Breast Cancer Tissues and Organoids BioBank: Constitution, Research Activities and Samples Access
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient’s Eligibility Criteria
2.2. Patient Derived Organoids (PDOs) Establishment
- (a)
- PDOs Establishment Protocol
- I.
- After collecting, the healthy (h) and tumor (t) tissue pieces were transferred separately to a 10 cm cell culture dish, keeping them moist with PBS/Pen/Strep.
- II.
- Each piece of tissue was chopped into small fragments of 1–3 mm, using scissors, making sure to remove non-epithelial components (i.e., adipose tissue, mainly present in breast tissue).
- III.
- One or two fragments, depending on the abundance of material available, from each minced piece, were fixed in 10% (wt/vol) neutral buffered formalin for their paraffin embedding.
- IV.
- Two or more fragments from each minced piece were also snap-frozen for molecular/biochemical analysis at −80 °C.
- V.
- The remaining minced tissue was transferred using a cell lifter to a 15 mL tube, containing the digestion buffer (10 mL/g of tissue; Supplementary Data, File S1) and subjected to enzymatic digestion for 60–120 min at 37 °C.
- VI.
- Digestion was monitored by microscope observation, transferring the digestion mixture to a MW-6 every 10–15 min. Each time the digested tissue was returned to the 15 mL tube, it was pipetted vigorously up and down using a P1000 pipette 30 times and then placed back at 37 °C to continue enzymatic digestion for as long as needed (usually this step takes no more than 60–120 min).
- VII.
- When the tissue was disrupted and the mixture became cloudy, the tissue was further broken up by mechanically pipetting up and down 10–20 times, using a P1000 pipette (Gilson, Milan, Italy).
- VIII.
- adDMEM/F12+++ medium (8 mL; Supplementary Data, File S1) was added to the mixture and then it was centrifuged at 300× g for 5 min at 4 °C. If the pellet contained a lot of fat, one or two additional washing steps were performed by using 10 mL of adDMEM/F12+++ medium.
- IX.
- The pellet was resuspended in 10 mL adDMEM/F12+++ medium and filtered using a 100 μm cell strainer (431752, Corning Gilson, Milan, Italy).
- X.
- If a red layer on the top of the pellet was observed, due to the presence of red blood cells, 1 mL of ACK Lysing Buffer was added and incubated for 10 min in a 37 °C water bath. Then, 10 mL of adDMEM/F12+++ medium was added to neutralize the red lysis buffer and a centrifuge (at 300× g for 5 min at 4 °C) was performed.
- XI.
- After aspirating the supernatant, the pellet was resuspended in Reduced Growth Factor Basement Membrane Extract (RGF BME), Type 2, previously kept on ice, to prevent it from solidifying.
- XII.
- Approximately 10,000 cells were plated in 20 μL of 80% BME (vol/vol) to ensure the formation of solid droplets into MW-48 plate, placing one drop for each well.
- XIII.
- The culture plate, flipped upside down, was then placed into a humidified incubator at 37 °C and 5% (vol/vol) CO2 for 30 min to let the BME solidify.
- XIV.
- The required amount of organoid medium (medium A), containing the growth factors listed in Table S2 (Supplementary Materials, File S1), 10 μM ROCK inhibitor and Primocin (100 μg/mL) was pre-heated in the 37 °C water bath.
- XV.
- Once the BME drops were solidified (~30 min), the plate was flipped upside down and 500 μL of organoid medium was carefully added to each well.
- XVI.
- Then, the culture plate was placed on the OrganoFlow L, Perfusion Rocker (L-191100104, Mimetas, 2342 DH Oegstgeest The Netherlands) in a humidified incubator at 37 °C and 5% (vol/vol) CO2.
- XVII.
- The medium was changed every 2–3 days, by carefully aspirating the medium from the wells and replacing it with fresh, pre-heated medium, containing ROCK inhibitor and Primocin in the culture medium for the first 5 days of the culture.
- XVIII.
- After five days, culture medium A was substituted with the B ones (Supplementary Materials, File S1).
