Dual Action of Bacillus and Lactobacillus spp.: Promoting Bean Cultivar Development and Suppressing Xanthomonas axonopodis pv. phaseoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Bacterial Growth and Pathogen Inoculation
2.3. Experimental Design and Disease Assessment
2.4. Statistical Analysis
3. Results
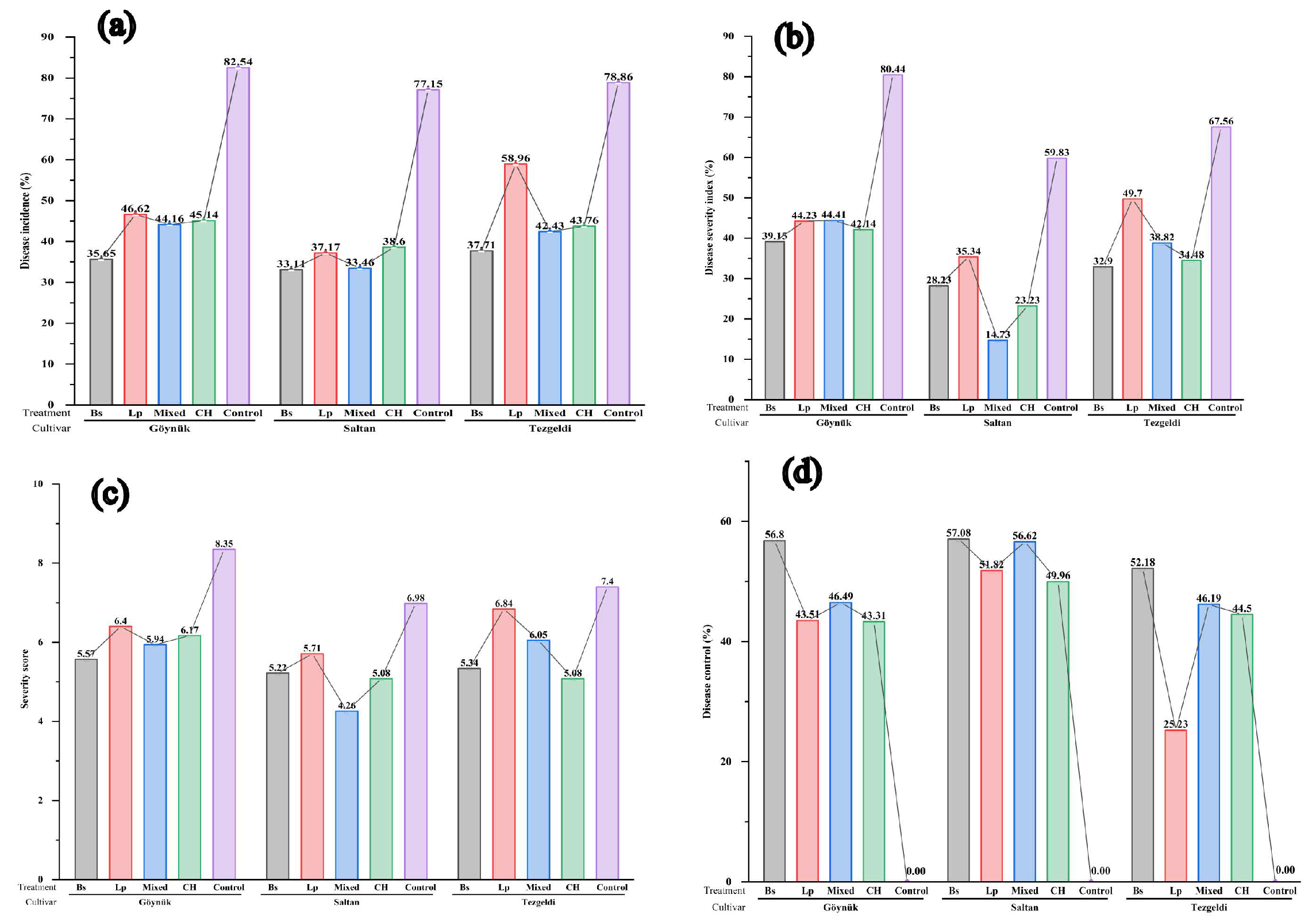
3.1. Disease Evaluation
3.2. Plant Growth Parameters
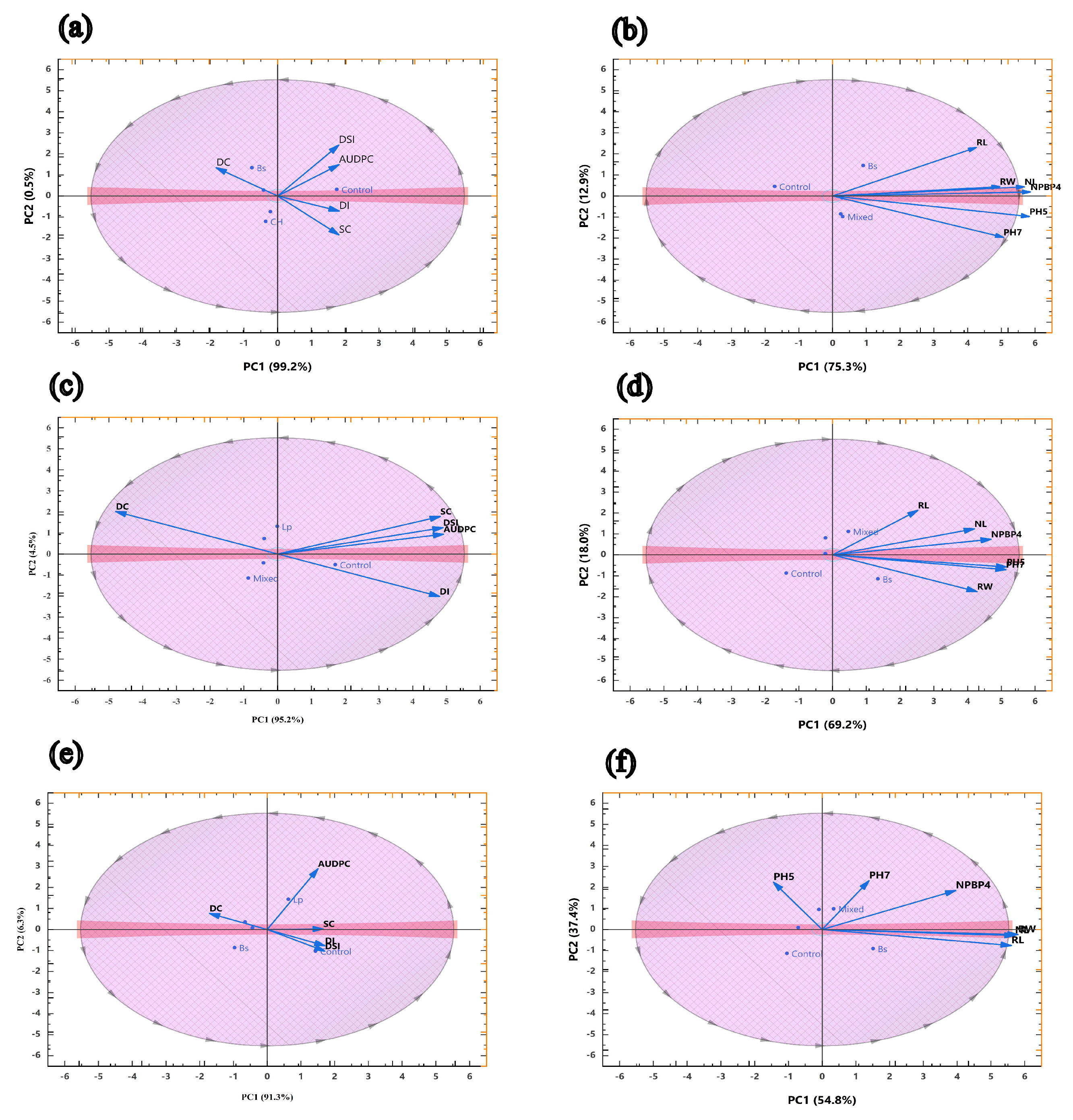
3.3. Principal Component Analysis of Disease Parameters and Growth Traits
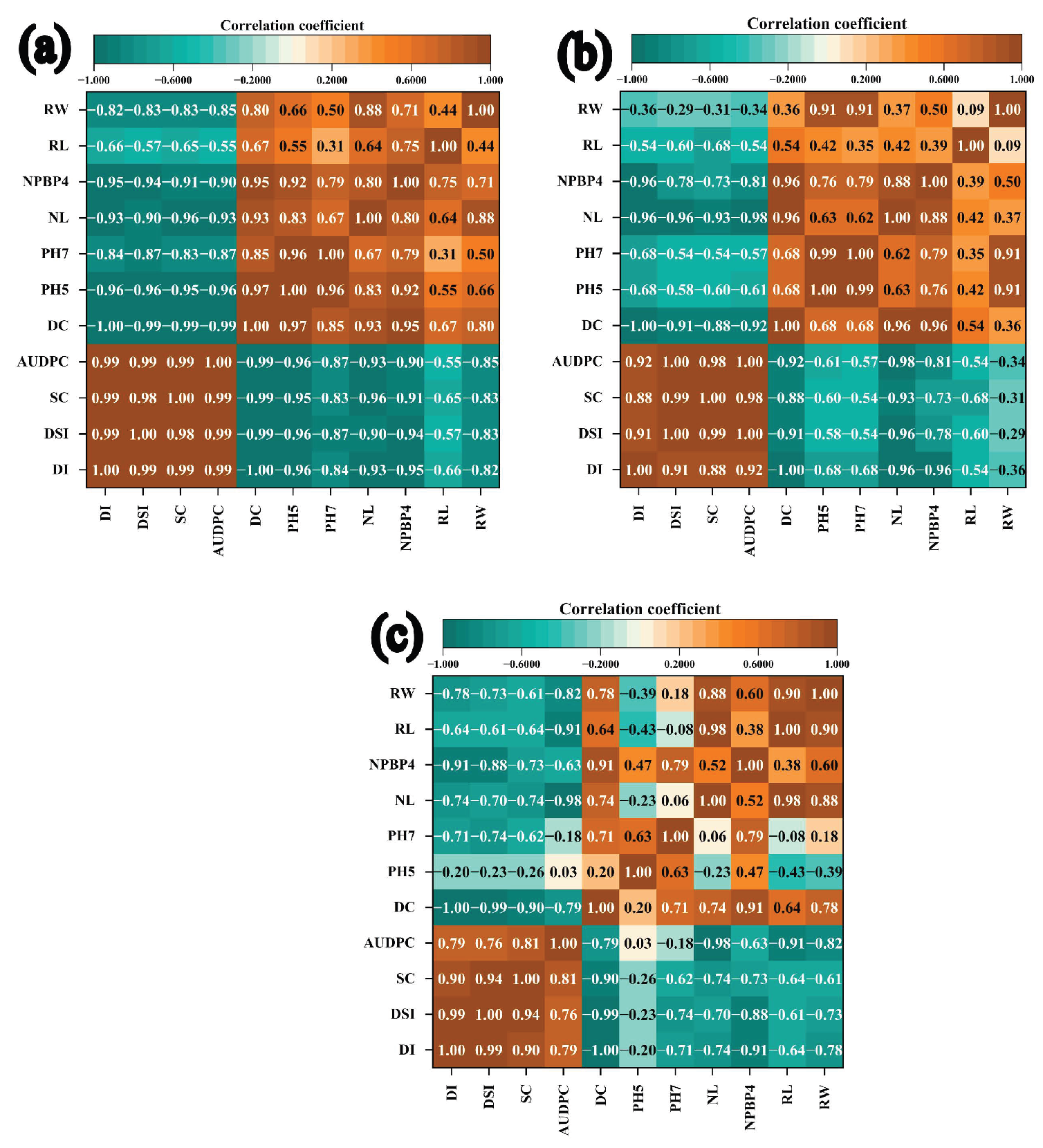
3.4. Disease-Growth Correlations in Bean Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Constantin, E.; Cleenwerck, I.; Maes, M.; Baeyen, S.; Van Malderghem, C.; De Vos, P.; Cottyn, B. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016, 65, 792–806. [Google Scholar] [CrossRef]
- Aritua, V.; Musoni, A.; Kabeja, A.; Butare, L.; Mukamuhirwa, F.; Gahakwa, D.; Kato, F.; Abang, M.M.; Buruchara, R.; Sapp, M. The draft genome sequence of Xanthomonas species strain