Abstract
Background: Lingual, labial and buccal weakness (LLBW) is a widespread consequence of several neurological insults. LLBW impact on oral motor functions such as speech production and swallowing is well documented in the literature. Therefore, it is important for the speech-language pathologists to have access to evidence-based approaches for treatment. Thus, it is imperative that the speech language pathology field search for effective treatment approaches and explore new treatment modalities that can improve therapy outcomes. One relatively new modality in this field is neuromuscular electrical stimulation (NMES). Aims: The purpose of this paper is fivefold: (a) to provide an overview of the general effects of NMES on skeletal muscles; (b) to review the effect of NMES on orofacial musculature evaluating the potential appropriateness of NMES for use in strengthening lingual, labial and buccal muscles; (c) to identify future directions for research with consideration of its potential role in improving speech intelligibility and the oral preparatory phase of swallowing in patients with oral motor weakness; (d) to provide a brief anatomic and physiologic bases of LLBW; (e) to provide background information for orofacial myologists who may encounter Individuals with LLBW. Main Contribution: NMES is a modality that Is commonly used In physical therapy and occupational therapy fields that assists in treating several motor and sensory muscular disorders including muscular weakness. The literature reviewed demonstrate that very limited data related to the use of NMES on orofacial muscles exist despite the fact that these muscles can be easily accessed by electrical stimulation from the surface. Conclusions: This review of the research using electrical stimulation of muscles highlights the need for experimental treatment studies that investigate the effect of NMES on orofacial weakness.
INTRODUCTION
One of the consequences following various neurological insults is paresis or paralysis to muscles of the mouth and face. Three of these muscle groups are known as lingual, labial, and buccal regions. Lingual, labial, and buccal weakness (LLBW) experienced by many individuals across the life span can lead to an increased risk of speech or swallowing impairment. Quality of life may also be affected when these individuals are unable to communicate using intelligible speech. These individuals may also, experience a life threatening disorder as a result of swallowing impairment that leads them to avoid previously enjoyed foods and beverages. Consequently, individuals with a swallowing disorder may avoid specific food consistencies that may pose a health risk and those with reduced speech intelligibility or oral phase dysphagia may avoid potentially embarrassing social situations.
The purpose of this paper is fivefold: (a) to provide an overview of the general effects of NMES on skeletal muscles; (b) to review the effect of NMES on orofacial musculature evaluating the potential appropriateness of NMES for use in strengthening lingual, labial and buccal muscles; (c) to identify future directions for research with consideration of its potential role in improving speech intelligibility and the oral preparatory phase of swallowing in patients with oral motor weakness; (d) to provide a brief anatomic and physiologic bases of LLBW; (e) to provide background information for orofacial myologists who may encounter individuals with LLBW.
- Anatomic and Physiologic Bases of LLB Muscles
The lingual, labial, and buccal (LLB) regions consist of many muscles that are active during speech and swallowing. The following is a brief anatomic and physiologic overview of muscles that are of interest to this review.
Labial muscles are considered part of the muscles of facial expression. A number of muscles constitute the lips and control their function (Figure 1)—i.e., Orbicularis oris, Levator labii superioris, Levator anquli oris, Zygomaticus minor, Zyqomaticus major, Risorius, Depressor anguli oris, Depressor labii inferioris, and Mentalis (Seikel, King & Drumright, 2009). These muscles receive motor supply via the facial nerve (Duffy, 2012) (Figure 4).
The buccal muscle is also considered part of the muscles of facial expression. The buccinator is a thin quadrilateral muscle that forms the buccal area and connects the maxilla and the mandible to form the anterior part of the cheek or the lateral wall of the oral cavity (Figure 1). This muscle is also innervated by the facial nerve (Seikel et al., 2009) (Figure 4). This muscle is active during mastci ation, and it assists in holding the cheek to the teeth during chewing (Duffy, 2012) to prevent food from entering the lateral sulcus of the oral cavity.
The tongue consists of eight intrinsic and extrinsic muscles (Figure 1 and Figure 2). Four intrinsic muscles that are not attached to any bone control the shape of the tongue, and four extrinsic muscles that are attached to bones control the positionof the tongue (Seikel et al., 2009). The extrinsic muscles are the genioglossus. which arises from the mandible and protrudes the tongue; hyoglossus, which arises from the hyoid bone and depresses the tongue; styloqlossus, which arises from the styloid process and elevates and retracts the tongue; palatoglossus, which arises from the palatine aponeurosis, and depresses the soft palate, moves the palatoglossal fold towards the midline, and elevates the back of the tongue (Seikel et al., 2009). The intrinsic muscles are the superior longitudinal muscle that runs along the superior surface of the tongue under the mucous membrane, and elevates. assists in retraction of, or deviates the tip of the tongue; the inferior longitudinal muscle that lines the sides of the tongue and is joined to the styloglossus muscle; the verticalis muscle that is located in the middle of the tongue and joins the superior and inferior longitudinal muscles; the transversus muscle that divides the tongue at the middle and is attached to the mucous membranes that run along the sides (Seikel et al., 2009). The motor functions of all intrinsic and extrinsic muscles of the tongue are supplied by the hypoglossal nerve (Figure 5), with the exception of the palatoglossus, which is innervated by the vagus nerve (Seikel et al., 2009) (Figure 5).
- Neurological bases for LLBW
LLBW can result from lesions to the muscles, the neuromuscular junction, the lower motor neurons of the cranial nerves, the upper motor neurons originating in the motor cortex or from damage to the extra pyramidalmotor system (Duffy, 2012; Webster, 1999; Brookshire, 1997). A brief overview of the effect of these lesions on LLBW is presented in the following section.
A. Upper motor neuron damage. Unilateral lesions of the motor cortex or pyramidal tract cause mild to moderate contralateral LLBW or paralysis. On the other hand, bilateral upper motor neuron lesions exhibit severe LLBW that leads to severe dysarthria, dysphagia and poorfy controlled laughing and crying (Duffy, 2012; Webster, 1999; Brookshire, 1997).
Upper motor neuron lesions occur in conditions affecting motor neurons in the brain or spinal cord such as stroke, multiple sclerosis, traumatic brain injury, and cerebral palsy. Symptoms include decreased control of active movement, muscular spasticity, and decreased control of active movement, particularfy slowness (Duffy, 2012; Webster, 1999; Brookshire, 1997).
B. Lower motor neuron damage. Damage to cranial nerves that innervate LLB muscles or their nuclei in the pons and medulla results in LLBW (Brookshire, 1997). As the cranial nerves lie very close to one another in the brainstem, a lesion will usually damage more than one pair of nuclei. Thus, a number of muscles may be affected bilaterally (Webb, Adler & Love, 2008). Symptoms include muscle paresis or paralysis, fibrillations, fasciculations, and hypotonia or flaccidity. Eventually these muscles become atrophied (Duffy, 2012; Webster, 1999; Brookshire, 1997). Damage affecting the hypoglossal, facial and trigeminal nerves result in weakness of lingual, labial, and buccal musculature and dysfunctions of speech and decreased eating and chewing capacity (Webb, Adler & Love, 2008).
