Abstract
Organoids have emerged as a powerful tool for studying organ development, disease modeling, and drug discovery due to their ability to mimic the in vivo structure and function of organs in a three-dimensional in vitro model. During in vivo organ maturation, the process of vascularization is crucial for the provision of nutrients and oxygen to cells and the removal of waste products as the organ increases in size. Similarly, organoids can grow to sizes greater than the millimeter scale, yet transport of oxygen and nutrients to the center becomes increasingly difficult, often resulting in the formation of a necrotic core. Herein, we provide a concise summary of the recent development of methods to initiate and maintain vascularization of organoids. Broadly, vascularization of organoids has been achieved primarily by two means: generating organoids that contain endothelial cells or employing the secretion of vascular growth factors to promote vascularization. Growth factors play a fundamental role in regulating blood vessel formation through chemical signals that cause changes in the cell–cell adhesions and ultimately the migration of endothelial cells. Furthermore, models with perfusable systems demonstrate that through the application of growth factors and cells, the vascular network in vascularization-based organoids can administer biological substances to the interior of the organoid, opening up new possibilities for long-term organoid culture in vitro. This goal is being realized through the development of bioengineering tools, such as vascularized organoids on a chip, which are currently tested for various organ systems, including the lung, brain, kidney, and tumors, with applications in cancer angiogenesis and metastasis research. Taken together, our review underlines the vast potential of vascularized organoids to improve the understanding of organ development, while also proposing exciting avenues of organoid-on-a-chip and disease modeling.
1. Introduction
Organoids are self-assembled, three-dimensional, in vitro units constructed from either stem cells or specific progenitor cells [1,2,3], which enable realistic micro-anatomy to form [4,5,6] through the provision of appropriate culture conditions and growth factors [7,8]. For instance, lung organoids serve as models for studying respiratory diseases [9,10], and blood-brain barrier (BBB) organoids recapitulate the selective diffusion function across the vessel wall of the in vivo BBB [11]. Organoids often grow into the millimeter scale [12], affording a greater likelihood of reproducing the complex cellular interactions and structures found in in vivo organs. However, the delivery of oxygen and nutrients to the center of the organoid relies solely on passive diffusion due to the lack of perfusive vasculature, leading to the development of a necrotic core. In vivo vasculature facilitates active transport of nutrients and oxygen while also removing waste and carbon dioxide [13,14,15,16,17]. Therefore, strategies to improve the vascularization of organoids are needed.
Vascularization involves the formation and development of new blood vessels in biological tissues [13,18]. In vitro, endothelial cells promote the development of nascent vascular structures which, with proper support and cues, can maturate into luminal-containing, branched vessels [19,20]. These cues arise from the co-culture with supporting cells such as pericytes and mesenchymal stem cells (MSCs), which make up the perivascular niche and wound repair response, respectively [21,22,23]. When endothelial cells and pericytes are co-cultured, pericytes form a close association with endothelial cell sprouts, leading to an increase in sprout number and vascular diameter compared to endothelial cells alone [23]. A similar effect is observed when MSCs are used in place of pericytes, where more endothelial-vessel-like structures were observed [24]. This is because these vascular support cells increase the secretion of angiogenic factors that promote angiogenesis, similar to the in vivo environment [21,24].
Theoretically, by integrating a vascularized organoid into a perfusable system, the overall size and lifespan of the organoid can be significantly increased [25]. Early work on this took advantage of in vivo implantation of pre-vascularized or vascular organoids, whereby the implanted organoid was eventually anastomosed with the host vasculature, resulting in organoid survival [25,26,27,28]. The establishment of perfusion systems in vitro [29,30,31], mostly by incorporating organoids into microfluidic bioreactor systems, provides perfusion to increase the delivery of nutrients [32]. Here, we refer to these systems collectively as “organoids-on-a-chip (OOCoid)”. OOCoid can be used to study and enhance the vascularization process as a result of the induced flow stresses on the engineered tissues which better mimic physiological conditions [32,33,34]. For example, BBB models generated from endothelial cells, pericytes and astrocytes co-cultured in a microfluidic system exhibit vasculature that is perfusable with selective microvessels exhibiting lower permeability than conventional in vitro models, which improves the function of BBB models [35].
Herein, we review the development of vascularized organoids. Firstly, we will briefly recap the angiogenesis process, highlighting the importance of vascular growth factor secretion in angiogenesis. Secondly, we will summarize co-culture works with endothelial cells, as well as the inclusion of vascular support cells such as pericytes, MSCs, and fibroblasts, which further promote the generation of vascularized organoids. Next, we describe in situ vascularization with vascular organoids and primary cell organoid implantation. Finally, we culminate in the state-of-the-art organ cultured on a chip.
2. Vascularization
Vascularization is used to describe the formation of blood vessels in a tissue or organ, including angiogenesis, the branching of existing vessels [36], vasculogenesis, and the development of new blood vessels [37,38,39], to ensure that tissues and cells receive adequate oxygen and nutrients and removal of waste products [14,25]. In this process, growth factors play a fundamental role in regulating vascularization through signaling that causes the release and migration of endothelial cells as they construct new microvessels.
2.1. Angiogenesis
Within a hypoxic tissue, a tissue in a state of low oxygenation, pre-existing blood vessels secrete pro-angiogenic factors, such as hypoxia-inducible factor-1 (HIF-1), which upregulates multiple target genes, such as vascular endothelial growth factor (VEGF) [40]. VEGF activates endothelial cells in a nearby blood vessel to loosen their endothelial tight junction bonds, causing static stalk endothelial cells to become migratory tip cells (Figure 1) [41,42]. Meanwhile, pericytes, which are present in the perivascular space, also disengage and migrate through the hypoxic region while secreting VEGF, resulting in the guidance of tip endothelial cells during sprouting [42,43,44,45,46]. Eventually, the tip cells give rise to more stalk cells which are highly proliferative and are responsible for establishing new tight junctions and the formation of the lumen of the new capillary [47,48]. The newly created vessels are unstable and require pericyte recruitment to ensure survival. For this, endothelial cells secrete platelet-derived growth factor (PDGF) to attract PDGFRβ-positive pericytes, enabling pericyte and endothelial cells’ interaction through various molecular mechanisms to initiate maturation by promoting the formation of a tight vascular barrier [49,50,51]. Additionally, transforming growth factor-β (TGF-β) can potentiate the effects of VEGF receptor signaling, as well as be involved in the formation of new blood vessels by affecting the synthesis and release of PDGF, thereby enhancing the process of angiogenesis. For example, experiments involving the knockout of TGF-β have demonstrated their role in endothelial cell proliferation, contributing to the formation of new blood vessels [52,53]. Furthermore, cellular membrane tension fluctuations caused by fluid flow, such as the induction of cellular stretching in the lumen, increase the longevity of resident endothelial cells [54]. Similarly, mechanical stimulation results in the release of endothelial nitric oxide synthase (eNOS), which influences endothelial cell polarity and angiogenic sprouting. Notably, the inhibition of eNOS significantly hinders VEGF-induced migration of endothelial cells while fluid flow can still lead to their polarization showing the dichotic role of mechanical regulation [55]. Readers interested about the role of mechanosensing in vascular development are referred to two excellent reviews on the topic by Gray et al. [56] and Tarbell et al. [57], which discuss in more detail the biochemical and biophysical cues that result from blood flow signaling on endothelial cells.
