Three-Dimensional Culture Systems in Neuroblastoma Research
Abstract
1. Introduction
2. Neuroblastoma 2D and In Vivo Models
2.1. Neuroblastoma
2.2. Neuroblastoma Cell Lines
2.3. Human Neuroblastoma Grown in Mice
2.4. Murine Neuroblastoma Grown in Mice
3. Neuroblastoma In Vitro 3D Models
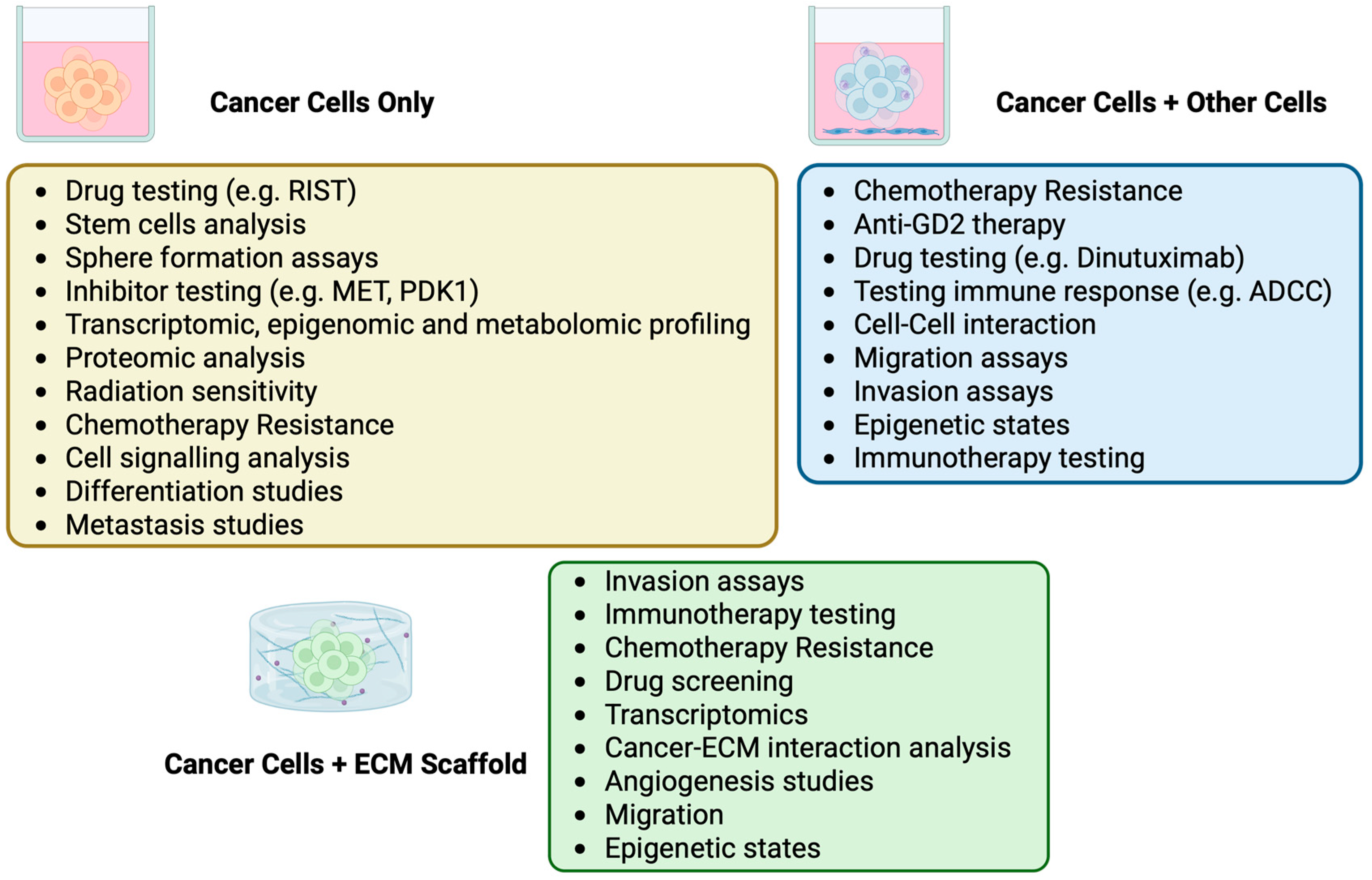
3.1. Human Neuroblastoma Cells Grown Alone in 3D
3.2. Human Neuroblastoma Cells Grown with Other Cell Types in 3D
3.3. Murine Neuroblastoma Cells Grown in 3D
3.4. Neuroblastoma Cells Grown with an Added Matrix

4. Limitations
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| CDX | Cell line-Derived Xenograft |
| CSTN | Childhood Solid Tumor Network |
| DBH | Dopamine β-Hydroxylase |
| ECM | Extracellular Matrix |
| gelMA | Gelatin Methacryloyl |
| GEMM | Genetically Engineered Mouse Models |
| HDAC | Histone Deacetylase |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| KSP | Kinesin Spindle Protein |
| NK | Natural Killer |
| NSG | NOD-Scid-Gamma |
| PAN | Polyacrylonitrile |
| PBMC | Peripheral Blood Mononuclear Cells |
| PDX | Patient-Derived Xenografts |
| PEG | Polyethylene Glycol |
| PIVOT | Pediatric Preclinical In Vivo Testing Consortium |
| PPTC | Pediatric Preclinical Testing Consortium |
| RIST | Rapamycin Irinotecan Sunitinib/Dasatinib Temozolomide |
| TH | Tyrosine Hydroxylase |
References
- Harrison, R.G. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 1910, 9, 787–846. [Google Scholar] [CrossRef]
- Keshishian, H. Ross Harrison’s ‘The outgrowth of the nerve fiber as a mode of protoplasmic movement’. J. Exp. Zool. A Comp. Exp. Biol. 2004, 301A, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Gey, G.O.; Coffman, W.D.; Kubicek, M.T. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952, 12, 264–265. [Google Scholar]
- Skloot, R. The Immortal Life of Henrietta Lacks; Crown Publishers: New York, NY, USA, 2010. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures-a comparison of different types of cancer cell cultures. Arch. Med. Sci. Off. AMS 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Voskoglou-Nomikos, T.; Pater, J.L.; Seymour, L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 2003, 9, 4227–4239. [Google Scholar]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Yamada, K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 2002, 14, 633–640. [Google Scholar] [CrossRef]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef]
- Balamurugan, K.; Poria, D.K.; Sehareen, S.W.; Krishnamurthy, S.; Tang, W.; McKennett, L.; Padmanaban, V.; Czarra, K.; Ewald, A.J.; Ueno, N.T.; et al. Stabilization of E-cadherin adhesions by COX-2/GSK3β signaling is a targetable pathway in metastatic breast cancer. JCI Insight 2023, 8, e156057. [Google Scholar] [CrossRef]
- Marconi, G.D.; Porcheri, C.; Trubiani, O.; Mitsiadis, T.A. Three-Dimensional Culture Systems for Dissecting Notch Signalling in Health and Disease. Int. J. Mol. Sci. 2021, 22, 12473. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chen, C.; Qin, J.; Liu, J.; Zheng, C. Genetic profiling reveals an alarming rate of cross-contamination among human cell lines used in China. FASEB J. 2015, 29, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.G.; Dirks, W.G.; Matsuo, Y.; MacLeod, R.A.F. False leukemia–lymphoma cell lines: An update on over 500 cell lines. Leukemia 2003, 17, 416–426. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A brief history of animal modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar] [PubMed]
- Rous, P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J. Exp. Med. 1911, 13, 397–411. [Google Scholar] [CrossRef]
- Yamagiwa, K.; Ichikawa, K. Experimental Study of the Pathogenesis of Carcinoma. CA Cancer J. Clin. 1977, 27, 174–181. [Google Scholar] [CrossRef]
- Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [CrossRef]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)evolution: Unveiling Tumor–Stroma Interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef]
- Corallo, D.; Frabetti, S.; Candini, O.; Gregianin, E.; Dominici, M.; Fischer, H.; Aveic, S. Emerging Neuroblastoma 3D In Vitro Models for Pre-Clinical Assessments. Front. Immunol. 2020, 11, 584214. [Google Scholar] [CrossRef]
- Nanki, Y.; Chiyoda, T.; Hirasawa, A.; Ookubo, A.; Itoh, M.; Ueno, M.; Akahane, T.; Kameyama, K.; Yamagami, W.; Kataoka, F.; et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci. Rep. 2020, 10, 12581. [Google Scholar] [CrossRef]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; De Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid Cultures Derived from Patients with Advanced Prostate Cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Crespo, M.; Vilar, E.; Tsai, S.-Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef]
- Hwang, H.J.; Oh, M.-S.; Lee, D.W.; Kuh, H.-J. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J. Exp. Clin. Cancer Res. 2019, 38, 258. [Google Scholar] [CrossRef]
- Balážová, K.; Clevers, H.; Dost, A.F. The role of macrophages in non-small cell lung cancer and advancements in 3D co-cultures. eLife 2023, 12, e82998. [Google Scholar] [CrossRef]
- Ravi, M.; Ramesh, A.; Pattabhi, A. Contributions of 3D Cell Cultures for Cancer Research. J. Cell Physiol. 2017, 232, 2679–2697. [Google Scholar] [CrossRef]
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative analysis between 2D and 3D colorectal cancer culture models for insights into cellular morphological and transcriptomic variations. Sci. Rep. 2023, 13, 18380. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, E.; Shang, Z. 3D Co-culture of Cancer-Associated Fibroblast with Oral Cancer Organoids. J. Dent. Res. 2021, 100, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Al Hrout, A.; Cervantes-Gracia, K.; Chahwan, R.; Amin, A. Modelling liver cancer microenvironment using a novel 3D culture system. Sci. Rep. 2022, 12, 8003. [Google Scholar] [CrossRef] [PubMed]