C. Extra pyramidal motor system damage. There are two major disorder complexes associated with disease of the extra pyramidal motor system: Parkinson’s disease (PD) and Huntington’s disease (HD). Parkinson’s disease results from the death of dopaminergic neurons in the substantia nigra pars compacta (Webb et al., 2008). It is characterized by a resting tremor, but the most debilitating symptom is severe bradykinesia or akinesia (Webb et al., 2008). Parkinsonism is characterized by varying degrees of (1) rigidity, (2) bradykinesia. (3) tremor, and (4) postural defects. Huntington’s disease is also known as Huntington’s Chorea because it is characterized by continuous choreiform movements of the body (especially the limbs and face) (Webb et al., 2008).
D. Neuromuscular junction diseases. In neuromuscular junction diseases, the end plate potential fails to effectively activate the muscle fiber due to an autoimmune reaction against acetylcholine receptors, resulting in muscle weakness and fatigue (Sha & Layzer, 2007). The most common example of this disorder is myasthenia gravis that is caused, commonly, by autoantibodies against the acetylcholine receptor (Sha & Layzer, 2007). Myasthenia gravis often causes a unique muscular weakness, in which the patient’s muscular strength deteriorates with prolonged use, but is recovered following a break from usage (Brookshire, 1997).

Figure 1.
Labial and Jaw Muscles.
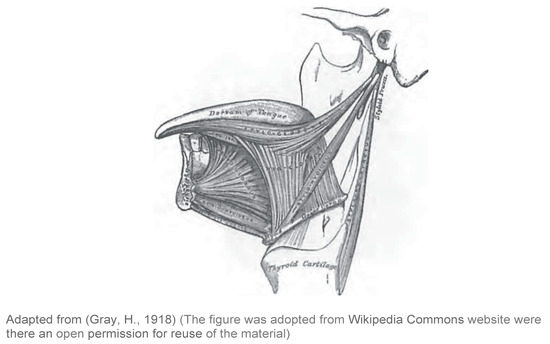
Figure 2.
Extrinsic Lingual Muscles.
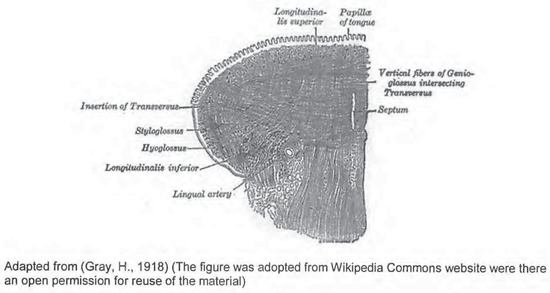
Figure 3.
Intrinsic and Extrinsic Lingual Muscles.
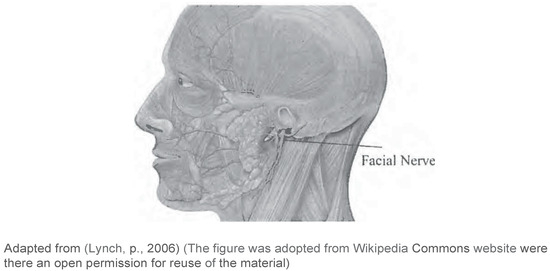
Figure 4.
Neural Innervation of Facial Muscles.
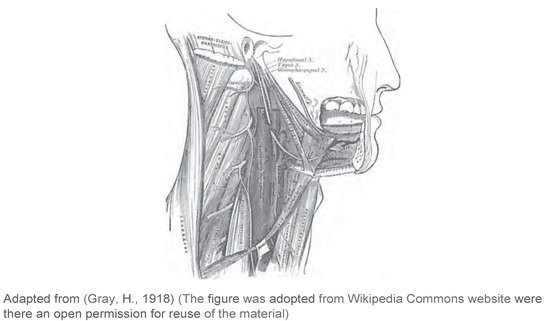
Figure 5.
Neural Innervation of Lingual Muscles.
- Prevalence of LLBW
To date, no reliable estimates exist on the incidence and prevalence of individuals who suffer from weakness of the labial, buccal and lingual muscles. However, the presence of lingual. labial and buccal weakness in a society can be inferred from the prevalence of disorders causing these weaknesses. Since LLBW is often associated with neurological impairments (Brookshire, 1997; Duffy, 2012) including neurological degenerative conditions such as Parkinson’s disease (McAuliffe, Ward, Murdoch & Farrell, 2005; Solomon, Robin & Luschei, 2000) and amyotrophic lateral sclerosis (ALS) (Dworkin, 1980; Cha & Patten, 1989; Khan & Prayson, 2003) as well as non progressive brain damage such as traumatic brain injury (TBI) (Theodoros, 2001; Goozee, Bruce, Murdoch, Deborah & Theodoros, 2001) and cerebrovascularaccidents (CVAs) (Brookshire, 1997; Duffy, 2012), the incidence may be inferred from data on these neurological disorders.
For example, studies estimate that in the United States, 1.5 to 2 million individuals sustain a TBI each year (Langlois, Kegler & Butler, 2003; Stierwalt & Murray, 2002; Coronado, 2011). A CDC survey in 2012 (CDC, 2012) indicates that 2.7% of non institutionalized U.S. adults (approximately 6.2 million persons) had a histories of stroke; consequently many of these individuals may present with LLBW.
There are several neurological degenerative conditions that may lead to LLBW, one of which is Parkinson’s disease (PD) (Ramig, Fox & Sapir, 2004). Samii, Nutta & Ranson (2004) estimate that 89% of individuals with PD have a speech or voice disorder that is caused by a neuromuscular impairment.
Additional studies estimated that the prevalence of PD in industrialized countries is 0.3% of the general population and about 1% of the population older than 60 years (Ramig, Fox & Sapir, 2004, Levine, Fahrbach & Siderowf, 2003). Another common cause for LLBW is Huntington’s disease. Individuals with this disorder present with an unpredictable and weak speech pattern that may severely compromise clarity of speech. Qin and Gu (2004) estimated that Huntington’s disease affects 5 out of 100,000 people and symptoms usually occur in the late 40 s.
Furthermore, LLBW is highly prevalent in patients with ALS (Kidney, Alexander, Corr, O’Toole & Hardiman, 2004). Because the disease usually does not affect cognitive abilities. patients with ALS are aware of their progressive loss of functioning and may become anxious and depressed. Khan and Prayson (2003) estimated that the disease’s worldwide prevalence ranges from 0.5 to 3 in 100,000, with a few areas having a higher prevalence.
Given the widespread nature of LLBW and its impact on oral motor functions such as speech production and swallowing, it is important for the speech-language pathologists to have access to evidence-based approaches for treatment. Thus, it’s imperative that the speech-language pathology field search for effective treatment approaches and explore new treatment modalities that can improve therapy outcomes.