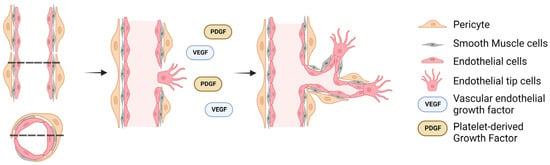
Figure 1.
Schematic diagram of the in vivo angiogenesis process. A precursor to the angiogenic process is the development of a hypoxic region which induces the release of pericytes into the perivascular space and the conformation change of stalk (resident) endothelial cells to tip (migratory) endothelial cells. The dashed lines indicate the cross-section of the blood vessel, with arrows indicating the temporal progression of angiogenesis.
2.2. Vascular Growth Factor Secretion
2.2.1. Exogenous Growth Factors
Growth-factor-directed angiogenesis has been exploited to great effect by incorporating growth factors into gels to enhance sprouting and promote the generation of vascular organoids. Hydrogels encapsulating exogenous VEGF stimulate the proliferation and migration of endothelial cells, leading to angiogenesis [58]. PDGF encapsulation within hydrogels has also been shown to promote angiogenesis and play a role in vascularization by promoting pericyte recruitment and stabilization of the newly formed blood vessels [59]. Similarly, in other trials, endothelial cell and capillary lumen formation in vitro were promoted by TGF-β1 at relatively low concentrations (100 pg/mL–1 ng/mL); however, lumen size in gels was markedly reduced at invasion-potentiating doses of TGF-β1 [60]. Hence, exogenous growth factors in hydrogel can influence the generation of vasculature for tissue engineering.
Exogenous trophic factors occur naturally in vivo, including VEGF and granulocyte-macrophage colony-stimulating factor, where following implantation they have been observed to mobilize endothelial progenitor cells from bone marrow and have been shown to contribute to neovascularization of ischemic tissues [61,62]. Similarly, in animal models, it has been shown that implanting autologous bone marrow cells, following hindlimb injury, results in the induction of angiogenesis and enhanced exercise capacity as a result of improved blood flow. The authors attributed this result to the secretion of VEGF [63]. Additionally, endothelial progenitor cells, activated by unidentified cellular signaling pathways, release cytokines that enhance the proliferation and migration of smooth muscle cells and vascular endothelial cells in the ischemic limb and promote the formation of collateral vessels [64].
2.2.2. Endogenous Growth Factors
VEGF, PDGF, and many other angiogenic factors are naturally secreted in vivo by the vascular cells, and as such the inclusion of vascular, wound repair and perivascular cells can be employed to facilitate angiogenesis [65,66,67,68]. For instance, Ding et al. demonstrated that the angiocrine trophogens Wnt2 and hepatocyte growth factor were released because of the activation of VEGFR2/inhibitor of differentiation 1 signaling, thereby promoting hepatocyte proliferation and tissue regeneration. The authors went on to show that, after 8 days post hepatectomy, genetic inhibition of VEGFR2 impairs hepatic and vascular recovery. They suggested that this is a result of the inhibition of the endothelial-cells-specific transcription factor, inhibitor of differentiation 1 [65]. Similarly, pulmonary capillary endothelial cells utilize VEGFR2, and after pneumonectomy, the production of MMP14 is restricted to VEGFR2-activated pulmonary capillary endothelial cells but not to other vascular-rich organs, such as the liver, spleen, heart and kidneys, highlighting a unique functional signature of pulmonary capillary endothelial cells in alveolar regeneration [66]. Additionally, when MSCs were co-cultured with endothelial cells and pancreatic organoids, VEGF-secreting MSCs promoted vascularization, as seen by the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms, which was verified by the enhanced expression of specific endothelial phenotype markers, including cluster of differentiation 31 (CD31) and von Willebrand factor [67]. VEGF secreted by MSCs has also been shown to enhance the cutaneous wound healing process in a mouse model. This is because autophagy increases the VEGF secretion from MSCs through regulating extracellular signal-regulated kinase phosphorylation. Kinase phosphorylation then acts on nearby endothelial cells to promote angiogenesis [68]. In tumor organoids, the progressive growth of the tumor eventually leads to intertumoral hypoxia, which induces HIF-1 [40]. HIF-1 upregulates VEGF and VEGF activates endothelial cells in a nearby blood vessel, which induces more sprouting within the tumor [36,47,69]. In similar studies, fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) pathways were shown to be significantly upregulated in human iPSC-derived hepatic endoderm cells when co-cultured with stromal cells [70]. The authors conclude that the FGF pathway promotes new vessel formation by stimulating endothelial cell proliferation and migration, while the BMP pathway is involved in the regulation of endothelial cell differentiation and tissue construction. Additionally, the BMP4 signaling pathway is essential for lung endothelial cell differentiation from bronchioalveolar stem cells along the alveolar lineage, which plays a role in defective alveolar injury repair. In null mice, it was observed that the repair was significantly inhibited illustrating the importance of endothelial repair mechanisms in addition to angiogenic potential [71].
3. Co-Culture of Endothelial Cells with Organoids
Endothelial cells regulate the secretion of vascular growth factors. Co-culturing with endothelial cells allows for the emulation of in vivo cellular interactions, thus enhancing the production of vascular growth factors. Therefore, the inclusion of these cells and factors is paramount to ensuring angiogenesis (Figure 2a). Therefore, the makeup of organoids should be derived from one or a few discrete cell types such as embryonic stem cells, induced pluripotent stem cells, or primary differentiated cells in conjunction with endothelial cells. These cells can self-organize in 3D culture owing to their adhesion strength and dynamic adhesion capacities which enables more in vivo-like structures to be constructed, such as the liver, lung, tumor organoids and more [72,73,74].
3.1. Co-Culture with Endothelial Cells
Endothelial cells, when co-cultured with other types of cells, such as pericytes and MSCs, enable the endogenous secretion of proangiogenic factors, which can promote sprouting and the formation of stable blood vessels [75,76,77]. For instance, co-culturing pericytes and astrocytes with endothelial cells resulted in more stable and shorter vessel branches, with more circular cross-sections and smaller vessel diameters compared to only culture endothelial cells [35]. Manocha et al. found that pericytes were able to induce angiogenesis in endothelial cell spheroids. Conditioned medium from pericytes led to the proliferation of endothelial cells, indicating their ability in angiogenesis stimulation (Figure 2b) [78]. Similarly, MSCs have also been shown to be able to play a role similar to perivascular cells allowing for tight BBB reformation with a vessel-constrictive capacity (Figure 2c) [79]. Endothelial cells/MSCs co-culture demonstrated significant enhancement effects on cell proliferation and angiogenic capacity in direct contact co-culture, and PDGF-BB treatment enhanced angiogenic capacity [80]. In particular, co-culturing spheroids of uniformly distributed endothelial cells and MSCs showed more vasculogenesis and cell–cell communication than endothelial cells seeded on the perimeter of the MSC core [81]. Shanbhag et al. encapsulated human umbilical vein endothelial cells with gingiva-derived progenitor cells as spheroids in a xeno-free environment. Endothelial cells revealed characteristic in vitro sprouting [82].
