Meniscus Repair: From In Vitro Research to Patients
Abstract
1. The Meniscus: Microarchitecture, Functions, and the Occurrence of Injuries
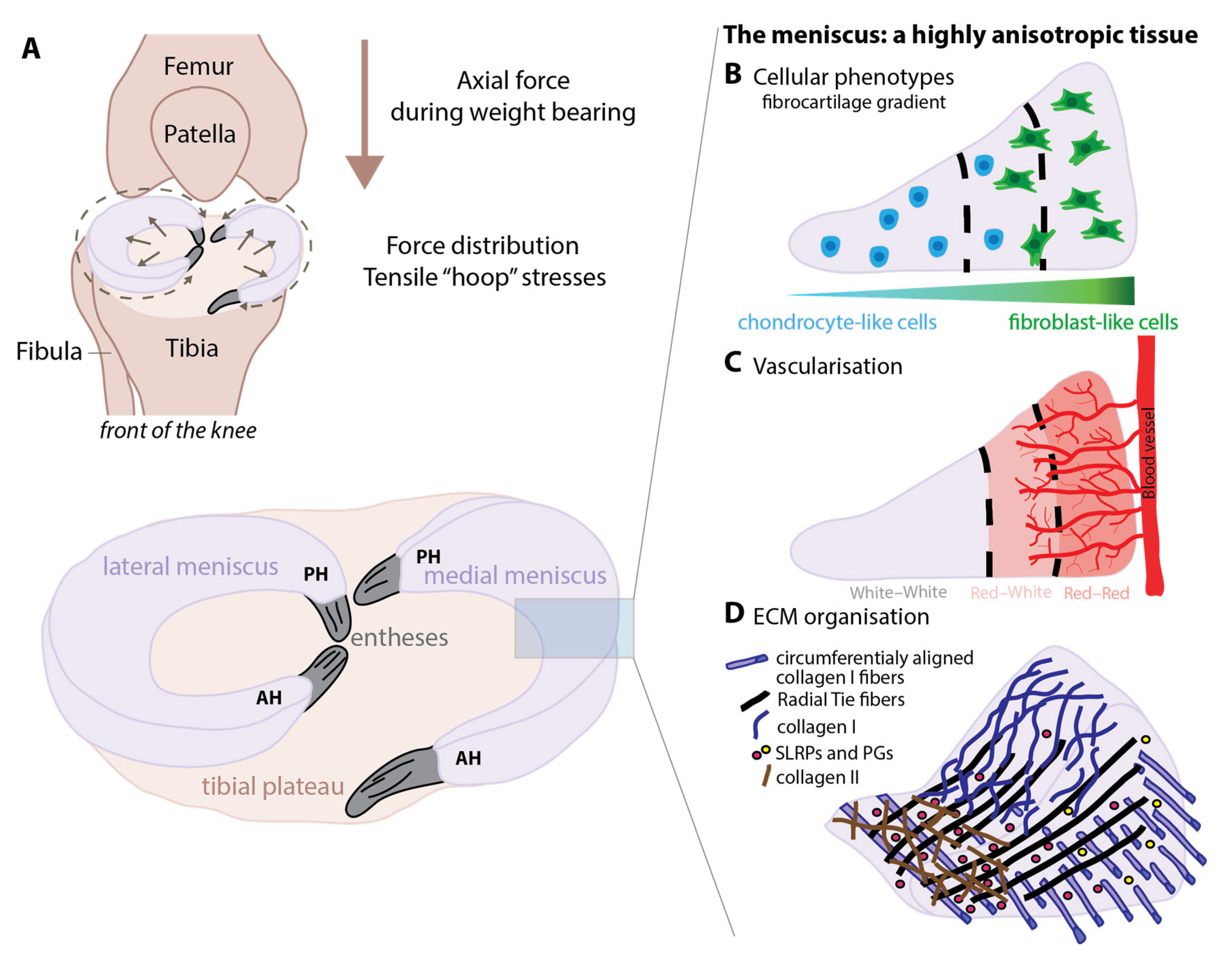
1.1. Microarchitecture
1.2. Mechanical Properties
1.3. Meniscus Tears
- -
- Traumatic meniscus injuries, due to an acute trauma in usually young and sportive patients and generally caused by a too high loading or twisting of the knee, often resulting in the onset of pain. Knee injuries correspond to approximately 40% of sport-related injuries and among them around 11% to medial meniscus injuries (more exposed to biomechanical forces compared to the lateral meniscus) and around 4% to lateral meniscus injuries [30]. Sports that are mainly at risk were reported to be: soccer, gymnastics, dancing, tennis, and jogging [30].
- -
- Degenerative meniscus injuries caused by slow degradation of the tissue in older patients.
2. Therapeutic Options: From Meniscectomy to Preservative Therapies
2.1. Meniscectomy/Partial Meniscectomy
2.2. Meniscal Allograft Transplantation (MAT)
2.3. Meniscus-Derived Scaffolds
2.4. Biological Augmentation Strategies and Synthetic Meniscus Scaffolds
- -
- for the fibrin glue as well as fibrin clots, it has been reported that scientific evidence studies are still lacking to evaluate the efficiency of this procedure.