One relatively new modality in this field is neuromuscular electrical stimulation (NMES). The effect of NMES on muscular strength and movement has been investigated in several areas of the head and neck region. Some examples include (1) the back of the tongue in sleep apnea (Isono, Tanaka & Nishina, 1999; Mezzanotte, Tangel & White, 1992; Miki, Hida, Shindoh, Kikuchi, Chonan, Taguchi, Inoue & Takishima, 1989, Oliven, Schnall, Pillar, Gavriely & Odeh, 2001; Randerath, 2006); (2) regions of the face associated with facial palsy (Kavanagh, Newellm, Hennessy & Sadick, 2012) and; (3) the larynx and submental areas associated with dysphagia (Humbert et al., 2006; Nam, Beom, Oh & Han, 2013; Carnaby-Mann & Crary, 2007; Ludlow et al., 2007). Although, the literature supports a positive correlation between the use of NMES and increased muscular strength and range of motion (Binder-Macleod, Halden & Jungles, 1995; Binder-Macleod & Lee, 1997; Doucet, Lam & Griffin 2012; Khan, 1987), limited research has investigated the effect of neuromuscular stimulation on the lingual, labial and buccal muscles in terms of speech production and intelligibility.
GENERAL ELECTRICAL STIMULATION DEFINITION AND ITS EFFECT ON THE SKELETAL MUSCLES
- Definition
Neuromuscular electrical stimulation (NEMS) is a treatment that uses small electrical current to activate nerves innervating muscles effected by paralysis resulting from spinal cord injury (SCI), head injury, stroke and other neurological disorders. The application of NMES causes muscles to contract as if they were exercising. NMES is delivered to muscles as a waveform of electrical current via electrodes.
Using electrical stimulation to produce human movement is not a novel approach. Cambridge (1997) noted that in 1790, Luigi Galvani first observed motion after applying electrical wires to leg muscles severed from the body of a frog. In 1831, Michael Faraday showed that electrical currents could stimulate nerves to create active movement (Cambridge, 1997). Liberson, Holmquest, Scot and Dow (1961) used electrical stimulation for muscle function—one of the early clinical experiments of NMES—to stimulate the peroneal nerve in the leg in an effort to correct foot drop during ambulation in persons with stroke-related hemiplegia.
There are, broadly, two types of NMES electrodes: surface (cutaneous) and intramuscular (percutaneous). In the rehabilitation field, surface NMES electrodes are most commonly used. The electrodes are generally incorporated in pads that adhere to the skin. The electrical current (in the NMES devices) has customizable characteristics which allow for manipulation of the stimulus frequency, amplitude, and pulse width of the electric current to be delivered. The amplitude and pulse width determine the number of muscle fibers that are activated (Sheffler & Chae, 2007).
- Benefits & Applications of NMES
NMES approaches are generally used in patients who have an intact peripheral and motor-neuron system but are unable to activate their musculature for volitional functions. Current therapeutic clinical application of NMES is limited to neurologic imparimentsthat involve the upper and lower motor neuron such as spinal cord injury (SCI), stroke, brain injury, multiple sclerosis, and cerebral palsy (Sheffler & Chae, 2007) and involve muscle weakness or paralysis.
According to Randerath (2006), “Muscle training using electrical neurostimulation (ENS) has been found to effectively strengthen skeletal muscles in pathological or posttraumatic situations. In healthy muscles, neuromuscular electrical stimulation can induce the activity of motor units which are difficult to activate voluntarily” (p. 161). The literature supports the therapeutic application of NMES to enhance muscle strength, retard muscle atrophy, and reduce spasticity (Binder-Macleod, Halden & Jungles, 1995; Binder-Macleod & Lee, 1997). NMES also has been found to help Improve muscle strength, increase range of motion, reduce edema, decrease atrophy, heal tissue, and decrease pain (Doucet, Lam & Griffin 2012; Khan, 1987).
Some types of electrical stimulation, such as functional electrical stimulation, provide therapeutic effects that persist when the NMES device is not in use (Daly et al., 1996). Daly et al. (1996) reported that clinicians observed improvements in voluntary neuromuscular function and improvements in the condition of soft tissue after using electrical stimulation. Additionally,therapists observed motor recovery in people with incomplete spinal cord injury, stroke, or traumatic brain injury after the use of motor prostheses (Daly et al., 1996).
Furthermore, Maffiuletti (2010) listed several additional applications for NMES in the rehabilitation field. These applications indude (1) the preservation of muscle mass and function during prolonged periodsof disuse or immobilization (Gibson et al., 1988), (2) for recovery of muscle mass and function following prolonged periods of disuse or immobilization (Snyder-Mackler et al., 1995), for improvement of muscle function in different healthy populations such as elderly subjects (Caggiano et al., 1994) and adult subjects (Currier & Mann, 1983) and (4) for use with recreational and competitive athletes (Babault et al., 2007; Delitto et al., 1989; Maffiuletti et al., 2002; Pichon et al., 1995).
Thus, according to Maffiuletti (2010), (re)training programs that utilized NMES have been used in the following areas:
- Cardiovascular medicine patients with chronic or refractory heart failure (Harris et al., 2003; Quittan et al., 2001), cardiac transplant (Vaquero et al., 1998), chronic obstructive pulmonary disease (Roig & Reid, 2009; Vivodtzev et al., 2008);
- Orthopedic medicine patients with anterior cruciate ligament reconstruction (Delitto et al., 1988; Eriksson & Haggmark, 1979; Fitzgerald et al., 2003; Lieber et al., 1996; Snyder-Mackler et al., 1991), bone fracture (Gibson et al., 1988), knee osteoarthritis (Gibson et al., 1989; Zizic et al., 1995), rheumatoid arthritis (Piva et al., 2007), total knee arthroplasty (Petterson & Snyder-Mackler, 2006; Stevens et al., 2004), total hip arthroplasty (Suetta et al., 2004), meniscectomy (Gould et al., 1983), patellofemoral pain (Callaghan et al., 2001);
- Neurological medicine patients following stroke (Glinsky et al., 2007; Newsam & Baker, 2004), spinal cord injury (Belanger et al., 2000; Crameri et al., 2000; Dudley et al., 1999), cerebral palsy (Merrill, 2009; Stackhouse et al., 2007);
- General medicine patients with hemophilia (Querol et al., 2006), cancer (Crevenna et al., 2006) and critically ill patients (Gerovasili et al., 2009;
- Geriatric medicine healthy (Amiridis et al., 2005; Caggiano et al., 1994) and unhealthy (Stevens et al., 2004), elderly subjects;
- Space medicine Astronauts (Convertino, 1996; Mayr et al., 1999), simulated microgravity (Duvoisin et al., 1989);
- Sports medicine healthy and injured athletes of individual and team sports (Delitto et al., 1989; Maffiuletti et al., 2006). (224). Table 1 presents a summary of studied NMES applications.

Table 1.
Summary of Studied NMES applications.
Table 1.
Summary of Studied NMES applications.
| Author | Aoolications |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although electrical stimulation has the capacity to produce movement in denervated, paralyzed, or spastic muscles, there is uncertainty concerning the mechanisms by which NMES produces these changes. However, it is obvious that neuromuscular electrical stimulation elicits muscle contraction by initiating action potentials in intramuscular nerve branches that mimic the action potential coming from the central nervous system (Hultman et al., 1983). The order of motor unit activation with NMES depends on two factors: (1) the combined effects of axon diameter (Clamann et al., 1974; Eccles et al., 1958); and (2) the distance between the axon and the active electrode (Gorman & Mortimer, 1983; Delitto & Snyder-Mackler, 1990).