3.2. Co-Culture of Endothelial Cells with iPSC-Derived Organoids
Mesodermal progenitor cells derived from induced pluripotent stem cells (iPSCs), which differentiate into cells within the vascular niche, have shown interesting vascular network formation in 3D organoid assembly. The inclusion encourages both the development of angiogenesis and vasculogenesis to improve overall organoid maturation [83,84]. Human pluripotent stem cell aggregates can differentiate into organoids by mesoderm induction; following the induction of the mesoderm, endothelial networks emerge through vessel sprouting and develop into well-established blood vessels in about two weeks, supported by pericytes and surrounded by a basement membrane for stability [84]. Wörsdörfer et al. initially incorporated iPSC-derived human mesodermal progenitors specifically into organoids. Since endothelial cells were differentiated from mesodermal progenitor cells treated with VEGF, the co-culture of mesodermal progenitor cells with spheroids resulted in the in vitro generation of vascularized organoids [27]. Likewise, hepatic endoderm cells can be prepared from human iPSCs, to construct three-dimensional vascularized liver buds. This system consists of hepatic endoderm cells with endothelial and mesenchymal lineages which gives rise to a collective structure that better recapitulates the in vivo structure [70].
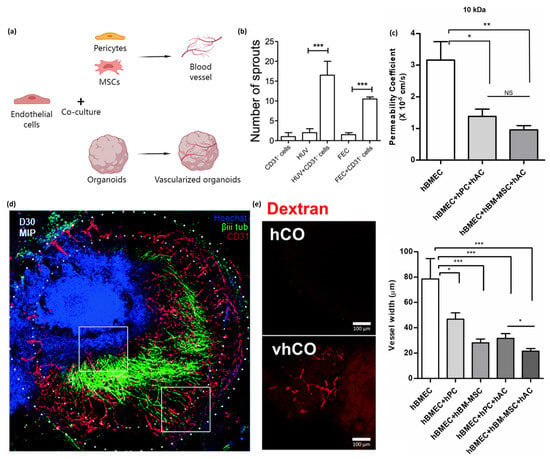
Figure 2.
Co-culturing endothelial cells with other types of cells promotes vasculature formation and vessel-constrictive capacity. Fusing endothelial cell spheroids and organoids results in the formation of vascularized organoids. (a) Schematic of endothelial cells co-cultured with pericytes and MSCs promoting vascular formation in planar culture or co-cultured with organoids to form vascularized organoids. (b) Sprout formation from endothelial cells and pericytes co-cultured with spheroids. The number of sprouts formed from CD31−pericytes or endothelial cells only (HUVECs or FEC), is less than those formed from pericytes and endothelial cells co-cultured with spheroids. Image is adapted with permission from Manocha et al. [78]. Statistical analysis was performed by one way ANOVA and Bonferroni’s post-test; * p ≤ 0.05, ** p < 0.01, *** p < 0.001. (c) Co-culturing with MSC allows for tight BBB reformation with vessel-constrictive capacity, a lower permeability coefficient, and less vessel width. The image is adapted with permission from Kim et al. [79]. All data are presented as mean ± SEM (* p < 0.05, ** p < 0.01, *** p < 0.001, Student’s t-test) (d) Stacked confocal images show the formation of a neurovascular organoid (30 days). The authors highlighted regions of invading endothelial cells (lower white box) and neurite infiltration (upper white box). Immunohistochemistry stained for Hoechst (blue), βiii tubulin (green), and CD31 (red). Endothelial cell spheroids and brain organoids were fused to obtain vascularized brain organoids. The image is adapted with permission from Salmon et al. [85]. (e) Vascularized human cortical organoids formed CD31+ endothelial-like networks on day 50 with 5% FBS supplementation. After incubation in 70 kDa Texas Red dextran containing medium, the tubular structure in vascularized human cortical organoids was visualized. Images are adapted with permission from Ahn et al. [86].
3.3. Vascular Niche Cells Further Promote the Generation of Vascularized Organoids
When vascularization is induced during the early stages of organoid development, it means that blood vessel formation is initiated concurrently with the formation of the organoid structure. On the other hand, inducing vascularization during later stages means that the organoid structure is already established before the vascularization process is initiated.
The former method aims to incorporate vascularization from the beginning, allowing for the development of a well-integrated vascular network throughout the organoid as it matures. During the early stages of this process, endothelial cells are introduced into the organoid culture along with the primary organoid cells. For instance, vascularized liver buds can be developed by seeding iPSC-derived hepatic endoderm, human umbilical vein endothelial cells, and MSCs on Matrigel [70]. The contractile properties of MSCs induced condensation within the mixed population of different cell types, leading to the emergence of spontaneously organized 3D spheroids, and endothelial cells developed early vascular networks in the spheroids [87].
The latter method involves adding or integrating blood vessels into existing organoids to enhance their vascularization. Endothelial cells can be incorporated to vascularize organoids that are generated based on embryoid body formation at a later time point, with some of the literature using a period of two weeks [88]. By fusing a BBB organoid and later stage tissue organoid, it is possible to obtain vascularized brain organoids [85,86]; the fused brain organoids were shown to be engrafted with robust vascular network-like structures, which prolong tissue culture in vitro [89]. Fusing BBB organoids and tissue organoids is another method to form vascular organoids. For instance, endothelial cell spheroids and brain organoids were created separately, and then the two types of organoids were fused to obtain vascularized brain organoids, the fused brain organoids were engrafted with robust vascular network-like structures (Figure 2d) [85,89]. Furthermore, Ahn et al. incorporated blood vessel organoids into brain organoids at day 5 and found vascular cells arising from blood vessel organoids penetrated the cerebral organoids and developed a vessel-like architecture composed of CD31+ endothelial vessels coated with SMA+ or PDGFR+ vascular support cells (Figure 2e). Molecular markers of the BBB were detected in the vascularized cerebral organoids [86]. Finally, the addition of endothelial cells promotes the development of nascent perivascular structures that maturate to generate vessel sprouting and lumen. It is noteworthy that, while the presence of vascular network in the organoids helps with diffusion of nutrients and oxygen, vascularized organoids still lack a perfusion system, which easily leads to the development of a necrotic core [90].
4. Implantation of Organoids In Vivo Leads to Host Anastomosis
Vascularized implants refer to the anastomosis of living vascular networks with artificial tissue constructs, which allows for the growth of large functional tissues owing to nutrient and oxygen delivery [91]. There are two methods to achieve this purpose. One is in situ vascularization of host ischemic wounds. This model is important as it verifies the required cells for angiogenesis, anastomosis and allows for a controlled model for the evaluation of chemokines involved in endothelial, fibroblast, immune, or MSC recruitment as it relates to the creation of a working vasculature in vivo for implants. The other is primary cell organoid implantation, which relies on the host’s vascular network to support the organoid’s vascularization (Figure 3).
4.1. In Situ Vascularization with Vascular Organoids
In situ implantation is done by creating local environments where vascularization is needed and then implanting vascular organoids comprised of vascularizing cell populations, aiming to ensure the incorporation of necessary cells such that anastomosis with the host system is achieved. For example, endothelialized organoids, containing human umbilical vein endothelial cells, were transplanted in the omental pouch of nude rats, whereby endothelialized parts were randomly assembled in vivo, resulting in the formation of channels among individual parts that remained stable for a minimum of two weeks, during which transplanted endothelial cells migrated and established primitive vessels within these channels [92]. Additionally, the implantation of vascular organoids into ischemic injuries such as myocardial infarction, or acute ischemic stroke has shown great potential for promoting neovascularization and tissue regeneration [93,94]. Similarly, myocardial patches, which increase the blood supply to the infarcted site thereby improving cardiac function, do so by promoting vascular proliferation while simultaneously reducing fibrosis [95]. Hindlimb ischemia is a common model of peripheral arterial disease and can lead to tissue necrosis and limb amputation. Wound repair is then initiated by the inclusion of pro-angiogenetic cells such as MSCs within, thereby increasing the tissue’s vascularization, perfusion, and limb retention [96,97,98].