- -
3. Tissue Engineering of the Meniscus and at a Larger Scale the Whole Knee Joint
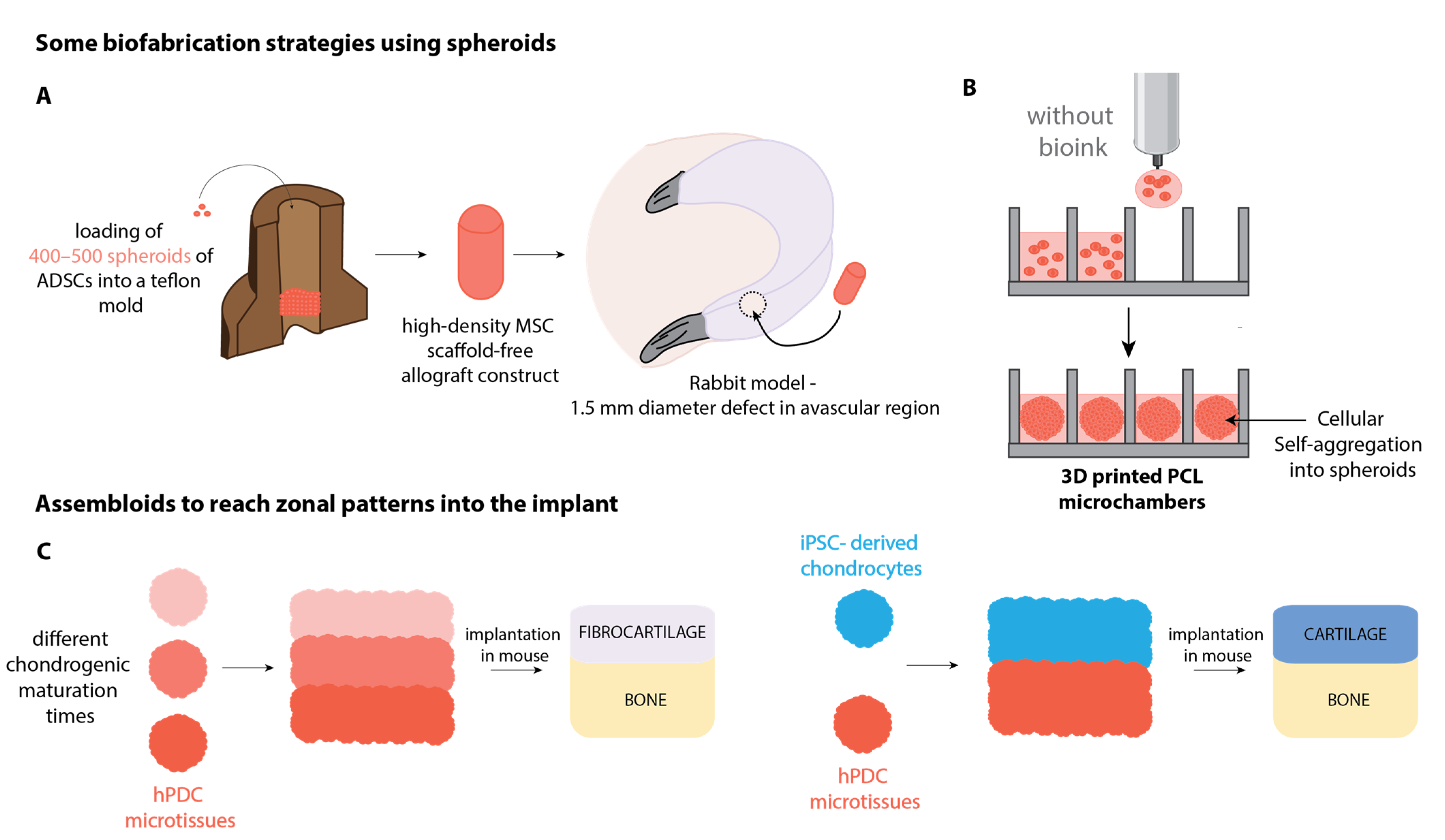
3.1. Cell-Based Biofabrication of Knee Joint Tissue Implants
3.2. Recapitulating the Zonal Complexity of Tissues
3.3. Optimization of the Mechanical Properties
3.4. Building Up an In Vitro 3D-Organoid Model of the Knee Joint
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beeler, S.; Jud, L.; Von Atzigen, M.; Sutter, R.; Fürnstahl, P.; Fucentese, S.F.; Vlachopoulos, L. Three-Dimensional Meniscus Allograft Sizing-a Study of 280 Healthy Menisci. J. Orthop. Surg. Res. 2020, 15, 74. [Google Scholar] [CrossRef]
- Aman, Z.S.; DePhillipo, N.N.; Storaci, H.W.; Moatshe, G.; Chahla, J.; Engebretsen, L.; LaPrade, R.F. Quantitative and Qualitative Assessment of Posterolateral Meniscal Anatomy: Defining the Popliteal Hiatus, Popliteomeniscal Fascicles, and the Lateral Meniscotibial Ligament. Am. J. Sports Med. 2019, 47, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- DePhillipo, N.N.; Moatshe, G.; Chahla, J.; Aman, Z.S.; Storaci, H.W.; Morris, E.R.; Robbins, C.M.; Engebretsen, L.; LaPrade, R.F. Quantitative and Qualitative Assessment of the Posterior Medial Meniscus Anatomy: Defining Meniscal Ramp Lesions. Am. J. Sports Med. 2019, 47, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Caridade, S.G.; Frias, A.M.; Silva-Correia, J.; Pereira, D.R.; Cengiz, I.F.; Mano, J.F.; Oliveira, J.M.; Espregueira-Mendes, J.; Reis, R.L. Biomechanical and Cellular Segmental Characterization of Human Meniscus: Building the Basis for Tissue Engineering Therapies. Osteoarthr. Cartil. 2014, 22, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Herwig, J.; Egner, E.; Buddecke, E. Chemical Changes of Human Knee Joint Menisci in Various Stages of Degeneration. Ann. Rheum. Dis. 1984, 43, 635–640. [Google Scholar] [CrossRef]
- Cheung, H.S. Distribution of Type I, II, III and V in the Pepsin Solubilized Collagens in Bovine Menisci. Connect. Tissue Res. 1987, 16, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Human Knee Menisci: Structure, Composition, and Function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef]
- Zhu, S.; Tong, G.; Xiang, J.P.; Qiu, S.; Yao, Z.; Zhou, X.; Lin, L.J. Microstructure Analysis and Reconstruction of a Meniscus. Orthop. Surg. 2021, 13, 306. [Google Scholar] [CrossRef]
- Lopez, S.G.; Bonassar, L.J. The Role of SLRPs and Large Aggregating Proteoglycans in Collagen Fibrillogenesis, Extracellular Matrix Assembly, and Mechanical Function of Fibrocartilage. Connect. Tissue Res. 2022, 63, 269–286. [Google Scholar] [CrossRef]
- Moyer, J.T.; Priest, R.; Bouman, T.; Abraham, A.C.; Haut Donahue, T.L. Indentation Properties and Glycosaminoglycan Content of Human Menisci in the Deep Zone. Acta Biomater. 2013, 9, 6624. [Google Scholar] [CrossRef]
- Folkesson, E.; Turkiewicz, A.; Rydén, M.; Hughes, H.V.; Ali, N.; Tjörnstrand, J.; Önnerfjord, P.; Englund, M. Proteomic Characterization of the Normal Human Medial Meniscus Body Using Data-independent Acquisition Mass Spectrometry. J. Orthop. Res. 2020, 38, 1735. [Google Scholar] [CrossRef] [PubMed]
- Aspberg, A. The Different Roles of Aggrecan Interaction Domains. J. Histochem. Cytochem. 2012, 60, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, Articular Cartilage, and Nucleus Pulposus: A Comparative Review of Cartilage-like Tissues in Anatomy, Development, and Function. Cell Tissue Res. 2017, 370, 53. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Tillmann, B. Age-Related Blood and Lymph Supply of the Knee Menisci. A Cadaver Study. Acta Orthop. Scand. 1995, 66, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Pufe, T.; Petersen, W.; Kurz, B.; Tsokos, M.; Tillmann, B.; Mentlein, R. Mechanical Factors Influence the Expression of Endostatin—An Inhibitor of Angiogenesis—In Tendons. J. Orthop. Res. 2003, 21, 610–616. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.J.; Miosge, N.; Goldring, M.B.; Mentlein, R.; Varoga, D.J.; Tillmann, B.N. Endostatin/Collagen XVIII—An Inhibitor of Angiogenesis—Is Expressed in Cartilage and Fibrocartilage. Matrix Biol. 2004, 23, 267–276. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Deponti, D.; Modina, S.; Tessaro, I.; Domeneghini, C.; Peretti, G.M. Age-related Modulation of Angiogenesis-regulating Factors in the Swine Meniscus. J. Cell. Mol. Med. 2017, 21, 3066. [Google Scholar] [CrossRef]