- Limitations of Electrical Stimulation
Doucet, Lam & Griffin (2012) noted that the most significant limitations of any non physiologically induced muscle activation is the overall decreased efficiency of contraction and the high tendency for development of neuromuscular fatigue. However, the application of NMES can be customized to reduce fatigue and optimize force output by adjusting the associated stimulation parameters. Therefore, strategies must be designed as part of electrical stimulation regimens to offset the high degree of fatigue associated with NMES.
NMES represents a non-physiologic means of muscle contraction that bypasses the processes associated with volition. Thus, Enoka (1988) suggested three lines of evidence that question the mechanisms by which NMES might elicit increases in strength secondary to the presence of strength related neural adaptations: (1) a time course of strength gains that precedes changes in muscle size, (2) a lower requisite training intensity compared with that necessary for voluntary training, and (3) increased strength of the non-exercised contralateral limb that accompanies the strengthening of the test limb with NMES.
Finally, another major limitation for the use of NMES modality is that the long-term effectiveness following discontinuation is not well investigated (Doucet, Lam & Griffin, 2012). According to Doucet et al. (2012), few studies have follow-up data after treatment. Therefore, NMES may not be a long-term intervention for muscle re-education or restoration of movement. However, for patients with spinal cord injuries, Shields and Dudley-Javoroski (2006) have suggested that only long-term use of NMES helps to offset the muscle atrophy and complications of disuse.
LINGUAL ELECTRICAL STIMULATION
Electrical stimulation has also been applied with the tongue. Application to the tongue is not a novel idea. Historically, electrical stimulation of the tongue has been used as ear1y as 1955 to examine the sensory effects of such stimulation (Pierrel, 1955). Pleasanton (1970) used electrical stimuli (delivered short electrical pulses) to investigate patterns of sensitivity to contrast the tip and dorsum, midline and lateral areas, and right and left sides of the tongue using electrodes that were placed on the tongue.
Most of the studies found in the literature related to lingual electrical stimulation investigated its effect on sleep apnea. Lingual neuromuscular electrical stimulation effects on airway patency were investigated in patients with obstructive sleep apnea (Isono, Tanaka & Nishina, 1999; Mezzanotte, Tangel & White, 1992; Miki et al., 1989; Oliven, Schnall, Pillar, Gavriely & Odeh, 2001; Randerath, 2006). In these studies, different approaches to the delivery methods for applying electrical pulses to the lingual muscles have been investigated using: (1) intramuscular NMES (Oliven et al., 2001; Oliven et al., 2009; Hu et al., 2008; Decker et al., 1993), (2) submental surface NMES (Steier et al., 2011; Yang, Meng & Zhu, 2000; Guilleminault et al., 1995; Decker et al., 1993), (3) lingual surface NMES (Isono et al., 1999; Schnall et al., 1995), and (4) sublingual NMES (Oliven, Schnall, Pillar, Gavriely & Odeh, 2001).
The aim of these studies was to explore the effect of NMES in maintaining open airway in patients with obstructive sleep apnea. The airway is typically obstructed in patients with sleep apnea due to pharyngeal and lingual weaknesses (Remmers et al., 1978; Oliven et al., 2003); thus, applying electrical stimulation to the genioglossus muscle (Oliven et al., 2003) pushes the tongue superiorly and anteriorly, leading to opening of the airway. Although these studies focused on the single time effect in relatlon to obstructive sleep apnea, they demonstrated that surface electrical stimulation can be safely utilized to stimulate extrinsic lingual muscles.
ELECTRICAL STIMULATION AND FACIAL MUSCLES
Electrical stimulation has also been investigated with facial muscles though not extensively. The facial muscles are superficial and would be targeted easily by electrical stimulation. However, few studies to date have investigated the effect of NMES on facial muscles, generally, and muscles of speech production and swallowing, specifically. In a study that targeted the zygomatic major muscle, Kavanagh et al. (2012) concluded that following a 12-week course of facial NMES, the thickness of the muscle was increased as measured by ultrasound and there were subjective improvements in facial characteirstics.
A limitednumber of studies have investigated the application of NMES within the facial palsy population (Cronin & Steenerson, 2003; Hyvarinen et al., 2008; Alakram & Puckree, 2011). In a retrospective case review of 24 patients with facial paralysis who received neuromuscular facial retraining, Cronin and Steenerson (2003) concluded that all patient groups made significant improvements in function with improved symmetry in dual channel electromyographic readings. The subjects also were found to have increased facial movement based on percentages of movement measured in the study.
In a study that targeted participants with chronic facial nerve paralysis with sensory level NMES, a significant improvement was observed in the upper branch of the facial nerve motor action potential distal latency on the affected side in all patients. An improvement of one grade on the House Brackmann scale was observed, and some patients also reported subjective improvement (Hyvarinen et al., 2008).
Another study investigated the NMES as a treatment approach for Bell’s palsy in the acute phase of the disorder in conjunction with other modalities (i.e., heat, massage, exercises). Results of the study demonstrated that the effects of electrical stimulation, as used in that study, were found to be clinically, but not statistically significant (Alakram & Puckree, 2011). To date, no studies exist in the field of communication sciences and disorders that have examined the effect of NMES on the strength of muscles that are involved in articulation and swallowing—such as labial muscles (i.e., the orbicularis oris, levator and depressor muscles), buccinator, and masseter—despite the fact that these muscles can be easily targeted by surface NMES.
5. ELECTRICAL STIMULATION AND DYSPHAGIA
Recently, surface electrical stimulation has been explored as treatment option in patients with neurogenic dysphagia, a disorder that involves oropharyngeal weakness. Most of the studies published are related to neuromuscular electrical stimulation and pharyngeal phase dysphagia and airway protection (Humbert et al., 2006; Ludlow et al., 2007; Nam et al., 2013). These studies investigated laryngeal and submental electrode placements. Systematic reviews presented a number of promising findings and findings having no benefit of NMES over traditional, non-electrical stimulation treatment. These reviews concluded that their findings warrant the need for more controlled trials to assess effectiveness of NMES (Carnaby-Mann & Crary, 2007; Clark, Lazarus, Arvedson, Schooling & Frymark, 2009; Ludlow, 2010).
In a study exploring the effect of electrical stimulation on swallowing in chronic pharyngeal dysphagia, Ludlow et al. (2007) noted that the depression in hyolaryngeal elevation due to electrical stimulationmay serve as resistanceduring muscle activation in swallowing, thus improving muscle strength. Humbert et al. (2006) and Nam et al. (2013) examined the effect of submental placement of electrodes on laryngeal movement. Humbert et al. (2006) showed that surface submental stimulation alone produced no elevation of the hyoid, with only some anterior movement. However, Nam et al. (2013) demonstrated that stimulating both the suprahyoid and the infrahyoid muscle resulted in a significant increase in superior laryngeal elevation but not in anterior hyoid excursion.
In a meta-analysis conducted by Carnaby-Mann & Crary (2007) that examined the evidence on neuromuscular electrical stimulation for swallowingrehabilitatio,nthe authors concluded that there is a statistically significant summary size effect that supports the use of neuromuscular electrical stimulation to treat swallowing disorders. Furthermore, clinicianswho are using surface NMES for dysphagia treatment reported positive clinical outcomes with no treatment-related complications and high patient and professional satisfaction (Crary et al., 2007) suggesting a functional benefit of this treatment modality.