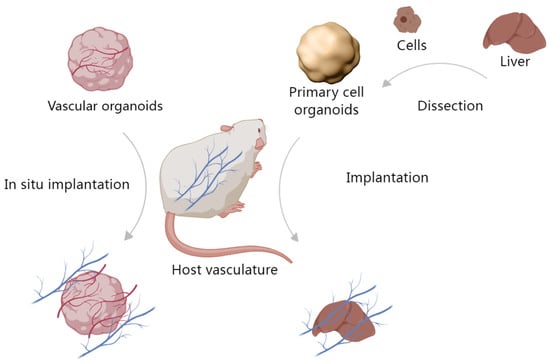
Figure 3.
Implantation of organoids in vivo. One method is in situ vascularization with vascular organoids, the other is primary cell organoid implantation.
4.2. Primary Cell Organoid Implantation
4.2.1. The Tissues Help Vascularize the Implanted Organoids
Primary cell organoids comprised of cells derived from the organ of interest, are typically pre-formed and cultured in vitro before being implanted into the target tissue or organ. Once implanted, they rely on the host tissue’s vascular network to provide the necessary blood supply for their survival and growth. For example, Low et al. introduced kidney organoids, which have cultured for four weeks, under the renal capsule of mice. Subsequently, the dimensions of the implant increased over time, as well as the count of vessels with perfusion. These kidney organoids, upon implantation into a host mouse, also showed gradual acquisition of glomeruli and mature architecture [99]. Takebe et al. showed the generation of vascularized and functional human liver from human iPSCs by transplantation of liver buds created in vitro, whereby the liver buds connected to the host vessels within 48 h, thereby becoming functional. These implants showed the formation of functional vasculature, which stimulated the maturation of liver buds into tissue resembling the adult liver [70]. Additionally, Mansour et al. developed an in vivo implantation model of human pluripotent stem cell-derived brain organoids. When the mouse brain was implanted with the brain organoid, the functional vascular system was established, promoting mature brain tissue generation in vivo [100].
4.2.2. Implanted Organoids Contribute to the Organism
Implanted cardiac organoids develop and secrete better than their ex vivo counterparts when integrated into the host vasculature [88,93]. Van den Berg et al. developed glomerular structures by transplanting kidney organoids derived from human iPSCs. These organoids were placed under the renal capsule, where they showed invasion of the host vasculature into the organoids. Implanted organoids also showed a greater degree of functionality compared with organoids that were not implanted [101]. Similarly, Li et al. generated 3D vascularized liver buds from human MSCs, MSC-derived hepatocytes, hepatic stellate, and sinusoidal endothelial cells. These organoids were then implanted into the mesentery of a murine liver failure model which they effectively rescued the animals from [102]. Pham et al. engrafted pre-vascularized, 54-day maturation, brain organoids into a mouse brain for 3–5 weeks, and observed after transplantation, human CD31-positive blood vessels between rosettes within the center of the organoid [88]. In vivo, perfusable implanted organoids can be incorporated into animal models, which can reflect complex biological environments.
5. Development of Organoids-on-a-Chip (OOCoid) within a Perfusable System
In vivo, perfusable implanted organoids can be incorporated into animals, but this results in a less controllable system. To control the in vitro environment and improve vascularization and tissue development, it is desirable to integrate the organoid into a perfusable system. Perfusion systems offer in vitro incorporation of organoids into microfluidic bioreactor systems which allow for external fluid flow to increase the delivery of nutrients [13,103,104] (Figure 4a). OOCoid demonstrates that the vascular network connected to external flow can deliver biological substances to the interior of the organoid, which decreases the necrotic core of organoids in vitro [103,105]. For example, Wang et al. proposed that perfusion resulting from chip-based flow would enhance cell viability of liver organoids compared to those under static culture. At day 30, organoids that had undergone dynamic culturing showed significantly less caspase-3 staining, which the author connects to the delivery of necessary nutrients [103].
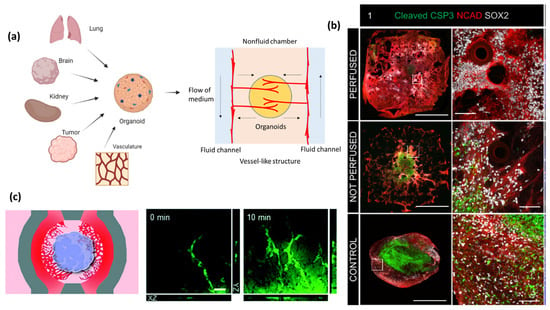
Figure 4.
Vascularized organoids (e.g., kidney, brain, and more) on-a-chip. The use of chips allows for the formation of functional vascular networks within the organoid. (a) A general schematic of the microfluidic device in OOCoids. The central gel region contains cells, which may be combined from multiple organ or tissue sources to construct an organoid. The central region also contains hydrogels and side channels that were filled with fluid cell culture medium. This design encourages in-growth of vascular cells from side chambers (arrows). (b) Fluorescent images of caspase-3 following 62 days of culture for perfused (top row), non-perfused (middle) and control (bottom row); cleaved caspase-3 (green), N-Cadherin (red) and Sox2 (white). Staining shows increased caspase-3/necrotic formation in the control and non-perfused organoids. The image is adapted with permission from Grebenyuk et al. [106]. (c) Vascular cells physically interact with the cerebral organoid, resulting in the formation of an integrated neurovascular organoid on the chip. The image is adapted with permission from Salmon et al. [85].
5.1. Perfusable System
OOCoids give rise to the combination of microfluidic techniques and organoid cultures resulting in “organoids-on-a-chip”, which helps to establish fluid renewal and stresses that induce in vivo-like vasculogenesis [103,104]. The flow aligns and maintains the endothelial cell layer within vessels in vivo, contributing to the structural integrity, while the pressure adjusts the vessel diameter to regulate the flow [107,108,109]. Through in vitro experiments, the impact of flow on vascularization can be simulated. For example, Rimal et al. proposed that 3D vascularized human skin models in flow culture promoted vascular openings as perfusable sites. Dynamic flow culture improved the skin barrier properties, facilitated the fabrication of thicker tissues, and enhanced wound closure [110]. The flow rate and pressure of fluid in the perfusable system can be tuned to further enhance vascularization and nutrient delivery to the organoid cells. Therefore, perfusable systems can improve tissue function and can be useful for drug screening and disease modeling [103,111]. Hydrogel is an important part of creating perfusable systems in vitro, as it gives support to the formation of blood vessels in a 3D space. The use of chips allows for the integration of multiple cell types and for the combination of vascular and tissue organoids, to promote the formation of functional vascular networks within the organoid.