- Subtil, B.; Iyer, K.K.; Poel, D.; Bakkerus, L.; Gorris, M.A.J.; Escalona, J.C.; Dries, K.V.D.; Cambi, A.; Verheul, H.M.W.; De Vries, I.J.M.; et al. Dendritic cell phenotype and function in a 3D co-culture model of patient-derived metastatic colorectal cancer organoids. Front. Immunol. 2023, 14, 1105244. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Lee, H.-K.; Noh, M.H.; Hong, S.-W.; Kim, S.-M.; Kim, S.H.; Kim, Y.S.; Broaddus, V.C.; Hur, D.Y. Erlotinib Activates Different Cell Death Pathways in EGFR-mutant Lung Cancer Cells Grown in 3D Versus 2D Culture Systems. Anticancer Res. 2021, 41, 1261–1269. [Google Scholar] [CrossRef]
- Berry, M.A.; Bland, A.R.; Major, G.S.; Ashton, J.C. Development of an ALK-positive Non-Small-Cell Lung Cancer in Vitro Tumor 3D Culture Model for Therapeutic Screening. J. Histochem. Cytochem. 2025, 73, 63–79. [Google Scholar] [CrossRef]
- Świerczewska, M.; Sterzyńska, K.; Ruciński, M.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. The response and resistance to drugs in ovarian cancer cell lines in 2D monolayers and 3D spheroids. Biomed. Pharmacother. 2023, 165, 115152. [Google Scholar] [CrossRef]
- Tadić, V.; Zhang, W.; Brozovic, A. The high-grade serous ovarian cancer metastasis and chemoresistance in 3D models. Biochim. Biophys. Acta BBA-Rev. Cancer 2024, 1879, 189052. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Whittle, J.R.; Vaillant, F.; Chen, H.-R.; Dawson, C.; Liu, K.; Geurts, M.H.; Herold, M.J.; Clevers, H.; Lindeman, G.J.; et al. Modeling Breast Cancer Using CRISPR-Cas9-Mediated Engineering of Human Breast Organoids. J. Natl. Cancer Inst. 2020, 112, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, A.; Juan, J.; Jacob, M.; Stehn, C.; Gardner, E.E.; Scherzer, M.T.; Schuman, S.; Van Veen, J.E.; Murphy, B.; Hackett, C.S.; et al. Transposon Mutagenesis Reveals RBMS3 Silencing as a Promoter of Malignant Progression of BRAFV600E-Driven Lung Tumorigenesis. Cancer Res. 2022, 82, 4261–4273. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.-H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Zhang, C.-Y.; Peng, K.-C.; Chen, Z.-X.; Su, J.-W.; Li, Y.-F.; Li, W.-F.; Gao, Q.-Y.; Zhang, S.-L.; Chen, Y.-Q.; et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: A real-world study. Cell Rep. Med. 2023, 4, 100911. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26.e6. [Google Scholar] [CrossRef]
- Herpers, B.; Eppink, B.; James, M.I.; Cortina, C.; Cañellas-Socias, A.; Boj, S.F.; Hernando-Momblona, X.; Glodzik, D.; Roovers, R.C.; van de Wetering, M.; et al. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat. Cancer 2022, 3, 418–436. [Google Scholar] [CrossRef]
- Fayette, J.; Clatot, F.; Brana, I.; Saada, E.; Van Herpen, C.M.L.-; Mazard, T.; Perez, C.A.; Tabernero, J.; Le Tourneau, C.; Hollebecque, A.; et al. Petosemtamab (MCLA-158) with pembrolizumab as first-line (1L) treatment of recurrent/metastatic (r/m) head and neck squamous cell carcinoma (HNSCC): Phase 2 study. J. Clin. Oncol. 2024, 42 (Suppl. 16), 6014. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primer 2016, 2, 16078. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Janoueix-Lerosey, I.; Delattre, O. Recent insights into the biology of neuroblastoma. Int. J. Cancer 2014, 135, 2249–2261. [Google Scholar] [CrossRef]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, D.A.; London, W.B.; Stephens, D.; Volchenboum, S.L.; Simon, T.; Nakagawara, A.; Shimada, H.; Schleiermacher, G.; Matthay, K.K.; Cohn, S.L.; et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur. J. Cancer 2016, 65, 1–10. [Google Scholar] [CrossRef]
- D’Angio, G.; Evans, A.; Koop, C.E. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet 1971, 297, 1046–1049. [Google Scholar] [CrossRef]
- Brodeur, G.M. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018, 372, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.L.; Ibragimova, S.; Ramachandran, R.; Philpott, A.; Ali, F.R. Tumoral heterogeneity in neuroblastoma. Biochim. Biophys. Acta BBA-Rev. Cancer 2022, 1877, 188805. [Google Scholar] [CrossRef]
- Murray, M.R.; Stout, A.P. Distinctive Characteristics of the Sympathicoblastoma Cultivated in Vitro: A Method for Prompt Diagnosis. Am. J. Pathol. 1947, 23, 429–441. [Google Scholar]
- Carol, J. Thiele Neuroblastoma Cell Lines. In Cell & Molecular Biology Section, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health; Springer: Berlin/Heidelberg, Germany, 1998; Volume 1, pp. 21–53. [Google Scholar]
- Cole, K.A.; Huggins, J.; Laquaglia, M.; Hulderman, C.E.; Russell, M.R.; Bosse, K.; Diskin, S.J.; Attiyeh, E.F.; Sennett, R.; Norris, G.; et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3336–3341. [Google Scholar] [CrossRef]
- Chen, L.; Alexe, G.; Dharia, N.V.; Ross, L.; Iniguez, A.B.; Conway, A.S.; Wang, E.J.; Veschi, V.; Lam, N.; Qi, J.; et al. CRISPR-Cas9 screen reveals a MYCN-amplified neuroblastoma dependency on EZH2. J. Clin. Investig. 2017, 128, 446–462. [Google Scholar] [CrossRef]
- Hahn, C.K.; Ross, K.N.; Warrington, I.M.; Mazitschek, R.; Kanegai, C.M.; Wright, R.D.; Kung, A.L.; Golub, T.R.; Stegmaier, K. Expression-based screening identifies the combination of histone deacetylase inhibitors and retinoids for neuroblastoma differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 9751–9756. [Google Scholar] [CrossRef]