Nyagatare, isolated from diseased bean in Rwanda. FEMS Microbiol. Lett. 2015, 362, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ambachew, D.; Joshua, J.; Mmbaga, M.T.; Blair, M.W. Sources of Resistance to Common Bacterial Blight and Charcoal Rot Disease for the Production of Mesoamerican Common Beans in the Southern United States. Plants 2021, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.W.; Singh, S.P.; Gilbertson, R.L. Interaction of common bacterial blight bacteria with disease resistance quantitative trait loci in common bean. Phytopathology 2011, 101, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Degu, T.; Alemu, T.; Desalegn, A.; Amsalu, B.; Assefa, A. Association of cropping practices, cropping areas, and foliar diseases of common bean (Phaseolus vulgaris L.) in Ethiopia. J. Agric. Food Res. 2023, 14, 100765. [Google Scholar] [CrossRef]
- Belete, T.; Bastas, K.K. Common bacterial blight (Xanthomonas axonopodis pv. phaseoli) of beans with special focus on Ethiopian condition. J. Plant Pathol. Microbiol. 2017, 8, 1000403. [Google Scholar] [CrossRef]
- Paiva, B.A.R.; Preveaux, A.; Darrasse, A.; Wendland, A.; Rossato, M.; Marques, A.S.A.; Ferreira, M.A.S.V.; Jacques, M.-A. A survey of common bacterial blight in Central Brazil reveals a third Xanthomonas species infecting common bean. Trop. Plant Pathol. 2024, 49, 566–572. [Google Scholar] [CrossRef]
- Suchita, S.; Kansal, S.; Sharma, R.; Parwan, S. Epidemiological dynamics of Xanthomonas axonopodis pv. phaseoli in french bean (Phaseolus vulgaris): Unraveling the factors governing bacterial blight pathogenesis. Int. J. Bio-Resour. Stress Manag. 2023, 14, 1472–1479. [Google Scholar] [CrossRef]
- Mtonga, A.; Maruthi, M.N. Diseases of Common Bean. In Handbook of Vegetable and Herb Diseases; Elmer, W.H., McGrath, M., McGovern, R.J., Eds.; Springer International Publishing: Cham, Switzerland, 2025; pp. 1–52. [Google Scholar]
- FAOSTAT. FAO Statistical Databases. Food and Agriculture Organization of the United Nations. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 December 2023).