Additionally, Ludlow and her colleagues (Martin et al., 2010) investigated the effect of intramuscular electrical stimulationin the larynx in 10 patients with dysphagia post radiation therapy and 9 healthy volunteers. This study demonstrated that intramuscular stimulationcan augment hyo-laryngeal elevation and might be able to increase airway protection during swallowing. Their data provided promising findings for the use of intramuscularelectrical stimulation in individualswith dysphagia.
The effect of NMES on swallowing in dysphagia studies is controversial, possibly because the laryngeal elevator extrinsic muscles are deep within the neck and cannot be easily accessed by surface NMES. However, studies have demonstrated that surface NMES placed in the laryngeal and submental region have reached the supralaryngealmuscles. Hence, NMES resulted in a movement of the hyoid bone despite any argument that this movement assisted in the physiology of swallowing or acts against it. Additionally, since intramuscular electrical stimulation is applied directly in the targeted muscles, it showed that direct electrical stimulationto the muscles produce favorable effects.
SUMMARY AND CONCLUSION
Lingual and facial muscles of mastication and expression are important for communication and swallowing. Speech-language pathologists are in need of modalities to assist in the treatment when muscle weaknesses exist. NMES is a modality that is commonly used in physical therapy and occupational therapy fields that assists in treating several motor and sensory muscular disorders including muscular weakness.
The literature reviewed demonstrate that very limiteddata related to the use of NMES on orofacial muscles exist despite the fact that these muscles can be easily accessed by electrical stimulation from the surface. Thus, the present review of the research using electrical stimulation of muscles highlights the need for experimental treatment studies that investigatethe effect of NMES on orofacial weakness. NMES has been shown to have promising potential as a treatment modality for muscle weakness.
Theoretically, this positive effect should translate into improved strengthwhen applied in the orofacial muscles. However, before incorporating this procedure into clinical practice, research is needed to investigate its effects on LLBW. Furthermore, research is needed to evaluate the impact of NMES on patients with diminished motor function of the labial, lingual and buccal muscles to demonstrate effectiveness, efficacy, and safety of this procedure. Additionally, there is a need for future studies that evaluate electrode placement effect of NMES on LLBW as well as the most effective characteristicsof the applied current used for stimulation.
References
- Alakram, P., and T. Puckree. 2011. Effects of electrical stimulation on house-brackmann scores in ear1y bells palsy. Physiotherapy Theory and Practice 26, 3: 160–166. [Google Scholar] [PubMed]
- Amiridis, I., F. Arabatzi, P. Violaris, E. Stavropoulos, and V. Hatzitaki. 2005. Static balance improvement in elderly after dorsiftexors electrostimulation training. European Journal of Applied Physiology 94, 4: 424–433. [Google Scholar]
- Babault, N., G. Cometti, M. Bernardin, M. Pousson, and J. C. Chatard. 2007. Effects of electromyostimulation training on musclestrength and power of elite rugby players. Journal of Strength and ConditioningResearch 21, 2: 431–437. [Google Scholar]
- Belanger, M., R. B. Stein, G. D. Wheeler, T. Gordon, and B. Leduc. 2000. Electrical stimulation: Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Archives of Physical Medicine and Rehabilitation 81, 8: 1090–1098. [Google Scholar] [PubMed]
- Binder-Macleod, S. A., E. E. Halden, and K. A. Jungles. 1995. Effects of stimulaiton intensity on the physiologci al responses of human motor units. Medicine & Science in Sports & Exercise 27, 4: 556–565. [Google Scholar]
- Binder-Macleod, S. A., and S. Lee. 1997. Assessment of the efficacy of functional electrical stimulation in patients with hemiplegla. Topics in Stroke Rehabilitaiotn 3: 88–98. [Google Scholar]
- Brookshire, R. H. 1997. Introduction to neurogenic communication disorders. ERIC. [Google Scholar]
- Burnett, T. A., E. A. Mann, S. A. Cornell, and C. L. Ludlow. 2003. Laryngeal elevation achieved by neuromuscular stimulationat rest. Journal of Applied Physiology (1985) 94, 1: 128–134. [Google Scholar]
- Caggiano, E., T. Emrey, S. Shirley, and R. L. Craik. 1994. Effects of electrical stimulation or voluntary contraction for strengthening the quadriceps femoris musclesin an aged male population. Journal of Orthopaedic and Sports Physical Therapy 20, 1: 22–28. [Google Scholar]
- Callaghan, M. J., J. A. Oldham, and J. Winstanley. 2001. A comparison of two types of electrical stimulation of the quadriceps in the treatment of patellofemoralpain syndrome. A pilot study. Clinical Rehabilitation 15, 6: 637–646. [Google Scholar]
- Cambridge, N. A. 1977. Electrical apparatus used in medicine before 1900. Journal of the Royal Society of Medicine 70, 9: 635–641. [Google Scholar]
- Carnaby-Mann, G. D., and M. A. Crary. 2007. Examining the evidence on neuromuscular electrical stimulation for swallowing: A meta-analysis. Archives of Otorhinolaryngology-Head & Neck Surgery 133, 6: 564–571. [Google Scholar]
- Cha, C. H., and B. M. Patten. 1989. Amyotrophic lateral sclerosis: Abnormalities of the tongue on magnetic resonance imaging. Annals Neurology 25, 5: 468–472. [Google Scholar]
- Clamann, H.P., J. D. Gillies, R. D. Skinner, and E. Henneman. 1974. Quantitative measures of output of a motoneuron pool during monosynaptic reflexes. Journal of Neurophysiology 37, 6: 1328–1337. [Google Scholar]
- Clark, H., C. Lazarus, J. Arvedson, T. Schooling, and T. Frymark. 2009. Evidence-based systematic review: Effects of neuromuscular electrical stimulation on swallowing and neural activation. American Journal of Speech Language Pathology 18, 4: 361–375. [Google Scholar] [PubMed]
- Connell, P. J., and C. K. Thompson. 1986. Flexibility of single-subject experimental designs. Part III: Using flexibility to design or modify experiments. Journal of Speech Hearing Disorders 51, 3: 214–225. [Google Scholar]
- Centers for Disease Control and Prevention. 2007. Prevalence of stroke—United States, 2005. MMWR Morbidity and Mortality Weekly Report 56, 19: 469. [Google Scholar]
- Centers for Disease Control and Prevention. 2012. Prevalence of stroke—United States, 2006–2010. MMWR Morbidity and Mortality Weekly Report 61, 20: 379. [Google Scholar]
- Convertino, V. A. 1996. Exercise as a countermeasure for physiological adaptation to prolonged spaceflight. Medicine & Science in Sports & Exercise 28, 8: 999–1014. [Google Scholar]
