Molecular Detection and Antibiogram of Bacteria and Fungi in Table Eggs Under Different Storage Durations with Organoleptic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Egg Samples
2.2. Processing of Egg Samples
2.2.1. External Surface
2.2.2. Internal Contents (Albumin, Yolk Membrane, Interior Content of Yolk)
2.3. Organoleptic Evaluation
2.4. Total Viable Count (TVC)
2.5. Isolation and Identification of E. coli, Salmonella, Staphylococcus, and Proteus
2.6. Isolation and Identification of Fungus (Yeast and Mold)
2.7. Molecular Identification of E. coli, Salmonella spp., Staphylococcus spp., and Fungus (Yeast and Mold) by PCR
2.8. Antibiotic Sensitivity Testing
3. Results
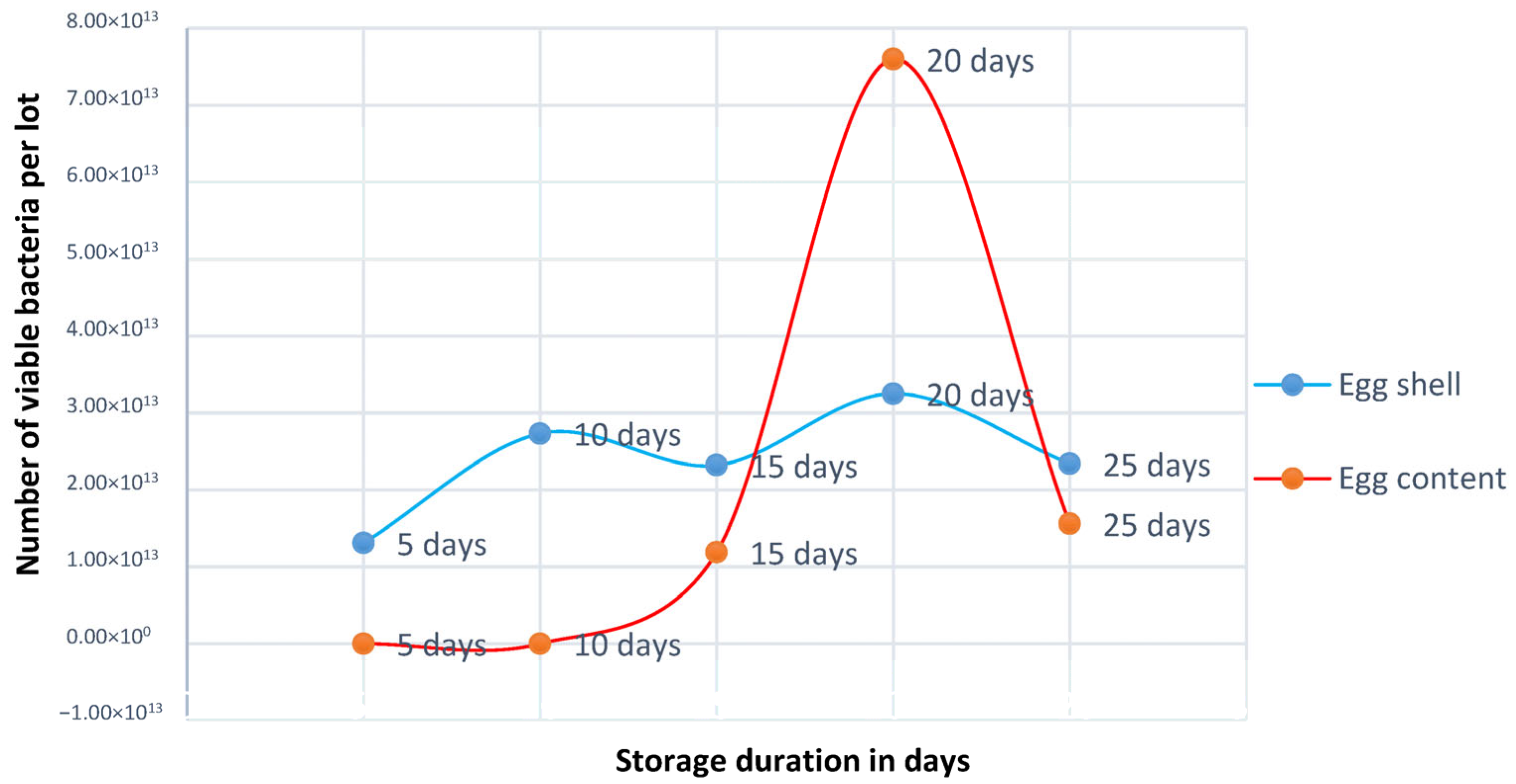
3.1. Summary of Total Viable Count and Prevalence of Bacteria After 5 Days, 10 Days, 15 Days, 20 Days, and 25 Days (Eggshell and Content)
3.2. Molecular Detection and Prevalence of Bacteria and Fungus
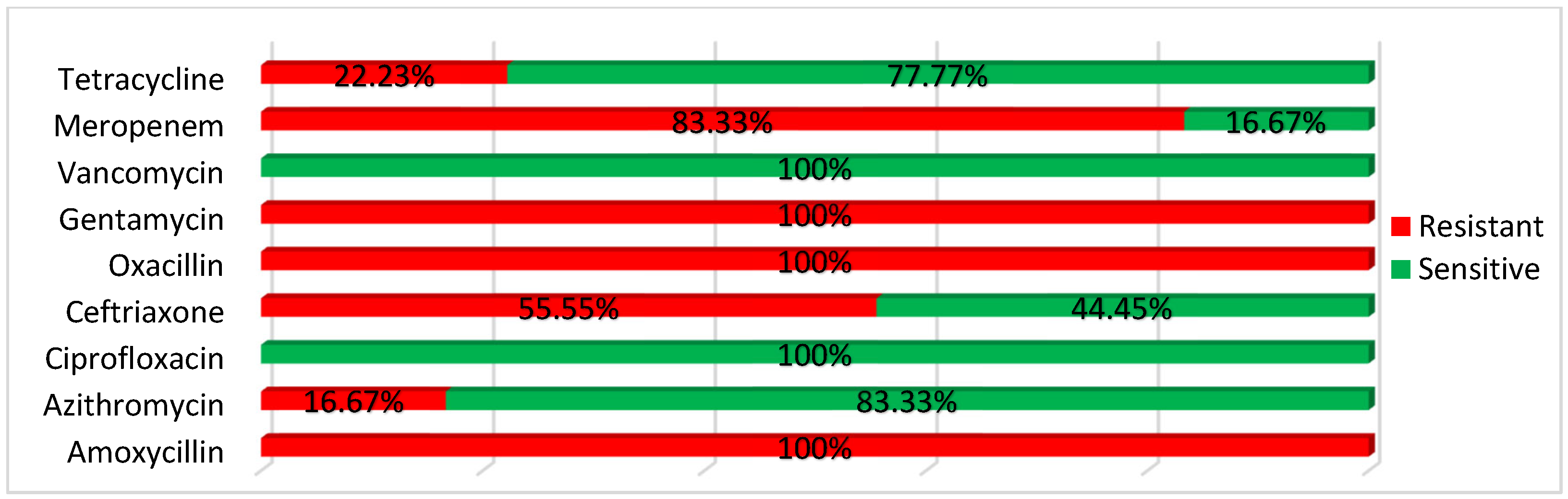
3.3. Results of Antibiotic Sensitivity Tests
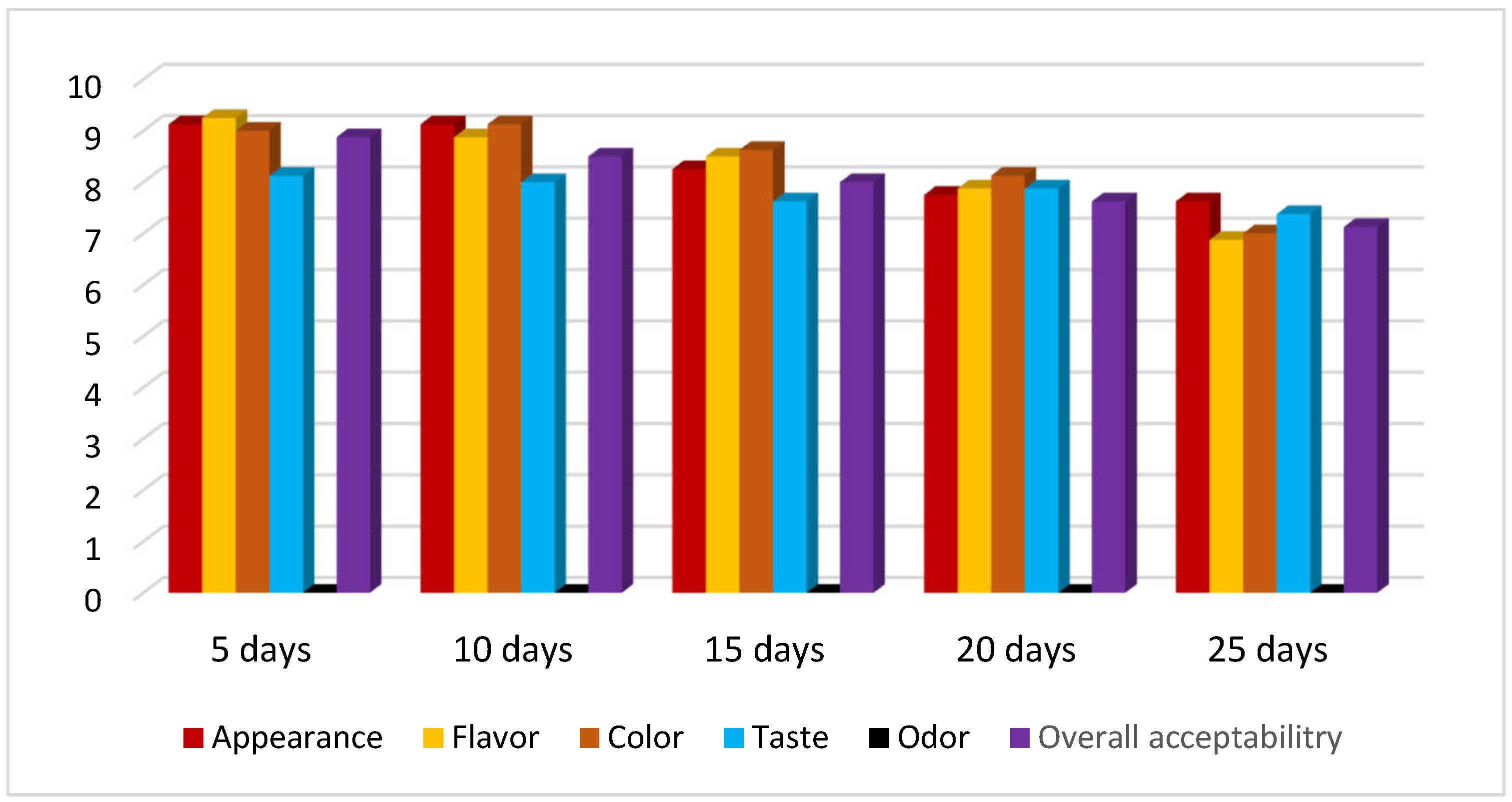
3.4. Result of Organoleptic Evaluation or Sensory Characters of Different Egg Samples Depending on Storage Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, A.H.; Nazir, K.H.M.N.H.; El Zowalaty, M.E.; Kabir, A.; Sarker, S.A.; Siddique, M.P.; Ashour, H.M. Molecular detection of vancomycin and methicillin resistance in Staphylococcus aureus isolated from food processing environments. One Health 2021, 13, 100276. [Google Scholar] [CrossRef]
- WHO. Food Safety and Foodborne Illness; World Health Organization: Geneva, Switzerland, 2007; Available online: https://foodhygiene2010.wordpress.com/wp-content/uploads/2010/06/who-food_safety_fact-sheet.pdf (accessed on 1 January 2025).
