Abstract
Boron supplementation may improve the musculoskeletal health of pullets before entering the lay phase. This study aimed to evaluate the effects of different boron amounts on the performance, muscle deposition, tibia cross-sectional area (CSA) and mineral density (BMD), ash percent, breaking strength, and bone mineralization (bone-specific alkaline phosphatase [BALP] and pro-collagen type 1 n-terminal propeptide [P1NP]) of a white-feathered strain of pullets. A total of 528 Hy-Line W-36 pullets were distributed across 24 pens and fed basal diets containing varying amounts of boron (C: 0 mg/kg; L: 50 mg/kg; M: 100 mg/kg; H: 150 mg/kg) for 17 weeks. Performance parameters (body weight, average daily weight gain/bird, and average daily feed intake/bird) were measured at weeks 4, 7, 10, 13, and 16, while all other measures were taken at 11 and 17 weeks of age. Performance was not impacted by boron supplementation. Pectoralis major weights were higher in H pullets at 11 weeks of age, and we also observed higher pectoralis major, minor, and leg muscle weights in H pullets at 17 weeks of age. Pullets fed the H diet had larger cortical CSA than the other treatment groups at 11 weeks of age. At 17 weeks of age, both the H and M groups had larger cortical CSA than the L and C groups, but the M group had slightly smaller cortical CSA. Pullets fed the H diet had higher BMD values than the other treatment groups at 11 weeks of age. At 17 weeks of age, pullets fed the H diet had the highest total BMD values compared to the other treatment groups, and cortical BMD increased with increasing boron inclusion. Pullets fed the H diet had the highest tibia ash percentages and concentrations of BALP and P1NP. Pullets fed the M and H diets had greater failure load and maximum bending moment than pullets fed the L or C diet at 11 weeks of age, with H pullets having greater stiffness values than other groups. At 17 weeks of age, pullets fed the H diet had greater failure load and maximum bending moment compared to all other treatment groups. Our results suggest that providing boron within the diet at 150 mg/kg can improve the musculoskeletal characteristics of Hy-Line W-36 pullets up to 17 weeks of age, without impacting performance parameters.
1. Introduction
Laying hens are prone to bone weakness and osteoporosis due to the intense demand for calcium for eggshell production [1]. Around 2.2 g of calcium is required to form just one eggshell, and a majority of this calcium is mobilized from the skeleton by osteoclasts [1]. Medullary bone is primarily made up of calcium and acts as a reservoir for the purpose of eggshell formation. However, osteoclasts do not discriminate, so some structural (i.e., cortical) bone is mobilized alongside the medullary bone [1,2]. Progressive loss of cortical bone is the main contributing factor to bone fracture and osteoporosis later in life [2,3]. Osteoporosis is an animal welfare concern because it can cause acute and chronic pain, reduced mobility, and reduced production.
Various nutritional interventions have been investigated as a solution to prevent osteoporosis. For example, supplementing the diet with vitamin D can facilitate intestinal absorption of calcium and phosphorus and maintain circulating calcium blood levels, which may prevent bone loss [4,5,6]. Furthermore, it is essential to provide nutritional supplementation for bone health during the pullet phase when the skeleton is still developing. This allows for the development of optimal bone quality before calcium resorption from the bone reserves begins during the laying phase.
Although boron is not an essential nutrient for poultry, it may present some benefits for the musculoskeletal health of laying hens. Older studies have suggested that boron may play a role in the metabolism of calcium, which helps improve bone strength and prevent fractures [7,8]. However, a majority of the studies in the literature have focused on broiler chicken health. For example, some studies have indicated that boron improves growth rate, nutritional efficiency, and calcium and phosphorus retention, and reduces the effects of vitamin D deficiency in broiler chickens [8,9,10,11,12]. Furthermore, a deficiency in boron may impact the normal development of bone and cartilage, bone ash content, and the concentrations of plasma calcium, phosphorus, and magnesium [12,13,14]. In laying hens, supplementing with boron has been shown to increase tibia calcium content [15], calcium retention [16], shear stress and ash content of the tibia [17], serum calcium concentration [18], bone resistance [19], femur bone strength, and tibia ash and calcium content [20]. In recent years, boron has not been evaluated as a proactive method to aid the bone health of pullets. To our knowledge, the only previous study performed in pullets was published in 1997, with inclusion rates of 50, 100, and 200 mg/kg boron [17]. This may be because some previous studies discovered negative effects of boron supplementation on measures of laying hen health and performance. For example, body weight was lower for Barred Rock hens fed 50, 100, and 200 mg/kg boron compared to the basal diet [20]. Also, the egg production, feed consumption, and body weight of White Leghorn hens decreased when fed 400 mg/kg compared to 50, 100, or 200 mg/kg boron [21,22]. However, in another study, boron did not affect the same measures at a lower inclusion rate of up to 250 mg/kg in Hisex Brown hybrids [18]. Because differing results have indicated that boron could negatively affect certain aspects of laying hen health, our objective was to establish a recent and relevant foundation for future research to determine appropriate boron inclusion rates. Furthermore, the limited availability of recent studies on boron supplementation in pullets warranted a cautious approach to incorporating boron into the diet due to uncertain outcomes. This comprehensive study is the first to evaluate the effect of boron as a feed supplement to improve the musculoskeletal health of Hy-Line W-36 pullets prior to entering the lay phase.
We hypothesized that pullets fed a diet supplemented with boron would show improved musculoskeletal health compared to pullets fed a control diet. The current study aimed to determine the optimal inclusion rate of boron within a commercial pullet diet and its effects on performance and musculoskeletal health.
2. Materials and Methods
2.1. Ethics
This project received approval from Clemson University’s Institutional Animal Care and Use Committee (protocol #AUP2021-0068).
