Abstract
With respect to the stomatognathic system, as a rule, speech therapy used to be restricted to the function of degtutition. At present, the importance of chewing and breathing for both the development and growth of the stomatognathic system is better understood. This study investigates the safety of APRELI DAEMON (AD) in healthy adults and children (i.e., showing no symptoms, signs or complaints of acute illness), and collects impressions on AD use and safety. Method: this is an intervention study with a convenience sample of 10 adults and 20 children. Exclusion criteria: lesions in the oral cavity, and use of orthodontic appliances. Subjects kept prototype device AD in the mouth for 30 uninterrupted minutes while performing routine activities. Participants and trained observers filled in questionnaires about the use of the device. A descriptive analysis with frequency distribution, measures of central tendency and of dispersion were carried out; qualitative data were assessed by reading all responses to the questionnaire, identifying core themes, and categorizing. Results: all volunteers kept AD in their mouths for 30 uninterrupted minutes; no adverse effects occurred during or after use. Discomfort at use was found in 40% of adults and 20% of children. Hypercontraction and lip sealing, lateralization and suction were observed during the use of the device. Conclusions: The device was safe in both age groups.
Introduction
For decades, speech therapy was restricted to the function of deglutition with regard to the stomatognathic system. Recently, other functions such as chewing and breathing have been understood as relevant to the development and growth of the stomatognathic system (Andrada e Silva, Natalini, Ramires & Ferreira, 2007; Marchesan, 2001; Tessitore, 2005; Douglas, 2006). Orofacial motricity is the area of Speech Therapy that studies, researches, prevents, evaluates, diagnoses, develops, promotes, improves and rehabilitates the structure and functions of the orofacial and cervical regions. It focuses on promoting health and preventing diseases of the orofacial myofunctional system with regard to breathing, suction, chewing, deglutition and speech in all life cycles, from the gestation period throughout the natural ageing process. It also involves the diagnosis and intervention in congenital or acquired disorders involving the orofacial myofunctional system and its functions—breathing, suction, chewing, deglutition and speech—in all life cycles (Comitê de motricidade orofacial, 2004; Hahn & Hahn, 1992; Umberger & Johnston, 1997).
The literature on the use of devices as an adjuvant resource to speech therapy focusing on jaw thrust, lip sealing, and tongue retraction is limited to Castillo Morales’ research (Carlstedt, Henningsson, McAllister & Dahllof, 2003; Castillo-Morales, 1999; Glatz-Noll & Berg, 1991; Limbrock, Fischer-Brandies & Avalle, 1991). The APRELI DAEMON (AD) device was developed taking into consideration the altered orofacial motricity in Down Syndrome (OS). The aims of the AD (Figure 1) converge with those of orofacial myofunctional therapy (OMT}, namely to attain normal interocclusal space at rest by developing lip competence, properly positioning the tongue at rest, retraining deglutition in tongue thrust, and breaking the habit of thumb, and finger sucking (Hanson & Mason, 2003). No studies on the use of any device similar to APRELI DAEMON (AD) as an adjuvant therapeutic resource in children with OS have been found. The prototype device was conceived to be a low cost, easy-to-use, adjuvant therapeutic resource to be used with OS children, for which future investigation with this audience is mandatory. A safety study was required and carried out with adults, and school and pre school children without syndromic dysmorphia, in order to investigate potential discomfort that might rule out the use of the device by the primary target audience OS. The present study aims to check AD safety in healthy adults and children, as well as its performance in orofacial motricity.
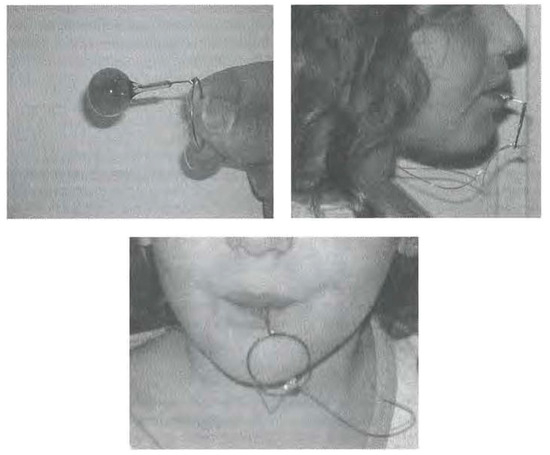
Figure 1.