- Fujii, M.; Furumatsu, T.; Yokoyama, Y.; Kanazawa, T.; Kajiki, Y.; Abe, N.; Ozaki, T. Chondromodulin-I Derived from the Inner Meniscus Prevents Endothelial Cell Proliferation. J. Orthop. Res. 2013, 31, 538–543. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The Knee Meniscus: Structure-Function, Pathophysiology, Current Repair Techniques, and Prospects for Regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Grogan, S.P.; Duffy, S.F.; Pauli, C.; Lotz, M.K.; D’Lima, D.D. Gene Expression Profiles of the Meniscus Avascular Phenotype: A Guide for Meniscus Tissue Engineering. J. Orthop. Res. 2018, 36, 1947–1958. [Google Scholar] [CrossRef]
- Meakin, J.R.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Finite Element Analysis of the Meniscus: The Influence of Geometry and Material Properties on Its Behaviour. Knee 2003, 10, 33–41. [Google Scholar] [CrossRef]
- De Rosa, M.; Filippone, G.; Best, T.M.; Jackson, A.R.; Travascio, F. Mechanical Properties of Meniscal Circumferential Fibers Using an Inverse Finite Element Analysis Approach. J. Mech. Behav. Biomed. Mater. 2022, 126, 105073. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.F.; Clarke, M.J.; Riches, D.P. Proteoglycans Exert a Significant Effect on Human Meniscal Stiffness through Ionic Effects. Clin. Biomech. 2020, 77, 105028. [Google Scholar] [CrossRef] [PubMed]
- Morejon, A.; Mantero, A.M.A.; Best, T.M.; Jackson, A.R.; Travascio, F. Mechanisms of Energy Dissipation and Relationship with Tissue Composition in Human Meniscus. Osteoarthr. Cartil. 2022, 30, 605–612. [Google Scholar] [CrossRef]
- Abdelgaied, A.; Stanley, M.; Galfe, M.; Berry, H.; Ingham, E.; Fisher, J. Comparison of the Biomechanical Tensile and Compressive Properties of Decellularised and Natural Porcine Meniscus. J. Biomech. 2015, 48, 1389–1396. [Google Scholar] [CrossRef]
- Maritz, J.; Agustoni, G.; Dragnevski, K.; Bordas, S.P.A.; Barrera, O. The Functionally Grading Elastic and Viscoelastic Properties of the Body Region of the Knee Meniscus. Ann. Biomed. Eng. 2021, 49, 2421–2429. [Google Scholar] [CrossRef]
- Agustoni, G.; Maritz, J.; Kennedy, J.; Bonomo, F.P.; Bordas, S.P.A.; Barrera, O. High Resolution Micro-Computed Tomography Reveals a Network of Collagen Channels in the Body Region of the Knee Meniscus. Ann. Biomed. Eng. 2021, 49, 2273–2281. [Google Scholar] [CrossRef]
- Walker, P.S.; Erkman, M.J. The Role of the Menisci in Force Transmission across the Knee. Clin. Orthop. Relat. Res. 1975, 109, 184–192. [Google Scholar] [CrossRef]
- Arno, S.; Hadley, S.; Campbell, K.A.; Bell, C.P.; Hall, M.; Beltran, L.S.; Recht, M.P.; Sherman, O.H.; Walker, P.S. The Effect of Arthroscopic Partial Medial Meniscectomy on Tibiofemoral Stability. Am. J. Sports Med. 2013, 41, 73–79. [Google Scholar] [CrossRef]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of Athletic Knee Injuries: A 10-Year Study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef]
- Henderson, B.S.; Cudworth, K.F.; Wale, M.E.; Siegel, D.N.; Lujan, T.J. Tensile Fatigue Strength and Endurance Limit of Human Meniscus. J. Mech. Behav. Biomed. Mater. 2022, 127, 105057. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Miller, L.M.; Patel, J.M.; Meadows, K.D.; Eby, M.R.; Saleh, K.S.; Martin, A.R.; Stoeckl, B.D.; Hast, M.W.; Elliott, D.M.; et al. Transection of the Medial Meniscus Anterior Horn Results in Cartilage Degeneration and Meniscus Remodeling in a Large Animal Model. J. Orthop. Res. 2020, 38, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The Surgical Destabilization of the Medial Meniscus (DMM) Model of Osteoarthritis in the 129/SvEv Mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The Long-Term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. eClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and Tissue Engineering Strategies for Articular Cartilage and Meniscus Repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Barber-Westin, S.D.; Noyes, F.R. Clinical Healing Rates of Meniscus Repairs of Tears in the Central-Third (Red-White) Zone. Arthroscopy 2014, 30, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Hoshino, Y.; Nagamune, K.; Yamamoto, T.; Nagai, K.; Araki, D.; Kanzaki, N.; Matsushita, T.; Kuroda, R. Radial Meniscal Tears Are Best Repaired by a Modified “Cross” Tie-Grip Suture Based on a Biomechanical Comparison of 4 Repair Techniques in a Porcine Model. Orthop. J. Sport. Med. 2020, 8, 2325967120935810. [Google Scholar] [CrossRef]