2.2. Animals and Housing
The study took place within a poultry house at the Morgan Poultry Center in Clemson, South Carolina, USA, where ventilation and temperature were carefully controlled. Day-old white Hy-Line W-36 chicks (n = 528) were randomly allocated across 24 pens (22 bird/pen) until 17 weeks of age. Each pen was 5.04 m2 with approximately three 7.6 cm clean pine wood shavings on the floor. During the initial 3 weeks, each pen was equipped with a focal electric brooder for heat, supplemented by a gas-fired brooder covering the entire facility. From 0 to 3 weeks, feed was given through tube feeders and water was accessible via gallon drinkers, with supplementary feed trays utilized during the first week. After the initial 3 weeks, circular adjustable hanging feeders and automatic cup drinkers were used. Both feed and water were available ad libitum throughout the duration of the study. The temperature was initially established at 35–36 °C on day 0, then gradually decreased by 2–3 °C each week until the chicks reached 3 weeks of age, at which point the brooders were removed. From this point, the temperature decreased on a weekly basis until the birds reached 6 weeks of age to eventually reach 21 °C, and then this temperature was maintained until the end of the experiment according to standard breed guidelines [23]. Chicks underwent vaccination against Marek’s disease, Newcastle disease (NDV), infectious bronchitis (IB), infectious bursal disease (IBD or Gumboro), avian encephalomyelitis (AE), and fowl pox according to standard breed guidelines [23] at the hatchery and throughout the trial period. Each pen was illuminated by a single 60-watt incandescent overhead lightbulb, with a decreasing light regimen of 20L:4D for the first week of age, reduced either by 1.5 or 3 h until 10L:14D was reached when the birds were 7 weeks old, adhering to established breed guidelines until the experiment’s conclusion at 17 weeks old [23].
2.3. Treatments
From 0 to 17 weeks of age, birds were phase-fed commercial mash pullet diets to meet nutritional needs, corresponding to the average bird body weight. The basal diet was formulated to meet or exceed requirements (Table 1), following the standard breed guidelines [23]. Starter 1 was provided during the first 3 weeks of life, starter 2 from 4–6 weeks, grower from 7–15 weeks, and pre-lay from 15–17 weeks of age. Diets were supplemented with varying levels of boron in the form of boric acid (Sigma-Aldrich Boric Acid B0394 (Burlington, MA, USA), containing 16.2% boron), resulting in four treatment groups (six pens/treatment): control (C; 0 mg/kg boron), low (L; 50 mg/kg boron), medium (M; 100 mg/kg boron), and high (H; 150 mg/kg boron).

Table 1.
Ingredient percentage and calculated nutrient analysis of the four basal diets used in the current experiment [24].
2.4. Performance
Body weight (BW) and average daily feed intake per bird (ADFI) were measured at weeks 4, 7, 10, 13, and 16 of age. Feed offered and refused were measured, and ADFI was calculated. The birds’ body weight was calculated using the following formulas to calculate the average daily body weight gain per bird (ADWG):
2.5. Computed Tomography (CT) Image Acquisition
At 11 and 17 weeks of age, 2 birds per pen per week (n = 48 birds/week) were euthanized on-farm by CO2 inhalation, placed in a cooler of ice, and immediately transported to Godley-Snell Research Center on Clemson University’s campus. CT image acquisition used a Toshiba Aquilion TSX-101A, 16-slice scanner (GE Healthcare, Chicago IL, USA) and followed the methodology described by Harrison et al. [25] and Anderson et al. [26]. Immediately following CT scan acquisition, birds were dissected and frozen at −29 °C for further testing.
2.6. Bone Cross-Sectional Area (CSA) and Bone Mineral Density (BMD)
Right tibiotarsal bone and muscle measurements were conducted utilizing a standardized CT image analysis method, as outlined in a protocol previously described by Harrison et al. [25].
2.7. Muscle Deposition
Birds were removed from the −29 °C freezer and allowed to thaw at refrigerated temperature for 24 h prior to dissection, which included obtaining weights of the biceps brachii, triceps brachii, pectoralis major, pectoralis minor, and leg muscle group. The separation of muscles followed procedures described by Anderson et al. [26] and Casey-Trott et al. [27], and was performed with help from a veterinarian (A.A.) to ensure consistent muscle specimen collection. The left tibiae were frozen at −29 °C for ash percentage, and the right tibiae were frozen at −20 °C for breaking strength measures.
2.8. Ash Percentage
The left tibiotarsi of the euthanized birds were thawed approximately 24 h before data collection. The bones were separated from surrounding muscles and soft tissues, and then processed according to the ashing procedure outlined by Anderson et al. [26].
2.9. Breaking Strength
The mechanical characteristics of the right tibiotarsi were evaluated via a three-point bending test following the guidelines outlined by the American National Standards Institute (ANSI) for conducting three-point bending tests on animal bones [28]. An Instron Dynamic and Static Material Test system (Model 5944, Instron Corp., Canton, MA, USA) equipped with a 500 N load cell and Automated Material Test System software was utilized for testing. Tibiae breaking strength was measured according to methods described by Anderson et al. [26]. Load and displacement data were collected and were used to obtain the breaking strength (N), stiffness (N/mm), and maximum bending moment (N/m).
2.10. Bone Mineralization
During weeks 11 and 17 of age, blood samples were collected from the brachial wing vein of 3 birds per pen per week (n = 72). Whole blood samples were transferred to 1.5 mL Eppendorf tubes, and serum was separated at 6000 rpm for 10 min at 4 °C. Serum samples were analyzed for levels of bone-specific alkaline phosphatase (BALP) and procollagen type 1 N-terminal propeptide (P1NP) using commercial ELISA kits from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China) and MyBioSource (San Diego, CA, USA), respectively.
2.11. Statistical Analysis
Statistical analyses were conducted utilizing the R software ‘stats’ package (version 4.3.2, R Core Team, 2023). Descriptive statistics were computed employing the “psych” package. Examination of data normality was performed via the Shapiro–Wilk test (p > 0.05) using the “shapiro.test” package and visual inspection of histograms using the “hist.” package, indicating normal distribution across all measurements. Generalized linear mixed models were constructed with the family set to “Poisson”, employing the lme4 package (Bates et al., 2014) to elucidate the impact of boron supplementation on performance parameters, CT parameters, muscle deposition, breaking strength, ash%, and bone mineralization over weeks of age, incorporating all potential interactions [29]. Dietary treatment and week of age were treated as the main effects, and unit and individual birds, where applicable, were considered random effects. Significance was determined at p ≤ 0.05, using the following model:
where Yijkl is the dependent variable, µ is the overall mean, Bi is the effect of the dietary treatment, Tj is the effect of the week of age, BTij is the interaction effect between Bi dietary treatment and Tj week of age, Ckl is the effect of individual birds within the unit of Bi, and across Tj weeks of age, and eijkl is the residual error.