APRELI DAEMON.
METHODS
This is an intervention study with a convenience sample of 10 healthy volunteer adults and 20 healthy volunteer children without dysmorphisms, and with no symptoms, sings or complaints of acute illness. Adults were graduate students attending a lecture within the Postgraduate Program on Internal Medicine of the Medical School of Federal University of Rio de Janeiro. Children are members of a School Club maintained by the School of Physical Education and Sports of Federal University of Rio de Janeiro. Exclusion criteria were presence of lesions in the oral cavity and/or use of fixed or mobile orthodontic appliances. The clinical research protocol has the approval of the Ethical Research Board of Martagao Gesteira Pediatric Institute, and complies with Resolution N°. 196/96 and further of the National Health Council, and the 1988 Code of Medical Ethics. All participants or their legal responsible party signed an informed consent.
Before it was manufactured, AD was submitted to four experts for evaluation—a pediatrician, an otorhinolaryngologist, a speech therapist specialized in oral motricity, and an orthopedist specialized in the maxilla. They stated their professional opinion on the device, its applications and potential hazards.
AD consists of a loop at one end (2.5 cm diameter), a stem (2.5 cm length), and a silicone ball at the other end (4.6 g weight). For greater safety, a silicone yarn was tied to the loop forming a necklace. The device was crafted by Prosthodontist CERN, CRO-TPD- 2012RO with the following material: a) 0.9 mm orthodontic springy stiff wire (CrNi), Morelli™, Anvisa Register N°. 10396830018; b) silver solder (56% Ag–650°C) for dental applications, Morelli™ , Anvisa Register N°. 10396830030; and c) dental resin (Methyl methacrylate), Classico Artigos Odontol6gicos ™, Anvisa Register N°. 10234680009. Patent protocol was issued in Brazil on October 25, 2011, INPI protocol N°. 020110109866.
The following variables were investigated: a) safety during use, safety being defined as absence of adverse reactions, such as gag reflex, cough, choking or aspiration; b) impressions on its use, provided by study subjects; c) discomfort at use, reported by study subjects; and d) uninterrupted use for an established period of time (30 uninterrupted minutes). Subjects were asked to use the prototype device for 30 uninterrupted minutes while performing routine activities: adults attended a lecture, and children attended gym class and/or played memory games. Adults were observed by three individuals: two researchers—speech therapist and physician, and one guest speech therapist. The observers were placed in a triangle position in the classroom. All volunteers answered a questionnaire at the end of the activity, without the observers’ interference. Children were observed by two individuals—researcher speech therapist, and a guest speech therapist who were placed opposite the study subjects. At the end of the activity, children answered a research questionnaire. Observers were entirely aware of the study protocol and of the activities to be performed by each group.
A descriptive analysis with frequency distribution, measures of central tendency and of dispersion was carried out. Qualitative data, gathered from participants’ responses to the questionnaires, were assessed through identification of core themes and later categorization (Santos, 1999). The questionnaire aimed to determine:
- Subjects’ impressions about the use of AD (taste, shape, size, etc.);
- Whether or not subjects felt discomfort at use, and if they did, a detailed description was requested;
- Whether or not the use of AD impaired attention to the activity;
- Comments and suggestions.
Data Collection Form (completed by observers) included:
- Subject’s initials
- Observer’s name
- Date;
- Time AD was placed in the oral cavity
- Time AD was removed from the oral cavity;
- Length of stay of AD in the oral cavity;
- Observer’s comments (free notes);
- Impressions on use (questions asked by the observers to research subjects);
- Discomfort at use (YES or NO) and subject’s comments;
- Impairment of attention to lecture (YES or NO) and subject’s comments;
- Subject’s critical comments
- Subject’s suggestions.
RESULTS
Adults
The sample consists of 10 adults (2 male, 8 female); mean age, 36.5 years (SD ± 9.7; median = 33 years; minimum age = 24 years; maximum age = 55 years):
- Regarding flavor: all volunteers stated that AD is flavor-free (Figure 2); so flavor did not interfere with use.