- (b)
- PDOs Culturing Protocol
- I.
- BME drops were dissolved, and the organoids were dissociated, by pipetting 20 times up and down the medium inside of each well into the plate with a P1000 filter tip with a (non-filtered) P100 tip on top to aid the disaggregation of the organoids. Then, they were transferred into a 15 mL tube.
- II.
- Each well was further washed twice using the ice-cold adDMEM/F12+++ medium
- III.
- Both washes were collected into the same 15 mL tube with the previously transferred BME drops containing the disaggregated organoids, and centrifuged at 300× g, T = 8 °C for 5 min.
- IV.
- The supernatant was discarded.
- V.
- The pellet was resuspended into the appropriate volume of 80% BME (vol/vol) to expand the culture 1:2–1:3, by following the seeding procedure described above.
- VI.
- Then, the plate was placed on the Perfusion Rocker, in a humidified incubator at 37 °C and 5% (vol/vol) CO2 for PDOs culturing.
- I.
- After the washing step of the PDOs mechanical dissociation procedure, the pellets were further dissociated using TrypLE dissociation buffer. Thus, after the centrifugation at step 3, 1 mL of TrypLE buffer was added.
- II.
- The pellet was then resuspended by pipetting up and down for 20 times, using a P1000 filter tip with a (non-filtered) P10 tip on top, followed by an incubation of 7 min at 37 °C.
- III.
- PDOs shearing was checked under the light microscope, to make sure that organoids were disaggregated.
- IV.
- Then, 10 mL of the adDMEM/F12+++ medium was added to neutralize TrypLE, and the sheared organoids were centrifuged at 300× g, T = 8 °C for 5 min.
- V.
- The supernatant was discarded.
- VI.
- The pellet was resuspended into the appropriate volume of 80% BME (vol/vol) to expand the organoid culture 1:2–1:3, by following the seeding procedure described above.
- VII.
- Then, the plate was placed on the Perfusion Rocker, in a humidified incubator at 37 °C and 5% (vol/vol) CO2 for PDOs culturing.
- (c)
- PDOs Freezing Protocol
- I.
- BME drops containing organoids from 12 wells of a 48-well plate were pooled in a 15 mL conical tube.
- II.
- To dissolve the BME gel, 10 mL of the ice-cold adDMEM/F12+++ medium was added, pipetting up and down several times with a 10 mL serological pipette in the tube placed in ice.
- III.
- The sample was incubated in ice for 10 min.
- IV.
- Then, the organoids were centrifuged at 300× g for 5 min at 4 °C.
- V.
- The supernatant was aspirated without disturbing the pellet.
- VI.
- The organoids were resuspended in 500 μL of the freezing medium (Supplementary Materials, File S1) in a sterile cryogenic vial.
- VII.
- The cryogenic vial was placed into a freezing container and stored at −80 °C for 24 h.
- VIII.
- Then, the cryo-vial was transferred into the liquid nitrogen tank (approximately −180 °C).
- (d)
- PDOs Thawing Protocol
- I.
- A 15 mL conical tube with 10 mL of the adDMEM/F12+++ medium was prepared at room temperature, before collecting the cryovial in dry ice from the liquid nitrogen tank.
- II.
- The cryogenic vial was incubated for 1–3 min in a water bath at 37 °C.
- III.
- A total of 1 mL of adDMEM/F12+++ medium was added dropwise into the cryogenic vial while mixing, and then the entire volume with the organoids in the vial was transferred to the 15 mL tube, previously prepared.
- IV.
- The organoids were centrifuged at 300× g for 5 min at 4 °C.
- V.
- The supernatant was removed without disturbing the pellet.
- VI.
- Then, organoids were resuspended into the appropriate volume of 80% BME (vol/vol) by following the seeding procedure described above.