- Coronado, V. G., L. Xu, S. V. Basavaraju, L. C. McGuire, M. M. Wald, M. D. Faul, and J. D. Hemphill. 2011. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. US Department of Health and Human Services, Centers for Disease Control and Prevention Atlanta. [Google Scholar]
- Crameri, R. M., A. R. Weston, S. Rutkowski, J. W. Middleton, G. M. Davis, and J. R. Sutton. 2000. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: A pilot study. European Journal of Applied Physiology 83, 4–5: 409–415. [Google Scholar]
- Crary, M. A., G. D. Carnaby-Mann, and A. Faunce. 2007. Electrical stimulation therapy for dysphagia: Descriptive results of two surveys. Dysphagia 22, 3: 165–173. [Google Scholar]
- Crevenna, R., C. Marosi, M. Schmidinger, and V. Fialka-Moser. 2006. Neuromuscular electrical stimulation for a patient with metastatic lung cancer—A case report. Support Care Cancer 14, 9: 970–973. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G. W., and R. L. Steenerson. 2003. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngology Head Neck Surgery 128, 4: 534–538. [Google Scholar] [CrossRef]
- Currier, D. P., and R. Mann. 1983. Muscular strength development by electrical stimulation in healthy individuals. Journal of Physical Therapy 63, 6: 915–921. [Google Scholar] [CrossRef] [PubMed]
- Daly, J. J., E. B. Marsolais, L. M. Mendell, W. Z. Rymer, A. Stefanovska, J. R. Wolpaw, and C. Kantor. 1996. Therapeutic neural effects of electrical stimulation. IEEE Transactions on Rehabilitation Engineering 4, 4: 218–230. [Google Scholar] [CrossRef]
- Decker, M. J., J. Haaga, J. L. Arnold, D. Atzberger, and K. P. Strohl. 1993. Functional electrical stimulation and respiration during sleep. Journal of Applied Physiology (1985) 75, 3: 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Delitto, A., M. Brown, M. J. Strube, S. J. Rose, and R. C. Lehman. 1989. Electrical stimulation of quadriceps femoris in an elite weight lifter: a single subject experiment. International Journal Sports Medicine 10, 3: 187–191. [Google Scholar] [CrossRef]
- Delitto, A., S. J. Rose, J. M. McKowen, R. C. Lehman, J. A. Thomas, and R. A. Shively. 1988. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Journal of Physical Therapy 68, 5: 660–663. [Google Scholar] [CrossRef]
- Delitto, A., and L. Snyder-Mackler. 1990. Two theories of muscle strength augmentation using percutaneous electrical stimulation. Journal of Physical Therapy 70, 3: 158–164. [Google Scholar] [CrossRef]
- Doucet, B. M., A. Lam, and L. Griffin. 2012. Neuromuscular electrical stimulation for skeletal muscle function. Yale Journal of Biology and Medicine 85, 2: 201–215. [Google Scholar]
- Dudley, G. A., M. J. Castro, S. Rogers, and D. F. Apple, Jr. 1999. A simple means of increasing muscle size after spinal cord injury: A pilot study. European Journal of Applied Physiology and Occupational Physiology 80, 4: 394–396. [Google Scholar] [CrossRef]
- Duffy, J. R. 2012. Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Duvoisin, M. R., V. A. Convertino, P. Buchanan, P. D. Gollnick, and G. A. Dudley. 1989. Characteristics and preliminary observations of the influence of electromyostimulation on the size and function of human skeletal muscle during 30 days of simulated microgravity. Aviation, Space, and Environmental Medicine 60, 7: 671–678. [Google Scholar]
- Dworkin, J. P. 1980. Tongue strength measurement in patients with amyotrophic lateral sclerosis: qualitative vs quantitative procedures. Archives of Physical Medicine and Rehabilitation 61, 9: 422–424. [Google Scholar]
- Eccles, J. C., R. M. Eccles, and A. Lundberg. 1958. The actionpotentials of the alpha motoneurones supplying fast and slow muscles. Journal of Physiology 142, 2: 275–291. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R. M. 1988. Muscle strength and its development: New perspectives. Sports Medicine 6, 3: 146–168. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E., and T. Haggmark. 1979. Comparison of isometric muscle training and electrical stimulation supplementing isometric muscle training in the recovery after major knee ligament surgery: A preliminary report. American Journal of Sports Medicine 7, 3: 169–171. [Google Scholar] [CrossRef]
- Fitzgerald, G. K., S. R. Piva, and J. J. Irrgang. 2003. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. Journal of Orthopaedic and Sports Physical Therapy 33, 9: 492–501. [Google Scholar] [CrossRef] [PubMed]
- Gerovasili, V., K. Stefanidis, K. Vitzilaios, E. Karatzanos, P. Politis, A. Koroneos, and S. Nanas. 2009. Electrical muscle stimulation preserves the muscle mass of critically ill patients: A randomized study. Critical Care 13, 5: R161. [Google Scholar] [CrossRef]
- Gibson, J. N., W. L. Morrison, C. M. Scrimgeour, K. Smith, P. J. Stoward, and M. J. Rennie. 1989. Effects of therapeutic percutaneous electrical stimulation of atrophic human quadriceps on muscle compositio,nprotein synthesis and contractile properties. European Journal of Clinical Investigation 19, 2: 206–212. [Google Scholar] [CrossRef]
- Gibson, J. N., K. Smith, and M. J. Rennie. 1988. Prevention of disuse muscle atrophy by means of electrical stimulaiton: maintenance of protein synthesis. Lancet 2, 8614: 767–770. [Google Scholar] [CrossRef]
- Glinsky, J., L. Harvey, and P. Van Es. 2007. Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: a systematic review. Physiotherapy Research International 12, 3: 175–194. [Google Scholar]
- Goozee, J. V., B. E. Murdoch, and D. G. Theodoros. 2001. Physiological assessment of tongue function in dysarthria following traumatic brain injury. Logopedics Phoniatrics Vocology 26, 2: 51–65. [Google Scholar]
- Gorman, P. H., and J. T. Mortimer. 1983. The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Transactions on Biomedical Engineering 30, 7: 407–414. [Google Scholar] [PubMed]
- Gould, N., D. Donnermeyer, G. G. Gammon, M. Pope, and T. Ashikaga. 1983. Transcutaneous muscle stimulation to retard disuse atrophy after open meniscectomy. Clinical Orthopedic Related Research 178: 190–197. [Google Scholar]
- Guilleminault, C., N. Powell, B. Bowman, and R. Stoohs. 1995. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 107, 1: 67–73. [Google Scholar]
- Harris, S., J. P. LeMaitre, G. Mackenzie, K. A. Fox, and M. A. Denvir. 2003. A randomised study of home-based electrical stimulation of the legs and conventional bicycle exercise training for patients with chronic heart failure. European Heart Journal 24, 9: 871–878. [Google Scholar]