- DEMİR, C. Determination of Energy Use Efficency and Indicators of Dry Bean (Phaseolus vulgaris L.) Production. ISPEC J. Agric. Sci. 2024, 8, 474–481. [Google Scholar] [CrossRef]
- Anonymous. Tarım ve Orman Bakanlığı, Bitkisel Üretim Genel Müdürlüğü Tarla ve Bahçe Bitkileri Daire Başkanlığı, Ürün Masaları. 2024. Available online: https://www.tarimorman.gov.tr/BUGEM/Belgeler/Bültenler/OCAK%202022/Kuru%20Fasulye%20Ocak%20Bülteni.pdf (accessed on 12 February 2024).
- Bastas, K.K.; Sahin, F. Evaluation of seedborne bacterial pathogens on common bean cultivars grown in central Anatolia region, Turkey. Eur. J. Plant Pathol. 2017, 147, 239–253. [Google Scholar] [CrossRef]
- Gedük, A.; Bastas, K.K.; Kordali, Ş.; Yılmaz, F. Fasulye Bakteriyel Adi Yaprak Yanıklığı Hastalığına Karşı Farklı Bor Bileşiklerinin Etkileri. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 226–233. [Google Scholar] [CrossRef]
- Fininsa, C. Relationship between common bacterial blight severity and bean yield loss in pure stand and bean?-?maize intercropping systems. Int. J. Pest Manag. 2003, 49, 177–185. [Google Scholar] [CrossRef]
- Fininsa, C.; Tefera, T. Effect of primary inoculum sources of bean common bacterial blight on early epidemics, seed yield and quality aspects. Int. J. Pest Manag. 2001, 47, 221–225. [Google Scholar] [CrossRef]
- Gillard, C.L.; Conner, R.L.; Howard, R.J.; Pauls, K.P.; Shaw, L.; Taran, B. The performance of dry bean cultivars with and without common bacterial blight resistance in field studies across Canada. Can. J. Plant Sci. 2009, 89, 405–410. [Google Scholar] [CrossRef]
- Neergaard, P. Seed Pathology; MacMillan Press: London, UK, 1979. [Google Scholar]
- Harveson, R.M.; Schwartz, H.F. Bacterial diseases of dry edible beans in the central high plains. Plant Health Prog. 2007, 8, 35. [Google Scholar] [CrossRef]
- Viteri, D.M.; Cregan, P.B.; Trapp, J.J.; Miklas, P.N.; Singh, S.P. A new common bacterial blight resistance QTL in VAX 1 common bean and interaction of the new QTL, SAP6, and SU91 with bacterial strains. Crop Sci. 2014, 54, 1598–1608. [Google Scholar] [CrossRef]
- Pawan, K.; Kumar, R.; Thakur, K.; Mahajan, D.; Brar, B.; Sharma, D.; Kumar, S.; Sharma, A.K. Impact of Pesticides Application on Aquatic Ecosystem and Biodiversity: A Review. Biol. Bull. 2023, 50, 1362–1375. [Google Scholar] [CrossRef]
- Rajak, P.; Roy, S.; Ganguly, A.; Mandi, M.; Dutta, A.; Das, K.; Nanda, S.; Ghanty, S.; Biswas, G. Agricultural pesticides—Friends or foes to biosphere? J. Hazard. Mater. Adv. 2023, 10, 100264. [Google Scholar] [CrossRef]
- Corrêa, B.O.; Soares, V.N.; Sangiogo, M.; de Oliveira, J.R.; Moura, A.B. Interaction between bacterial biocontrol agents and strains of Xanthomonas axonopodis pv. phaseoli effects on biocontrol efficacy of common blight in beans. Afr. J. Microbiol. Res. 2017, 11, 1294–1302. [Google Scholar] [CrossRef]
- Mutlu, N.; Vidaver, A.K.; Coyne, D.P.; Steadman, J.R.; Lambrecht, P.A.; Reiser, J. Differential Pathogenicity of Xanthomonas campestris pv. phaseoli and X. fuscans subsp. fuscans Strains on Bean Genotypes with Common Blight Resistance. Plant Dis. 2008, 92, 546–554. [Google Scholar] [CrossRef]
- Ali, I.I. Bacillus spp. as Bioagents- Applications and Effective Strategies in Plant Bacterial Disease Management: A Review. Agric. Rev. 2025, 1–7. [Google Scholar] [CrossRef]
- Yang, R.; Du, X.; Khojasteh, M.; Ali Shah, S.M.; Peng, Y.; Zhu, Z.; Xu, Z.; Chen, G. Green guardians: The biocontrol potential of Pseudomonas-derived metabolites for sustainable agriculture. Biol. Control 2025, 201, 105699. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Kulkova, I.; Kowalczyk, P.; Kramkowski, K. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol. 2023, 14, 1194606. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Ali, I.I.; Akraym, H.M.; Bastas, K.K. Exploring Effective Biocontrol Strategies to Combat Soft Rot Disease in Tomato Cultivars. In Proceedings of the 4 th International Conference on Sustainable Ecological Agriculture, Nicosia, Northern Cyprus, 25–27 April 2025. [Google Scholar]