Significant effects were subjected to additional analysis using Tukey’s honestly significant difference (HSD) multiple comparison procedure implemented via the “multcomp” package [30]. Tukey’s HSD was utilized to identify significant differences between pairwise comparisons, denoted in figures or tables by distinct superscript letters. Data are expressed as mean ± standard error of the mean (SEM), accompanied by p values of the pairwise comparisons.
3. Results
3.1. Performance
At weeks 4, 7, 10, 13, and 16 weeks of age, there were no differences in individual body weight, average daily weight gain per bird, or average daily feed intake per bird between the treatment groups (p > 0.05; Table 2).

Table 2.
Body weight, average daily weight gain, and average daily feed intake per bird (g) fed (mean ± SEM) a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at weeks 4, 7, 10, 13, and 16 weeks of age.
3.2. Bone Cross-Sectional Area (CSA) and Bone Mineral Density (BMD)
At week 11 of age, pullets from H pens had the highest cortical CSA compared to other treatment groups (proximal: M = 0.036, L = 0.026, C = 0.019; middle: M = 0.031, L = 0.022, C = 0.016; Table 3), except at the distal location. There were no differences between treatments for total CSA (p > 0.05; Table 3). Also at week 11 of age, hens from H pens had consistently higher total BMD than the other treatment groups (middle: M = 0.031, L = 0.021, C = 0.011; distal: M = 0.026, L = 0.016, C = 0.001), except for the proximal location, where C pens had the lowest total BMD compared to other treatments (proximal: H = 0.003, M = 0.016, L = 0.026; Table 3). Hens from H pens had higher cortical BMD than other treatments (proximal: M = 0.031, L = 0.024, C = 0.021; distal: M = 0.013, L = 0.029, C = 0.041), except at the middle location (middle: L = 0.025, C = 0.016; Table 3), where it was not significantly different from M pens.

Table 3.
Tibia cross-sectional area (CSA; mm2) and bone mineral density (BMD; mg/cm3) of pullets (mean ± SEM) fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at week 11 of age (n = 48). a–d Means with different superscripts within columns differ at p < 0.05.