- Meghla, N.S.; Mridha, D.; Rana, M.S.; Shahid, M.A.H.; Mahmud, M.M. Isolation, identification and antibiogram of verotoxin producing Escherichia coli from raw salad vegetables at Jashore, Bangladesh. Afr. J. Microbiol. Res. 2021, 15, 401–407. [Google Scholar] [CrossRef]
- Kakon, M.T.A.; Shahid, M.A.H.; Dutta, A.; Pinky, S.A.; Islam, M.S.; Hasnath, M.R. Comparative fertility and hatchability of broiler grandparent stocks in Bangladesh, Asian-Australas. J. Food Saf. Secur. 2024, 8, 48–57. [Google Scholar] [CrossRef]
- Howard, Z.R.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Salmonella Enteritidis in shell eggs: Current issues and prospects for control. Food Res. Int. 2012, 45, 755–764. [Google Scholar] [CrossRef]
- Gole, V.C.; Chousalkar, K.K.; Roberts, J.R.; Sexton, M.; May, D.; Tan, J.; Kiermeier, A. Effect of Egg Washing and Correlation between Eggshell Characteristics and Egg Penetration by Various Salmonella Typhimurium Strains. PLoS ONE 2014, 9, e90987. [Google Scholar] [CrossRef] [PubMed]
- Adesiyun, A.; Offiah, N.; Seepersadsingh, N.; Rodrigo, S.; Lashley, V.; Musai, L.; Georges, K. Microbial health risk posed by table eggs in Trinidad. Epidemiol. Infect. 2005, 133, 1049. [Google Scholar] [CrossRef]
- Gast, R.K.; Guard, J.; Guraya, R.; Locatelli, A. Multiplication in Egg Yolk and Survival in Egg Albumen of Genetically and Phenotypically Characterized Salmonella Enteritidis Strains. J. Food Prot. 2018, 81, 876–880. [Google Scholar] [CrossRef]
- Cumeras, R.; Aksenov, A.A.; Pasamontes, A.; Fung, A.G.; Cianchetta, A.N.; Doan, H.; Davis, R.M.; Davis, C.E. Identification of fungal metabolites from inside Gallus gallus domesticus eggshells by non-invasively detecting volatile organic compounds (VOCs). Anal. Bioanal. Chem. 2016, 408, 6649–6658. [Google Scholar] [CrossRef]
- Kobir, M.A.; Akther, L.; Hasan, I.; Shahid, M.A.H.; Haque, Z.; Karim, M.R. Effects of Imidacloprid-Contaminated Feed Exposure on Hematological Parameters in Adult Rabbits (Oryctolagus Cuniculus). Res. Agric. Livest. Fish. 2020, 7, 439–444. [Google Scholar] [CrossRef]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial Drug Resistance in Escherichia coli from Humans and Food Animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef]