- Butt, H.; Bastas, K.K. Antagonistic Activity of Bacillus spp. Against Fire Blight Disease In vitro and In planta. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 2486–2492. [Google Scholar] [CrossRef]
- Belete, T.; Bastas, K.K.; Francesconi, S.; Balestra, G.M. Biological effectiveness of Bacillus subtilis on common bean bacterial blight. J. Plant Pathol. 2021, 103, 249–258. [Google Scholar] [CrossRef]
- Limanska, N.; Ivanytsia, T.; Basiul, O.; Krylova, K.; Biscola, V.; Chobert, J.-M.; Ivanytsia, V.; Haertlé, T. Effect of Lactobacillus plantarum on germination and growth of tomato seedlings. Acta Physiol. Plant. 2013, 35, 1587–1595. [Google Scholar] [CrossRef]
- Zengin, M.; Karakaplan, S.; Ersoy, İ. Determination of Irrigation Water Quality of Lake Beyşehir and Other Water Sources Used in Irrigation of Çumra Plain. Selcuk J. Agric. Food Sci. 2002, 16, 72–78. [Google Scholar]
- Draper, S.R. International Rules for Seed Testing. Seed Sci. Technol. 1995, 3, 331. [Google Scholar]
- Schoonhoven, A.V.; Pastor-Corrales, M.A. Standard System for the Evaluation of Bean Germplasm; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 1987; 56p. [Google Scholar]
- Ararsa, L.; Fikre, L.; Getachew, A. Evaluation of Integrated Management of Common Bacterial Blight of Common Bean in Central Rift Valley of Ethiopia. Am. J. Phytomed. Clin. Ther. 2018, 6, 3. [Google Scholar] [CrossRef]
- Mengesha, G.G.; Yetayew, H.T. Distribution and association of factors influencing bean common bacterial blight (Xanthomonas axonopodis pv. phaseoli) epidemics in Southern Ethiopia. Arch. Phytopathol. Plant Prot. 2018, 51, 1066–1089. [Google Scholar] [CrossRef]
- Darrasse, A.; Bureau, C.; Samson, R.; Morris, C.E.; Jacques, M.-A. Contamination of bean seeds by Xanthomonas axonopodis pv. phaseoli associated with low bacterial densities in the phyllosphere under field and greenhouse conditions. Eur. J. Plant Pathol. 2007, 119, 203–215. [Google Scholar] [CrossRef]
- İslamoğlu, M. The place and importance of biological control in Turkey and some application examples. In Agricultural Researches Resourcebook; Iksad Publishing House: Ankara, Turkey, 2021; p. 159. [Google Scholar]
- Akilli, S.; Katircioğlu, Y.Z.; Maden, S. Biological control of chestnut canker, caused by Cryphonectria parasitica, by antagonistic organisms and hypovirulent isolates. Turk. J. Agric. For. 2011, 35, 515–523. [Google Scholar] [CrossRef]
- Umarusman, M.A.; Baştaş, K.K. Potential Biological Control Agents against Soft Rot Diseases Caused by Pectobacteria on Some Sugar Beet Cultivars. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 2601–2608. [Google Scholar] [CrossRef]
- Rees, H.J.; Drakulic, J.; Cromey, M.G.; Bailey, A.M.; Foster, G.D. Endophytic Trichoderma spp. can protect strawberry and privet plants from infection by the fungus Armillaria mellea. PLoS ONE 2022, 17, e0271622. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases–What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Butt, H.; Bastas, K.K. Environment-Friendly Management of Plant Diseases by Bacillus Through Molecular Pathways. In Microbial Biocontrol: Molecular Perspective in Plant Disease Management; Bastas, K.K., Kumar, A., Sivakumar, U., Eds.; Springer Nature: Singapore, 2023; pp. 217–241. [Google Scholar]
