac. Res. 2022, 12, 639–644, Erratum in J. Oral Biol. Craniofac. Res. 2024, 14, 358–359. https://doi.org/10.1016/j.jobcr.2024.05.010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Zheng, L.; Huang, X.; Li, C.; Liu, J.; Hu, Y. Effects of mouth breathing on facial skeletal development in children: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chirakalwasan, N.; Ruxrungtham, K. The linkage of allergic rhinitis and obstructive sleep apnea. Asian Pac. J. Allergy Immunol. 2014, 32, 276–286. [Google Scholar] [PubMed]
- Machado Júnior, A.J.; Zancanella, E.; Evangelisti, M.; Villa, M.P. OSAS treatments: Is treating shape enough? Sleep Med. 2021, 79, 122–123. [Google Scholar] [CrossRef]
- Paskay, L.C. Instrumentation and measurement procedures in orofacial myology. Int. J. Orofac. Myol. 2006, 32, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Khurana, A. Artificial intelligence in obstructive sleep apnea: Transforming diagnosis and management. Respir. Med. 2025, 243, 108100. [Google Scholar] [CrossRef] [PubMed]
- Machado Júnior, A.J.; Crespo, A.N. Influence of mandibular morphology on the hyoid bone in atypical deglutition: A correlational study. Int. J. Orofac. Myol. 2011, 37, 39–46. [Google Scholar] [CrossRef]
- Machado Almiro, J.; Crespo, A.N. Pediatric obstructive sleep apnea: Beyond adenotonsillectomy. Sleep Med. 2019, 66, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Tourne, L.P. The long face syndrome and impairment of the nasopharyngeal airway. Angle Orthod. 1990, 60, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.R.; Xie, S.Q.; Yang, X.; Chen, J.L. The Effects of Orofacial Myofunctional Therapy on Children with OSAHS’s Craniomaxillofacial Growth: A Systematic Review. Children 2023, 10, 670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer, L.J.; Tessitore, A.; Machado Júnior, A.J.; Sakano, E. Clinical and Surface Electromyography Evaluation Pre and Post Orofacial Myology Therapy. Int. J. Orofac. Myol. Myofunct. Ther. 2020, 46, 5–12. [Google Scholar] [CrossRef]
- Machado-Júnior, A.J.; Zancanella, E.; Crespo, A.N. Rapid maxillary expansion and obstructive sleep apnea: A review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e465–e469. [Google Scholar] [CrossRef]
- D’Elia, C.; Gozal, D.; Bruni, O.; Goudouris, E.; Meira ECruz, M. Allergic rhinitis and sleep disorders in children-coexistence and reciprocal interactions. J. Pediatr. 2022, 98, 444–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, R.M.; Tricco, A.C.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny, C.; et al. Reporting guideline for overviews of reviews of healthcare interventions: Development of the PRIOR statement. BMJ 2022, 378, e070849. [Google Scholar] [CrossRef] [PubMed]
| Study Reference | Protocol Registered | Included Studies Quality | Clear Inclusion/Exclusion Criteria | Adequate Database Search | Risk of Bias Assessment | Discussion on Bias Impact | Final Rating |
|---|---|---|---|---|---|---|---|
| Barbosa et al. (2023) [9] | Yes | Moderate | Yes | Yes | Yes | Yes | High confidence |
| Ji et al. (2021) [10] | No | Moderate | Yes | Yes | Yes | Yes | Moderate confidence |
| Lumeng & Chervin (2008) [5] | No | High | Yes | Yes | Yes | Yes | High confidence |
| Mohammed et al. (2021) [6] | No | Low | Yes | Yes | Yes | No | Low confidence |
| Neiva et al. (2018) [7] | No | Low | Yes | Yes | Yes | No | Low confidence |
| Zhao et al. (2021) [21] | Yes | Moderate | Yes | Yes | Yes | Yes | Moderate confidence |
| Category | Key Findings | References | Limitations |
|---|---|---|---|
| Epidemiology and Diagnosis | OSA prevalence ranges from 0.1% to 13%; lack of standardized diagnostic criteria | Lumeng & Chervin (2008) [5] | Inconsistent diagnostic methods |
| Child Development | Craniofacial alterations, postural changes, and speech/language impairments associated with mouth breathing | Zhao et al. (2021) [21]; Neiva et al. (2018) [7]; Mohammed et al. (2021) [6] | Small sample sizes, selection bias |
| Therapeutic Interventions | RME and montelukast show promising results, but evidence remains limited | Barbosa et al. (2023) [9]; Ji et al. (2021) [10] | High heterogeneity, potential adverse effects |
| Study Reference: | Total Studies Included: | Population (Sample): | Metrics: | Main Findings: |
|---|---|---|---|---|
| Barbosa, D. et al., 2023 [9] | 8 | 0–18 years | AHI; SaO2; LSAT; AI; | The studies presented high heterogeneity, compromising the uniformity of results. Despite some improvements in sleep after RME, the evidence is limited for definitive recommendations. The study highlights the importance of assessing the disease phenotype prior to intervention and reinforces the need for preventive strategies for childhood respiratory disorders. |