- Cheung, B.B.; Kleynhans, A.; Mittra, R.; Kim, P.Y.; Holien, J.K.; Nagy, Z.; Ciampa, O.C.; Seneviratne, J.A.; Mayoh, C.; Raipuria, M.; et al. A novel combination therapy targeting ubiquitin-specific protease 5 in MYCN-driven neuroblastoma. Oncogene 2021, 40, 2367–2381. [Google Scholar] [CrossRef]
- Chakrabarti, L. Reversible adaptive plasticity: A mechanism for neuroblastoma cell heterogeneity and chemo-resistance. Front. Oncol. 2012, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Spengler, B.A.; Biedler, J.L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J. Natl. Cancer Inst. 1983, 71, 741–747. [Google Scholar]
- Corey, J.M.; Gertz, C.C.; Sutton, T.J.; Chen, Q.; Mycek, K.B.; Wang, B.; Martin, A.A.; Johnson, S.L.; Feldman, E.L. Patterning N-type and S-type neuroblastoma cells with Pluronic F108 and ECM proteins. J. Biomed. Mater. Res. A 2010, 93A, 673–686. [Google Scholar] [CrossRef]
- Ross, R.A.; Biedler, J.L.; Spengler, B.A. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003, 197, 35–39. [Google Scholar] [CrossRef]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef]
- Van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Durbin, A.D.; Zimmerman, M.W.; Dharia, N.V.; Abraham, B.J.; Iniguez, A.B.; Weichert-Leahey, N.; He, S.; Krill-Burger, J.M.; Root, D.E.; Vazquez, F.; et al. Selective gene dependencies in MYCN-amplified neuroblastoma include the core transcriptional regulatory circuitry. Nat. Genet. 2018, 50, 1240–1246. [Google Scholar] [CrossRef]
- Kendsersky, N.M.; Odrobina, M.; Mabe, N.W.; Farrel, A.; Grossmann, L.; Tsang, M.; Groff, D.; Wolpaw, A.J.; Narch, A.; Zammarchi, F.; et al. Lineage dependence of the neuroblastoma surfaceome defines tumor cell state-dependent and -independent immunotherapeutic targets. Neuro-Oncol. 2025, noaf012. [Google Scholar] [CrossRef]
- Wolpaw, A.J.; Grossmann, L.D.; Dessau, J.L.; Dong, M.M.; Aaron, B.J.; Brafford, P.A.; Volgina, D.; Pascual-Pasto, G.; Rodriguez-Garcia, A.; Uzun, Y.; et al. Epigenetic state determines inflammatory sensing in neuroblastoma. Proc. Natl. Acad. Sci. USA 2022, 119, e2102358119. [Google Scholar] [CrossRef]
- Sengupta, S.; Das, S.; Crespo, A.C.; Cornel, A.M.; Patel, A.G.; Mahadevan, N.R.; Campisi, M.; Ali, A.K.; Sharma, B.; Rowe, J.H.; et al. Mesenchymal and adrenergic cell lineage states in neuroblastoma possess distinct immunogenic phenotypes. Nat. Cancer 2022, 3, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.G.; Ashenberg, O.; Collins, N.B.; Segerstolpe, Å.; Jiang, S.; Slyper, M.; Huang, X.; Caraccio, C.; Jin, H.; Sheppard, H.; et al. A spatial cell atlas of neuroblastoma reveals developmental, epigenetic and spatial axis of tumor heterogeneity. BioRxiv 2024. [Google Scholar] [CrossRef]
- Gartlgruber, M.; Sharma, A.K.; Quintero, A.; Dreidax, D.; Jansky, S.; Park, Y.-G.; Kreth, S.; Meder, J.; Doncevic, D.; Saary, P.; et al. Super enhancers define regulatory subtypes and cell identity in neuroblastoma. Nat. Cancer 2020, 2, 114–128. [Google Scholar] [CrossRef]
- Nolan, J.C.; Frawley, T.; Tighe, J.; Soh, H.; Curtin, C.; Piskareva, O. Preclinical models for neuroblastoma: Advances and challenges. Cancer Lett. 2020, 474, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Houghton, P.J.; Lock, R.B.; Maris, J.M.; Gorlick, R.; Kurmasheva, R.T.; Li, X.-N.; Teicher, B.A.; Chuang, J.H.; Dela Cruz, F.S.; et al. Lessons learned from 20 years of preclinical testing in pediatric cancers. Pharmacol. Ther. 2024, 264, 108742. [Google Scholar] [CrossRef]
- Teitz, T.; Stanke, J.J.; Federico, S.; Bradley, C.L.; Brennan, R.; Zhang, J.; Johnson, M.D.; Sedlacik, J.; Inoue, M.; Zhang, Z.M.; et al. Preclinical Models for Neuroblastoma: Establishing a Baseline for Treatment. PLoS ONE 2011, 6, e19133. [Google Scholar] [CrossRef]
- Stewart, E.; Shelat, A.; Bradley, C.; Chen, X.; Federico, S.; Thiagarajan, S.; Shirinifard, A.; Bahrami, A.; Pappo, A.; Qu, C.; et al. Development and characterization of a human orthotopic neuroblastoma xenograft. Dev. Biol. 2015, 407, 344–355. [Google Scholar] [CrossRef]
- Khanna, C.; Jaboin, J.J.; Drakos, E.; Tsokos, M.; Thiele, C.J. Biologically relevant orthotopic neuroblastoma xenograft models: Primary adrenal tumor growth and spontaneous distant metastasis. In Vivo Athens Greece 2002, 16, 77–85. [Google Scholar]
- Patterson, D.M.; Shohet, J.M.; Kim, E.S. Preclinical Models of Pediatric Solid Tumors (Neuroblastoma) and Their Use in Drug Discovery. Curr. Protoc. Pharmacol. 2011, 52, 14.17.1–14.17.18. [Google Scholar] [CrossRef]
- Braekeveldt, N.; Bexell, D. Patient-derived xenografts as preclinical neuroblastoma models. Cell Tissue Res. 2018, 372, 233–243. [Google Scholar] [CrossRef]
- Braekeveldt, N.; Wigerup, C.; Gisselsson, D.; Mohlin, S.; Merselius, M.; Beckman, S.; Jonson, T.; Börjesson, A.; Backman, T.; Tadeo, I.; et al. Neuroblastoma patient-derived orthotopic xenografts retain metastatic patterns and geno- and phenotypes of patient tumours. Int. J. Cancer 2015, 136, E252–E261. [Google Scholar] [CrossRef] [PubMed]
- Rokita, J.L.; Rathi, K.S.; Cardenas, M.F.; Upton, K.A.; Jayaseelan, J.; Cross, K.L.; Pfeil, J.; Egolf, L.E.; Way, G.P.; Farrel, A.; et al. Genomic Profiling of Childhood Tumor Patient-Derived Xenograft Models to Enable Rational Clinical Trial Design. Cell Rep. 2019, 29, 1675–1689.e9. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Federico, S.M.; Chen, X.; Shelat, A.A.; Bradley, C.; Gordon, B.; Karlstrom, A.; Twarog, N.R.; Clay, M.R.; Bahrami, A.; et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 2017, 549, 96–100. [Google Scholar] [CrossRef]
- The Childhood Cancer Repository. Available online: https://www.cccells.org/xenografts.php (accessed on 30 April 2025).