At week 17 of age, pullets from H pens had the highest cortical CSA compared to other treatment groups (middle: M = 0.029, L = 0.016, C = 0.021; distal: M = 0.031, L = 0.036, C = 0.027), except at the proximal location (proximal: L = 0.025, C = 0.019; Table 4), where it was not significantly different from M pens. There were no differences between treatments for total CSA (p > 0.05; Table 4). Similarly, hens from H pens had higher total BMD than the other treatments (proximal: M = 0.023, L = 0.019, C = 0.009; middle: M = 0.026, L = 0.021, C = 0.005; distal: M = 0.036, L = 0.019, C = 0.026; Table 4), and higher cortical BMD (middle: M = 0.041, L = 0.026, C = 0.021; distal: M = 0.043, L = 0.039, C = 0.024), except at the proximal location (proximal: L = 0.023, C = 0.036; Table 4), where it was not significantly different from M pens.

Table 4.
Tibia cross-sectional area (CSA; mm2) and bone mineral density (BMD; mg/cm3) of pullets (mean ± SEM) fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at week 17 of age (n = 48). a–d Means with different superscripts within columns differ at p < 0.05.
3.3. Muscle Deposition
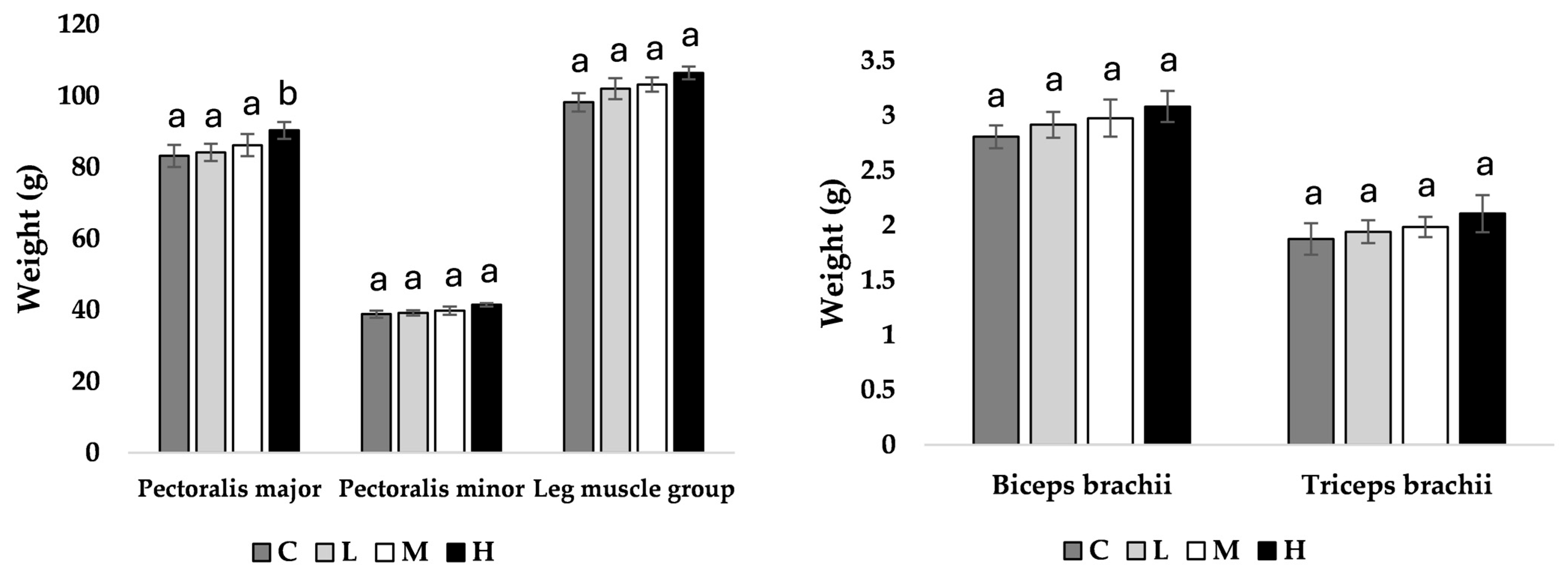
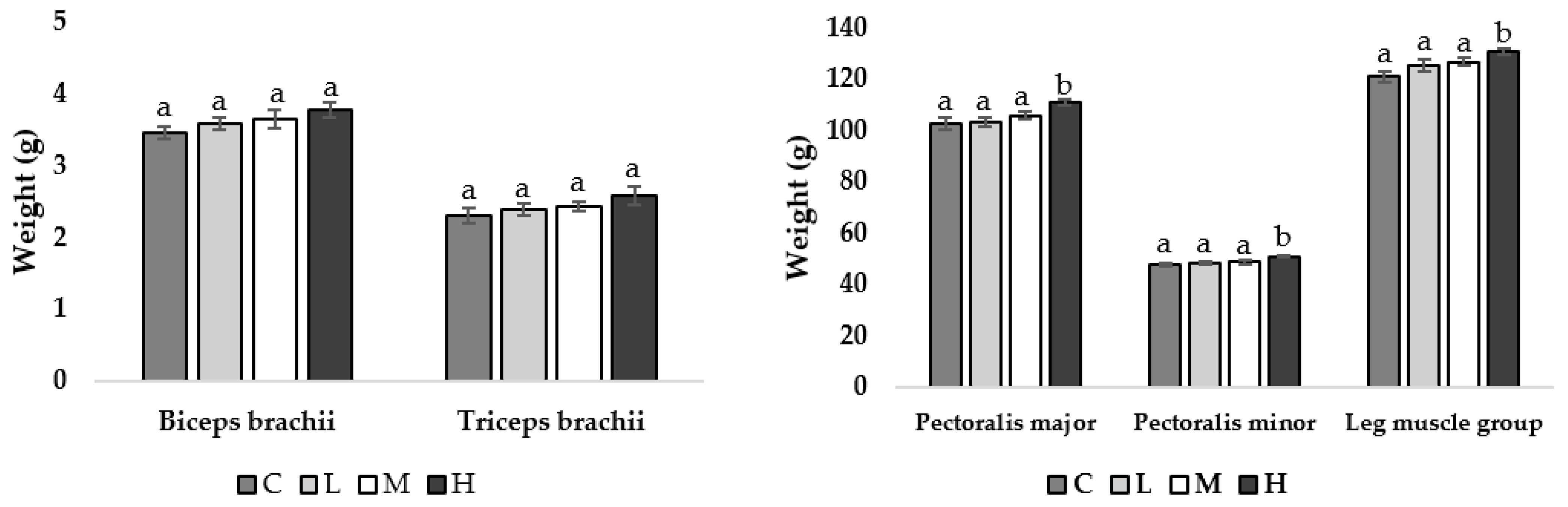
At week 11 of age, pullets fed the H diet had heavier pectoralis major muscles compared to all other treatment groups (M = 0.039, L = 0.031, C = 0.028; Figure 1), with no other observed differences. At week 17 of age, pullets fed the H diet had heavier pectoralis major (M = 0.043, L = 0.036, C = 0.034), pectoralis minor (M = 0.044, L = 0.040, C = 0.037), and leg muscle groups (M = 0.041, L = 0.033, C = 0.036) compared to all other treatment groups (Figure 2).
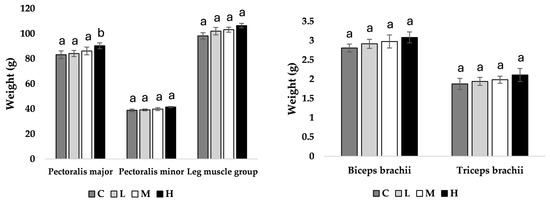
Figure 1.
Muscle mean weight (g) ± SEM of pullets fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at week 11 of age (n = 48 birds). a–b Means with different superscripts differ at p < 0.05.
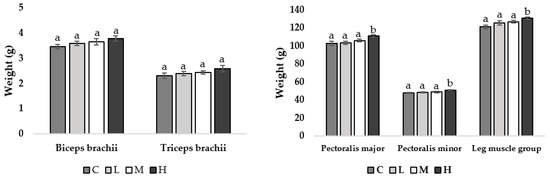
Figure 2.
Muscle mean weight (g) ±SEM of pullets fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at week 17 of age (n = 48 birds). a–b Means with different superscripts differ at p < 0.05.