5.2. Vascularized Organoids on a Chip
5.2.1. Lung Organoids
Recently, lung organoids on a chip have been used to investigate lung diseases such as chronic obstructive pulmonary disease and acute respiratory disease by simulating the alveolar–capillary barrier [10,112]. The self-assembly of lung fibroblasts with human umbilical vein endothelial cells results in the formation of vascular networks that undergo angiogenic sprouting [104]. In addition, Ko et al. developed a novel bioreactor that contained a circular, fenestrated inner chamber, which only allowed media and nutrients to bypass, while restricting cells to the fenestrations. This layout recreates the endothelial lining of vessels found in vivo. Endothelial cells co-cultured with lung fibroblasts on the chip can reproduce a 3D vascular network model that controls the sprouting direction of the angiogenic response in all directions [113].
5.2.2. Brain and Blood-Brain Barrier (BBB) Organoids
Perfusive BBB and brain OOCoid models have shown impressive vascularization potential due to the inclusion of endothelial cells in either the support matrix or the organoid. In these models, proper alignment of the cells even gives rise to barrier-like function similar to that found in vivo [85,106]. For example, Grebenyuk et al. used a 2-photon-mediated, 3D microfluidics device (Figure 4b), which enabled them to construct neural spheroids. These spheroids were cultured for 5 days before they were added to Matrigel systems that were then either perfused or held static. By day 62, the author observed that the organoids had exceeded 2 mm in scale. Extensive necrosis, by way of caspase-3 staining, was observed in the core of organoids in the non-perfused group, while those in the perfused group showed minimal caspase-3 staining [106]. In addition, Salmon et al. designed an open well microfluidic chip with fenestrated, microfluidic channels flanking the central organoid chamber for seeding vascular cells via an inlet. Vascular cells physically interact with the cerebral organoid, resulting in the formation of an integrated neurovascular organoid on the chip. Fluorescein-40kDa dextran was employed to validate BBB integrity and observe vascular channels. A strong fluorescent signal was observed within the vascular network (Figure 4c) [85]. The BBB is a highly selective and protective barrier that separates blood circulation from the brain and the central nervous system, which plays a crucial role in maintaining the brain’s microenvironment and protecting it from potentially harmful substances [114]. Nashimoto et al. established a spheroid model that contained a multi-chamber system. Endothelial cells were cultured in a central fibrin-collagen coated chamber, which the authors suggest recapitulates the in vivo ECM. Endothelial cells were also in adjoining chambers thereby creating a system by which cells could migrate between the spheroid and the adjoining chambers [115]. Numerous advantages are observed from using chip-based systems for the analysis of brain organoids. One is the simplification of the evaluation of BBB permeability, for example, in a BBB-glioma-on-a-chip model high levels of barrier function, fluorescein, and structure similar to that found in vivo were observed [11]. Additionally, OOCoid devices allow for the detailed analysis of BBB functions such as calcium related neuronal activity [116]. Incorporation of neural and vascular cells results in the formation of neurovascular units, which leads to vascularization and production of essential neurotransmitters such as glutamate and gamma-aminobutyric acid [117]. Dysfunctional BBB is also linked to Alzheimer’s, Huntington’s, Parkinson’s disease, and multiple sclerosis; therefore, a vascularized brain model would be invaluable for improving the assessment and understanding of these diseases [118].
5.2.3. Kidney Organoids
Kidneys function by taking in arterial blood and filtering it to regulate nitrogen/urea, glucose, electrolytes and hypostasis. Filtration results from the complex structure of glomerular units comprised of vascular capillaries and excretory tubules within the Bowman’s capsule. Due to the importance of this organ to body function, kidney organoids are typically glomeruli-on-a-chip or tubules-on-a-chip models [111,119]. The chip-based system has been able to establish primitive blood filtration, re-absorption, and urine production, which are key functions of the kidney [120]. Canonically, models were constructed from primary kidney cells and endothelial cells, which were incorporated into hydrogel-based chip systems to achieve diffusive filtration through the kidney unit [30]. Rayner et al. proposed a filtration that occurs across the endothelial–epithelial surface in the glomerulus and reactor systems utilize a collagen membrane as an interface for exchange. This structural design recapitulates the in vivo dynamic conditions of the kidney. This is because it employs a cell-remodelable hydrogel and customizable perfusion flow which recreates the human renal vascular–tubular unit. The authors prove this by showing valid quantification of the selective reabsorption of albumin [121]. Furthermore, flow can enhance tubular and glomerular maturation within the organoid following vascular perfusion [122]. Hiratsuka et al. utilized organoid-on-a-chip to generate a patient autosomal recessive polycystic kidney disease pathogenesis model. With this model, they made a comparison between flow and static culture organoids and revealed more mature nephron structures that developed underflow. Additionally, by employing low-molecular-weight dextran glomerular-like structures, tubular lumen formation was observed in the perfusion condition. This indicates the presence of tubular luminal flow, which activates mechanosensing in organoid tubules, which the author proposes is important for autosomal recessive polycystic kidney disease pathogenesis [54].
5.2.4. Tumor Organoids
Attempts at understanding tumor progression have been advanced by the generation of 3D vascularized tumor organoids comprised of a mixture of tumor and endothelial cells within a 3D gel environment containing fibroblasts [123]. This construction results in the formation of self-assembled tumor microvasculature, which penetrates the tumor organoids and extends into the surrounding gel [69]. However, these organoids were generated in the static culture portion of the chip, and therefore lack interstitial flow resulting in improper vascular lumen formation. So, when malignant glioblastoma cells are seeded in an adjacent compartment, the tumor-cell-derived soluble factors increase angiogenesis of the tumor in the microfluidic component of the chip [124]. In a similar study, it was observed that vessel sprouting within a collagen gel in a flow-directed, 3D human renal cell carcinoma-on-a-chip could be enhanced by the inclusion of renal cell carcinoma cells [125]. These examples illustrate the importance of perfusion even in tumor systems, where it is shown to elevate the tumor model to a more in vivo-like system.
Microvessel invasion of tumors and tumor migration towards microvessels is a key feature of metastasis whereby cells enter the vasculature and seed other organ systems [123,126,127]. Tumor cell migration, like endothelial cell migration, has been linked to the occurrence of morphogens such as VEGF gradients within the interstitial flow. This has been explored in multichambered chip systems in which tumor and microvascular assemblies reside in adjoining chambers. Over time tumor cells invade the vascular assemblies and penetrate inside [123]. These types of systems have also been used to simulate the role of natural killer leukocytes to destroy cancer. In these systems, natural killer cells actively migrate across the endothelial barrier of the vasculature to destroy the tumor cells [114,128]. Understanding the crosstalk between endothelial, immune and cancer cells is vital as it has been shown that tumor cells that have been in contact with immune macrophages have an enhanced intravasation rate on microfluidic devices. The literature suggests that this is a result of secreted TNFα, which increases microvasculature permeability [126]. Additionally, the use of tumor-on-chip models improves the in vitro recapitulation of the complex 3D microenvironment of tumors. This opens the doors to reducing obstacles in creating cancer treatments, thereby simultaneously reducing cost and time [129].