- Scheerlinck, J.-P.Y. Cytokine Species-Specificity and Humanized Mice. In Humanized Mice for HIV Research; Poluektova, L.Y., Garcia, J.V., Koyanagi, Y., Manz, M.G., Tager, A.M., Eds.; Springer: New York, NY, USA, 2014; pp. 93–108. [Google Scholar] [CrossRef]
- Nguyen, R.; Patel, A.G.; Griffiths, L.M.; Dapper, J.; Stewart, E.A.; Houston, J.; Johnson, M.; Akers, W.J.; Furman, W.L.; Dyer, M.A. Next-generation humanized patient-derived xenograft mouse model for pre-clinical antibody studies in neuroblastoma. Cancer Immunol. Immunother. 2021, 70, 721–732. [Google Scholar] [CrossRef]
- Huang, M.; Fang, W.; Farrel, A.; Li, L.; Chronopoulos, A.; Nasholm, N.; Cheng, B.; Zheng, T.; Yoda, H.; Barata, M.J.; et al. ALK upregulates POSTN and WNT signaling to drive neuroblastoma. Cell Rep. 2024, 43, 113927. [Google Scholar] [CrossRef]
- Cohen, M.A.; Zhang, S.; Sengupta, S.; Ma, H.; Bell, G.W.; Horton, B.; Sharma, B.; George, R.E.; Spranger, S.; Jaenisch, R. Formation of Human Neuroblastoma in Mouse-Human Neural Crest Chimeras. Cell Stem Cell 2020, 26, 579–592.e6. [Google Scholar] [CrossRef]
- Guerin, M.V.; Finisguerra, V.; Van Den Eynde, B.J.; Bercovici, N.; Trautmann, A. Preclinical murine tumor models: A structural and functional perspective. eLife 2020, 9, e50740. [Google Scholar] [CrossRef]
- Chesler, L.; Weiss, W.A. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin. Cancer Biol. 2011, 21, 245–255. [Google Scholar] [CrossRef]
- Weiss, W.A. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997, 16, 2985–2995. [Google Scholar] [CrossRef]
- McNerney, K.; Karageorgos, S.; Ferry, G.; Wolpaw, A.; Burudpakdee, C.; Khurana, P.; Toland, C.; Vemu, R.; Vu, A.; Hogarty, M.; et al. TH-MYCN tumors, but not tumor-derived cell lines, are adrenergic lineage, GD2+, and responsive to anti-GD2 antibody therapy. OncoImmunology 2022, 11, 2075204. [Google Scholar] [CrossRef]
- Yogev, O.; Barker, K.; Sikka, A.; Almeida, G.S.; Hallsworth, A.; Smith, L.M.; Jamin, Y.; Ruddle, R.; Koers, A.; Webber, H.T.; et al. p53 Loss in MYC-Driven Neuroblastoma Leads to Metabolic Adaptations Supporting Radioresistance. Cancer Res. 2016, 76, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Chesler, L.; Goldenberg, D.D.; Collins, R.; Grimmer, M.; Kim, G.E.; Tihan, T.; Nguyen, K.; Yakovenko, S.; Matthay, K.K.; Weiss, W.A. Chemotherapy-Induced Apoptosis in a Transgenic Model of Neuroblastoma Proceeds Through p53 Induction. Neoplasia 2008, 10, 1268–1274, IN33–IN34. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALKF1174L Mutation Potentiates the Oncogenic Activity of MYCN in Neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Teitz, T.; Inoue, M.; Valentine, M.B.; Zhu, K.; Rehg, J.E.; Zhao, W.; Finkelstein, D.; Wang, Y.-D.; Johnson, M.D.; Calabrese, C.; et al. Th-MYCN Mice with Caspase-8 Deficiency Develop Advanced Neuroblastoma with Bone Marrow Metastasis. Cancer Res. 2013, 73, 4086–4097. [Google Scholar] [CrossRef]
- Iwakura, H.; Ariyasu, H.; Kanamoto, N.; Hosoda, K.; Nakao, K.; Kangawa, K.; Akamizu, T. Establishment of a novel neuroblastoma mouse model. Int. J. Oncol. 2008, 33, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Hadjidaniel, M.D.; Muthugounder, S.; Hung, L.T.; Sheard, M.A.; Shirinbak, S.; Chan, R.Y.; Nakata, R.; Borriello, L.; Malvar, J.; Kennedy, R.J.; et al. Tumor-associated macrophages promote neuroblastoma via STAT3 phosphorylation and up-regulation of c-MYC. Oncotarget 2017, 8, 91516–91529. [Google Scholar] [CrossRef]
- Althoff, K.; Beckers, A.; Bell, E.; Nortmeyer, M.; Thor, T.; Sprüssel, A.; Lindner, S.; De Preter, K.; Florin, A.; Heukamp, L.C.; et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene 2015, 34, 3357–3368. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Domingo-Fernández, R.; Ebus, M.E.; Lindner, S.; Koster, J.; Drabek, K.; Mestdagh, P.; Van Sluis, P.; Valentijn, L.J.; Van Nes, J.; et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet. 2012, 44, 1199–1206. [Google Scholar] [CrossRef]
- Ornell, K.J.; Coburn, J.M. Developing preclinical models of neuroblastoma: Driving therapeutic testing. BMC Biomed. Eng. 2019, 1, 33. [Google Scholar] [CrossRef]
- Kroesen, M.; Brok, I.C.; Reijnen, D.; Van Hout-Kuijer, M.A.; Zeelenberg, I.S.; Den Brok, M.H.; Hoogerbrugge, P.M.; Adema, G.J. Intra-adrenal murine TH-MYCN neuroblastoma tumors grow more aggressive and exhibit a distinct tumor microenvironment relative to their subcutaneous equivalents. Cancer Immunol. Immunother. 2015, 64, 563–572. [Google Scholar] [CrossRef]
- Yuhas, J.M.; Li, A.P.; Martinez, A.O.; Ladman, A.J. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977, 37, 3639–3643. [Google Scholar] [PubMed]
- Hofmann, S.; Cohen-Harazi, R.; Maizels, Y.; Koman, I. Patient-derived tumor spheroid cultures as a promising tool to assist personalized therapeutic decisions in breast cancer. Transl. Cancer Res. 2022, 11, 134–147. [Google Scholar] [CrossRef]
- Tang, J.; Shi, J.; Liu, J. Editorial: Advances in 3D cell culture for drug screening and toxicology evaluation. Front. Bioeng. Biotechnol. 2023, 11, 1266506. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Little, J.B.; Tomkinson, K.; Evans, S.; Yuhas, J. Repair of fractionated radiation in plateau phase cultures of human tumor cells and human multicellular tumor spheroids. Radiother. Oncol. 1984, 2, 41–47. [Google Scholar] [CrossRef]