3.4. Ash Percentage
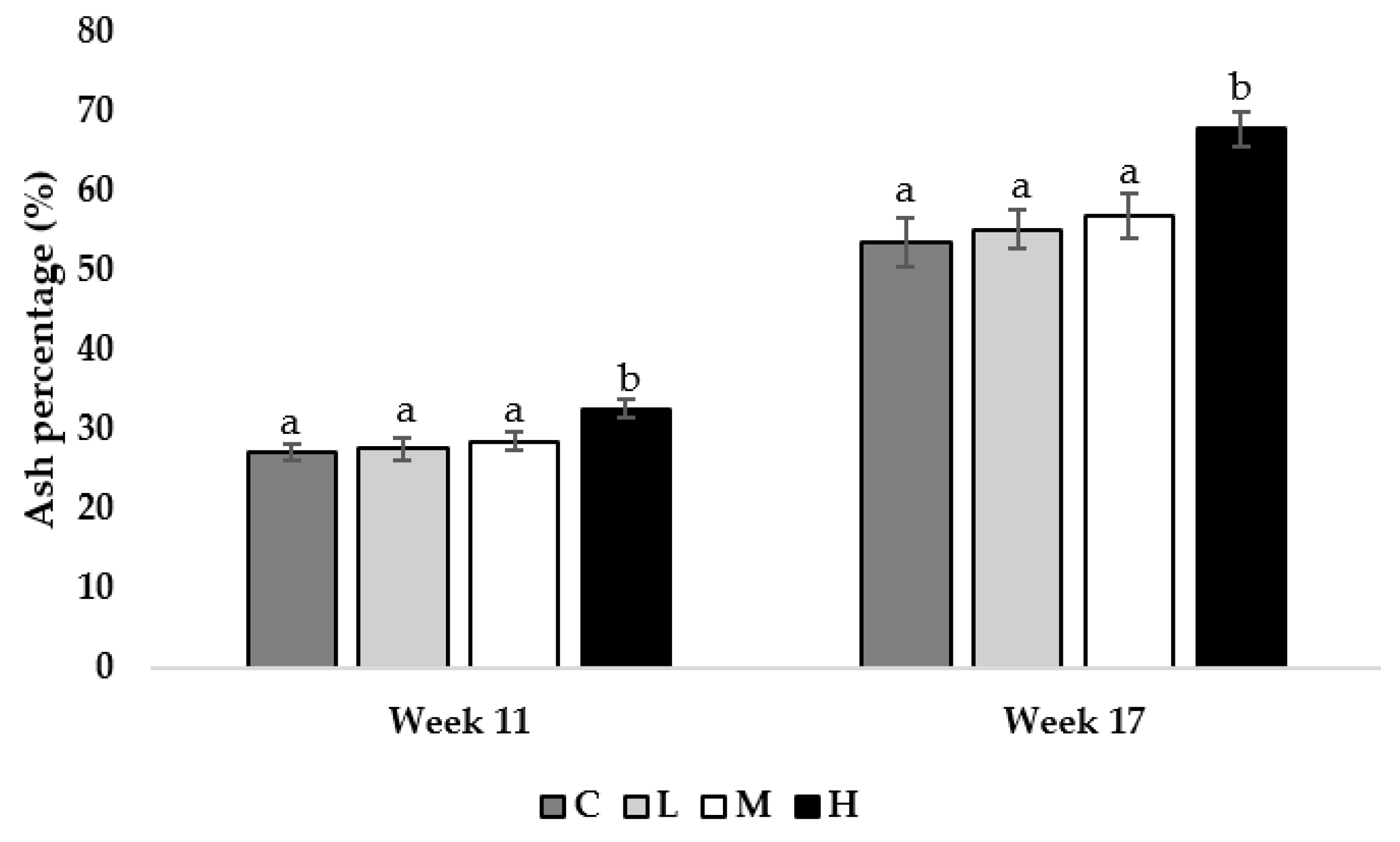
At 11 and 17 weeks of age, pullets fed the H diet had greater tibia ash percentages compared to all other treatment groups (Week 11; M = 0.013, L = 0.009, C = 0.001, week 17; M = 0.021, L = 0.015, C = 0.009; Figure 3).
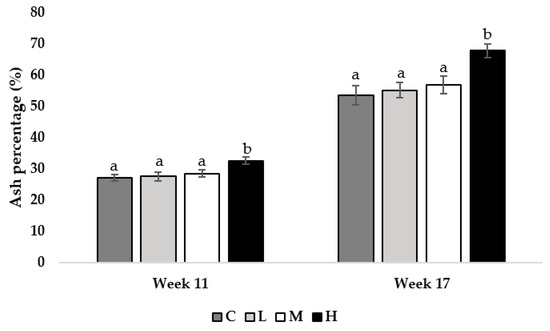
Figure 3.
Tibia ash percentage of pullets fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at weeks 11 and 17 of age (n = 48 birds/week). a–b Means with different superscripts differ at p < 0.05.
3.5. Breaking Strength
The tibiae of pullets fed the M or H diets had greater failure load (M; L = 0.032, C = 0.025, H; L = 0.012, C = 0.009) and maximum bending moment (M; L = 0.033, C = 0.012, H; L = 0.008, C = 0.002) compared to pullets fed the L or C diet at 11 weeks of age (Table 5). Furthermore, pullets fed the H diet had greater stiffness values compared to pullets fed the M diet (week 11; 0.036, week 17; 0.029), with the lowest stiffness in L and C pullets at both 11 and 17 weeks of age (week 11; 0.031, 0.023, week 17; 0.011, 0.021, respectively; Table 5). At 17 weeks of age, pullets fed the H diet had greater failure load (M = 0.033, L = 0.019, C = 0.003) and maximum bending moment (M = 0.029, L = 0.009, C = 0.001) compared to all other treatment groups (Table 5).

Table 5.
Tibia breaking strength (N), stiffness (N/mm), and maximum bending moment (N/m) of pullets fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at weeks 11 and 17 of age (n = 48 birds/week). a–c Means with different superscripts within columns differ at p < 0.05.