- Gur, A.; Bastas, K.K. Effectiveness of Phosphorous acid, Bacillus subtilis and Copper Compounds on Apple cv. Gala with M9 Rootstock in the Control of Fire Blight. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 2595–2600. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Factories 2009, 8, 63. [Google Scholar] [CrossRef]
- Bottone, E.J.; Peluso, R.W. Production by Bacillus pumilus (MSH) of an antifungal compound that is active against Mucoraceae and Aspergillus species: Preliminary report. J. Med. Microbiol. 2003, 52, 69–74. [Google Scholar] [CrossRef]
- Fousia, S.; Paplomatas, E.J.; Tjamos, S.E. Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv. tomato and induces plant defence-related genes. J. Phytopathol. 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Sunyar, B.; Yeşildağ, M.F.; Alma, M.H. Effectiveness of Bacillus and Pseudomonas Strains in Biological Control of Common Bacterial Blight Disease in Common Bean (Phaseolus vulgaris L.). J. Crop Health 2024, 76, 1357–1372. [Google Scholar] [CrossRef]
- Punja, Z.K.; Rodriguez, G.; Tirajoh, A. Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 2016, 84, 98–104. [Google Scholar] [CrossRef]
- Zare, D.; Aryaee, H.; Mirdamadi, S.; Shirkhan, F. The Benefits and Applications of Lactobacillus plantarum in Food and Health: A Narrative Review. Iran. J. Public Health 2024, 53, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, A.; Rashwan, N.; Vasani, S.; Alkhawashki, A.; Wu, T.T.; Lu, X.; Castillo, D.A.; Xiao, J. The Health Benefits of Probiotic Lactiplantibacillus plantarum: A Systematic Review and Meta-Analysis. Probiotics Antimicrob. Proteins 2024, 17, 3358–3377. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Wei, X.; Zhu, Y.; Chen, W.; Han, Y. Postharvest biological control of Botrytis cinerea and the mechanisms underlying the induction of disease resistance in grapes by Lactobacillus plantarum CM-3. Biol. Control 2022, 172, 104982. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J. Anim. Sci. Biotechnol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Motyl, I.; Berłowska, J.; Czyżowska, A.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Lactic acid bacteria as biocontrol agents against potato (Solanum tuberosum L.) pathogens. Appl. Sci. 2022, 12, 7763. [Google Scholar] [CrossRef]
- Jaffar, N.S.; Jawan, R.; Chong, K.P. The potential of lactic acid bacteria in mediating the control of plant diseases and plant growth stimulation in crop production—A mini review. Front. Plant Sci. 2023, 13, 1047945. [Google Scholar] [CrossRef]
- Fhoula, I.; Najjari, A.; Turki, Y.; Jaballah, S.; Boudabous, A.; Ouzari, H. Diversity and antimicrobial properties of lactic acid bacteria isolated from rhizosphere of olive trees and desert truffles of Tunisia. Biomed Res. Int. 2013, 2013, 405708. [Google Scholar] [CrossRef]
- Shrestha, A.; Kim, B.S.; Park, D.H. Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 2014, 24, 763–779. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Montesinos, E.; Badosa, E. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int. Microbiol. 2008, 11, 231. [Google Scholar]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Gwiazdowski, R.; Kubiak, K.; Juś, K.; Marchwińska, K.; Gwiazdowska, D. The Biocontrol of Plant Pathogenic Fungi by Selected Lactic Acid Bacteria: From Laboratory to Field Study. Agriculture 2024, 14, 61. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-based biocontrol solutions for fruits and vegetables: Recent insight, patents, and innovative trends. Recent Pat. Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef]