6. Conclusions
Organoids hold great potential for studying the development of organ systems and for the establishment of donor organs for transplantation; however, a major limitation in organoid execution is due to the small size and incapability of long-term culture. Both of these points can be addressed by the inclusion of perfusable vasculature which will provide the cells with the needed nutrients to grow and maintain viability. Initially, studies focused on cells that made up the primary tissue, but this view has shifted to encompass the use of vascular niche cells such as endothelial cells. The inclusion of these cells has been shown to create nascent vasculature within the organoid. Additionally, the implantation of these organoids shows their capability to connect with the host vasculature. Following these successes, the need for greater in vitro perfusibility was explored. This has been mostly covered by the use of vascularizable hydrogel systems. The merging of the pre-vascularized and vascular hydrogels has resulted in the creation of perfusable in vitro organoid systems that will enable an improved view of how organ systems organize and develop in vivo. Much work is still needed to further the development of these systems to better recapitulate the in vivo system such as the inclusion of greater nutrient availability, growth factor inclusion/secretion, and culture environment optimization; however, the results so far have been very promising.
Author Contributions
Conceptualization, X.W. and B.M.B.; writing—original draft preparation, X.W. and B.M.B.; writing—review and editing, X.W., B.M.B. and N.A.K.; Visualization, X.W.; supervision, N.A.K.; funding acquisition, N.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received financial support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (CoEvolve, grant no. 851960, N.A.K.) the Gravitation Program “Materials-Driven Regeneration”, funded by the Netherlands Organization for Scientific Research (024.003.013, N.A.K.), and the China Scholarship Council (X.W.). The APC for this invited review article is funded by Organoids, MDPI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicble.
Acknowledgments
The authors thank Aref Saberi and Chunling Tang for insightful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 2013, 493, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Pacitti, D.; Privolizzi, R.; Bax, B.E. Organs to Cells and Cells to Organoids: The Evolution of in vitro Central Nervous System Modelling. Front. Cell. Neurosci. 2019, 13, 129. [Google Scholar] [CrossRef]
- Alhaque, S.; Themis, M.; Rashidi, H. Three-dimensional cell culture: From evolution to revolution. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170216. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Meng, T.; Yang, J.; Hu, N.; Zhao, H.; Tian, T. Three-dimensional in vitro tissue culture models of brain organoids. Exp. Neurol. 2021, 339, 113619. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, Y.; Liao, L.; Tian, W. Oral Organoids: Progress and Challenges. J. Dent. Res. 2021, 100, 454–463. [Google Scholar] [CrossRef]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Huh, D.; Fujioka, H.; Tung, Y.-C.; Futai, N.; Paine, R.; Grotberg, J.B.; Takayama, S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl. Acad. Sci. USA 2007, 104, 18886–18891. [Google Scholar] [CrossRef]
- Mejías, J.C.; Nelson, M.R.; Liseth, O.; Roy, K. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip 2020, 20, 3601–3611. [Google Scholar] [CrossRef]
- Shi, Y.; He, X.; Wang, H.; Dai, J.; Fang, J.; He, Y.; Chen, X.; Hong, Z.; Chai, Y. Construction of a novel blood brain barrier-glioma microfluidic chip model: Applications in the evaluation of permeability and anti-glioma activity of traditional Chinese medicine components. Talanta 2023, 253, 123971. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, S.; Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.S.; Kim, S.J.; Oh, S.T.; Bae, C.M.; Choi, W.-Y.; Baek, K.M.; Kim, J.S.; Lee, M.Y. A simple method to improve the quality and yield of human pluripotent stem cell-derived cerebral organoids. Heliyon 2021, 7, e07350. [Google Scholar] [CrossRef]
- Biju, T.S.; Priya, V.V.; Francis, A.P. Role of three-dimensional cell culture in therapeutics and diagnostics: An updated review. Drug Deliv. Transl. Res. 2023, 13, 2239–2253. [Google Scholar] [CrossRef]
- Gonçalves, R.C.; Banfi, A.; Oliveira, M.B.; Mano, J.F. Strategies for re-vascularization and promotion of angiogenesis in trauma and disease. Biomaterials 2021, 269, 120628. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting vasculature: Materials, cells and emergent techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Sainson, R.C.A.; Aoto, J.N.; Taylor, K.L.; Aitkenhead, M.; Pérez-Del-Pulgar, S.; Carpenter, P.M.; Hughes, C.C.W. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and Angiopoietin-1. Microvasc. Res. 2003, 66, 102–112. [Google Scholar] [CrossRef]
- Vokes, S.A.; Yatskievych, T.A.; Heimark, R.L.; McMahon, J.; McMahon, A.P.; Antin, P.B.; Krieg, P.A. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development 2004, 131, 4371–4380. [Google Scholar] [CrossRef]
- Rao, R.R.; Peterson, A.W.; Ceccarelli, J.; Putnam, A.J.; Stegemann, J.P. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, 253–264. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, A.C.; Kielty, C.M. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int. J. Biochem. Cell Biol. 2004, 36, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, I.W.; Du, J.; Cong, Z.; Cho, B.S.; Klein, A.M.; Dieck, C.L.; Chaudhri, R.A.; Cuervo, H.; Herts, J.H.; Kitajewski, J. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis 2016, 19, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chuah, Y.J.; Fu, J.; Zhu, W.; Wang, D.-A. Co-culture of human umbilical vein endothelial cells and human bone marrow stromal cells into a micro-cavitary gelatin-methacrylate hydrogel system to enhance angiogenesis. Mater. Sci. Eng. C 2019, 102, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Z.; Kamm, R.D. Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature. Lab Chip 2021, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, L.; Zhao, L.; Zou, T.; Xu, H. Toward the next generation of vascularized human neural organoids. Med. Res. Rev. 2022, 43, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Bhushan, A.; Senutovitch, N.; Bale, S.S.; McCarty, W.J.; Hegde, M.; Jindal, R.; Golberg, I.; Usta, O.B.; Yarmush, M.L.; Vernetti, L.; et al. Towards a three-dimensional microfluidic liver platform for predicting drug efficacy and toxicity in humans. Stem Cell Res. Ther. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-Chip. Front. Bioeng. Biotechnol. 2021, 9, 637048. [Google Scholar] [CrossRef]
- Mazio, C.; Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Recapitulating spatiotemporal tumor heterogeneity in vitro through engineered breast cancer microtissues. Acta Biomater. 2018, 73, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.F.L.; Lu, R.X.Z.; Huyer, L.D.; Kakinoki, S.; Yazbeck, J.; Wang, E.Y.; Wu, Q.; Zhang, B.; Radisic, M. A well plate–based multiplexed platform for incorporation of organoids into an organ-on-a-chip system with a perfusable vasculature. Nat. Protoc. 2021, 16, 2158–2189. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Isu, G.; Lemme, M.; Conficconi, C.; Oertle, P.; Räz, C.; Visone, R.; Cerino, G.; Plodinec, M.; Rasponi, M.; et al. A three-dimensional in vitro dynamic micro-tissue model of cardiac scar formation. Integr. Biol. 2018, 10, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Srinivasan, B.; Esch, M.B.; McLamb, W.T.; Bernabini, C.; Shuler, M.L.; Hickman, J.J. Using PBPK guided ‘Body-on-a-Chip’ Systems to Predict Mammalian Response to Drug and Chemical Exposure. Exp. Biol. Med. 2014, 239, 1225–1239. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Karamysheva, A.F. Mechanisms of angiogenesis. Biochemistry 2008, 73, 751–762. [Google Scholar] [CrossRef]