3.6. Bone Mineralization
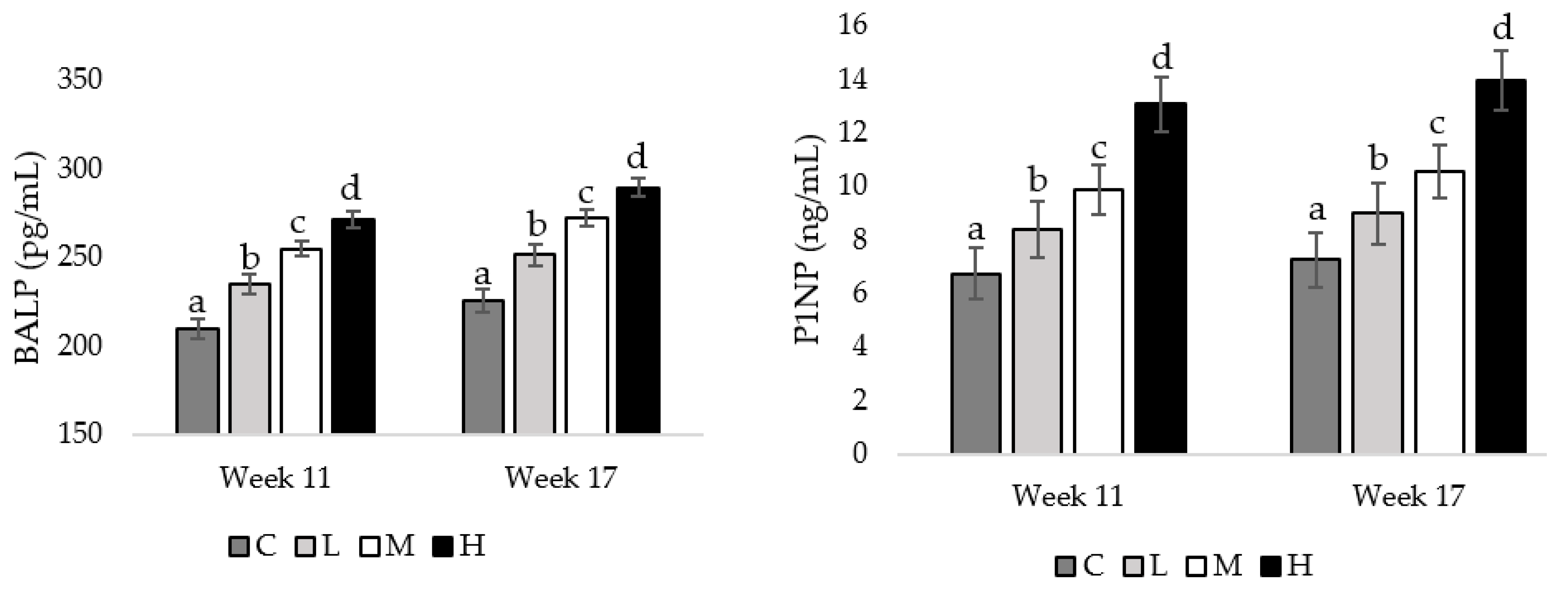
At weeks 11 and 17 of age, pullets fed the H diet had the greatest concentrations of BALP, compared to pullets fed the M diet (week 11 = 0.026, week17 = 0.033), followed by pullets fed the L diet (week 11 = 0.013, week 17 = 0.027), and pullets fed the C diet had the lowest BALP concentration (week 11 = 0.008, week 17 = 0.020; Figure 4). Also, at both the mentioned weeks of age, pullets fed the H diet had the greatest P1NP concentrations compared to pullets fed the M diet (week 11 = 0.036, week 17 = 0.021), with pullets fed the L (week 11 = 0.023, week 17 = 0.019) and C diets having the lowest P1NP concentrations (week 11 = 0.009, week 17 = 0.001; Figure 4).
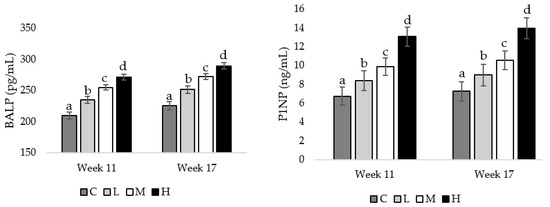
Figure 4.
Serum concentrations of bone-specific alkaline phosphatase (BALP) and procollagen type 1 N-terminal propeptide (P1NP) in pullets fed a control diet (C), or a basal diet supplemented with low (L; 50 mg/kg), medium (M; 100 mg/kg), or high (H; 150 mg/kg) boron at weeks 11 and 17 of age (n = 72 birds/week). a–d Means with different superscripts differ at p < 0.05.
4. Discussion
There were no differences in individual body weight, average daily weight gain per bird, or average daily feed intake per bird between the control diet and any of the diets supplemented with boron. This indicates that boron supplementation has no effect on pullet performance parameters. Previous studies in pullets and laying hens are in agreement with the current results, as they reported no difference in body weight as a result of boron supplementation [17,18]. However, a previous study reported that 64-week-old laying hens fed dietary boron had lower body weights than the control group [20]. Moreover, results from the current study contrast with some previous work in broiler chickens, where male broilers supplemented with 5 ppm boron resulted in heavier broilers [8]. Similarly, broilers fed 37.4 ppm boron experienced increased weight gain from 1 to 21 days of age without impacting the feed conversion ratio [10]. Perhaps the difference in weight gain of broilers in previous studies and the lack of difference in pullets in our study is due to broilers’ intense genetic selection for feed consumption and conversion into body mass. Since broilers gain a large amount of weight in a small amount of time, we may observe greater performance differences compared to pullets that do not gain weight rapidly and are not highly motivated to feed compared to broilers. Our results suggest that boron supplementation at 50, 100, or 150 mg/kg does not impact pullet performance parameters.
This is the first study to apply computed tomography to evaluate bone cross-sectional area and mineral density as a result of dietary boron supplementation in pullets. There were no differences between any of the treatment groups in total CSA at week 11 or week 17 of age, indicating that adding boron to the diet does not impact the overall size of the tibia. However, it was observed that pullets fed the H diet exhibited larger cortical CSA, and higher total and cortical BMD, than the other treatment groups at 11 weeks of age. By 17 weeks of age, both the H and M treatment groups had larger cortical CSA than the L and C groups, albeit the M group had slightly smaller cortical CSA than the H group. Additionally, pullets fed the H diet exhibited the highest total BMD values compared to the other treatment groups. Notably, cortical BMD increased with increasing boron inclusion, peaking in the H group and declining in the C group. Previous studies have suggested that boron supplementation can enhance cortical bone area and strength, as evidenced by ostrich chicks supplemented with 400 mg/L boron in drinking water showing significantly higher tibial cross-sectional areas compared to the control group or those given lower doses [31]. Also, in that study, ostrich chicks supplemented with 200 mg/L boron in drinking water displayed higher tibial bone mineral densities and stronger bones compared to the other treatment groups [31]. Boron is known to facilitate the resorption of minerals such as calcium and phosphorus, which are found in the bones in large quantities [16]. Moreover, boron acts with vitamin D, calcium, and magnesium, all of which are vital for bone metabolism [32]. For example, a deficiency of boron in the diet diminishes bone development and can cause bone irregularities in chicks that are deficient in vitamin D [33]. Supplementing the diet with boric acid and vitamin D has been shown to elevate tibia calcium levels in laying hens [15], suggesting a higher mineral content within the bones. Trace element distribution in laying hens is promoted with boron supplementation at 60, 120, or 240 mg/kg without negatively impacting bone calcium, phosphorus, or magnesium [19]. It is believed that boron influences bone metabolism by affecting bone composition and physical attributes through its stimulation of the formation of the organic matrix, which serves as the foundation for the calcification of bone [15,34]. Building up cortical bone reserves in pullets before they enter reproductive activity is especially important, as they draw calcium from bones for eggshell formation [3,35]. So, these findings, alongside previous studies, support the notion that adding boron to the diet at 100 or 150 mg/kg is beneficial in enhancing bone characteristics, particularly mineral content and the cortical area of the tibia, potentially reducing the risk of bone fractures later in life.