2.3. Formalin-Fixed Paraffin Embedded (FFPE) PDOs Procedure
2.4. PDOs if on a Microfluidic OrganoPlate
2.5. Reagents
- –
- RGF BME Type 2, Cultrex reduced growth factor BME type 2 (BIOTECHNE, Milan, Italy; cat. no. 3533-010-02);
- –
- BSA (Sigma-Aldrich, Milan, Italy; cat. no. A9418);
- –
- Dispase (Gibco, cat. no. 17105-041);
- –
- Collagenase type 2 (Wortington, cat. no. LS004176);
- –
- GlutaMAX supplement, 100× (Thermo Fisher Scientific, Milan, Italy, cat. no. 35050-061);
- –
- PBS (Sigma-Aldrich, cat. no. P4417);
- –
- DMSO (Sigma-Aldrich, cat. no. D2650);
- –
- TrypLE Express Enzyme (1×), phenol red (Thermo Fisher Scientific, cat. no. 12605-010);
- –
- Hydrocortisone (Sigma-Aldrich, cat. no. H0135);
- –
- Gentamycin (Euroclone, Milan, Italy; cat. no. ECM0011B);
- –
- ACK Lysing Buffer (Invitrogen, Milan, Italy; cat. no. A1049201);
- –
- Rabbit monoclonal Estrogen Receptor (SP1) antibody (Ventana, Basel, Switzerland; (92) 790-4324);
- –
- Progepharm mouse Progesteron Receptor antibody (Dako, Santa Clara, CA, USA; cat. no. 10128534);
- –
- Rabbit anti-human HER2 protein (HerceptTestTM, Agilent, Santa Clara, CA, USA; cat. no. SK001);