- Abram, S.G.F.; Judge, A.; Beard, D.J.; Price, A.J. Adverse Outcomes after Arthroscopic Partial Meniscectomy: A Study of 700 000 Procedures in the National Hospital Episode Statistics Database for England. Lancet 2018, 392, 2194–2202. [Google Scholar] [CrossRef]
- Lee, S.J.; Aadalen, K.J.; Malaviya, P.; Lorenz, E.P.; Hayden, J.K.; Farr, J.; Kang, R.W.; Cole, B.J. Tibiofemoral Contact Mechanics after Serial Medial Meniscectomies in the Human Cadaveric Knee. Am. J. Sports Med. 2006, 34, 1334–1344. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kwoh, C.K.; Hannon, M.J.; Hunter, D.J.; Eckstein, F.; Grago, J.; Boudreau, R.M.; Englund, M.; Guermazi, A. Partial Meniscectomy Is Associated with Increased Risk of Incident Radiographic Osteoarthritis and Worsening Cartilage Damage in the Following Year. Eur. Radiol. 2017, 27, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, R.; Paavola, M.; Malmivaara, A.; Itälä, A.; Joukainen, A.; Kalske, J.; Nurmi, H.; Kumm, J.; Sillanpaä, N.; Kiekara, T.; et al. Arthroscopic Partial Meniscectomy for a Degenerative Meniscus Tear: A 5 Year Follow-up of the Placebo-Surgery Controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) Trial. Br. J. Sports Med. 2020, 54, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Shrestha, S.; Losina, E.; Jones, M.H.; Marx, R.G.; Mandl, L.A.; Levy, B.A.; MacFarlane, L.A.; Spindler, K.P.; Silva, G.S.; et al. Five-Year Outcome of Operative and Nonoperative Management of Meniscal Tear in Persons Older Than Forty-Five Years. Arthritis Rheumatol. 2020, 72, 273–281. [Google Scholar] [CrossRef]
- Collins, J.E.; Losina, E.; Marx, R.G.; Guermazi, A.; Jarraya, M.; Jones, M.H.; Levy, B.A.; Mandl, L.A.; Martin, S.D.; Wright, R.W.; et al. Early Magnetic Resonance Imaging-Based Changes in Patients with Meniscal Tear and Osteoarthritis: Eighteen-Month Data from a Randomized Controlled Trial of Arthroscopic Partial Meniscectomy Versus Physical Therapy. Arthritis Care Res. 2020, 72, 630–640. [Google Scholar] [CrossRef]
- Jacquet, C.; Pujol, N.; Pauly, V.; Beaufils, P.; Ollivier, M. Analysis of the Trends in Arthroscopic Meniscectomy and Meniscus Repair Procedures in France from 2005 to 2017. Orthop. Traumatol. Surg. Res. 2019, 105, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Turkiewicz, A.; Bergkvist, D.; Neuman, P.; Englund, M. The Risk of Symptomatic Knee Osteoarthritis after Arthroscopic Meniscus Repair vs Partial Meniscectomy vs the General Population. Osteoarthr. Cartil. 2018, 26, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of Traumatic Meniscus Tears: The 2019 ESSKA Meniscus Consensus. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1177–1194. [Google Scholar] [CrossRef]
- Milachowski, K.A.; Weismeier, K.; Wirth, C.J. Homologous Meniscus Transplantation. Int. Orthop. 1989, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A. Meniscal Allografts—Where Do We Stand? Am. J. Sports Med. 2001, 29, 246–261. [Google Scholar] [CrossRef]
- Beeler, S.; Vlachopoulos, L.; Jud, L.; Sutter, R.; Fürnstahl, P.; Fucentese, S.F. Contralateral MRI Scan Can Be Used Reliably for Three-Dimensional Meniscus Sizing—Retrospective Analysis of 160 Healthy Menisci. Knee 2019, 26, 954–961. [Google Scholar] [CrossRef]
- Stone, K.R.; Walgenbach, A.W.; Turek, T.J.; Freyer, A.; Hill, M.D. Meniscus Allograft Survival in Patients with Moderate to Severe Unicompartmental Arthritis: A 2- to 7-Year Follow-Up. Arthroscopy 2006, 22, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.; Figueroa, D.; Calvo, R.; Vaisman, A.; Espregueira-Mendes, J. Meniscus Allograft Transplantation: Indications, Techniques and Outcomes. EFORT Open Rev. 2019, 4, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Gelber, P.E.; Erquicia, J.I.; Pelfort, X.; Gonzalez-Lucena, G.; Monllau, J.C. Suture-Only Fixation Technique Leads to a Higher Degree of Extrusion than Bony Fixation in Meniscal Allograft Transplantation. Am. J. Sports Med. 2012, 40, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Ambra, L.F.; Mestriner, A.B.; Ackermann, J.; Phan, A.T.; Farr, J.; Gomoll, A.H. Bone-Plug Versus Soft Tissue Fixation of Medial Meniscal Allograft Transplants: A Biomechanical Study. Am. J. Sports Med. 2019, 47, 2960–2965. [Google Scholar] [CrossRef]