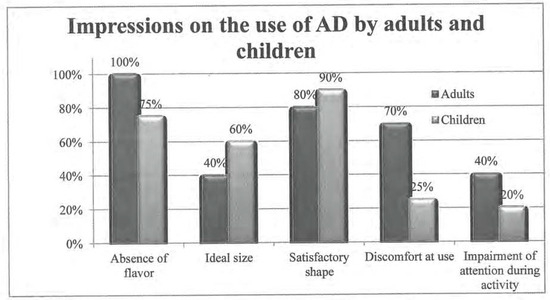 Figure 2. Impressions on the use of APRELI DAEMON.
Figure 2. Impressions on the use of APRELI DAEMON. - Regarding size: 5 subjects found the device large (5/10; 50%), 4 said size is appropriate (4/10; 40%), and 1 did not reply (1/10; 10%) (Figure 2).
- Regarding shape: 8 subjects found it adequate (8/10; 80%), and 2 did not reply (2/10; 20%).
- Discomfort at use was indicated by 7 volunteers (7/10; 70%) (Figure 2). The types of discomfort (Figure 2) were: hypersalivation, (7/10; 70%), discomfort at deglutition (6/10; 60%), nausea (1/10; 10%), and difficulty keeping the lips together throughout use (1/10; 10%). Observers reported that 4 subjects (4/10; 40%) displayed discomfort at use.
- Impairment of attention was reported by 40% (4/10) of the sample, which coincides with observers’ notes.
- Observers noted that 5 subjects touched the device (5/10; 50%); among those, 4 moved it with their hands; suction was observed in 3 subjects (3/10; 30%); lip and chin hypercontraction in 3; and lip parting in 1 (1/10; 10%).
- Critical comments on AD were stated by 8 participants: 7 reported having thought that they were not supposed to move the device, thus there was accumulation of saliva in the oral cavity; and 2 considered the device too large. Impaired speech during use (1), discomfort caused by the silicone yarn (1), perceived need to “pout” during use (1), and the possibility that patients chew the device and might never use it (1) were also reported.
- Suggestions for improvement were provided by 4 individuals: a thinner stem (2/10; 20%), added flavor (1/10; 10%), and flattened ball (1/10; 10%).
- No adverse effects were found in this group.
Children
The sample consists of 20 participants (6 male, 14 fernale; 6 in pre-school school, 14 in elementary in school; mean age= 7.2years (SD ± 1.67; median = 10.0 years; minimum age = 4 years; maximum age = 10 years); 9 participants attended rhythmic gymnastics class, and 11 performed guided recreational activities:
- Regarding flavor: 15 participants responded that AD is flavor-free (Figure 2), so flavor did not interfere with the use (15/20; 75%); 2 individuals disliked the flavor—1 of them found it nauseating.
- Regarding size (Figure 2): 12 subjects found it adequate (12/20; 60%); 5 found it large (5/20; 25%), and 3 found it small.
- Regarding shape: 18 individuals (18/20; 90%) reported to like it, for it was familiar (lollypop-shaped); 1 fairly liked it; and 1 disliked it.
- Discomfort at use (Figure 2) was reported by 5 individuals (5/20; 25%). The types of discomfort were: difficulty in deglutition (3/18; 16.6%), hypersalivation (1/18; 5.5%); and willingness to remove the device from the oral cavity (1/18; 5.5%). Observers noted discomfort at use in 10% (2/20) of the sample.
- Impairment of attention was indicated by 2 children (2/20; 10%). Observers noticed that 1 child showed impaired attention to the activity while using the device (1/20; 5%).
- The following was also noted: lateralization was found in 11 children (11/20; 55%); lip sealing in 11 (11/20; 55%); touching the device with their hands in 10 (10/20; 50%); suction movements in 8 (8/20; 40%); contraction of the orbicularis oris muscle in 8 (8/20; 40%); moving the device with their tongue in 3 (3/20; 15%), and parted lips in 3 (3/20; 15%).