- –
- Rabbit Polyclonal Ki-67/MK167 antibody (Novus, Milan, Italy; cat. no. NB500-170);
- –
- Mouse Cytokeratin, Multi (AE1/AE3) antibody (Leica, Milan, Italy; cat. no. PA0094 AE1/AE3);
- –
- Mouse Monoclonal E-Cadherin antibody [4A2] (Abcam, Cambridge, UK; cat. no. ab231303);
- –
- AlexafluorTM 488 GAR (Invitrogen, cat. no. R37116);
- –
- AlexafluorTM 594 GAR (Invitrogen, cat. no. R37117);
- –
- AlexafluorTM 488 GAM (Invitrogen, cat. no. R37120);
- –
- AlexafluorTM 594 GAM (Invitrogen, cat. no. R37121);
- –
- Hoectsh 33342 (Invitrogen, Milan, Italy; cat. no. H3570);
- –
- Fetal Bovin Serum (Gibco cat. no. A5670701);
- –
- Normal Goat Serum (NGS, Gibco, cat. no. 16210-064).
3. Results
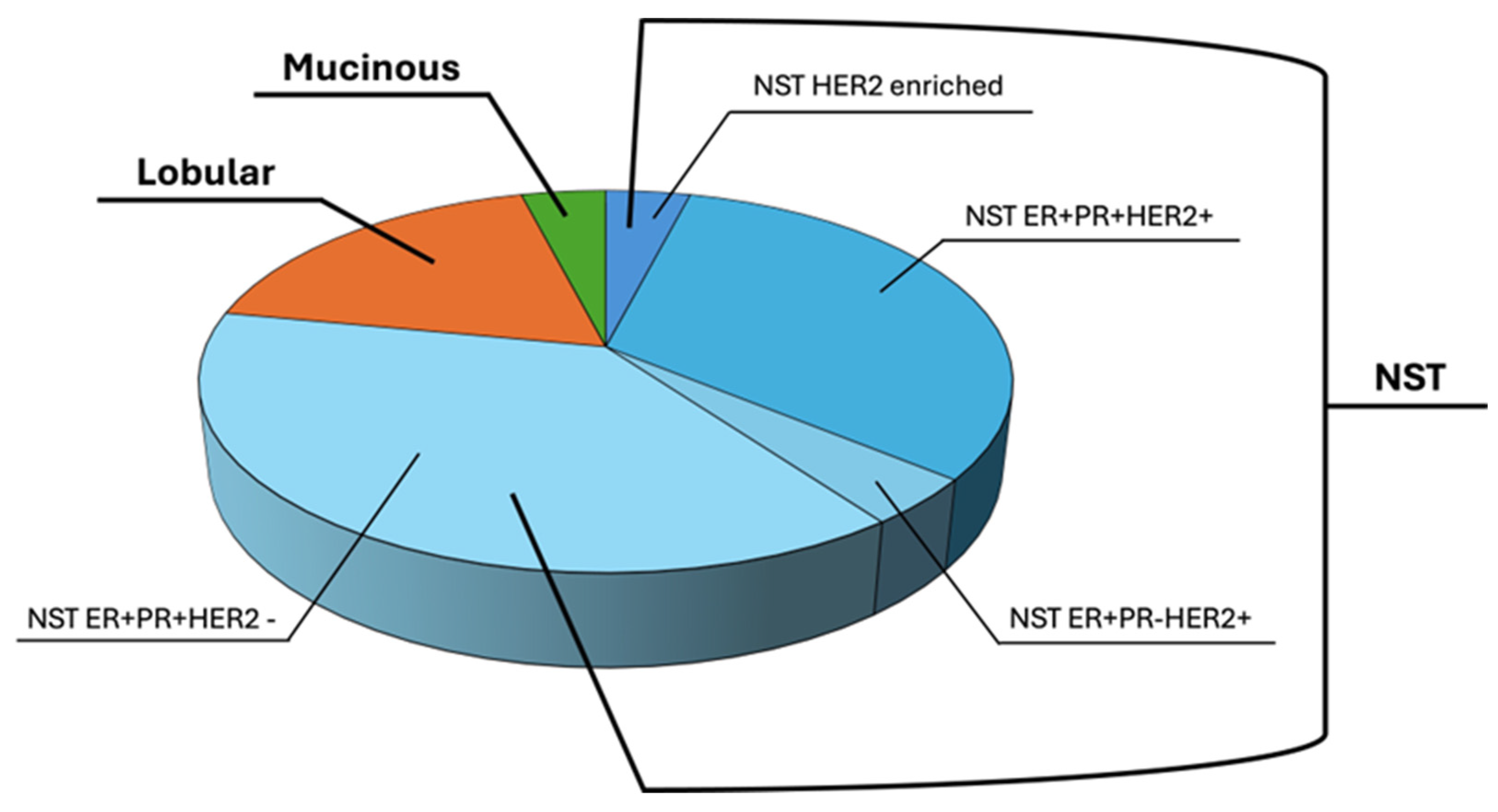
3.1. Patient Samples and Clinical Data
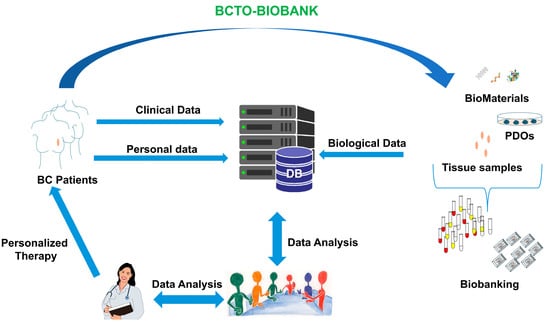
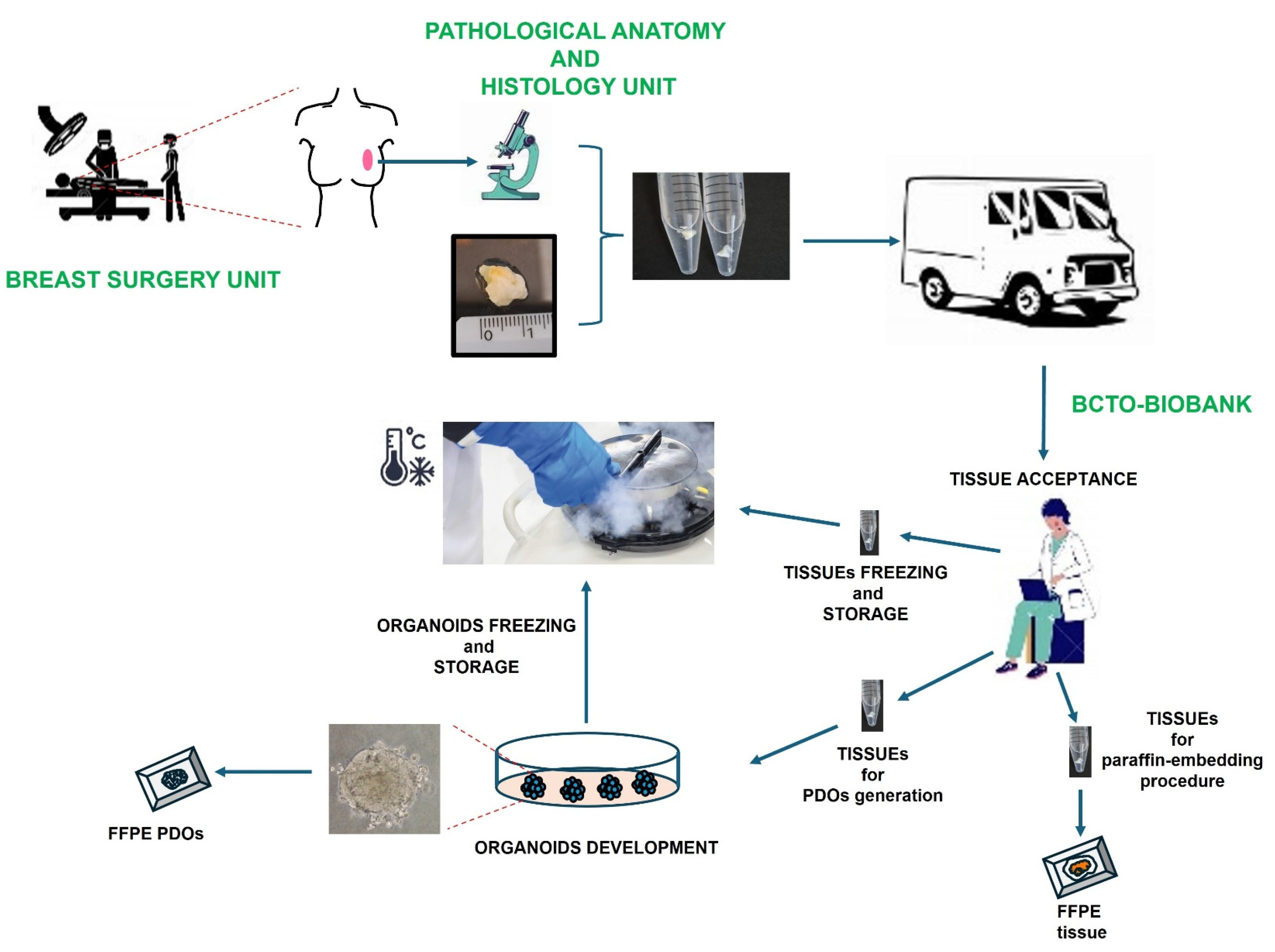
3.2. Establishment of Organoids for Their Storage in Breast Cancer Tissues and Organoids Biobank (BCTO BioBank)
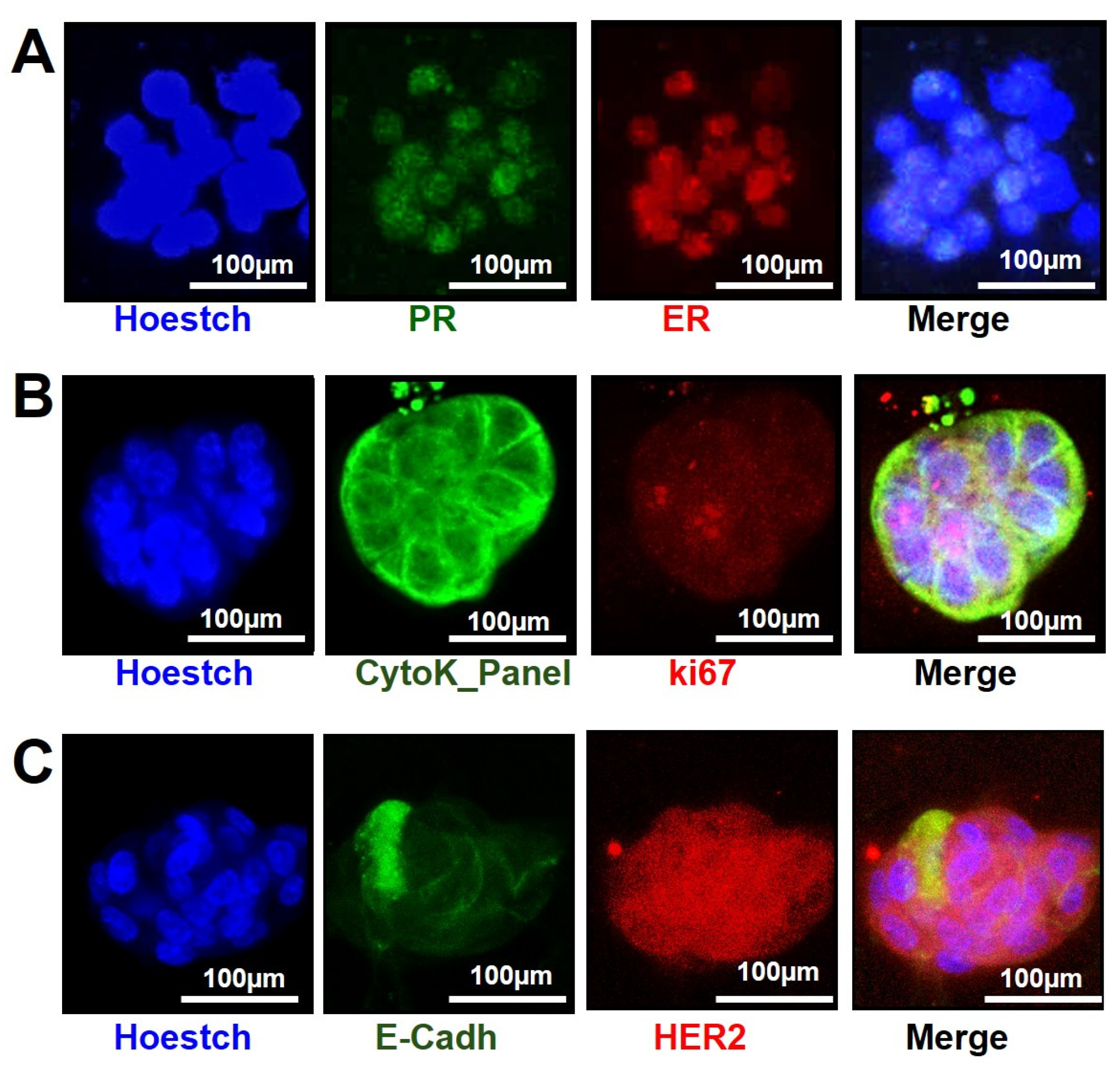
3.3. Organoids Characterization for Their Receptor Status by Immunofluorescence on Microfluidic 2-Lane OrganoPlate