- Risau, W. Embryonic angiogenesis factors. Pharmacol. Ther. 1991, 51, 371–376. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and Angiogenesis BT. In Angiogenesis in Brain Tumors; Kirsch, M., Black, P.M., Eds.; Springer: Boston, MA, USA, 2004; pp. 3–32. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Gaengel, K.; Niaudet, C.; Hagikura, K.; Laviña, B.; Muhl, L.; Hofmann, J.J.; Ebarasi, L.; Nyström, S.; Rymo, S.; Chen, L.L.; et al. The Sphingosine-1-Phosphate Receptor S1PR1 Restricts Sprouting Angiogenesis by Regulating the Interplay between VE-Cadherin and VEGFR2. Dev. Cell 2012, 23, 587–599. [Google Scholar] [CrossRef]
- Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 2008, 4, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fraisl, P.; Mazzone, M.; Schmidt, T.; Carmeliet, P. Regulation of Angiogenesis by Oxygen and Metabolism. Dev. Cell 2009, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Nehls, V.; Denzer, K.; Drenckhahn, D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992, 270, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Amselgruber, W.M.; Schäfer, M.; Sinowatz, F. Angiogenesis in the bovine corpus luteum: An immunocytochemical and ultrastructural study. Anat. Histol. Embryol. 1999, 28, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, K.Y.; Brekken, R.A. Recruitment and retention: Factors that affect pericyte migration. Cell. Mol. Life Sci. 2013, 71, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.M.; van Dijk, C.G.; Kramann, R.; Verhaar, M.C.; Cheng, C. Implementation of Pericytes in Vascular Regeneration Strategies. Tissue Eng. Part B Rev. 2022, 28, 1–21. [Google Scholar] [CrossRef]
- Phng, L.-K.; Gerhardt, H. Angiogenesis: A Team Effort Coordinated by Notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef]
- Draoui, N.; de Zeeuw, P.; Carmeliet, P. Angiogenesis revisited from a metabolic perspective: Role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017, 7, 170219. [Google Scholar] [CrossRef]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arter. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2012, 3, a006569. [Google Scholar] [CrossRef]
- Goumans, M.-J.; Lebrin, F.; Valdimarsdottir, G. Controlling the angiogenic switch: A balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovasc. Med. 2003, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Arany, P.R.; Philip, A.; McLean, S.; Di Guglielmo, G.M.; Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A.; Boo, S.; et al. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv. Wound Care 2013, 2, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, K.; Miyoshi, T.; Kroll, K.T.; Gupta, N.R.; Valerius, M.T.; Ferrante, T.; Yamashita, M.; Lewis, J.A.; Morizane, R. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci. Adv. 2022, 8, eabq0866. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Oubaha, M.; Cagnone, G.; Boscher, C.; Kim, J.S.; El Bakkouri, Y.; Zhang, Y.; Chidiac, R.; Corriveau, J.; Delisle, C.; et al. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell. Mol. Life Sci. 2021, 79, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Stroka, K.M. Vascular endothelial cell mechanosensing: New insights gained from biomimetic microfluidic models. Semin. Cell Dev. Biol. 2017, 71, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Simon, S.I.; Curry, F.-R.E. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 2014, 16, 505–532. [Google Scholar] [CrossRef]
- Belair, D.G.; Schwartz, M.P.; Knudsen, T.; Murphy, W.L. Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta Biomater. 2016, 39, 12–24. [Google Scholar] [CrossRef]
- Saik, J.E.; Gould, D.J.; Watkins, E.M.; Dickinson, M.E.; West, J.L. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomater. 2011, 7, 133–143. [Google Scholar] [CrossRef]
- Pepper, M.; Vassalli, J.-D.; Orci, L.; Montesano, R. Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp. Cell Res. 1993, 204, 356–363. [Google Scholar] [CrossRef]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, S.; Hamano, K.; Nishida, M.; Kobayashi, T.; Li, T.-S.; Kobayashi, S.; Matsuzaki, M.; Zempo, N.; Esato, K. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J. Surg. Res. 2001, 96, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Hu, X.; Pan, L.; Han, S.; Cao, C.; Jia, Z.; Li, M. Human primary CD34+ cells transplantation for critical limb ischemia. J. Clin. Lab. Anal. 2018, 32, e22569. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.-S.; Nolan, D.J.; Butler, J.M.; James, D.; Babazadeh, A.O.; Rosenwaks, Z.; Mittal, V.; Kobayashi, H.; Shido, K.; Lyden, D.; et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010, 468, 310–315. [Google Scholar] [CrossRef]
- Ding, B.-S. Endothelial-derived inductive angiocrine signals initiate and sustain regenerative lung alveolarization. Cell 2011, 147, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by Mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2017, 17, 1667–1675. [Google Scholar] [CrossRef]
- An, Y.; Liu, W.J.; Xue, P.; Ma, Y.; Zhang, L.Q.; Zhu, B.; Qi, M.; Li, L.Y.; Zhang, Y.J.; Wang, Q.T.; et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Ehsan, S.M.; Welch-Reardon, K.M.; Waterman, M.L.; Hughes, C.C.W.; George, S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 2014, 6, 603–610. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Lee, J.-H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.-H.; Ryeom, S.; Kim, C.F. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell 2014, 156, 440–455. [Google Scholar] [CrossRef]
- van der Vaart, J.; Clevers, H. Airway organoids as models of human disease. J. Intern. Med. 2020, 289, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Pinel, L.; Cyr, D.G. Self-renewal and differentiation of rat epididymal basal cells using a novel in vitro organoid model. Biol. Reprod. 2021, 105, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Ogoke, O.; Maloy, M.; Parashurama, N. The science and engineering of stem cell-derived organoids-examples from hepatic, biliary, and pancreatic tissues. Biol. Rev. 2020, 96, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yoshitomi, H.; Rossant, J.; Zaret, K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001, 294, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Narayanan, K.; Leong, M.F.; Wan, A.C. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 2014, 35, 6006–6014. [Google Scholar] [CrossRef] [PubMed]
- Baranski, J.D.; Chaturvedi, R.R.; Stevens, K.R.; Eyckmans, J.; Carvalho, B.; Solorzano, R.D.; Yang, M.T.; Miller, J.S.; Bhatia, S.N.; Chen, C.S. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 2013, 110, 7586–7591. [Google Scholar] [CrossRef] [PubMed]
- Manocha, E.; Consonni, A.; Baggi, F.; Ciusani, E.; Cocce, V.; Paino, F.; Tremolada, C.; Caruso, A.; Alessandri, G. CD146+ Pericytes Subset Isolated from Human Micro-Fragmented Fat Tissue Display a Strong Interaction with Endothelial Cells: A Potential Cell Target for Therapeutic Angiogenesis. Int. J. Mol. Sci. 2022, 23, 5806. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lim, J.; Choi, H.; Kang, H.; Jeon, N.L.; Son, Y. Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials 2021, 279, 121210. [Google Scholar] [CrossRef]