- Hu, L., X. Xu, Y. Gong, X. Fan, L. Wang, J. Zhang, and Y. Zeng. 2008. Percutaneous biphaslc electrical stimulation for treatment of obstructive sleep apnea syndrome. IEEE Transactions on Biomedical Engineering 55, 1: 181–187. [Google Scholar]
- Hultman, E., H. Sjoholm, I. Jaderholm-Ek, and J. Krynicki. 1983. Evaluation of methods for electrical stimulation of human skeletal muscle in situ. Pflugers Arch 398, 2: 139–141. [Google Scholar] [PubMed]
- Humbert, I. A., C. J. Poletto, K. G. Saxon, P. R. Kearney, L. Crujido, W. Wright-Harp, and C. L. Ludlow. 2006. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. Journal of Applied Physiology (1985) 101, 6: 1657–1663. [Google Scholar]
- Hyvarinen, A., I. M. Tarkka, E. Mervaala, A. Paakkonen, H. Valtonen, and J. Nuutinen. 2008. Cutaneous electrical stimulation treatment in unresolved facial nerve paralysis: An exploratory study. American Journal of Physical Medicine and Rehabilitation 87, 12: 992–997. [Google Scholar]
- Isono, S., A. Tanaka, and T. Nishino. 1999. Effects of tongue electrical stimulation on pharyngeal mechanics in anaesthetized patients with obstructive sleep apnea. European Respiratory Journal 14, 6: 1258–1265. [Google Scholar]
- Khan, J. 1987. Principles and practiceof electrotherapy. Churchill Livingstone New York. [Google Scholar]
- Kavanagh, S., J. Newell, M. Hennessy, and N. Sadick. 2012. Use of a neuromuscular electrical stimulation device for facial muscle toning: A randomized, controlled trial. Journal of Cosmetic Dermatology 11, 4: 261–266. [Google Scholar] [PubMed]
- Khan, T. S., and R. A. Prayson. 2003. Pathool gic quiz case: A 50-year-old man with progressive worsening of neurological symptoms. Archives of Pathology & Laboratory Medicine 127, 2: E113–E114. [Google Scholar]
- Kidney, D., M. Alexander, B. Corr, O. O’Toole, and O. Hardiman. 2004. Oropharyngeal dysphagia in amyotrophic lateral sclerosis: Neurological and dysphagia specific rating scales. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 5, 3: 150–153. [Google Scholar] [PubMed]
- Langlois, J. A., S. R. Kegler, J. A. Butler, K. E. Gotsch, R. L. Johnson, A. A. Reichard, and D. J. Thurman. 2003. Traumatic brain injury-related hospital discharges. MMWR Surveillance Summaries 52, 4: 1–20. [Google Scholar]
- Levine, C. B., K. R. Fahrbach, A. D. Siderowf, R. P. Estok, V. M. Ludensky, and S. D. Ross. 2003. Diagnosis and treatment of Parkinson’s disease: A systematic review of the literature. Evidence Report/Technology Assessment (Summary) 57: 1–4. [Google Scholar]
- Liberson, W. T., H. J. Holmquest, D. Scot, and M. Dow. 1961. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Archives of Physical Medicine and Rehabilitation 42: 101–105. [Google Scholar]
- Lieber, R. l., P. D. Silva, and D. M. Daniel. 1996. Equal effectiveness of electrical and volitional strength training for quadriceps femoris muscles after anterior cruciate ligament surgery. Journal of Orthopedic Research 14, 1: 131–138. [Google Scholar]
- Ludlow, C. L. 2010. Electrical neuromuscular stimulationin dysphagia: current status. Current Opinion in Otolaryngology & Head and Neck Surgery 18, 3: 159–164. [Google Scholar]
- Ludlow, C. L., J. Hoit, R. Kent, L. O. Ramig, R. Shrivastav, E. Strand, and C. M. Sapienza. 2008. Translating principles of neural plasticity into research on speech motor control recovery and rehabilitation. Journal of Speech, Language, and Hearing Research 51, 1: S240–S258. [Google Scholar]
- Ludlow, C. L., I. Humbert, K. Saxon, C. Poletto, B. Sonies, and L. Crujido. 2007. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal Dysphagia. Dysphagia 22, 1: 1–10. [Google Scholar]
- Maffiuletti, N. A. 2010. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. European Journal of Applied Physiology 110, 2: 223–234. [Google Scholar] [PubMed]
- Maffiuletti, N. A., S. Dugnani, M. Folz, E. Di Pierno, and F. Mauro. 2002. Effect of combined electrostimulation and plyometric training on vertical jump height. Medicine & Science in Sports & Exercise 34, 10: 1638–1644. [Google Scholar]
- Maffiuletti, N. A., R. Zory, D. Miotti, M. A. Pellegrino, M. Jubeau, and R. Bottinelli. 2006. Neuromuscular adaptations to electrostimulation resistance training. American Journal of Physical Medicine and Rehabilitation 85, 2: 167–175. [Google Scholar] [PubMed]
- Martin, M., B. Chung, C. Bratlund, B. Mathews, P. Kearney, K. Dietrich-Bums, and C. Ludlow. 2010. Movement of the Hyo-Laryngeal Complex with Intramuscular stimulation in Dysphagia Following Chemo/Radiation Therapy. 18th Annual Meeting for the Dysphagia Research Society, San Diego, CA, USA, March 3–6. [Google Scholar]
- Mayr, W., M. Bijak, W. Girsch, C. Hofer, H. Lanmuller, D. Rafolt, and G. Freilinger. 1999. MYOSTIM-FES to prevent muscle atrophy in microgravity and bed rest: preliminary report. Artificial Organs 23, 5: 428–431. [Google Scholar]
- McAuliffe, M. J., E. C. Ward, B. E. Murdoch, and A. M. Farrell. 2005. A nonspeech investigation of tongue function in Parkinson’s disease. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 60, 5: 667–674. [Google Scholar] [PubMed]
- Merrill, D. R. 2009. Review of electrical stimulation in cerebral palsy and recommendations for future directions. Developmental Medicine & Child Neurology 51, Suppl 4: 154–165. [Google Scholar]
- Mezzanotte, W. S., D. J. Tangel, and D. P. White. 1992. Waking genioglossal electromyogramin sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). Journal of Clinical Investigation 89, 5: 1571–1579. [Google Scholar]
- Miki, H., W. Hida, T. Chonan, Y. Kikuchi, and T. Takishima. 1989. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. The American Review of Respiratory Disease 140, 5: 1285–1289. [Google Scholar]
- Nam, H. S., J. Beom, 8. M. Oh, and T. R. Han. 2013. Kinematic effects of hyolaryngeal electrical stimulation therapy on hyoid excursion and laryngeal elevation. Dysphagia 28, 4: 548–556. [Google Scholar]
- Newsam, C. J., and L. L. Baker. 2004. Effect of an electric stimulation facilitation program on quadriceps motor unit recruitment after stroke. Archives of Physical Medicine and Rehabilitation 85, 12: 2040–2045. [Google Scholar]
- Oliven, A., D. J. O’Hearn, A. Boudewyns, M. Odeh, W. De Backer, P. van de Heyning, and A. R. Schwartz. 2003. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. Journal of Applied Physiology (1985) 95, 5: 2023–2029. [Google Scholar] [PubMed]
- Oliven, A., R. P. Schnall, G. Pillar, N. Gavriely, and M. Odeh. 2001. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respiration Physiology 127, 2–3: 217–226. [Google Scholar]