| Ji, T. et al., 2021 [10] | 4 | 0–18 years | AHI; ODI; AI; SpO2; PSQ; OSA-18; | Montelukast, when combined with conventional treatments, showed improvements in sleep parameters, such as apnea-hypopnea index and oxygen saturation. However, concerns remain regarding serious side effects reported by the FDA. The quality of evidence was considered moderate due to methodological limitations and inconsistencies across studies. |
| Lumeng et al., 2008 [5] | 48 | 0–18 years | AHI; ODI; SDB Score; | The study highlights the lack of standardization in diagnostic criteria for pediatric OSA, resulting in wide variability in prevalence (0.1% to 13%). It points out the limitations of alternative methods, such as questionnaires and home recordings, and reinforces the need for standardized diagnoses to improve identification and intervention in at-risk children. |
| Mohammed, D. et al., 2021 [6] | 6 | 2–13 years | PSG; Behavior Rating Inventory of Executive Function–Preschool Version (BRIEF-P); Child Behavior Checklist (CBCL 1.5–5); Caregiver-Teacher Report Form (C-TRF 1.5–5); Behavior Assessment System for Children, 2nd Ed. (BASC-II) Parent/Primary; Psychological tests, such as the Token Test (TT); Physical examinations to assess adenotonsillar hypertrophy and malocclusion. | It was concluded that speech and language difficulties are common in these children, with an impact associated with low oxygen saturation during sleep, affecting neurocognitive and neurobehavioral functions. However, the quality of evidence was considered very low due to methodological limitations and small sample sizes. |
| Neiva, P. D. et al., 2018 [7] | 10 | 5–14 years | New York Postural Assessment Scale; Photography; Motion capture; Postural Analysis Software (SAPO); Goniometer; ALC image; MATLAB (version 2.1) program routines. | The review indicates a possible association between mouth breathing and postural changes in children, such as forward head posture and body misalignment. However, all studies analyzed presented low methodological quality, with shortcomings in participant description and in the standardization of assessment methods. Thus, no conclusive evidence exists, underscoring the need for more rigorous research to validate this relationship. |
| Zhao, Z. et al., 2021 [21] | 10 | 2–14 years | SNA (Sella–Nasion–A); SNB (Sella–Nasion–B); ANB; SN-OP (Sella–Nasion–Occlusal Plane); SN-PP (Sella–Nasion–Palatal Plane); PP-MP (Palatal Plane–Mandibular Plane); SNGoGn (Sella–Nasion–Gonion); 1-NA (Tooth 1–Nasion); 1-NB (Tooth 1–B); Overjet; Overbite; SPAS; PAS; C3-H; | The findings showed that children with mouth breathing had unique facial characteristics, such as posterior mandibular rotation, steeper occlusal plane, and limited upper arch width, commonly referred to as an “adenoid face.” Additionally, this group presented higher frequencies of malocclusion, especially Class II and crossbite. The study also demonstrated that nasal stenosis exacerbates these changes. Comparisons were made using control groups, and statistically significant differences in facial growth were identified, emphasizing the need for early diagnosis and timely intervention in pediatric patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Association of Orofacial Myology (IAOM). Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscuola, M.P.C.; Lobato, D.M.; Machado Júnior, A.J. Mouth Breathing and Obstructive Sleep Apnea in Children: An Umbrella Review. Int. J. Orofac. Myol. Myofunct. Ther. 2025, 51, 13. https://doi.org/10.3390/ijom51020013
Biscuola MPC, Lobato DM, Machado Júnior AJ. Mouth Breathing and Obstructive Sleep Apnea in Children: An Umbrella Review. International Journal of Orofacial Myology and Myofunctional Therapy. 2025; 51(2):13. https://doi.org/10.3390/ijom51020013
Chicago/Turabian StyleBiscuola, Mariana Pires Comune, Daniel Mendes Lobato, and Almiro José Machado Júnior. 2025. "Mouth Breathing and Obstructive Sleep Apnea in Children: An Umbrella Review" International Journal of Orofacial Myology and Myofunctional Therapy 51, no. 2: 13. https://doi.org/10.3390/ijom51020013
APA StyleBiscuola, M. P. C., Lobato, D. M., & Machado Júnior, A. J. (2025). Mouth Breathing and Obstructive Sleep Apnea in Children: An Umbrella Review. International Journal of Orofacial Myology and Myofunctional Therapy, 51(2), 13. https://doi.org/10.3390/ijom51020013