- Bate-Eya, L.T.; Ebus, M.E.; Koster, J.; Den Hartog, I.J.M.; Zwijnenburg, D.A.; Schild, L.; Van Der Ploeg, I.; Dolman, M.E.M.; Caron, H.N.; Versteeg, R.; et al. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. Eur. J. Cancer 2014, 50, 628–637. [Google Scholar] [CrossRef]
- Strijker, J.G.M.; Pscheid, R.; Drent, E.; Van Der Hoek, J.J.F.; Koopmans, B.; Ober, K.; Van Hooff, S.R.; Kholosy, W.M.; Cornel, A.M.; Coomans, C.; et al. αβ-T Cells Engineered to Express γδ-T Cell Receptors Can Kill Neuroblastoma Organoids Independent of MHC-I Expression. J. Pers. Med. 2021, 11, 923. [Google Scholar] [CrossRef]
- Cornel, A.M.; Dunnebach, E.; Hofman, D.A.; Das, S.; Sengupta, S.; van den Ham, F.; Wienke, J.; Strijker, J.G.M.; van den Beemt, D.A.M.H.; Essing, A.H.W.; et al. Epigenetic modulation of neuroblastoma enhances T cell and NK cell immunogenicity by inducing a tumor-cell lineage switch. J. Immunother. Cancer 2022, 10, e005002. [Google Scholar] [CrossRef]
- Chan, C.; Stip, M.; Nederend, M.; Jansen, M.; Passchier, E.; Van Den Ham, F.; Wienke, J.; Van Tetering, G.; Leusen, J. Enhancing IgA-mediated neutrophil cytotoxicity against neuroblastoma by CD47 blockade. J. Immunother. Cancer 2024, 12, e008478. [Google Scholar] [CrossRef]
- Sundaramoorthy, S.; Colombo, D.F.; Sanalkumar, R.; Broye, L.; Balmas Bourloud, K.; Boulay, G.; Cironi, L.; Stamenkovic, I.; Renella, R.; Kuttler, F.; et al. Preclinical spheroid models identify BMX as a therapeutic target for metastatic MYCN nonamplified neuroblastoma. JCI Insight 2024, 9, e169647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, H.; Yuan, T.; Zhang, Z.; Kaul, S.C.; Wadhwa, R. Molecular Characterization of Cancer Preventive and Therapeutic Potential of Three Antistress Compounds, Triethylene Glycol, Withanone, and Withaferin A. Int. J. Mol. Sci. 2025, 26, 493. [Google Scholar] [CrossRef] [PubMed]
- Challagundla, K.B.; Pathania, A.S.; Chava, H.; Kantem, N.M.; Dronadula, V.M.; Coulter, D.W.; Clarke, M. FOXJ3, a novel tumor suppressor in neuroblastoma. Mol. Ther. Oncol. 2025, 33, 200914. [Google Scholar] [CrossRef] [PubMed]
- Brignole, C.; Calarco, E.; Bensa, V.; Giusto, E.; Perri, P.; Ciampi, E.; Corrias, M.V.; Astigiano, S.; Cilli, M.; Loo, D.; et al. Antitumor activity of the investigational B7-H3 antibody-drug conjugate, vobramitamab duocarmazine, in preclinical models of neuroblastoma. J. Immunother. Cancer 2023, 11, e007174. [Google Scholar] [CrossRef]
- Lukoseviciute, M.; Need, E.; Holzhauser, S.; Dalianis, T.; Kostopoulou, O.N. Combined targeted therapy with PI3K and CDK4/6, or FGFR inhibitors show synergistic effects in a neuroblastoma spheroid culture model. Biomed. Pharmacother. 2024, 177, 116993. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. Repurposing of c-MET Inhibitor Tivantinib Inhibits Pediatric Neuroblastoma Cellular Growth. Pharmaceuticals 2024, 17, 1350. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Rouse, D.C.; Yu, Y.; Kabir, A.S.; Muth, A.; Yang, J.; Lipton, J.M.; Agarwal, S. BX-795 inhibits neuroblastoma growth and enhances sensitivity towards chemotherapy. Transl. Oncol. 2022, 15, 101272. [Google Scholar] [CrossRef]
- Sumer-Bayraktar, Z.; Fife, C.M.; Byrne, F.L.; Kavallaris, M.; Packer, N.H. Membrane glycome is impacted by the cell culturing mode of neuroblastoma cells with differing migration and invasion potential. Glycobiology 2022, 32, 588–599. [Google Scholar] [CrossRef]
- Redden, R.A.; Doolin, E.J. Microgravity assay of neuroblastoma: In vitro aggregation kinetics and organoid morphology correlate with MYCN expression. Vitr. Cell. Dev. Biol.-Anim. 2011, 47, 312–317. [Google Scholar] [CrossRef]
- Troschke-Meurer, S.; Zumpe, M.; Meißner, L.; Siebert, N.; Grabarczyk, P.; Forkel, H.; Maletzki, C.; Bekeschus, S.; Lode, H.N. Chemotherapeutics Used for High-Risk Neuroblastoma Therapy Improve the Efficacy of Anti-GD2 Antibody Dinutuximab Beta in Preclinical Spheroid Models. Cancers 2023, 15, 904. [Google Scholar] [CrossRef]
- Lundsten, S.; Berglund, H.; Jha, P.; Krona, C.; Hariri, M.; Nelander, S.; Lane, D.P.; Nestor, M. p53-Mediated Radiosensitization of 177Lu-DOTATATE in Neuroblastoma Tumor Spheroids. Biomolecules 2021, 11, 1695. [Google Scholar] [CrossRef]
- Kaess, C.; Matthes, M.; Gross, J.; Waetzig, R.; Heise, T.; Corbacioglu, S.; Sommer, G. Evaluating the RIST Molecular-Targeted Regimen in a Three-Dimensional Neuroblastoma Spheroid Cell Culture Model. Cancers 2023, 15, 1749. [Google Scholar] [CrossRef]
- Zheng, X.; Naiditch, J.; Czurylo, M.; Jie, C.; Lautz, T.; Clark, S.; Jafari, N.; Qiu, Y.; Chu, F.; Madonna, M.B. Differential effect of long-term drug selection with doxorubicin and vorinostat on neuroblastoma cells with cancer stem cell characteristics. Cell Death Dis. 2013, 4, e740. [Google Scholar] [CrossRef]
- Mahller, Y.Y.; Williams, J.P.; Baird, W.H.; Mitton, B.; Grossheim, J.; Saeki, Y.; Cancelas, J.A.; Ratner, N.; Cripe, T.P. Neuroblastoma Cell Lines Contain Pluripotent Tumor Initiating Cells That Are Susceptible to a Targeted Oncolytic Virus. PLoS ONE 2009, 4, e4235. [Google Scholar] [CrossRef] [PubMed]
- Marayati, R.; Williams, A.P.; Bownes, L.V.; Quinn, C.H.; Stewart, J.E.; Mroczek-Musulman, E.; Atigadda, V.R.; Beierle, E.A. Novel retinoic acid derivative induces differentiation and growth arrest in neuroblastoma. J. Pediatr. Surg. 2020, 55, 1072–1080. [Google Scholar] [CrossRef]