- Roselló, G.; Bonaterra, A.; Francés, J.; Montesinos, L.; Badosa, E.; Montesinos, E. Biological control of fire blight of apple and pear with antagonistic Lactobacillus plantarum. Eur. J. Plant Pathol. 2013, 137, 621–633. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yuan, Z.; Feng, J.; Chen, S.; Sun, G.; Wei, Z.; Hu, T. Effects of microbial fertilizer and irrigation amount on growth, physiology and water use efficiency of tomato in greenhouse. Sci. Hortic. 2024, 323, 112553. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial fertilizers improve soil quality and crop yield in coastal saline soils by regulating soil bacterial and fungal community structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, K.; Chang, T.; Shaghaleh, H.; Qi, Z.; Zhang, J.; Ye, H.; Hamoud, Y.A. Interactive Effects of Microbial Fertilizer and Soil Salinity on the Hydraulic Properties of Salt-Affected Soil. Plants 2024, 13, 473. [Google Scholar] [CrossRef]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef] [PubMed]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.-Y.; Zhou, X.-K.; Yang, C.-G.; Zheng, S.-C.; Duo, J.-L.; Mo, M.-H. Biological control tobacco bacterial wilt and black shank and root colonization by bio-organic fertilizer containing bacterium Pseudomonas aeruginosa NXHG29. Appl. Soil Ecol. 2018, 129, 136–144. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Weselowski, B. Influence of foliar sprays of Bacillus subtilis QST 713 on development of early blight disease and yield of field tomatoes in Ontario. Can. J. Plant Pathol. 2014, 36, 170–178. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Saleh, A.A.; El Komy, M.H.; Al Saleh, M.A. Bacillus subtilis QST 713, copper hydroxide, and their tank mixes for control of bacterial citrus canker in Saudi Arabia. J. Citrus Pathol. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- La Fuente, L.D.; Thomashow, L.; Weller, D.; Bajsa, N.; Quagliotto, L.; Chernin, L.; Arias, A. Pseudomonas Fluorescens UP61 Isolated From Birdsfoot Trefoil Rhizosphere Produces Multiple Antibiotics and Exerts a Broad Spectrum of Biocontrol Activity. Eur. J. Plant Pathol. 2004, 110, 671–681. [Google Scholar] [CrossRef]



| Ranks | Country | Production (Tons) | World Share (%) |
|---|---|---|---|
| 1 | India | 6,491,362.2 | 22.77% |
| 2 | Brazil | 2,899,043.0 | 10.17% |
| 3 | Myanmar | 2,683,918.8 | 9.42% |
| 4 | Tanzania | 1,484,000.0 | 5.21% |
| 5 | China | 1,304,639.86 | 4.58% |
| 6 | USA | 1,071,613.0 | 3.76% |
| 7 | Uganda | 865,202.84 | 3.04% |
| 8 | Kenya | 860,973.0 | 3.02% |
| 9 | Burundi | 834,214.01 | 2.93% |
| 10 | Argentina | 792,564.0 | 2.78% |
| Cultivar | Treatment | 8 DAI | DI (%) 15 DAI | 21 DAI | DSI (%) | SC | AUDPC | DC (%) |
|---|---|---|---|---|---|---|---|---|
| Göynük | Bst26 | 23.77 ± 1.4 c | 30.26 ± 1.9 c | 35.65 ± 1.7 c | 39.15 ± 5.0 defg | 5.57 ± 0.5 cde | 651.26 ± 39.0 f | 56.80 |
| Lpkb10 | 31.84 ± 3.7 bc | 39.77 ± 4.6 bc | 46.62 ± 4.6 bc | 44.23 ± 1.9 de | 6.40 ± 0.5 bcd | 910.25 ± 9.8 d | 43.51 | |
| Mixed (Bst26 + Lpkb10) | 29.38 ± 6.0 c | 37.26 ± 6.6 c | 44.16 ± 6.6 c | 44.41 ± 5.0 de | 5.94 ± 0.3 bcd | 730.10 ± 38.31 ef | 46.49 | |
| CH | 31.43 ± 9.5 bc | 38.47 ± 9.6 bc | 45.14 ± 9.4 bc | 42.14 ± 1.0 def | 6.17 ± 0.16 bcd | 748.63 ± 28.9 e | 43.31 | |
| Control (SDW) | 57.70 ± 2.3 a | 70.85 ± 1.0 a | 82.54 ± 2.7 a | 80.44 ± 1.3 a | 8.35 ± 0.5 a | 2023.64 ± 8.0 a | 0.00 | |
| Saltan | Bst26 | 22.69 ± 2.9 c | 28.51 ± 3.2 c | 33.11 ± 3.5 c | 28.23 ± 1.6 gh | 5.22 ± 0.1 de | 516.86 ± 12.6 g | 57.08 |
| Lpkb10 | 27.11 ± 3.7 c | 32.83 ± 3.8 c | 37.17 ± 3.4 c | 35.34 ± 2.8 efg | 5.71 ± 0.5 cde | 714.00 ± 12.8 ef | 51.82 | |
| Mixed (Bst26 + Lpkb10) | 23.55 ± 5.3 c | 29.59 ± 5.0 c | 33.46 ± 5.1 c | 14.73 ± 1.0 i | 4.26 ± 0.6 e | 307.63 ± 5.0 i | 56.62 | |
| CH | 28.30 ± 3.2 c | 33.90 ± 3.1 c | 38.60 ± 2.4 c | 23.23 ± 2.5 hi | 5.08 ± 0.9 de | 425.36 ± 15.2 h | 49.96 | |
| Control (SDW) | 56.79 ± 2.1 a | 67.15 ± 1.5 a | 77.15 ± 2.6 a | 59.83 ± 1.0 bc | 6.98 ± 0.1 abc | 1235.89 ± 32.2 b | 0.00 | |