- Critical comments on AD were stated by 1 child, who reported discomfort at speaking and said that the loop was large.
- Suggestions for improvement were: add flavor (strawberry and lollypop flavors: 3/20; 15%), change shape (heart-shaped and square: 3/20; 15%) and reduce loop (1/20; 5%).
- All volunteers kept the device in their mouths for 30 uninterrupted minutes; no adverse effects occurred either during or immediately after use.
DISCUSSION
The study demonstrates that AD is safe and showed no harmful side effects. It was manufactured as suggested by a dedicated multidisciplinary team. The results found by the observers—e.g., lip hypercontraction, lip closure, lateralization and suction—reinforce goals proposed by orofacial motricity experts (Mason, 2008; Bianchini, 2001; Marchesan, 2001; Schievano, Rontani & Berzin, 1999). These goals include lip closure, tongue lateralization, nasal breathing, and suction. These studies show that lip sealing is an important factor for nasal breathing, for retraining of deglutition in tongue thrust, and for improved appearance. They also underline that poor posture of the lips, tongue and Jaw may lead to malocclusion and to compensations such as clenching and hyperfunction of the orbicularis oris muscle and chin muscle, respectively, which are common in individuals with lip incompetence, a significant factor to be treated in speech therapy (Mason, 2008; Bianchini, 2001; Marchesan, 2001; Schievano et al., 1999).
This study found prevalence of 70% of discomfort in adults during AD use. Hypersalivation was found in 70% of the adult sample. A possible explanation is that volunteers believed they were not supposed to move their tongue, despite observers’ and researchers’ instructions that they were asked to keep the device in the oral cavity but were not forbidden to do anything. The presence of a foreign body in the oral cavity increases saliva production, with consequent need for deglutition.
Discomfort at deglutition was found in 60% of the adult sample. Indeed, the device can impede normal deglutition pattern; however, in OS individuals (the intended population for AD, for which further study is required) AD may promote tongue retraction and decrease tongue thrust. Nausea and difficulty to keep the oral cavity closed were reported by only one subject, and it was not possible to determine whether there was increased fntraoral sensitivity, Out of the 20 volunteer children, only 25% showed discomfort at use. Discomfort at deglutition (16.6%) and hypersalivation (5.5%) may be a consequence of increased salivation due to the presence of a foreign body in the oral cavity. Observers noted that only 10% of the sample showed discomfort at use.
Impairment of attention to the ongoing activity in 40% of the adults and 20% of the children does not impede the use of AD by DS individuals. The device is not meant to be used in activities demanding the OS individuals’ attention, but as adjuvant of speech therapy in recreational activities.
Results suggest that AD will contribute to improved tonus, posture, direction and mobility of the perioral and intraoral regions in children with hypotonia and consequent open-mouth posture and mouth breathing. In addition to helping to keep the tongue within the oral cavity and leading to closed-mouth posture, AD use promotes nasal breathing, as well as improves non-nutritive suction and deglutition, as it is known that the orbicularis oris muscle is phonetlcally important in children with open mouth posture (Silva, 2007).
Children with DS often present with tongue thrust, open mouth and parted lips, vertical mastication, and delayed head control, which impairs the development of feeding skills. As part of the speech therapy treatment, and in order to decrease tongue thrust, exerting vigorous lateral vibrations with a finger, along with downward compression of the tongue, is an effective measure to improve the tongue’s motor function.
According to Castillo-Morales (1999), therapeutic intervention in the orofacial complex aims at accumulating normal and standard muscle functions throughout development promoted by a specific orofacial rnyofunctional therapy program. Castillo Morales’ palatal plate devised for DS children enhances the effects of speech therapy treatment, and it should be ideally introduced in the first months of life. It aims at adequate positioning of the tongue, activation of the orbicularis oris muscle, and increased nasal breathing, as well as physiological improvement of suction and deglutition, with decreased tongue thrust. AD pursues the same goals: activation of the orbicularis oris muscle, elevation of the jaw with lip closure, and decreased tongue thrust with tongue at rest within the oral cavity.