- Langenberg, K.P.S.; van Hooff, S.R.; Koopmans, B.; Strijker, J.G.M.; Kholosy, W.M.; Ober, K.; Zwijnenburg, D.A.; van der Hoek, J.J.F.; Keller, K.M.; Vernooij, L.; et al. Exploring high-throughput drug sensitivity testing in neuroblastoma cell lines and patient-derived tumor organoids in the era of precision medicine. Eur. J. Cancer. 2025, 218, 115275. [Google Scholar] [CrossRef]
- Rossi, M.; Blasi, P. Multicellular Tumor Spheroids in Nanomedicine Research: A Perspective. Front. Med. Technol. 2022, 4, 909943. [Google Scholar] [CrossRef]
- Coulon, A.; Flahaut, M.; Mühlethaler-Mottet, A.; Meier, R.; Liberman, J.; Balmas-Bourloud, K.; Nardou, K.; Yan, P.; Tercier, S.; Joseph, J.-M.; et al. Functional Sphere Profiling Reveals the Complexity of Neuroblastoma Tumor-Initiating Cell Model. Neoplasia 2011, 13, 991–1004, IN30. [Google Scholar] [CrossRef] [PubMed]
- Besançon, O.G.; Tytgat, G.A.M.; Meinsma, R.; Leen, R.; Hoebink, J.; Kalayda, G.V.; Jaehde, U.; Caron, H.N.; Van Kuilenburg, A.B.P. Synergistic interaction between cisplatin and gemcitabine in neuroblastoma cell lines and multicellular tumor spheroids. Cancer Lett. 2012, 319, 23–30. [Google Scholar] [CrossRef]
- Mañas, A.; Aaltonen, K.; Andersson, N.; Hansson, K.; Adamska, A.; Seger, A.; Yasui, H.; Van Den Bos, H.; Radke, K.; Esfandyari, J.; et al. Clinically relevant treatment of PDX models reveals patterns of neuroblastoma chemoresistance. Sci. Adv. 2022, 8, eabq4617. [Google Scholar] [CrossRef]
- Radke, K.; Hansson, K.; Sjölund, J.; Wolska, M.; Karlsson, J.; Esfandyari, J.; Pietras, K.; Aaltonen, K.; Gisselsson, D.; Bexell, D. Anti-tumor effects of rigosertib in high-risk neuroblastoma. Transl. Oncol. 2021, 14, 101149. [Google Scholar] [CrossRef]
- Cuperus, R.; Tytgat, G.A.M.; Leen, R.; Brites, P.; Bras, J.; Caron, H.N.; Van Kuilenburg, A.B.P. Pleiotropic effects of fenretinide in neuroblastoma cell lines and multicellular tumor spheroids. Int. J. Oncol. 2008, 32, 1011–1019. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Lode, H.; Ellinger, S.; Zeman, F.; Suttorp, M.; Escherich, G.; Bochennek, K.; Gruhn, B.; Lang, P.; Rohde, M.; et al. Irinotecan and temozolomide in combination with dasatinib and rapamycin versus irinotecan and temozolomide for patients with relapsed or refractory neuroblastoma (RIST-rNB-2011): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2024, 25, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.; Radke, K.; Aaltonen, K.; Saarela, J.; Mañas, A.; Sjölund, J.; Smith, E.M.; Pietras, K.; Påhlman, S.; Wennerberg, K.; et al. Therapeutic targeting of KSP in preclinical models of high-risk neuroblastoma. Sci. Transl. Med. 2020, 12, eaba4434. [Google Scholar] [CrossRef]
- Sidarovich, V.; De Mariano, M.; Aveic, S.; Pancher, M.; Adami, V.; Gatto, P.; Pizzini, S.; Pasini, L.; Croce, M.; Parodi, F.; et al. A High-Content Screening of Anticancer Compounds Suggests the Multiple Tyrosine Kinase Inhibitor Ponatinib for Repurposing in Neuroblastoma Therapy. Mol. Cancer Ther. 2018, 17, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Kholosy, W.M.; Derieppe, M.; Van Den Ham, F.; Ober, K.; Su, Y.; Custers, L.; Schild, L.; Van Zogchel, L.M.J.; Wellens, L.M.; Ariese, H.R.; et al. Neuroblastoma and DIPG Organoid Coculture System for Personalized Assessment of Novel Anticancer Immunotherapies. J. Pers. Med. 2021, 11, 869. [Google Scholar] [CrossRef]
- Nguyen, R.; Moustaki, A.; Norrie, J.L.; Brown, S.; Akers, W.J.; Shirinifard, A.; Dyer, M.A. Interleukin-15 Enhances Anti-GD2 Antibody-Mediated Cytotoxicity in an Orthotopic PDX Model of Neuroblastoma. Clin. Cancer Res. 2019, 25, 7554–7564. [Google Scholar] [CrossRef]
- Veneziani, I.; Infante, P.; Ferretti, E.; Melaiu, O.; Battistelli, C.; Lucarini, V.; Compagnone, M.; Nicoletti, C.; Castellano, A.; Petrini, S.; et al. Nutlin-3a Enhances Natural Killer Cell–Mediated Killing of Neuroblastoma by Restoring p53-Dependent Expression of Ligands for NKG2D and DNAM-1 Receptors. Cancer Immunol. Res. 2021, 9, 170–183. [Google Scholar] [CrossRef]
- Blavier, L.; Yang, R.-M.; DeClerck, Y.A. The Tumor Microenvironment in Neuroblastoma: New Players, New Mechanisms of Interaction and New Perspectives. Cancers 2020, 12, 2912. [Google Scholar] [CrossRef]
- Zaghmi, A.; Aybay, E.; Jiang, L.; Shang, M.; Steinmetz-Späh, J.; Wermeling, F.; Kogner, P.; Korotkova, M.; Östling, P.; Jakobsson, P.; et al. High-content screening of drug combinations of an mPGES -1 inhibitor in multicellular tumor spheroids leads to mechanistic insights into neuroblastoma chemoresistance. Mol. Oncol. 2024, 18, 317–335. [Google Scholar] [CrossRef]
- Kock, A.; Bergqvist, F.; Steinmetz, J.; Elfman, L.H.M.; Korotkova, M.; Johnsen, J.I.; Jakobsson, P.; Kogner, P.; Larsson, K. Establishment of an in vitro 3D model for neuroblastoma enables preclinical investigation of combined tumor-stroma drug targeting. FASEB J. 2020, 34, 11101–11114. [Google Scholar] [CrossRef] [PubMed]
- Corallo, D.; Nardelli, C.; Pantile, M.; Menegazzo, S.; Biffi, A.; Aveic, S. Exploring the Role of Fibroblasts in Promoting Neuroblastoma Cell Migration and Invasion. J. Nanotheranostics 2024, 5, 212–227. [Google Scholar] [CrossRef]