- Boonyasiri, A.; Tangkoskul, T.; Seenama, C.; Saiyarin, J.; Tiengrim, S.; Thamlikitkul, V. Prevalence of antibiotic resistant bacteria in healthy adults, foods, food animals, and the environment in selected areas in Thailand. Pathog. Glob. Health 2014, 108, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ripon, J.; Shahid, M.; Mahmud, M.; Das, S.; Rahman, M.; Nazir, K. Isolation and molecular characterization of Shiga-toxin producing Escherichia coli from betel leaf (Piper betel L.). Vet. Res. Notes 2021, 1, 12–16. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Nys, Y.; Bain, M. Improving the Safety and Quality of Eggs and Egg Products: Egg Safety and Nutritional Quality; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jones, D.R.; Musgrove, M.T.; Northcutt, J.K. Variations in External and Internal Microbial Populations in Shell Eggs during Extended Storage. J. Food Prot. 2004, 67, 2657–2660. [Google Scholar] [CrossRef]
- Fluck, A.C.; Cardinal, K.M.; Costa, O.A.D.; De Borba, L.P.; Da Silva Pires, P.G. Yolk and eggshell colour: Are these the parameters that influence egg purchasing? A systematic review. Worlds Poult. Sci. J. 2023, 79, 551–562. [Google Scholar] [CrossRef]
- Amerine, M.A.; Pangborn, R.M.; Roessler, E.B. Principles of Sensory Evaluation of Food; Elsevier: Amsterdam, The Netherlands, 1965. [Google Scholar] [CrossRef]
- Afrin, S.; Hossain, M.; Khan, M.; Hossain, M. Microbial assessment of beef in selected areas of Mymensingh district in Bangladesh. Bangladesh J. Anim. Sci. 2018, 46, 244–248. [Google Scholar] [CrossRef][Green Version]
- Khanam, S.; Shahid, M.; Mahmud, M.; Hossain, M.; Khatun, M.; Nazir, K. Isolation and molecular identification of Candida spp. and Saccharomyces spp. from bakery products and their impact on public health. Vet. Res. Notes 2021, 1, 1–5. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahid, M.A.H.; Nazir, K.H.M.N.H. Molecular Characterization of Bacillus anthracis from Selected Districts of Bangladesh. Acta Microbiol. Hell. 2025, 70, 17. [Google Scholar] [CrossRef]
- Khan, M.; Pavel, M.; Keya, A.; Shahid, M.; Ferdausi, T.; Siddique, M.; Hossain, M.; Nazir, K.; Rahman, M.; Rahman, M. Comparative molecular analysis of contemporary isolates of duck plague virus from haor areas of Bangladesh. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 44–52. [Google Scholar] [CrossRef]
- Paião, F.G.; Arisitides, L.G.A.; Murate, L.S.; Vilas-Bôas, G.T.; Vilas-Boas, L.A.; Shimokomaki, M. Detection of Salmonella spp, Salmonella Enteritidis and Typhimurium in naturally infected broiler chickens by a multiplex PCR-based assay. Braz. J. Microbiol. 2013, 44, 37–41. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing 26th Edition CLSI Supplement M 100s Wayne; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2016. [Google Scholar]