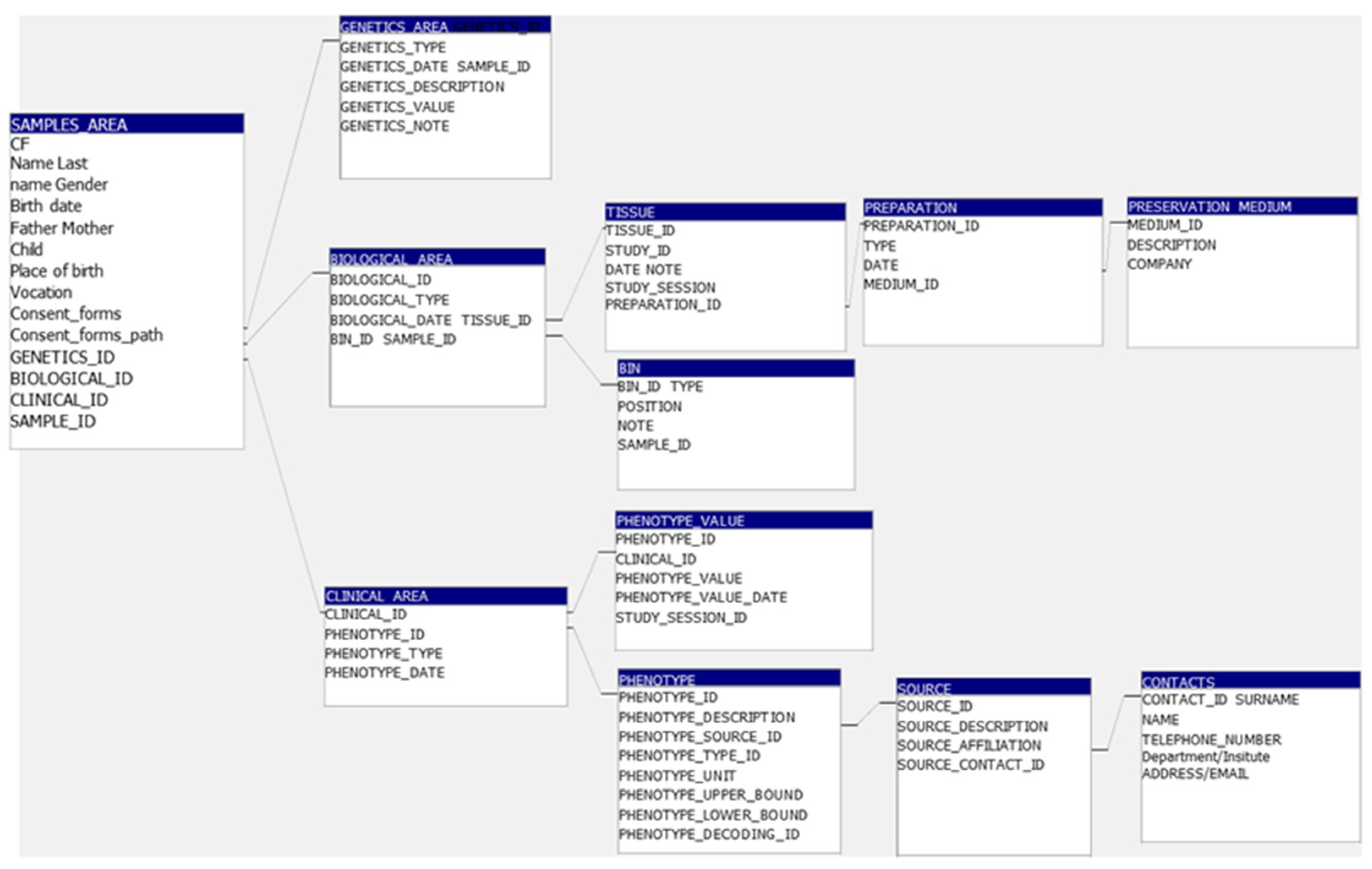
3.4. BCTO BioBank Database
3.5. BCTO BioBank Data and Samples Access
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Haber, D.A.; Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 2010, 10, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Korn, J.M.; Ferretti, S.; Monahan, J.E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015, 21, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Rueda, O.M.; Greenwood, W.; Batra, A.S.; Callari, M.; Batra, R.N.; Pogrebniak, K.; Sandoval, J.; Cassidy, J.W.; Tufegdzic-Vidakovic, A.; et al. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell 2016, 167, 260–274.e22. [Google Scholar] [CrossRef]
- Fitzpatrick, P.A.; Akrap, N.; Söderberg, E.M.V.; Harrison, H.; Thomson, G.J.; Landberg, G. Robotic Mammosphere Assay for High-Throughput Screening in Triple-Negative Breast Cancer. SLAS Discov. 2017, 22, 827–836. [Google Scholar] [CrossRef]
- Muthuswamy, S.K. Bringing together the organoid field: From early beginnings to the road ahead. Development 2017, 144, 963–967. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Schipper, L.