- Treis, D.; Lundberg, K.I.; Bell, N.; Polychronopoulos, P.A.; Tümmler, C.; Åkerlund, E.; Aliverti, S.; Lilienthal, I.; Pepich, A.; Seashore-Ludlow, B.; et al. Targeted inhibition of WIP1 and histone H3K27 demethylase activity synergistically suppresses neuroblastoma growth. Cell Death Dis. 2025, 16, 318. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liang, Z. Epigallocatechin-3-gallate inhibits the growth of three-dimensional in vitro models of neuroblastoma cell SH-SY5Y. Mol. Cell Biochem. 2021, 476, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.; Zhao, Y. Tumor Microenvironment Based on Extracellular Matrix Hydrogels for On-Chip Drug Screening. Biosensors 2024, 14, 429. [Google Scholar] [CrossRef]
- Villasante, A.; Lopez-Martinez, M.J.; Quiñonero, G.; Garcia-Lizarribar, A.; Peng, X.; Samitier, J. Microfluidic model of the alternative vasculature in neuroblastoma. In Vitro Models 2024, 3, 49–63. [Google Scholar] [CrossRef]
- Ning, L.; Shim, J.; Tomov, M.L.; Liu, R.; Mehta, R.; Mingee, A.; Hwang, B.; Jin, L.; Mantalaris, A.; Xu, C.; et al. A 3D Bioprinted in vitro Model of Neuroblastoma Recapitulates Dynamic Tumor-Endothelial Cell Interactions Contributing to Solid Tumor Aggressive Behavior. Adv. Sci. 2022, 9, 2200244. [Google Scholar] [CrossRef]
- Nothdurfter, D.; Ploner, C.; Coraça-Huber, D.C.; Wilflingseder, D.; Müller, T.; Hermann, M.; Hagenbuchner, J.; Ausserlechner, M.J. 3D bioprinted, vascularized neuroblastoma tumor environment in fluidic chip devices for precision medicine drug testing. Biofabrication 2022, 14, 035002. [Google Scholar] [CrossRef]
- Amoli, M.S.; Rezapourdamanab, S.; Jin, L.; Cadena, M.A.; Kaw, K.; Sridhar, V.; Meselhe, M.; Krikor, S.; Mahmoudi, M.; Ning, L.; et al. Protocol for 3D bioprinting of a 3D in vitro model of neuroblastoma. STAR Protoc. 2025, 6, 103725. [Google Scholar] [CrossRef]
- Abou-Antoun, T.J.; Nazarian, J.; Ghanem, A.; Vukmanovic, S.; Sandler, A.D. Molecular and functional analysis of anchorage independent, treatment-evasive neuroblastoma tumorspheres with enhanced malignant properties: A possible explanation for radio-therapy resistance. PLoS ONE 2018, 13, e0189711. [Google Scholar] [CrossRef]
- Lucarini, V.; Melaiu, O.; D’Amico, S.; Pastorino, F.; Tempora, P.; Scarsella, M.; Pezzullo, M.; De Ninno, A.; D’Oria, V.; Cilli, M.; et al. Combined mitoxantrone and anti-TGFβ treatment with PD-1 blockade enhances antitumor immunity by remodelling the tumor immune landscape in neuroblastoma. J. Exp. Clin. Cancer Res. 2022, 41, 326. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Melaiu, O.; Gragera, P.; Król, K.; Scaldaferri, V.; Damiani, V.; De Ninno, A.; Nardozi, D.; Businaro, L.; Masuelli, L.; et al. Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors. Int. J. Mol. Sci. 2025, 26, 1613. [Google Scholar] [CrossRef]
- Liu, M.; Xia, Y.; Ding, J.; Ye, B.; Zhao, E.; Choi, J.-H.; Alptekin, A.; Yan, C.; Dong, Z.; Huang, S.; et al. Transcriptional Profiling Reveals a Common Metabolic Program in High-Risk Human Neuroblastoma and Mouse Neuroblastoma Sphere-Forming Cells. Cell Rep. 2016, 17, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.-Y.; Hansen, J.N.; He, M.; Dai, S.-K.; Choi, Y.; Fulton, M.D.; Lloyd, S.M.; Szemes, M.; Sen, J.; Ding, H.-F.; et al. PRMT1 promotes neuroblastoma cell survival through ATF5. Oncogenesis 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kishida, S.; Huang, P.; Mu, P.; Tsubota, S.; Mizuno, M.; Kadomatsu, K. A New Tumorsphere Culture Condition Restores Potentials of Self-Renewal and Metastasis of Primary Neuroblastoma in a Mouse Neuroblastoma Model. PLoS ONE 2014, 9, e86813. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Kiyonari, S.; Kishida, S.; Cao, D.; Murakami-Tonami, Y.; Ichikawa, H.; Takeuchi, I.; Nakamura, S.; Kadomatsu, K. Nucleolar protein PES 1 is a marker of neuroblastoma outcome and is associated with neuroblastoma differentiation. Cancer Sci. 2015, 106, 237–243. [Google Scholar] [CrossRef]
- Embaie, B.T.; Sarkar, H.; Alchahin, A.M.; Otte, J.; Olsen, T.K.; Tümmler, C.; Kameneva, P.; Artemov, A.V.; Akkuratova, N.; Adameyko, I.; et al. Comparative Single-Cell Transcriptomics of Human Neuroblastoma and Preclinical Models Reveals Conservation of an Adrenergic Cell State. Cancer Res. 2025, 85, 1015–1034. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef]
- Mitchell, C.B.; O’Neill, G.M. Rac GTPase regulation of 3D invasion in neuroblastomas lacking MYCN amplification. Cell Adhes. Migr. 2017, 11, 68–79. [Google Scholar] [CrossRef]
- Curtin, C.; Nolan, J.C.; Conlon, R.; Deneweth, L.; Gallagher, C.; Tan, Y.J.; Cavanagh, B.L.; Asraf, A.Z.; Harvey, H.; Miller-Delaney, S.; et al. A physiologically relevant 3D collagen-based scaffold–neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomater. 2018, 70, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Murphy, C.; Kelly, G.; O’Brien, F.J.; Piskareva, O. Three-dimensional In Vitro Biomimetic Model of Neuroblastoma using Collagen-based Scaffolds. J. Vis. Exp. 2021, 62627. [Google Scholar] [CrossRef]
- Bova, L.; Maggiotto, F.; Micheli, S.; Giomo, M.; Sgarbossa, P.; Gagliano, O.; Falcone, D.; Cimetta, E. A Porous Gelatin Methacrylate-Based Material for 3D Cell-Laden Constructs. Macromol. Biosci. 2023, 23, 2200357. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Fusco, P.; Parisatto, B.; Rampazzo, E.; Persano, L.; Frasson, C.; Di Meglio, A.; Leslz, A.; Santoro, L.; Cafferata, B.; Zin, A.; et al. Patient-derived organoids (PDOs) as a novel in vitro model for neuroblastoma tumours. BMC Cancer 2019, 19, 970. [Google Scholar] [CrossRef]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cells Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Torresan, V.; Dedroog, L.M.; Deschaume, O.; Koos, E.; Lettinga, M.P.; Gandin, A.; Pelosin, M.; Zanconato, F.; Brusatin, G.; Bartic, C. Nanocellulose-collagen composites as advanced biomaterials for 3D in-vitro neuronal model systems. Carbohydr. Polym. 2025, 348, 122901. [Google Scholar] [CrossRef]