- Teo, S.J.; Tan, M.W.P.; Koh, D.T.S.; Lee, K.H. Medial Meniscal Allograft Transplantation with Bone Plugs Using a 3-Tunnel Technique. Arthrosc. Tech. 2022, 11, e217–e222. [Google Scholar] [CrossRef]
- Struijk, C.; Van Genechten, W.; Verdonk, P.; Krych, A.J.; Dietz, A.B.; van Wijnen, A.J.; Saris, D.B.F. Human Meniscus Allograft Augmentation by Allogeneic Mesenchymal Stromal/Stem Cell Injections. J. Orthop. Res. 2022, 40, 712–726. [Google Scholar] [CrossRef]
- Noyes, F.; Barber-Westin, S.D. Meniscus Transplantation: Indications, Techniques, Clinical Outcomes. AAOS Instr. Course Lect. 2005, 54, 341. [Google Scholar]
- Sandmann, G.H.; Eichhorn, S.; Vogt, S.; Adamczyk, C.; Aryee, S.; Hoberg, M.; Milz, S.; Imhoff, A.B.; Tischer, T. Generation and Characterization of a Human Acellular Meniscus Scaffold for Tissue Engineering. J. Biomed. Mater. Res. Part A 2009, 91, 567–574. [Google Scholar] [CrossRef]
- Shimomura, K.; Rothrauff, B.B.; Tuan, R.S. Region-Specific Effect of the Decellularized Meniscus Extracellular Matrix on Mesenchymal Stem Cell-Based Meniscus Tissue Engineering. Am. J. Sports Med. 2017, 45, 604–611. [Google Scholar] [CrossRef]
- Qu, F.; Guilak, F.; Mauck, R.L. Cell Migration: Implications for Repair and Regeneration in Joint Disease. Nat. Rev. Rheumatol. 2019, 15, 167–179. [Google Scholar] [CrossRef]
- Ruprecht, J.C.; Waanders, T.D.; Rowland, C.R.; Nishimuta, J.F.; Glass, K.A.; Stencel, J.; DeFrate, L.E.; Guilak, F.; Weinberg, J.B.; McNulty, A.L. Meniscus-Derived Matrix Scaffolds Promote the Integrative Repair of Meniscal Defects. Sci. Rep. 2019, 9, 8719. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.P.; Perea, S.H.; Weinberg, J.B.; Wittstein, J.R.; McNulty, A.L. Meniscus-Derived Matrix Bioscaffolds: Effects of Concentration and Cross-Linking on Meniscus Cellular Responses and Tissue Repair. Int. J. Mol. Sci. 2020, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Zadeh, L.; Chevrier, A.; Farr, J.; Rodeo, S.A.; Buschmann, M.D. Augmentation Techniques for Meniscus Repair. J. Knee Surg. 2018, 31, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Kennedy, N.I.; Geeslin, A.G.; Moatshe, G.; Cinque, M.E.; DePhillipo, N.N.; LaPrade, R.F. Meniscal Repair with Fibrin Clot Augmentation. Arthrosc. Tech. 2017, 6, e2065–e2069. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, M.S.; Gwathmey, F.W. Circumferential Suture Repair of Isolated Horizontal Meniscal Tears Augmented with Fibrin Clot. Arthrosc. Tech. 2017, 6, e1567–e1572. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Babu, S.S.; Lal, J.V.; Kaushik, Y.S.; Lukose, A.M.; Sandesh, G.M.; Amaravathi, R.S. Fibrin Clot Augmented Repair of Longitudinal Tear of Medial Meniscus. Arthrosc. Tech. 2021, 10, e2449–e2455. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, Y.; Kato, T.; Akao, M.; Takata, T.; Kobayakawa, K.; Deie, M. Meniscal Repair Using Fibrin Clots Made from Bone Marrow Blood Wrapped in a Polyglycolic Acid Sheet. Arthrosc. Tech. 2021, 10, e2541–e2546. [Google Scholar] [CrossRef]
- Griffin, J.W.; Hadeed, M.M.; Werner, B.C.; Diduch, D.R.; Carson, E.W.; Miller, M.D. Platelet-Rich Plasma in Meniscal Repair: Does Augmentation Improve Surgical Outcomes? Clin. Orthop. Relat. Res. 2015, 473, 1665–1672. [Google Scholar] [CrossRef]
- Kluyskens, L.; Debieux, P.; Wong, K.L.; Krych, A.J.; Saris, D.B.F. Biomaterials for Meniscus and Cartilage in Knee Surgery: State of the Art. J. ISAKOS 2022, 7, 67–77. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G. Tissue-Engineered Collagen Meniscus Implants: 5- to 6-Year Feasibility Study Results. Arthroscopy 2005, 21, 515–525. [Google Scholar] [CrossRef]
- Baynat, C.; Andro, C.; Vincent, J.P.; Schiele, P.; Buisson, P.; Dubrana, F.; Gunepin, F.X. Actifit Synthetic Meniscal Substitute: Experience with 18 Patients in Brest, France. Orthop. Traumatol. Surg. Res. 2014, 100, S385–S389. [Google Scholar] [CrossRef] [PubMed]
- Reale, D.; Previtali, D.; Andriolo, L.; Grassi, A.; Candrian, C.; Zaffagnini, S.; Filardo, G. No Differences in Clinical Outcome between CMI and Actifit Meniscal Scaffolds: A Systematic Review and Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Martinek, V.; Ueblacker, P.; Bräun, K.; Nitschke, S.; Mannhardt, R.; Specht, K.; Gansbacher, B.; Imhoff, A.B. Second Generation of Meniscus Transplantation: In-Vivo Study with Tissue Engineered Meniscus Replacement. Arch. Orthop. Trauma Surg. 2006, 126, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bulgheroni, E.; Grassi, A.; Campagnolo, M.; Bulgheroni, P.; Mudhigere, A.; Gobbi, A. Comparative Study of Collagen versus Synthetic-Based Meniscal Scaffolds in Treating Meniscal Deficiency in Young Active Population. Cartilage 2016, 7, 29–38. [Google Scholar] [CrossRef]