The current study found that pullets fed the H diet had higher tibia ash percentages than the other treatment groups. Similarly, in a previous study with White Leghorn pullets, tibia ash content increased at 50, 100, and 200 mg/kg of boron supplementation [17]. Also, laying hen femur and tibia ash content increased at 25 and 50 mg/kg boron [20]. Boron has been suggested to play a role in the metabolism of certain minerals, such as calcium and phosphorus [7,8]. These macro-minerals are known to play integral roles in normal skeletal development and functioning [36]. The higher tibia ash percentages and BMD values found in pullets fed the H diet indicate that boron supplementation aids in retaining macro-minerals within the tibia of 11- and 17-week-old Hy-Line W-36 pullets. This indicates that 150 mg/kg boron supplementation increases tibia mineral content at 11 and 17 weeks of age, which is in agreement with the CSA and BMD results obtained from CT scans. We also observed greater concentrations of BALP and P1NP, which are indicators of bone formation and indicate higher rates of bone mineralization by osteoblasts [37,38]. Although these are novel biomarkers of bone mineralization in poultry and have not previously been tested, the higher concentrations of BALP and P1NP, together with the bone mineral density and ash percent data, indicate that boron supplementation at 150 mg/kg increases osteoblast activity and therefore, bone mineralization.
Although body weights and feed efficiency did not differ between treatment groups, we did observe differences between pectoralis major muscle weights at 11 weeks of age, with pullets fed the H diet having heavier muscles compared to all other treatment groups. Also, at week 17 of age, pullets fed the H diet had heavier pectoralis major muscles, as well as heavier pectoralis minor and leg muscle groups, than the other treatment groups. The primary factors influencing adaptations to bone are locally acting stressors and strains generated by intrinsic muscle forces, as well as external loads [39]. The physical loading of bones directs the deposition of bone materials to areas experiencing the highest physical stress [39,40]. White-feathered strains are known to be flighty [41,42], and anecdotal observations indicate that the pullets used in this study frequently flew to areas at the top of the pens. Almost all pullets roosted on elevated structures within the pen at night, which were at least 2 m from the ground. Reaching such high areas within the pen would have required pullets to jump and use their wings to fly the distance to the elevated surface, imposing mechanical load on the breast and leg muscles [43]. This may explain why we observed an increase in breast muscle mass, as this is where the flight muscles attach, and leg muscles, as the legs are used in flight, take off, and landing [44,45]. However, this effect on muscle deposition was only observed in pullets fed the H diet. It is possible that the reciprocal relationship between bone density and muscle mass could explain this difference, together with the beneficial effect of boron supplementation on bone characteristics in a compounding manner [40]. Weight-bearing activity can increase bone density and muscle mass [46]. When muscles exert force on the bones during this activity, bone formation is stimulated, leading to increased bone density alongside increased bone mineralization, strength, and cross-sectional area, observed as a result of boron supplementation [46]. Casey-Trott et al. [27] hypothesized that the increased keel bone size of pullets reared in an aviary system was due to increased wing-assisted activity. Wing use involves the breast muscles (i.e., pectoralis major and minor), which are attached to the keel. Therefore, increased wing use increases pectoralis muscle contraction against the keel, which could induce keel bone formation. Alternatively, the increased muscle deposition seen in pullets fed the H diet may be a compensatory mechanism due to the denser bones. The denser tibiotarsal bones observed in pullets fed the H diet may direct the deposition of tissues in those areas, such as the leg muscle group, by 17 weeks of age as a compensatory mechanism since all birds were housed in identical environments, with access to the same resources and space per bird.
The present study utilized a three-point bend test to measure failure load, stiffness, and maximum bending moment. Failure load indicates the bone’s breaking strength, with a higher failure load indicating a stronger bone that requires more force to break [47]. Stiffness refers to the resistance of a bone to an applied force during elastic deformation (i.e., deformation disappears when the external force is removed), where a stiffer bone is more rigid and able to withstand increased force without permanently deforming [48]. In this study, tibiae of 11-week-old pullets fed the M and H diets had greater failure load and maximum bending moment compared to pullets fed the L and C diet, indicating the tibiae were stronger and more elastic in 11-week-old pullets fed a diet supplemented with 100 or 150 mg/kg boron. At 17 weeks of age, only pullets fed the H diet had greater failure load and maximum bending moment compared to all other treatment groups. This may suggest that only the highest level of boron inclusion sustained the beneficial results observed on tibia strength and elasticity beyond 11 and up to 17 weeks of age. At 11 and 17 weeks of age, the tibiae of pullets fed the H diet showed greater stiffness values than those fed the M diet, with the lowest stiffness values in L and C pullets. Our results are in line with previous studies, which noted that boron supplementation has a beneficial impact on bone strength characteristics via optimal calcium absorption [31,32,49]. For example, shear stress of the tibia and shear fracture of the femur in White Leghorn pullets increased when fed 50 and 100 mg/kg boron [17], and boron supplementation at 60, 120, or 240 mg/kg for 16 weeks increased shear force, stress, and fracture energy of the tibia in 26-week-old Lohmann hens [19], and femur bone strength increased in 64-week-old Barred Rock hens fed 25 and 50 mg/kg boron for 60 weeks [20]. One study investigating the long-term impact of boron supplementation found that the shear fracture energy of the tibia and radius increased in 72-week-old White Leghorns fed 200 mg/kg boron starting at 32 weeks of age, indicating a decrease in bone brittleness by the end of the lay period [22]. However, one study found no impact of boron on bone strength characteristics [50]. This lack of difference may be because this study was performed in the last 28 days of production, when hens were around 60 weeks of age. At this late point in production, when the bone calcium reserves are already quite depleted, as well as providing the nutritional intervention for only 28 days, it would be difficult to observe any beneficial impact of boron on skeletal health. Our results suggest that providing boron at 150 mg/kg improves bone strength characteristics in 11- and 17-week-old Hy-Line W-36 pullets.
