Antitumor Potential of Bioactive Crude Extracts Derived from Actinomycetes
Abstract
1. Introduction
2. Materials and Methods
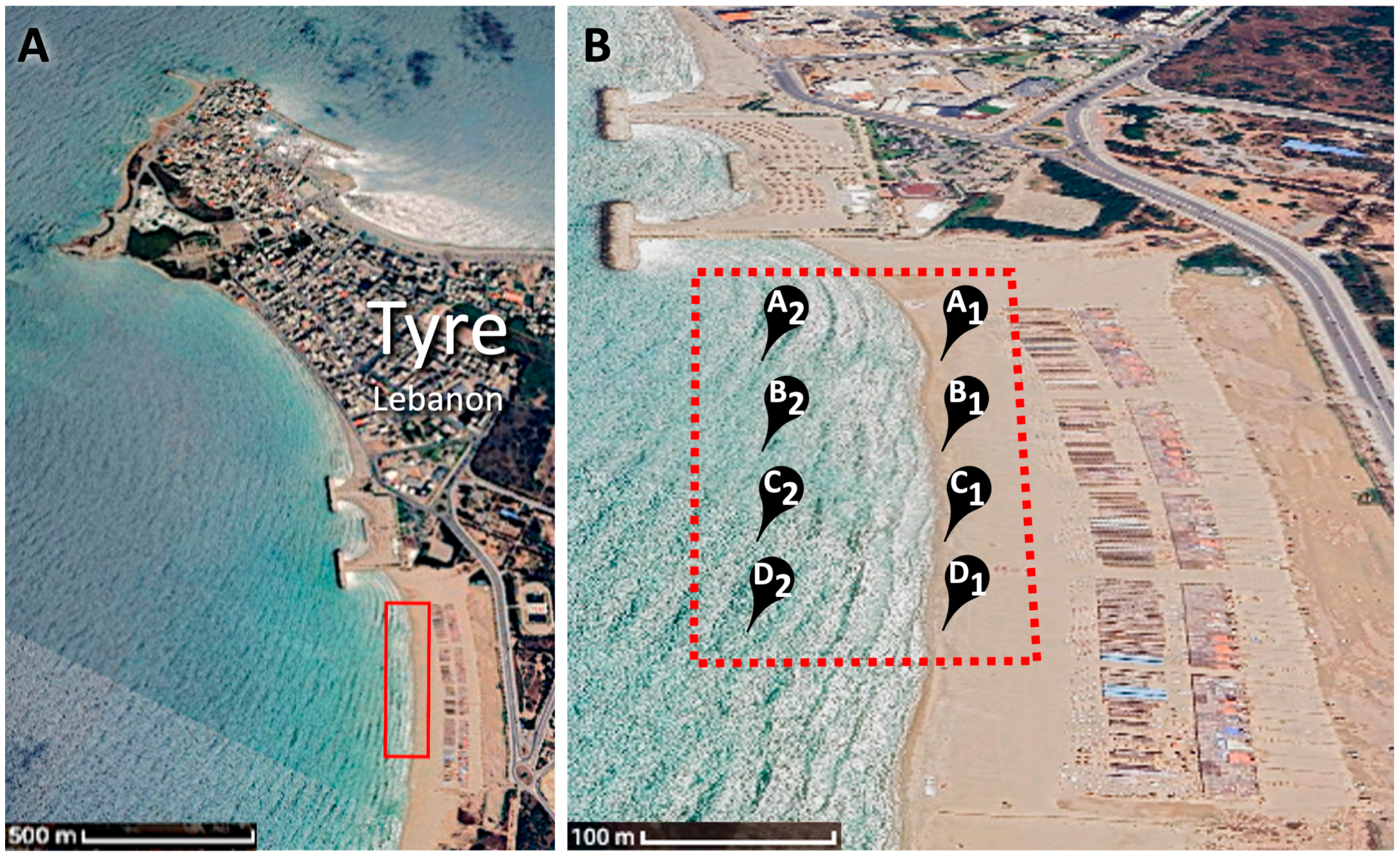
2.1. Sample Preparation and Identification
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Western Blot Assay
2.5. Statistical Analysis
3. Results
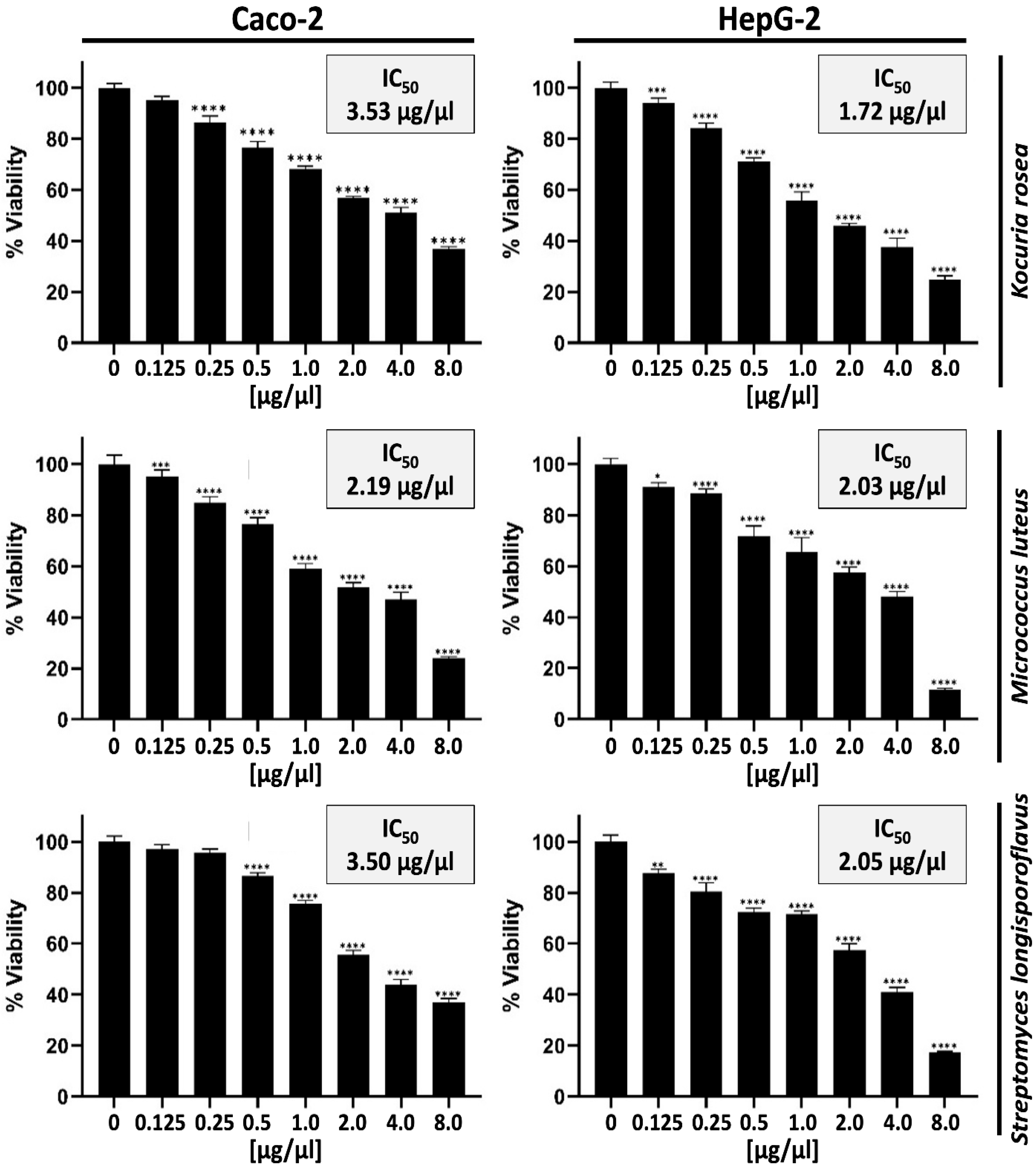
3.1. Effect of Extracts Derived from Kocuria Rosea, Micrococcus luteus, and Streptomyces longisporoflavus on the Proliferation and Viability of Caco-2 and HepG-2 Cells
3.2. Effect of Bacterial Extracts on the Induction of Mitochondrial-Dependent Apoptosis in Caco-2 and HepG-2 Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; El-Bondkly, A.M.A.; Shaaban, M.T. Streptomyces spp. with antitumor activity: Isolation, identification and biological evaluation. Res. J. Pharm. Biol. Chem. Sci. 2008, 1, 1–12. [Google Scholar]
- Genilloud, O. Streptomyces as a source of novel antibiotics. Antibiotics 2017, 6, 21. [Google Scholar]
- Kouroshnia, A.; Zeinali, S.; Irani, S.; Sadeghi, A. Induction of apoptosis and cell cycle arrest in colorectal cancer cells by novel anticancer metabolites of Streptomyces sp. 801. Cancer Cell Int. 2022, 22, 235. [Google Scholar] [CrossRef]
- Osama, N.; Bakeer, W.; Raslan, M.; Soliman, H.A.; Abdelmohsen, U.R.; Sebak, M. Anti-cancer and antimicrobial potential of five soil Streptomycetes: A metabolomics-based study. R. Soc. Open Sci. 2022, 9, 211509. [Google Scholar] [CrossRef]