- Gole, V.C.; Chousalkar, K.K.; Roberts, J.R. Survey of Enterobacteriaceae contamination of table eggs collected from layer flocks in Australia. Int. J. Food Microbiol. 2013, 164, 161–165. [Google Scholar] [CrossRef]
- Mamun, M.M.; Parvej, M.S.; Ahamed, S.; Hassan, J.; Nazir, K.H.M.N.H.; Nishikawa, Y.; Rahman, M.T. Prevalence and Characterization of Shigatoxigenic Escherichia Coli in Broiler Birds in Mymensingh. Bangladesh J. Vet. Med. 2016, 14, 5–8. [Google Scholar] [CrossRef]
- Hassan, J.; Parvej, M.S.; Rahman, M.B.; Khan, M.S.R.; Rahman, M.T.; Kamal, T.; Nazir, K.N.H. Prevalence and Characterization of Escherichia coli from Rectal Swab of Apparently Healthy Cattle in Mymensingh, Bangladesh. Microbes Health 2014, 3, 12–14. [Google Scholar] [CrossRef]
- Stepien, P.D. Occurrence of Gram-negative bacteria in hens’ eggs depending on their source and storage conditions. Pol. J. Vet. Sci. 2010, 13, 507–513. [Google Scholar]
- Musgrove, M.T.; Jones, D.R.; Northcutt, J.K.; Cox, N.A.; Harrison, M.A. Identification of Enterobacteriaceae from Washed and Unwashed Commercial Shell Eggs. J. Food Prot. 2004, 67, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Verma, S. A Microbiological Analysis of Egg Shell Bacteria. Vantage J. Themat. Anal. 2023, 4, 34–46. [Google Scholar] [CrossRef]
- Jones, D.R.; Musgrove, M.T. Pathogen Prevalence and Microbial Levels Associated with Restricted Shell Eggs. J. Food Prot. 2007, 70, 2004–2007. [Google Scholar] [CrossRef]
- Higenyi, J.; Kabasa, J.D. Microbial contamination Load of Hatching Eggs in Butaleja, Eastern Uganda. Anim. Vet. Sci. 2014, 2, 22–30. [Google Scholar] [CrossRef]
- Damena, A.; Mikru, A.; Adane, M.; Dobo, B. Microbial Profile and Safety of Chicken Eggs from a Poultry Farm and Small-Scale Vendors in Hawassa, Southern Ethiopia. J. Food Qual. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Cader, S.; Goburdhun, D.; Neetoo, H. Assessment of the Microbial Safety and Quality of Eggs from Small and Large-Scale Hen Breeders. J. Worlds Poult. Res. 2014, 4, 75–81. [Google Scholar]
- Alleoni, A.C.C.; Antunes, A.J. Perfil de textura e umidade espremível de géis do albume de ovos recobertos com soro de leite. Ciência Tecnol. Aliment. 2005, 25, 153–157. [Google Scholar] [CrossRef][Green Version]
- Huang, T.-M.; Lin, T.L.; Wu, C.C. Antimicrobial Susceptibility and Resistance of Chicken Escherichia Coli, Salmonella spp., and Pasteurella Multocida Isolates. Avian Dis. 2009, 53, 89–93. [Google Scholar] [CrossRef]