- Chemmarappally, J.M.; Pegram, H.C.N.; Abeywickrama, N.; Fornari, E.; Hargreaves, A.J.; De Girolamo, L.A.; Stevens, B. A co-culture nanofibre scaffold model of neural cell degeneration in relevance to Parkinson’s disease. Sci. Rep. 2020, 10, 2767. [Google Scholar] [CrossRef]
- Monferrer, E.; Dobre, O.; Trujillo, S.; González Oliva, M.A.; Trubert-Paneli, A.; Acevedo-León, D.; Noguera, R.; Salmeron-Sanchez, M. Vitronectin-based hydrogels recapitulate neuroblastoma growth conditions. Front. Cell Dev. Biol. 2022, 10, 988699. [Google Scholar] [CrossRef]
- Ornell, K.J.; Mistretta, K.S.; Ralston, C.Q.; Coburn, J.M. Development of a stacked, porous silk scaffold neuroblastoma model for investigating spatial differences in cell and drug responsiveness. Biomater. Sci. 2021, 9, 1272–1290. [Google Scholar] [CrossRef] [PubMed]
- Granados-Aparici, S.; Vieco-Martí, I.; López-Carrasco, A.; Navarro, S.; Noguera, R. Real-time morphometric analysis of targeted therapy for neuroblastoma cells in monolayer and 3D hydrogels using digital holographic microscopy. iScience 2024, 27, 111231. [Google Scholar] [CrossRef]
- Mistretta, K.S.; Coburn, J.M. Three-dimensional silk fibroin scaffolded co-culture of human neuroblastoma and innate immune cells. Exp. Cell Res. 2024, 443, 114289. [Google Scholar] [CrossRef] [PubMed]
- Barberio, C.; Saez, J.; Withers, A.; Nair, M.; Tamagnini, F.; Owens, R.M. Conducting Polymer-ECM Scaffolds for Human Neuronal Cell Differentiation. Adv. Healthc. Mater. 2022, 11, 2200941. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.H.; Beierle, A.M.; Julson, J.R.; Erwin, M.E.; Alrefai, H.; Markert, H.R.; Stewart, J.E.; Hutchins, S.C.; Bownes, L.V.; Aye, J.M.; et al. Using 3D-bioprinted models to study pediatric neural crest-derived tumors. Int. J. Bioprinting 2023, 9, 723. [Google Scholar] [CrossRef]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef]
- Aveic, S.; Seidelmann, M.; Davtalab, R.; Corallo, D.; Vogt, M.; Rütten, S.; Fischer, H. Three-dimensional in vitro model of bone metastases of neuroblastoma as a tool for pharmacological evaluations. Nanotheranostics 2024, 8, 1–11. [Google Scholar] [CrossRef]
- Aveic, S.; Janßen, S.; Nasehi, R.; Seidelmann, M.; Vogt, M.; Pantile, M.; Rütten, S.; Fischer, H. A 3D printed in vitro bone model for the assessment of molecular and cellular cues in metastatic neuroblastoma. Biomater. Sci. 2021, 9, 1716–1727. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Asthana, A.; Kisaalita, W.S. Microtissue size and hypoxia in HTS with 3D cultures. Drug Discov. Today 2012, 17, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Vega, V.F.; Yang, D.; Jordán, L.O.; Ye, F.; Conway, L.; Chen, L.Y.; Shumate, J.; Baillargeon, P.; Scampavia, L.; Parker, C.; et al. Protocol for 3D screening of lung cancer spheroids using natural products. SLAS Discov. 2023, 28, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Koo, I.-S.; Hwang, H.J.; Lee, D.W. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discov. 2023, 28, 119–137. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Valdés, J.; Pérez-Navarro, Y.; Mandujano-Lazaro, G.; Marchat, L.A.; Ramos-Payán, R.; Nuñez-Olvera, S.I.; Pérez-Plascencia, C.; López-Camarillo, C. Three-Dimensional 3D Culture Models in Gynecological and Breast Cancer Research. Front. Oncol. 2022, 12, 826113. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez De La Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Lovitt, C.; Shelper, T.; Avery, V. Advanced Cell Culture Techniques for Cancer Drug Discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef]
- Kim, S.-Y.; van de Wetering, M.; Clevers, H.; Sanders, K. The future of tumor organoids in precision therapy. Trends Cancer, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Narasimhan, V.; Wright, J.A.; Churchill, M.; Wang, T.; Rosati, R.; Lannagan, T.R.M.; Vrbanac, L.; Richardson, A.B.; Kobayashi, H.; Price, T.; et al. Medium-throughput Drug Screening of Patient-derived Organoids from Colorectal Peritoneal Metastases to Direct Personalized Therapy. Clin. Cancer Res. 2020, 26, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yin, S.-Y.; Bie, Z.-X.; Li, Y.-M.; Qi, J.; Ma, Y.-D.; Wang, Z.; Xi, J.J.; Li, X.-G. Personalized drug screening of patient-derived tumor-like cell clusters based on specimens obtained from percutaneous transthoracic needle biopsy in patients with lung malignancy: A real-world study. BMC Cancer 2025, 25, 649. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, P.; Wolpaw, A.J. Three-Dimensional Culture Systems in Neuroblastoma Research. Organoids 2025, 4, 10. https://doi.org/10.3390/organoids4020010
Jung P, Wolpaw AJ. Three-Dimensional Culture Systems in Neuroblastoma Research. Organoids. 2025; 4(2):10. https://doi.org/10.3390/organoids4020010
Chicago/Turabian StyleJung, Piotr, and Adam J. Wolpaw. 2025. "Three-Dimensional Culture Systems in Neuroblastoma Research" Organoids 4, no. 2: 10. https://doi.org/10.3390/organoids4020010
APA StyleJung, P., & Wolpaw, A. J. (2025). Three-Dimensional Culture Systems in Neuroblastoma Research. Organoids, 4(2), 10. https://doi.org/10.3390/organoids4020010