CONCLUSIONS
Further research on the use of devices as adjuvant resource to traditional speech therapy is required, specifically with regard to studies focusing on tongue and lip posture in DS children. Results suggest that APRELI DAEMON is safe as an adjuvant resource to traditional speech therapy.
The use of AD must be prescribed exclusively by the speech therapist in charge of the treatment as an adjuvant resource to traditional speech therapy. The speech therapist should know the ideal/adequate moment to introduce AD. Patients must use AD only in speech therapy.
References
- Andrada e Silva, M. A, V. Natalini, R. R. Ramires, and L. P. Ferreira. 2007. Análise comparativa da mastigação de crianças respiradoras nasais e orais com dentição decídua. Revista CEFAC 9, 2: 190–198. [Google Scholar]
- Bianchini, E. M. G. 2001. Avaliaçao fonoaudiológica da motricidade oral-disturbios miofuncionais orafaciais ou situaçöes adaptativas. In Revista Dental Press de Ortodontia e Ortopedia Facial. vol. 6, pp. 73–82. [Google Scholar]
- Carlstedt, K., G. Henningsson, A. McAllister, and G. Dahllof. 2003. Long-term effects of palatal plate therapy on oral motor function in children with Down syndrome evaluated by video registration. Acta Odontologica Scandinavica 59: 63–68. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Morales, R. 1999. Terapia de Regularção orofacial. São Paulo: Memnon: pp. 121–124. [Google Scholar]
- Comitê de motricidade orofacial. 2004. Motricidade orofacial: como atuam os especialistas. São José dos Campos: Pulso Editorial. [Google Scholar]
- Douglas, C. R. 2006. Fisiologia da mastigação. In Fisiologia aplicada a Fonoaudiologia. Rio de Janeiro: Ed. Guanabara Koogan: pp. 325–350. [Google Scholar]
- Glatz-Noll, E., and R. Berg. 1991. Oral dysfunction in children with Down syndrome: an evaluation of treatment effects by means of video registration. European Journal of Orthodontics 13: 446–451. [Google Scholar] [PubMed]
- Hahn, V., and H. Hahn. 1992. Efficacy of oral myofunctional therapy. International Journal of Orofacial Myology 18: 21–23. [Google Scholar]
- Hanson, M. L., and R. M. Mason. 2003. Orofacial myology: International perspectives. Springfield, IL: Charles C. Thomas Publisher: 476p. [Google Scholar]
- Limbrock, G. J., H. Fischer-Brandies, and C. Avalle. 1991. Castillo morales’ orofacial therapy: Treatment of 67 children with down syndrome. Developmental Medicine & Child Neurology 33: 296–303. [Google Scholar]
- Marchesan, I. Q. 2001. Manual pratico de motricidade orofacial, 5th ed. Rio de Janeiro: Ed Revinter: p. 1. [Google Scholar]
- Mason, R. M. 2008. A retrospective and prospective view of orofacial myology. International Journal of Orofacial Myology 34: 7–9. [Google Scholar] [CrossRef]
- Santos, S. R. 1999. Métodos qualitativos e quantitativos na pesquisa biomédica. Jornal de Pediatria (Rio de Janeiro) 75, 6: 401–406. [Google Scholar]
- Schievano, D., R. M. P. Rontani, and F. Berzin. 1999. Influence of myofunctional therapy on the perioral muscles. Clinical and electromyographic evaluations. Journal Oral Rehabilitation 26: 564–569. [Google Scholar]
- Silva, R. G. 2007. Efficacy of rehabilitation in oropharyngeal dysphagia. Pró-Fono Revista de Atualização Científica, Barueri (SP) 19, 1: 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A. 2005. Regulação Orofacial: Sua importância no equilíbrio das Funções. Estomatognáticas. Anais do 16° Conclave Internacional de Campinas. [Google Scholar]
- Umberger, F. G., and R. Johnston. 1997. The efficacy of oral myofunctional and coarticulation therapy. Intemational Journal of Orofacial Myology 23: 3–9. [Google Scholar] [CrossRef]
© 2015 by the author. 2015 Moacyr Daemon Henriques Filho, Alexandra Prufer De Queiroz Campos Araujo, Marcia Goncalves Ribeiro