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van de Haar, J.; Prevoo, W.; van Werkhoven, E.; Snaebjornsson, P.; et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open 2021, 6, 100103. [Google Scholar] [CrossRef]
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 2019, 573, 439–444. [Google Scholar] [CrossRef]
- Bajrami, I.; Marlow, R.; van de Ven, M.; Brough, R.; Pemberton, H.N.; Frankum, J.; Song, F.; Rafiq, R.; Konde, A.; Krastev, D.B.; et al. E-Cadherin/ROS1 Inhibitor Synthetic Lethality in Breast Cancer. Cancer Discov. 2018, 8, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Malainou, C.P.; Stachika, N.; Damianou, A.K.; Anastopoulos, A.; Ploumaki, I.; Triantafyllou, E.; Drougkas, K.; Gomatou, G.; Kotteas, E. Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Curr. Oncol. 2023, 30, 9734–9745. [Google Scholar] [CrossRef]
- Fan, H.; Demirci, U.; Chen, P. Emerging organoid models: Leaping forward in cancer research. J. Hematol. Oncol. 2019, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Silva-Almeida, C.; Ewart, M.A.; Wilde, C. 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin. Cell Dev. Biol. 2020, 98, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sleire, L.; Forde, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P.O. Drug repurposing in cancer. Pharmacol. Res. 2017, 124, 74–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Piccotti, F.; Allevi, R.; Truffi, M.; Sorrentino, L.; Russo, L.; Agozzino, M.; Signati, L.; Bonizzi, A.; Villani, L.; et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Russo, S.; Naik, P.; Bhatia, S.; Spector, D.L. Establishment and Culture of Patient-Derived Breast Organoids. J. Vis. Exp. 2023, 192, 10-3791. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Sinati, L.; Messa, L.; Franceschini, A.; Bonizzi, A.; Castagnoli, L.; Gasparini, P.; Consolandi, C.; Mangano, E.; Pelucchi, P.; et al. Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution. Cancer Cell Int. 2024, 24, 220. [Google Scholar] [CrossRef] [PubMed]
- Sellick, J. Enhancing the protection of animals used for scientific purposes. Environ. Law Manag. 2011, 23, 75–82. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, L.; Mandrich, L.; Massa, S.; Nutile, T.; Crovella, C.; De Rosa, I.; Lucci, R.; De Rosa, F.; Somma, P.; Mercadante, V.; et al. Breast Cancer Tissues and Organoids BioBank: Constitution, Research Activities and Samples Access. Organoids 2025, 4, 5. https://doi.org/10.3390/organoids4010005
Miranda L, Mandrich L, Massa S, Nutile T, Crovella C, De Rosa I, Lucci R, De Rosa F, Somma P, Mercadante V, et al. Breast Cancer Tissues and Organoids BioBank: Constitution, Research Activities and Samples Access. Organoids. 2025; 4(1):5. https://doi.org/10.3390/organoids4010005
Chicago/Turabian StyleMiranda, Lucia, Luigi Mandrich, Simona Massa, Teresa Nutile, Clotilde Crovella, Ilaria De Rosa, Raffaella Lucci, Filippo De Rosa, Pasquale Somma, Vincenzo Mercadante, and et al. 2025. "Breast Cancer Tissues and Organoids BioBank: Constitution, Research Activities and Samples Access" Organoids 4, no. 1: 5. https://doi.org/10.3390/organoids4010005
APA StyleMiranda, L., Mandrich, L., Massa, S., Nutile, T., Crovella, C., De Rosa, I., Lucci, R., De Rosa, F., Somma, P., Mercadante, V., Abate, C., Arbucci, S., Panico, L., & Caputo, E. (2025). Breast Cancer Tissues and Organoids BioBank: Constitution, Research Activities and Samples Access. Organoids, 4(1), 5. https://doi.org/10.3390/organoids4010005