- Reguzzoni, M.; Manelli, A.; Ronga, M.; Raspanti, M.; Grassi, F.A. Histology and Ultrastructure of a Tissue-Engineered Collagen Meniscus before and after Implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 808–816. [Google Scholar] [CrossRef]
- Baek, J.; Lotz, M.K.; D’Lima, D.D. Core-Shell Nanofibrous Scaffolds for Repair of Meniscus Tears. Tissue Eng. Part A 2019, 25, 1577–1590. [Google Scholar] [CrossRef]
- Shemesh, M.; Asher, R.; Zylberberg, E.; Guilak, F.; Linder-Ganz, E.; Elsner, J.J. Viscoelastic Properties of a Synthetic Meniscus Implant. J. Mech. Behav. Biomed. Mater. 2014, 29, 42–55. [Google Scholar] [CrossRef]
- Elsner, J.J.; Portnoy, S.; Zur, G.; Guilak, F.; Shterling, A.; Linder-Ganz, E. Design of a Free-Floating Polycarbonate-Urethane Meniscal Implant Using Finite Element Modeling and Experimental Validation. J. Biomech. Eng. 2010, 132, 095001. [Google Scholar] [CrossRef]
- Balint, E.; Gatt, C.J.; Dunn, M.G. Design and Mechanical Evaluation of a Novel Fiber-Reinforced Scaffold for Meniscus Replacement. J. Biomed. Mater. Res. A 2012, 100, 195–202. [Google Scholar] [CrossRef]
- Cojocaru, D.G.; Hondke, S.; Krüger, J.P.; Bosch, C.; Croicu, C.; Florescu, S.; Lazarescu, A.; Patrascu, J.M.; Patrascu, J.M.; Dauner, M.; et al. Meniscus-Shaped Cell-Free Polyglycolic Acid Scaffold for Meniscal Repair in a Sheep Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 809–818. [Google Scholar] [CrossRef]
- Amiri, F.; Babaei, M.; Jamshidi, N.; Agheb, M.; Rafienia, M.; Kazemi, M. Fabrication and Assessment of a Novel Hybrid Scaffold Consisted of Polyurethane-Gellan Gum-Hyaluronic Acid-Glucosamine for Meniscus Tissue Engineering. Int. J. Biol. Macromol. 2022, 203, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, K.I.; Ra, H.J.; Lotz, M.K.; D’Lima, D.D. Collagen Fibrous Scaffolds for Sustained Delivery of Growth Factors for Meniscal Tissue Engineering. Nanomedicine 2022, 17, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.R.; Howells, N.R.; Parry, M.C.; Austin, E.; Kafienah, W.; Brady, K.; Goodship, A.E.; Eldridge, J.D.; Blom, A.W.; Hollander, A.P. Repair of Torn Avascular Meniscal Cartilage Using Undifferentiated Autologous Mesenchymal Stem Cells: From In Vitro Optimization to a First-in-Human Study. Stem Cells Transl. Med. 2017, 6, 1237. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef]
- Rivron, N.C.; Frias-Aldeguer, J.; Vrij, E.J.; Boisset, J.C.; Korving, J.; Vivié, J.; Truckenmüller, R.K.; Van Oudenaarden, A.; Van Blitterswijk, C.A.; Geijsen, N. Blastocyst-like Structures Generated Solely from Stem Cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ Printing: Tissue Spheroids as Building Blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- McDermott, A.M.; Herberg, S.; Mason, D.E.; Collins, J.M.; Pearson, H.B.; Dawahare, J.H.; Tang, R.; Patwa, A.N.; Grinstaff, M.W.; Kelly, D.J.; et al. Recapitulating Bone Development through Engineered Mesenchymal Condensations and Mechanical Cues for Tissue Regeneration. Sci. Transl. Med. 2019, 11, eaav7756. [Google Scholar] [CrossRef]
- Yang, Q.; Xue, S.L.; Chan, C.J.; Rempfler, M.; Vischi, D.; Maurer-Gutierrez, F.; Hiiragi, T.; Hannezo, E.; Liberali, P. Cell Fate Coordinates Mechano-Osmotic Forces in Intestinal Crypt Formation. Nat. Cell Biol. 2021, 23, 733–744. [Google Scholar] [CrossRef]
- Lukonin, I.; Zinner, M.; Liberali, P. Organoids in Image-Based Phenotypic Chemical Screens. Exp. Mol. Med. 2021, 53, 1495–1502. [Google Scholar] [CrossRef]
- Favreau, H.; Pijnenburg, L.; Seitlinger, J.; Fioretti, F.; Keller, L.; Scipioni, D.; Adriaensen, H.; Kuchler-Bopp, S.; Ehlinger, M.; Mainard, D.; et al. Osteochondral Repair Combining Therapeutics Implant with Mesenchymal Stem Cells Spheroids. Nanomedicine 2020, 29, 102253. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Wagner, Q.; Schwinté, P.; Benkirane-Jessel, N. Double Compartmented and Hybrid Implant Outfitted with Well-Organized 3D Stem Cells for Osteochondral Regenerative Nanomedicine. Nanomedicine 2015, 10, 2833–2845. [Google Scholar] [CrossRef] [PubMed]