- Oliven, A., N. Tov, M. Odeh, U. Steinfeld, R. Oliven, A. Schwartz, and L. Gaitini. 2009. [Electrical stimulation of the genioglossus to improve pharyngeal patency in obstructive sleep apnea: comparison of results obtained during sleep and anesthesia]. Harefuah 148, 5: 315–319, 350, 349. [Google Scholar] [PubMed]
- Petterson, S., and L. Snyder-Mackler. 2006. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. Journal of Orthopedic Sports and Physical Therapy 36, 9: 678–685. [Google Scholar]
- Pichon, F., J.-C. Chatard, A. Martin, and G. Cometti. 1995. Electrical stimulation and swimming performance. Medicine & Science in Sports & E xercise 27, 12: 1671–1676. [Google Scholar]
- Pierrel, R. 1955. Taste effects resulting from intermittent electrical stimulation of the tongue. Journal of Experimental Psychology 49, 5: 374–380. [Google Scholar]
- Piva, S. R., E. A. Goodnite, K. Azuma, J. D. Woollard, B. H. Goodpaster, M. C. Wasko, and G. K. Fitzgerald. 2007. Neuromuscular electrical stimulation and volitional exercise for individuals with rheumatoid arthritis: a multiple-patient case report. Physical Therapy 87, 8: 1064–1077. [Google Scholar]
- Pleasanton, A. K. 1970. Sensitivity of the tongue to electrical stimulation. Journal of Speech and Hearing Research 13, 3: 635. [Google Scholar]
- Qin, Z. H., and Z. L. Gu. 2004. Huntingtin processing in pathogenesis of Huntington disease. Acta Pharmacologica Sinica 25, 10: 1243–1249. [Google Scholar]
- Querol, F., J. E. Gallach, J. L. Toca-Herrera, M. Gomis, and L. M. Gonzalez. 2006. Surface electrical stimulation of the quadriceps femoris in patients affected by haemophilia A. Haemophilia 12, 6: 629–632. [Google Scholar]
- Quittan, M., G. F. Wiesinger, B. Sturm, S. Puig, W. Mayr, A. Sochor, and V. Fialka-Moser. 2001. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: A single-blind, randomized, controlled trial. American Journal of Physical Medicine Rehabilitation 80, 3: 206–214, quiz 215–206, 224. [Google Scholar] [PubMed]
- Ramig, L. O., C. Fox, and S. Sapir. 2004. Parkinson’s disease: Speech and voice disorders and their treatment with the Lee Silverman Voice Treatment. Seminar in Speech Language 25, 2: 169–180. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W. 2006. Electrical stimulation of the upper airwaysmuscles.
- Remmers, J., E. Sauerland, and A. Anch. 1978. Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology 44, 6: 931–938. [Google Scholar] [CrossRef]
- Samii, A., J. G. Nutt, and B. R. Ransom. 2004. Parkinson’s disease. Lancet 363, 9423: 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Schnall, R. P., G. Pillar, S. G. Kelsen, and A. Oliven. 1995. Dilatory effects of upper airway muscle contraction induced by electrical stimulation in awake humans. Journal of Applied Physiology (1985) 78, 5: 1950–1956. [Google Scholar] [CrossRef]
- Seikel, J., D. King, and D. Drumright. 2009. Anatomy & physiology for speech, language, and hearing. Cengage Learning. [Google Scholar]
- Sha, S. J., and R. B. Layzer. 2007. Myasthenia gravis and Lambert-Eaton myasthenic syndrome in the same patient. Muscle & Nerve 36, 1: 115–117. [Google Scholar]
- Sheffler, L. R., and J. Chae. 2007. Neuromuscular electrical stimulation in neurorehaiblitation. Muscle & Nerve 35, 5: 562–590. [Google Scholar]
- Shields, R. K., and S. Dudley-Javoroski. 2006. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. Journal of Neurophysiology 95, 4: 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Mackler, L., A. Delitto, S. L. Bailey, and S. W. Stralka. 1995. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. Journal of Bone & Joint Surgery, American Volume 77, 8: 1166–1173. [Google Scholar]
- Snyder-Mackler, L., Z. Ladin, A. A. Schepsis, and J. C. Young. 1991. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. Effects of electrically elicited contraction of the quadriceps fernoris and hamstring muscles on gait and on strength of the thigh muscles. Journal of Bone & Joint Surgery, American Volume 73, 7: 1025–1036. [Google Scholar]
- Solomon, N. P., D. A. Robin, and E. S. Luschei. 2000. Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech Language Hearing Research 43, 1: 256–267. [Google Scholar]
- Stackhouse, S. K., S. A. Binder-Macleod, C. A. Stackhouse, J. J. McCarthy, L. A. Prosser, and S. C. Lee. 2007. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: A preliminary study. Neurorehabil Neural Repair 21, 6: 475–485. [Google Scholar]
- Steier, J., J. Seymour, G. F. Rafferty, C. J. Jolley, E. Solomon, Y. Luo, and J. Moxham. 2011. Continuous Transcutaneous Submental Electrical Stimulation in Obstructive Sleep Apnea Genioglossus Stimulation in Sleep ApneaA Feasibility Study. CHEST Journal 140, 4: 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J. E., R. L. Mizner, and L. Snyder-Mackler. 2004. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplast:ya case series. Journal of Orthopaedic & Sports Physical Therapy 34, 1: 21–29. [Google Scholar]
- Stierwalt, J. A., and L. L. Murray. 2002. Attention impairment following traumatic brain injury. Seminar Speech Language 23, 2: 129–138. [Google Scholar]
- Suetta, C., P. Aagaard, A. Rosted, A. K. Jakobsen, B. Duus, M. Kjaer, and S. P. Magnusson. 2004. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. Journal of Applied Physiology 97, 5: 1954–1961. [Google Scholar] [PubMed]
- Theodoros, D. G. 2001. Traumatic brain injury: Associated speech, language, and swallowing disorders. Cengage Learning. [Google Scholar]
- van Boxtel, A., P. Goudswaard, G. M. van der Molen, and W. E. van den Bosch. 1983. Changes in electromyogrampower spectra of facial and jaw-elevator muscles during fatigue. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 54, 1: 51–58. [Google Scholar] [PubMed]
- Vaquero, A. F., J. L. Chicharro, L. Gil, M. P. Ruiz, V. Sanchez, A. Lucia, and M. A. Gomez. 1998. Effects of muscle electrical stimulation on peak VO2 in cardiac transplant patients. International Journal of Sports Medicine 19, 5: 317–322. [Google Scholar] [CrossRef]
- Vivodtzev, I., Y. Lacasse, and F. Maltais. 2008. Neuromuscular electrical stimulation of the lower limbs in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation and Prevention 28, 2: 79–91. [Google Scholar] [CrossRef]
- Webb, W. G., R. K. Adler, and R. J. Love. 2008. Neurology for the speech-language pathologist. Mosby/Elsevier. [Google Scholar]
- Webster, D. B. 1999. Neuroscience of communication. Singular Publishing Group. [Google Scholar]
- Yang, H., X. Meng, and Y. Zhu. 2000. [The position of submaxil/ary transcutaneous electrical stimulation for obstructive sleep apnea syndrome]. Zhonghua er bi yan hou ke za zhi 35, 1: 55–58. [Google Scholar]
© 2014 by the author. 2014 Mohammed F. Safi, Wilhelmina Wright-Harp, Jay R. Lucker, Joan C. Payne, Ovetta Harris