- Islam, M.K.; Kabir, S.M.L.; Haque, A.K.M.Z.; Sarker, Y.A.; Sikder, M.H. Molecular detection and characterization of Escherichia coli, Salmonella spp. and Campylobacter spp. isolated from broiler meat in Jamalpur, Tangail, Netrokona and Kishoreganj districts of Bangladesh. Afr. J. Microbiol. Res. 2018, 12, 761–770. [Google Scholar] [CrossRef]
- Wouafo, M.; Nzouankeu, A.; Kinfack, J.A.; Fonkoua, M.-C.; Ejenguele, G.; Njine, T.; Ngandjio, A. Prevalence and Antimicrobial Resistance of Salmonella Serotypes in Chickens from Retail Markets in Yaounde (Cameroon). Microb. Drug Resist. 2010, 16, 171–176. [Google Scholar] [CrossRef]
- De Jong, A.; Thomas, V.; Simjee, S.; Godinho, K.; Schiessl, B.; Klein, U.; Butty, P.; Valle, M.; Marion, H.; Shryock, T.R. Pan-European monitoring of susceptibility to human-use antimicrobial agents in enteric bacteria isolated from healthy food-producing animals. J. Antimicrob. Chemother. 2012, 67, 638–651. [Google Scholar] [CrossRef]
- Hyeon, J.-Y.; Chon, J.-W.; Hwang, I.-G.; Kwak, H.-S.; Kim, M.-S.; Kim, S.-K.; Choi, I.-S.; Song, C.-S.; Park, C.; Seo, K.-H. Prevalence, Antibiotic Resistance, and Molecular Characterizatio of Salmonella Serovars in Retail Meat Products. J. Food Prot. 2011, 74, 161–166. [Google Scholar] [CrossRef]
- Pyzik, E.; Marek, A. Plasmid profile analysis and evaluation of antibiotic susceptibility of Staphylococcus aureus strains isolated from table chicken eggs. Pol. J. Vet. Sci. 2013, 16, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, N.E.; Erginkaya, Z.; Ünal, E. Antibiotic resistance of enterococci, coagulase negative staphylococci and Staphylococcus aureus isolated from chicken meat. Czech J. Food Sci. 2013, 31, 14–19. [Google Scholar] [CrossRef]
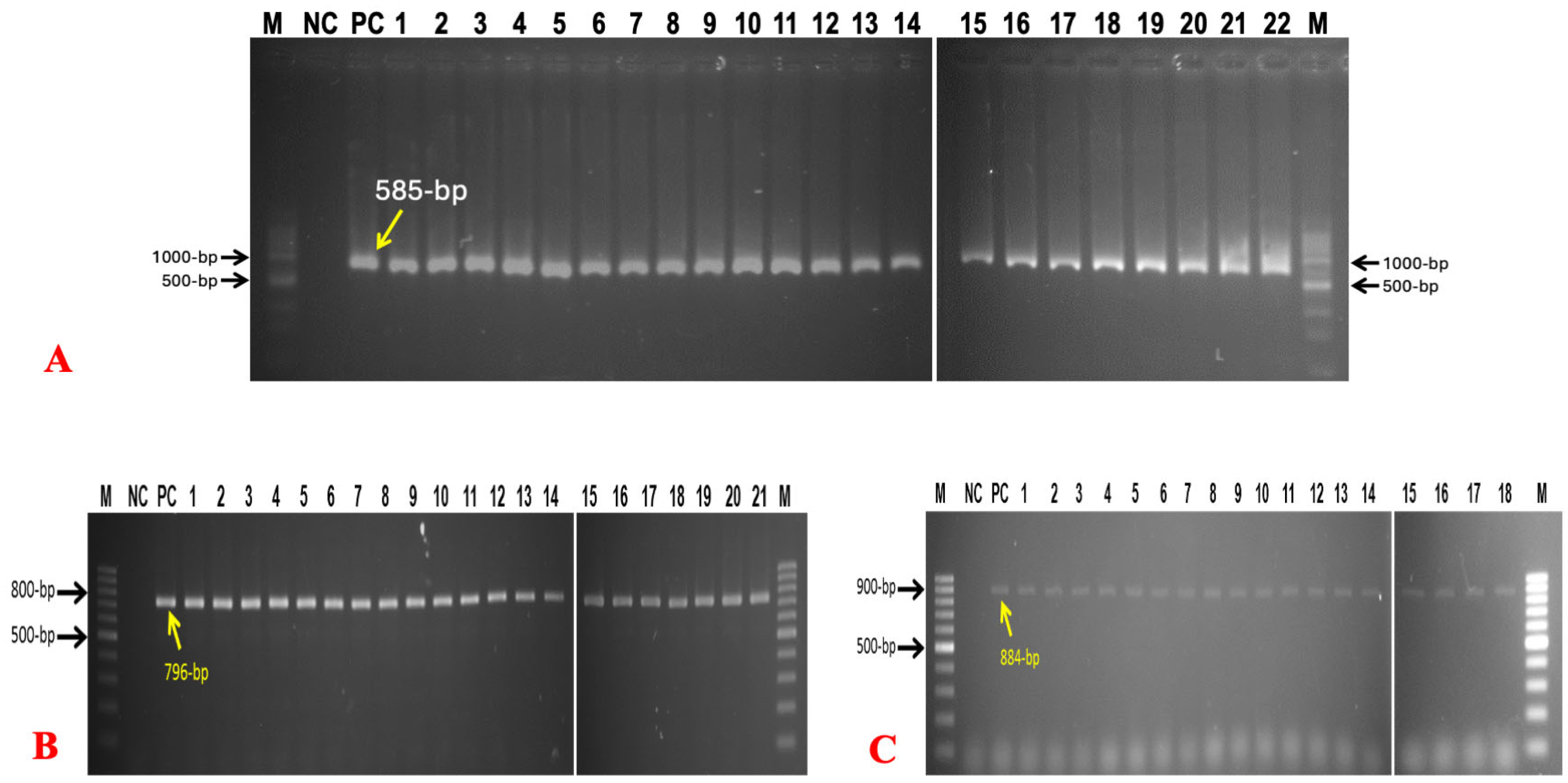



| Primer Name | Ologonucleotide Sequence (5′–3′) | Targeted Gene | Amplicon Size (bp) | References |
|---|---|---|---|---|
| 16SrRNA F | GACCTCGGTTTAGTTCACAGA | 16SrRNA | 585 | [14] |
| 16SrRNA R | CACACGCTGACGCTGACCA | |||
| Inv-A F | CGG TGG TTT TAA GCG TAC TCT T | inv-A | 796 | [23] |
| Inv-A R | CGA ATA TGC TCC ACA AGG TTA | |||
| Tseq271 | AAY ATG ATI ACI GGI GCI GCI CAR ATG GA | Tuf gene | 884 | [1] |
| Tseq1138 | CCI ACI GTI CKI CCR CCY TCR CG | |||
| ITS 1 | TCC GTA GGT GAA CCT GCG G | Simplicillium spp. | ~606 | |
| ITS 4 | TCC TCC GCT TAT TGA TAT GC | |||
| ITS 1 | TCC GTA GGT GAA CCT GCG G | Saccharomyces spp. | ~550 | [20] |