- Toratani, T.; Nakase, J.; Numata, H.; Oshima, T.; Takata, Y.; Nakayama, K.; Tsuchiya, H. Scaffold-Free Tissue-Engineered Allogenic Adipose-Derived Stem Cells Promote Meniscus Healing. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nakayama, K.; Akieda, S.; Matsuda, S.; Iwamoto, Y. Simultaneous Regeneration of Full-Thickness Cartilage and Subchondral Bone Defects In vivo Using a Three-Dimensional Scaffold-Free Autologous Construct Derived from High-Density Bone Marrow-Derived Mesenchymal Stem Cells. J. Orthop. Surg. Res. 2014, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Gaston, J.; Ho, V.B. A Review of Strategies for Development of Tissue Engineered Meniscal Implants. Biomater. Biosyst. 2021, 4, 100026. [Google Scholar] [CrossRef]
- Patel, J.M.; Brzezinski, A.; Ghodbane, S.A.; Tarapore, R.; Lu, T.M.; Gatt, C.J.; Dunn, M.G. Personalized Fiber-Reinforcement Networks for Meniscus Reconstruction. J. Biomech. Eng. 2020, 142, 051008. [Google Scholar] [CrossRef]
- Filardo, G.; Petretta, M.; Cavallo, C.; Roseti, L.; Durante, S.; Albisinni, U.; Grigolo, B. Patient-Specific Meniscus Prototype Based on 3D Bioprinting of Human Cell-Laden Scaffold. Bone Jt. Res. 2019, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Kelly, D.J. Biofabrication of Spatially Organised Tissues by Directing the Growth of Cellular Spheroids within 3D Printed Polymeric Microchambers. Biomaterials 2019, 197, 194–206. [Google Scholar] [CrossRef]
- Kunisch, E.; Knauf, A.-K.; Hesse, E.; Freudenberg, U.; Werner, C.; Bothe, F.; Diederichs, S.; Richter, W. StarPEG/Heparin-Hydrogel Based in Vivo Engineering of Stable Bizonal Cartilage with a Calcified Bottom Layer. Biofabrication 2018, 11, 015001. [Google Scholar] [CrossRef]
- Hall, G.N.; Tam, W.L.; Andrikopoulos, K.S.; Casas-Fraile, L.; Voyiatzis, G.A.; Geris, L.; Luyten, F.P.; Papantoniou, I. Patterned, Organoid-Based Cartilaginous Implants Exhibit Zone Specific Functionality Forming Osteochondral-like Tissues in Vivo. Biomaterials 2021, 273, 120820. [Google Scholar] [CrossRef]
- Gardner, E.; O’Rahilly, R. The Early Development of the Knee Joint in Staged Human Embryos. J. Anat. 1968, 102, 289–299. [Google Scholar] [PubMed]
- Uhthoff, H.K.; Kumagai, J. Embryology of Human Meniscus. In Trends in Research and Treatment of Joint Diseases; Hirohata, K., Mizuno, K., Matsubara, T., Eds.; Springer: Tokyo, Japan, 1992; pp. 135–141. ISBN 9784431681922. [Google Scholar]
- Pazin, D.E.; Gamer, L.W.; Capelo, L.P.; Cox, K.A.; Rosen, V. Gene Signature of the Embryonic Meniscus. J. Orthop. Res. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Higashioka, M.M.; Chen, J.A.; Hu, J.C.; Athanasiou, K.A. Building an Anisotropic Meniscus with Zonal Variations. Tissue Eng. Part A 2014, 20, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Puetzer, J.L.; Koo, E.; Bonassar, L.J. Induction of Fiber Alignment and Mechanical Anisotropy in Tissue Engineered Menisci with Mechanical Anchoring. J. Biomech. 2015, 48, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Puetzer, J.L.; Bonassar, L.J. Physiologically Distributed Loading Patterns Drive the Formation of Zonally Organized Collagen Structures in Tissue-Engineered Meniscus. Tissue Eng. Part A 2016, 22, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zitnay, J.L.; Reese, S.P.; Tran, G.; Farhang, N.; Bowles, R.D.; Weiss, J.A. Fabrication of Dense Anisotropic Collagen Scaffolds Using Biaxial Compression. Acta Biomater. 2018, 65, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Leon, E.A.; Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Engineering Self-Assembled Neomenisci through Combination of Matrix Augmentation and Directional Remodeling. Acta Biomater. 2020, 109, 73–81. [Google Scholar] [CrossRef]
- MacBarb, R.F.; Makris, E.A.; Hu, J.C.; Athanasiou, K.A. A Chondroitinase-ABC and TGF-Β1 Treatment Regimen for Enhancing the Mechanical Properties of Tissue-Engineered Fibrocartilage. Acta Biomater. 2013, 9, 4626–4634. [Google Scholar] [CrossRef]
- Makris, E.A.; Responte, D.J.; Paschos, N.K.; Hu, J.C.; Athanasiou, K.A. Developing Functional Musculoskeletal Tissues through Hypoxia and Lysyl Oxidase-Induced Collagen Cross-Linking. Proc. Natl. Acad. Sci. USA 2014, 111, E4832–E4841. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Chen, Y.-R.; Wang, S.-J.; Zhao, F.; Wang, X.-G.; Yang, F.; Shi, J.-J.; Ge, Z.-G.; Ding, W.-Y.; Yang, Y.-C.; et al. Orchestrated Biomechanical, Structural, and Biochemical Stimuli for Engineering Anisotropic Meniscus. Sci. Transl. Med. 2019, 11, eaao0750. [Google Scholar] [CrossRef]