- Ye, X.; Anjum, K.; Song, T.; Wang, W.; Liang, Y.; Chen, M.; Huang, H.; Lian, Y.; Zhang, Z. Antiproliferative cyclodepsipeptides from the marine actinomycete Streptomyces sp. P11-23B downregulating the tumor metabolic enzymes of glycolysis, glutaminolysis, and lipogenesis. Phytochemistry 2017, 135, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Saurav, K.; Krishnan, K. Diversity and Optimization of Process Parameters for the Growth of Streptomyces VITSVK9 spp. İsoled From Bay of Bengal, İndia. J. Nat. Environ. Sci. 2010, 1, 9–18. [Google Scholar]
- Dhaini, H.K.; Khalil, M.I.; El Hajj, R. The Antimicrobial Potential of Actinomycetes Isolated from Marine Soils in Tyre City Beach, Lebanon: A Promising Source of Novel Bioactive Metabolites. Appl. Microbiol. 2025, 5, 27. [Google Scholar] [CrossRef]
- Selvameenal, L.; Radhakrishnan, M.; Balagurunathan, R. Antibiotic pigment from desert soil actinomycetes; biological activity, purification and chemical screening. Indian J. Pharm. Sci. 2009, 71, 499–504. [Google Scholar]
- Janardhan, A.; Kumar, A.P.; Viswanath, B.; Saigopal, D.V.R.; Narasimha, G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol. Res. Int. 2014, 20, 217030. [Google Scholar] [CrossRef]
- Kuzmich, A.S.; Romanenko, L.A.; Kokoulin, M.S. Cell-cycle arrest and mitochondria-dependent apoptosis induction in T-47D cells by the capsular polysaccharide from the marine bacterium Kangiella japonica KMM 3897. Carbohydr. Polym. 2023, 320, 121237. [Google Scholar] [CrossRef]
- Ngamcharungchit, C.; Chaimusik, N.; Panbangred, W.; Euanorasetr, J.; Intra, B. Bioactive Metabolites from Terrestrial and Marine Actinomycetes. Molecules 2023, 28, 5915. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S.V.; Manemann, E.M.; Rowe, S.E.; Callender, M.C.; Soto, W. Marine Actinomycetes, New Sources of Biotechnological Products. Mar. Drugs 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Rammali, S.; Idir, A.; Aherkou, M.; Ciobică, A.; Kamal, F.Z.; El Aalaoui, M.; Rahim, A.; Khattabi, A.; Abdelmajid, Z.; Aasfar, A.; et al. In vitro and computational investigation of antioxidant and anticancer properties of Streptomyces coeruleofuscus SCJ extract on MDA-MB-468 triple-negative breast cancer cells. Sci. Rep. 2024, 14, 25251. [Google Scholar] [CrossRef]
- Kanchanasin, P.; Salahong, T.; Sripreechasak, P.; Suriyachadkun, C.; Harunari, E.; Igarashi, Y.; Tanasupawat, S.; Tawinwung, S.; Vimolmangkang, S.; Chaotham, C.; et al. Discovery of two new actinobacteria, Micromonospora palythoicola sp. nov. and Streptomyces poriticola sp. nov., isolated from marine invertebrates. Sci. Rep. 2024, 14, 22140. [Google Scholar] [CrossRef]
- Su, Z.; Li, K.; Luo, X.; Zhu, Y.; Mai, S.-Y.; Zhu, Q.; Yang, B.; Zhou, X.; Tao, H. Aromatic Acids and Leucine Derivatives Produced from the Deep-Sea Actinomycetes Streptomyces chumphonensis SCSIO15079 with Antihyperlipidemic Activities. Mar. Drugs 2022, 20, 259. [Google Scholar] [CrossRef] [PubMed]
- Jelali, H.; Mansour, L.; Deniau, E.; Sauthier, M.; Hamdi, N. An Efficient Synthesis of Phthalimides and Their Biological Activities. Polycycl. Aromat. Compd. 2022, 42, 1806–1813. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Xiang, X.; Xie, J. Mycobacterium tuberculosis effectors interfering host apoptosis signaling. Apoptosis 2015, 20, 883–891. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, J.; Xu, H.; Xue, K.; Liu, X.; Zhang, Z.; Liu, J.; Liu, Y. Staphylococcus aureus extracellular vesicles induce apoptosis and restrain mitophagy-mediated degradation of damaged mitochondria. Microbiol. Res. 2023, 273, 127421. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dashwood, W.M.; Zhong, X.; Nakagama, H.; Dashwood, R.H. Bcl-2 overexpression in PhIP-induced colon tumors: Cloning of the rat Bcl-2 promoter and characterization of a pathway involving β-catenin, c-Myc and E2F1. Oncogene 2007, 26, 6194–6202. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K. Identification of Bcl2 as a Stably Expressed qPCR Reference Gene for Human Colon Cancer Cells Treated with Cottonseed-Derived Gossypol and Bioactive Extracts and Bacteria-Derived Lipopolysaccharides. Molecules 2022, 27, 7560. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Deng, S.; Jiang, T. Recent developments in the identification and biosynthesis of anticancer metabolites from actinomycetes. Front. Microbiol. 2022, 13, 11611020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaini, H.K.; Hassanieh, B.F.; El Hajj, R.; Khalil, M.I. Antitumor Potential of Bioactive Crude Extracts Derived from Actinomycetes. Bacteria 2025, 4, 51. https://doi.org/10.3390/bacteria4040051
Dhaini HK, Hassanieh BF, El Hajj R, Khalil MI. Antitumor Potential of Bioactive Crude Extracts Derived from Actinomycetes. Bacteria. 2025; 4(4):51. https://doi.org/10.3390/bacteria4040051
Chicago/Turabian StyleDhaini, Hassan K., Bahaa Fahed Hassanieh, Rana El Hajj, and Mahmoud I. Khalil. 2025. "Antitumor Potential of Bioactive Crude Extracts Derived from Actinomycetes" Bacteria 4, no. 4: 51. https://doi.org/10.3390/bacteria4040051
APA StyleDhaini, H. K., Hassanieh, B. F., El Hajj, R., & Khalil, M. I. (2025). Antitumor Potential of Bioactive Crude Extracts Derived from Actinomycetes. Bacteria, 4(4), 51. https://doi.org/10.3390/bacteria4040051