| ITS 4 | TCC TCC GCT TAT TGA TAT GC |
| Days | E. coli | Salmonella spp. | Staphylococcus spp. | Proteus spp. | Fungus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eggshell | Egg Content | Eggshell | Egg Content | Eggshell | Egg Content | Eggshell | Egg Content | Eggshell | Egg Content | |
| 5 days (4 samples) | 0 | 0 | 0 | 0 | 25% | 0 | 0 | 0 | 100% | 0 |
| 10 days (4 samples) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% | 0 |
| 15 days (8 samples) | 100% | 0 | 50% | 50% | 50% | 50% | 3.13% | 0 | 100% | 0 |
| 20 days (8 samples) | 62.5% | 12.5% | 12.5% | 50% | 50% | 12.5% | 0 | 0 | 100% | 0 |
| 25 days (8 samples) | 50% | 50% | 100% | 0 | 50% | 0 | 0 | 0 | 100% | 0 |
| Parameter | Presence of E. coli | Presence of Salmonella spp. | Presence of Staphylococcus spp. | Presence of Proteus spp. | Presence of Fungus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eggshell (32) | Egg Content (32) | Eggshell (32) | Egg Content (32) | Eggshell (32) | Egg Content (32) | Eggshell (32) | Egg Content (32) | Eggshell (32) | Egg Content (32) | |
| 17 | 5 | 13 | 8 | 13 | 5 | 1 | 0 | 32 | 0 | |
| Prevalence | 53.13% | 15.63% | 40.63% | 25% | 40.63% | 15.63% | 3.13% | 0 | 100% | 0 |
| p value | 0.0016 * | 0.183 | 0.026 * | 0.313 | 0.000 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.S.; Shahid, M.A.H.; Saiduzzaman; Rahman, M.; Nazir, K.H.M.N.H. Molecular Detection and Antibiogram of Bacteria and Fungi in Table Eggs Under Different Storage Durations with Organoleptic Properties. Bacteria 2025, 4, 40. https://doi.org/10.3390/bacteria4030040
Uddin MS, Shahid MAH, Saiduzzaman, Rahman M, Nazir KHMNH. Molecular Detection and Antibiogram of Bacteria and Fungi in Table Eggs Under Different Storage Durations with Organoleptic Properties. Bacteria. 2025; 4(3):40. https://doi.org/10.3390/bacteria4030040
Chicago/Turabian StyleUddin, Md Shahab, Md Ahosanul Haque Shahid, Saiduzzaman, Marzia Rahman, and K. H. M. Nazmul Hussain Nazir. 2025. "Molecular Detection and Antibiogram of Bacteria and Fungi in Table Eggs Under Different Storage Durations with Organoleptic Properties" Bacteria 4, no. 3: 40. https://doi.org/10.3390/bacteria4030040
APA StyleUddin, M. S., Shahid, M. A. H., Saiduzzaman, Rahman, M., & Nazir, K. H. M. N. H. (2025). Molecular Detection and Antibiogram of Bacteria and Fungi in Table Eggs Under Different Storage Durations with Organoleptic Properties. Bacteria, 4(3), 40. https://doi.org/10.3390/bacteria4030040