- Bigoni, M.; Turati, M.; Sacerdote, P.; Gaddi, D.; Piatti, M.; Castelnuovo, A.; Franchi, S.; Gandolla, M.; Pedrocchi, A.; Omeljaniuk, R.J.; et al. Characterization of Synovial Fluid Cytokine Profiles in Chronic Meniscal Tear of the Knee. J. Orthop. Res. 2017, 35, 340–346. [Google Scholar] [CrossRef] [PubMed]
- McNulty, A.L.; Estes, B.T.; Wilusz, R.E.; Weinberg, J.B.; Guilak, F. Dynamic Loading Enhances Integrative Meniscal Repair in the Presence of Interleukin-1. Osteoarthr. Cartil. 2010, 18, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Andress, B.D.; Irwin, R.M.; Puranam, I.; Hoffman, B.D.; McNulty, A.L. A Tale of Two Loads: Modulation of IL-1 Induced Inflammatory Responses of Meniscal Cells in Two Models of Dynamic Physiologic Loading. Front. Bioeng. Biotechnol. 2022, 10, 837619. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-Mediated Calcium Signaling in Mesenchymal Stem Cells Regulates Aligned Collagen Matrix Formation and Vinculin Tension. Proc. Natl. Acad. Sci. USA 2019, 116, 1992–1997. [Google Scholar] [CrossRef]
- Lee, K.I.; Choi, S.; Matsuzaki, T.; Alvarez-Garcia, O.; Olmer, M.; Grogan, S.P.; D’Lima, D.D.; Lotz, M.K. FOXO1 and FOXO3 Transcription Factors Have Unique Functions in Meniscus Development and Homeostasis during Aging and Osteoarthritis. Proc. Natl. Acad. Sci. USA 2020, 117, 3135–3143. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Dai, K.; You, Y.; Jiang, W. Generating 3D-Cultured Organoids for Pre-Clinical Modeling and Treatment of Degenerative Joint Disease. Signal Transduct. Target. Ther. 2021, 6, 380. [Google Scholar] [CrossRef]
- Keller, L.; Pijnenburg, L.; Idoux-Gillet, Y.; Bornert, F.; Benameur, L.; Tabrizian, M.; Auvray, P.; Rosset, P.; María Gonzalo-Daganzo, R.; Gómez Barrena, E.; et al. Preclinical Safety Study of a Combined Therapeutic Bone Wound Dressing for Osteoarticular Regeneration. Nat. Commun. 2019, 10, 2156. [Google Scholar] [CrossRef]
- Keller, L.; Schwinté, P.; Gomez-Barrena, E.; Arruebo, M.; Benkirane-Jessel, N. Smart Implants as a Novel Strategy to Regenerate Well-Founded Cartilage. Trends Biotechnol. 2017, 35, 8–11. [Google Scholar] [CrossRef]
- O’Connor, S.K.; Katz, D.B.; Oswald, S.J.; Groneck, L.; Guilak, F. Formation of Osteochondral Organoids from Murine Induced Pluripotent Stem Cells. Tissue Eng. Part A 2021, 27, 1099–1109. [Google Scholar] [CrossRef]
- Paggi, C.; Venzac, B.; Leijten, J.; Teixeira Leijten, L.M.; Le Gac, S.; Karperien, M. Cartilage-on-Chip: A Multi-Modal Platform to Study Human Chondrocyte’s Response to Mechanical Stimuli. Osteoarthr. Cartil. 2020, 28, S176–S177. [Google Scholar] [CrossRef]
- Collison, J. Cartilage-on-a-Chip to Aid OA Drug Development. Nat. Rev. Rheumatol. 2019, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Paggi, C.A.; Hendriks, J.; Karperien, M.; Le Gac, S. Emulating the Chondrocyte Microenvironment Using Multi-Directional Mechanical Stimulation in a Cartilage-on-Chip. Lab Chip 2022, 22, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Rothbauer, M.; Byrne, R.A.; Schobesberger, S.; Calvo, I.O.; Fischer, A.; Reihs, E.I.; Spitz, S.; Bachmann, B.; Sevelda, F.; Holinka, J.; et al. Establishment of a Human Three-Dimensional Chip-Based Chondro-Synovial Coculture Joint Model for Reciprocal Cross Talk Studies in Arthritis Research. Lab Chip 2021, 21, 4128–4143. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignes, H.; Conzatti, G.; Hua, G.; Benkirane-Jessel, N. Meniscus Repair: From In Vitro Research to Patients. Organoids 2022, 1, 116-134. https://doi.org/10.3390/organoids1020010
Vignes H, Conzatti G, Hua G, Benkirane-Jessel N. Meniscus Repair: From In Vitro Research to Patients. Organoids. 2022; 1(2):116-134. https://doi.org/10.3390/organoids1020010
Chicago/Turabian StyleVignes, Hélène, Guillaume Conzatti, Guoqiang Hua, and Nadia Benkirane-Jessel. 2022. "Meniscus Repair: From In Vitro Research to Patients" Organoids 1, no. 2: 116-134. https://doi.org/10.3390/organoids1020010
APA StyleVignes, H., Conzatti, G., Hua, G., & Benkirane-Jessel, N. (2022). Meniscus Repair: From In Vitro Research to Patients. Organoids, 1(2), 116-134. https://doi.org/10.3390/organoids1020010