- Liang, T.; Zhu, L.; Gao, W.; Gong, M.; Ren, J.; Yao, H.; Wang, K.; Shi, D. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio 2017, 7, 1722–1736. [Google Scholar] [CrossRef]
- Vorwald, C.E.; Joshee, S.; Leach, J.K. Spatial localization of endothelial cells in heterotypic spheroids influences Notch signaling. J. Mol. Med. 2020, 98, 425–435. [Google Scholar] [CrossRef]
- Shanbhag, S.; Rashad, A.; Nymark, E.H.; Suliman, S.; de Lange Davies, C.; Stavropoulos, A.; Bolstad, A.I.; Mustafa, K. Spheroid Coculture of Human Gingiva-Derived Progenitor Cells with Endothelial Cells in Modified Platelet Lysate Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 739225. [Google Scholar] [CrossRef]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef] [PubMed]
- Salmon, I.; Grebenyuk, S.; Fattah, A.R.A.; Rustandi, G.; Pilkington, T.; Verfaillie, C.; Ranga, A. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip 2022, 22, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; An, J.-H.; Yang, H.-J.; Gil Lee, D.; Kim, J.; Koh, H.; Park, Y.-H.; Song, B.-S.; Sim, B.-W.; Lee, H.J.; et al. Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells 2021, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef]
- Phama, B.M.T.; Pollocka, K.M.; Rosea, M.D.; Carya, W.A.; Stewarta, H.R.; Zhoua, P.; Noltaa, J.A.; Waldaua, B. Generation of human vascularized brain organoids. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G.; et al. Generation of vascularized brain organoids to study neurovascular interactions. eLife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Zhang, S.; Kan, E.L.; Kamm, R.D. Integrating functional vasculature into organoid culture: A biomechanical perspective. APL Bioeng. 2022, 6, 030401. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Du, J.; Wang, T.; Li, Y.; Zeng, W. Application of stem cells in engineered vascular graft and vascularized organs. Semin. Cell Dev. Biol. 2022, 144, 31–40. [Google Scholar] [CrossRef]
- Gupta, R.; Van Rooijen, N.; Sefton, M.V. Fate of endothelialized modular constructs implanted in an omental pouch in nude rats. Tissue Eng. Part A 2009, 15, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Y.; Gao, J.; Yang, L.; Mao, D.; Stefanitsch, C.; Li, Y.; Zhang, J.; Ou, L.; Kong, D.; et al. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 2015, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, M.; Li, P.; Zhao, S.; Yang, G.; Zhang, W.; Yang, Y. Implantation and repair of 3D printed myocardial patch in rabbit model of myocardial infarction. Bioprinting 2021, 24, e00165. [Google Scholar] [CrossRef]
- Qin, J.; Yuan, F.; Peng, Z.; Ye, K.; Yang, X.; Huang, L.; Jiang, M.; Lu, X. Periostin enhances adipose-derived stem cell adhesion, migration, and therapeutic efficiency in Apo E deficient mice with hind limb ischemia. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plock, J.A.; Schnider, J.T.; Zhang, W.; Schweizer, R.; Tsuji, W.; Kostereva, N.; Fanzio, P.M.; Ravuri, S.; Solari, M.G.; Cheng, H.-Y.; et al. Adipose- and Bone Marrow–Derived Mesenchymal Stem Cells Prolong Graft Survival in Vascularized Composite Allotransplantation. Transplantation 2015, 99, 1765–1773. [Google Scholar] [CrossRef]
- Plock, J.A.; Schnider, J.T.; Schweizer, R.; Zhang, W.; Tsuji, W.; Waldner, M.; Solari, M.G.; Marra, K.G.; Rubin, J.P.; Gorantla, V.S. The influence of timing and frequency of adipose-derived mesenchymal stem cell therapy on immunomodulation outcomes after vascularized composite allotransplantation. Transplantation 2017, 101, e1–e11. [Google Scholar] [CrossRef]
- Low, J.H.; Li, P.; Chew, E.G.Y.; Zhou, B.; Suzuki, K.; Zhang, T.; Lian, M.M.; Liu, M.; Aizawa, E.; Esteban, C.R.; et al. Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell Stem Cell 2019, 25, 373–387.e9. [Google Scholar] [CrossRef]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Berg, C.W.v.D.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; Berg, B.M.v.D.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J.; et al. Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Rep. 2018, 10, 751–765. [Google Scholar] [CrossRef]
- Li, J.; Xing, F.; Chen, F.; He, L.; So, K.-F.; Liu, Y.; Xiao, J. Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages. Cell Transplant. 2018, 28, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018, 18, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.-S.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef]
- Licata, J.P.; Schwab, K.H.; Har-El, Y.-E.; Gerstenhaber, J.A.; Lelkes, P.I. Bioreactor Technologies for Enhanced Organoid Culture. Int. J. Mol. Sci. 2023, 24, 11427. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, S.; Fattah, A.R.A.; Kumar, M.; Toprakhisar, B.; Rustandi, G.; Vananroye, A.; Salmon, I.; Verfaillie, C.; Grillo, M.; Ranga, A. Large-scale perfused tissues via synthetic 3D soft microfluidics. Nat. Commun. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Dessalles, C.A.; Leclech, C.; Castagnino, A.; Barakat, A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Whited, B.M.; Rylander, M.N. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2013, 111, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Determinants of tumor blood flow: A review. Cancer Res. 1988, 48, 2641–2658. [Google Scholar]
- Rimal, R.; Marquardt, Y.; Nevolianis, T.; Djeljadini, S.; Marquez, A.B.; Huth, S.; Chigrin, D.N.; Wessling, M.; Baron, J.M.; Möller, M.; et al. Dynamic flow enables long-term maintenance of 3-D vascularized human skin models. Appl. Mater. Today 2021, 25, 101213. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Lee, Y.; Lee, S.; Lee, S.; Jeon, N.L. Human Ocular Angiogenesis-Inspired Vascular Models on an Injection-Molded Microfluidic Chip. Adv. Healthc. Mater. 2019, 8, e1900328. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.; George, S.C. Engineering Vascularized Organoid-on-a-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2019, 229, 119547. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; A FitzGerald, E.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.T.; Bender, R.H.F.; Andrejecsk, J.W.; Sobrino, A.; Hachey, S.J.; George, S.C.; Hughes, C.C. Blood–brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood–central nervous system interface. Exp. Biol. Med. 2017, 242, 1669–1678. [Google Scholar] [CrossRef]
- Petrosyan, A.; Cravedi, P.; Villani, V.; Angeletti, A.; Manrique, J.; Renieri, A.; De Filippo, R.E.; Perin, L.; Da Sacco, S. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Weinberg, E.; Kaazempur-Mofrad, M.; Borenstein, J. Concept and computational design for a bioartificial nephron-on-a-chip. Int. J. Artif. Organs 2008, 31, 508–514. [Google Scholar] [CrossRef]
- Rayner, S.G.; Phong, K.T.; Xue, J.; Lih, D.; Shankland, S.J.; Kelly, E.J.; Himmelfarb, J.; Zheng, Y. Reconstructing the Human Renal Vascular–Tubular Unit In Vitro. Adv. Healthc. Mater. 2018, 7, e1801120. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.P.; Tsuchida, C.; Zheng, Y.; Himmelfarb, J.; Akilesh, S. A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis. Neoplasia 2018, 20, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [PubMed]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Truttschel, R.; Gong, M.M.; Humayun, M.; Virumbrales-Munoz, M.; Vitek, R.; Felder, M.; Gillies, S.D.; Sondel, P.; Wisinski, K.B.; et al. Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. Oncoimmunology 2018, 8, 1553477. [Google Scholar] [CrossRef]
- Subia, B.; Dahiya, U.R.; Mishra, S.; Ayache, J.; Casquillas, G.V.; Caballero, D.; Reis, R.L.; Kundu, S.C. Breast tumor-on-chip models: From disease modeling to personalized drug screening. J. Control. Release 2021, 331, 103–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).