| Tezgeldi | Bst26 | 26.67 ± 1.8 c | 33.27 ± 1.8 c | 37.71 ± 2.6 c | 32.90 ± 2.0 fgh | 5.34 ± 0.7 de | 731.49 ± 6.7 ef | 52.18 |
| Lpkb10 | 42.53 ± 4.2 b | 51.18 ± 5.0 b | 58.96 ± 5.0 b | 49.70 ± 6.7 cd | 6.84 ± 0.2 bc | 1228.61 ± 34.6 b | 25.23 | |
| Mixed (Bst26 + Lpkb10) | 29.00 ± 4.5 c | 35.92 ± 5.2 c | 42.43 ± 6.4 c | 38.82 ± 7.0 defg | 6.05 ± 0.5 bcd | 905.37 ± 51.9 d | 46.19 | |
| CH | 30.09 ± 4.5 bc | 37.19 ± 4.7 bc | 43.76 ± 4.5 c | 34.48 ± 5.0 efg | 5.08 ± 0.1 de | 928.79 ± 50.0 d | 44.50 | |
| Control (SDW) | 59.65 ± 1.0 a | 69.16 ± 1.0 a | 78.86 ± 1.7 a | 67.56 ± 1.5 b | 7.40 ± 0.3 ab | 1131.81 ± 25.3 c | 0.00 | |
| CV (%) | 14.76 | 13.54 | 13.36 | 16.41 | 12.35 | 29.12 | - |
| Cultivar | Treatment | PH5 (cm) | PH7 (cm) | NPBPP (co) | NL (co) | RL (cm) | RW (g) |
|---|---|---|---|---|---|---|---|
| Göynük | Bst26 | 52.25 ± 5.5 a | 68.76 ± 0.5 bc | 3.36 ± 0.7 a | 17.66 ± 1.5 ab | 24.06 ± 1.7 ab | 3.85 ± 0.1 cd |
| Lpkb10 | 52.50 ± 6.5 a | 74.47 ± 2.1 a | 3.09 ± 0.5 a | 13.99 ± 1.5 bcde | 20.66 ± 2.0 b | 2.79 ± 0.3 cd | |
| Mixed (Bst26 + Lpkb10) | 52.33 ± 2.5 a | 74.00 ± 1.0 ab | 2.51 ± 1.1 ab | 16.58 ± 2.5 abc | 19.78 ± 6.0 b | 3.31 ± 0.8 cd | |
| CH | 48.55 ± 0.9 ab | 68.11 ± 0.5 cd | 2.75 ± 0.5 ab | 16.25 ± 1.0 abc | 19.77 ± 0.8 b | 4.39 ± 0.5 bcd | |
| Control (SDW) | 35.77 ± 2.2 d | 56.10 ± 2.2 hi | 1.31 ± 0.6 b | 11.00 ± 1.0 def | 18.88 ±1.0 b | 1.85 ± 0.4 d | |
| Saltan | Bst26 | 49.46 ± 6.5 ab | 67.07 ± 2.0 cd | 2.98 ± 0.5 ab | 15.46 ± 1.5 abc | 20.55 ± 1.3 b | 7.47 ± 1.0 ab |
| Lpkb10 | 40.37 ± 1.0 bcd | 58.78 ± 2.1 fghi | 2.77 ± 0.5 ab | 14.41 ± 0.6 bcd | 21.21 ± 0.7 b | 3.26 ± 1.5 cd | |
| Mixed (Bst26 + Lpkb10) | 44.24 ± 4.4 abcd | 60.85 ± 1.5 efgh | 2.64 ± 0.3 ab | 15.66 ± 1.0 abc | 23.66 ± 1.0 ab | 4.66 ± 0.4 bcd | |
| CH | 40.19 ± 1.0 bcd | 58.08 ± 1.7 ghi | 2.64 ± 0.2 ab | 15.83 ± 1.5 abc | 18.99 ± 1.5 b | 4.10 ± 0.1 bcd | |
| Control (SDW) | 37.39 ± 2.0 cd | 55.15 ± 1.0 i | 1.84 ± 0.1 ab | 11.25 ± 1.0 def | 19.11 ± 0.5 b | 3.85 ± 1.5 cd | |
| Tezgeldi | Bst26 | 40.74 ± 2.1 bcd | 61.32 ± 2.4 efgh | 2.51 ± 0.6 ab | 18.66 ± 1.0 a | 29.62 ± 0.6 a | 10.29 ± 1.7 a |
| Lpkb10 | 43.12 ± 2.5 abcd | 62.97 ± 0.5 defg | 2.10 ± 0.6 ab | 8.50 ± 1.0 f | 17.73 ± 3.6 b | 5.98 ± 2.0 bc | |
| Mixed (Bst26 + Lpkb10) | 46.93 ± 5.2 abc | 63.40 ± 1.8 def | 3.07 ± 0.5 a | 13.60 ± 2.3 bcde | 21.66 ± 2.9 b | 7.51 ± 1.5 ab | |
| CH | 47.31 ± 2.1 abc | 64.08 ± 3.0 cde | 2.54 ± 0.4 ab | 13.08 ± 1.0 cde | 21.33 ± 1.2 b | 5.85 ± 1.1 bc | |
| Control (SDW) | 43.14 ± 2.5 abcd | 58.73 ± 1.1 fghi | 1.34 ± 0.2 b | 10.24 ± 0.6 ef | 19.50 ± 5.4 b | 4.49 ± 1.1 bcd | |
| CV (%) | 14.47 | 10.02 | 23.68 | 18.40 | 20.98 | 10.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.I.; Bastas, K.K. Dual Action of Bacillus and Lactobacillus spp.: Promoting Bean Cultivar Development and Suppressing Xanthomonas axonopodis pv. phaseoli. Bacteria 2025, 4, 56. https://doi.org/10.3390/bacteria4040056
Ali II, Bastas KK. Dual Action of Bacillus and Lactobacillus spp.: Promoting Bean Cultivar Development and Suppressing Xanthomonas axonopodis pv. phaseoli. Bacteria. 2025; 4(4):56. https://doi.org/10.3390/bacteria4040056
Chicago/Turabian StyleAli, Ibrahim Isse, and Kubilay Kurtulus Bastas. 2025. "Dual Action of Bacillus and Lactobacillus spp.: Promoting Bean Cultivar Development and Suppressing Xanthomonas axonopodis pv. phaseoli" Bacteria 4, no. 4: 56. https://doi.org/10.3390/bacteria4040056
APA StyleAli, I. I., & Bastas, K. K. (2025). Dual Action of Bacillus and Lactobacillus spp.: Promoting Bean Cultivar Development and Suppressing Xanthomonas axonopodis pv. phaseoli. Bacteria, 4(4), 56. https://doi.org/10.3390/bacteria4040056