5. Conclusions
Measures of musculoskeletal health in Hy-Line W-36 pullets were beneficially altered up to 17 weeks of age as a result of supplementing the diet with 150 mg/kg boron. Our results highlight the beneficial effect of boron supplementation on muscle deposition, tibia ash content and breaking strength, and bone mineralization, without impacting performance parameters. Providing boron at 100 mg/kg showed some beneficial effects, such as improved bone-cross sectional area and bone mineral density, although this effect was not as pronounced as what was observed in pullets fed 150 mg/kg boron. We also observed enhanced bone strength characteristics in pullets fed 100 mg/kg boron at 11 weeks of age; however, this effect dissipated by 17 weeks of age. Future studies should focus on investigating the long-term impact of feeding dietary boron to pullets on their musculoskeletal health, as well as the egg production and quality of laying hens.
Author Contributions
Conceptualization, M.G.A. and A.A.; methodology, M.G.A., A.C., C.H. and A.A.; formal analysis, M.G.A. and A.A.; investigation, M.G.A., A.C., A.M.J., C.H., M.A.-R. and A.A.; data curation, M.G.A., A.C., A.M.J., C.H. and A.A.; writing—original draft preparation, M.G.A. and A.A.; writing—review and editing, M.G.A., M.A.-R. and A.A.; supervision, M.A.-R. and A.A.; project ad-ministration, M.G.A., A.C. and A.M.J.; funding acquisition, M.A.-R. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the United Sorghum Checkoff Program (project # RG002-21). Technical Contribution No. 7279 of the Clemson University Experiment Station, this material is based upon work supported by NIFA/USDA, under project number SC-1029. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Clemson University’s Institutional Animal Care and Use Committee (protocol #: AUP2021-0068; November 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the employees and student workers at the Morgan Poultry Center for their help, care, and troubleshooting. We would also like to thank our undergraduate volunteers, especially Jose Charre-Perales, Zoie Morrow, Ari Bragg, and Shaunie Cruz.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Whitehead, C.C. Overview of Bone Biology in the Egg-Laying Hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C. Bone Biology and Skeletal Disorders in Poultry; Poultry Science Symposium Series; Carfax Publishing Co.: Abingdon, UK, 1992. [Google Scholar]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in Cage Layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Chen, C.; Turner, B.; Applegate, T.J.; Litta, G.; Kim, W.K. Role of Long-Term Supplementation of 25-Hydroxyvitamin D3 on Egg Production and Egg Quality of Laying Hen. Poult. Sci. 2020, 99, 6899–6906. [Google Scholar] [CrossRef]
- Liu, D.; Veit, H.; Wilson, J.; Denbow, D. Long-Term Supplementation of Various Dietary Lipids Alters Bone Mineral Content, Mechanical Properties and Histological Characteristics of Japanese Quail. Poult. Sci. 2003, 82, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, Calcium, Parathyroid Hormone, and Sex Steroids in Bone Health and Effects of Aging. J. Osteoporos 2020, 2020, 9324505. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.A.; Edwards, H.M. Studies to Determine Whether an Interaction Exists Among Boron, Calcium, and Cholecalciferol on the Skeletal Development of Broiler Chickens. Poult. Sci. 1992, 71, 677–690. [Google Scholar] [CrossRef]
- Rossi, A.F.; Miles, R.D.; Damron, B.L.; Flunker, L.K. Effects of Dietary Boron Supplementation on Broilers. Poult. Sci. 1993, 72, 2124–2130. [Google Scholar] [CrossRef]
- Hunt, C.D. Dietary Boron Modified the Effects of Magnesium and Molybdenum on Mineral Metabolism in the Cholecalciferol-Deficient Chick. Biol. Trace Elem. Res. 1989, 22, 201–220. [Google Scholar] [CrossRef]
- Fassani, E.; Bertechini, A.; Brito, J.; Kato, R.; Fialho, E.; Geraldo, A. Boron Supplementation in Broiler Diets. Rev. Bras. Cienc. Avic 2004, 6, 213–217. [Google Scholar] [CrossRef]
- Kurtoğlu, F.; Kurtoğlu, V.; Çelik, İ.; Keçeci, T.; Nizamlioğlu, M. Effects of Dietary Boron Supplementation on Some Biochemical Parameters, Peripheral Blood Lymphocytes, Splenic Plasma Cells and Bone Characteristics of Broiler Chicks given Diets with Adequate or Inadequate Cholecalciferol (Vitamin D). Br. Poult. Sci. 2005, 46, 87–96. [Google Scholar] [CrossRef]
- Kurtoĝlu, V.; Kurtoĝlu, F.; Coşkun, B. Effects of Boron Supplementation of Adequate and Inadequate Vitamin D3-Containing Diet on Performance and Serum Biochemical Characters of Broiler Chickens. Res. Vet. Sci. 2001, 71, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Shuler, T.R. Studies of the Interaction between Boron and Calcium, and Its Modification by Magnesium and Potassium, in Rats. Biol. Trace Elem. Res. 1992, 35, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. The justification for providing dietary guidance for the nutritional intake of boron. Biol. Trace Elem. Res. 1998, 66, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sizmaz, O.; Yildiz, G. Influence of Dietary Boric Acid and Ascorbic Acid on Performance, Egg Traits, Cholesterol and Bone Parameters of Laying Hens. Ankara Üniv. Vet. Fak. Derg. 2016, 63, 151–156. [Google Scholar]
- Adarsh, V.; Dintaran, P.; Shivakumar, G.N.K.; Vijayarangam, E.A.; Kumar, D.D.; Nagaraj, K.; Eknath, J.S. Effect of Boron Supplementation on Laying Performance of White Leghorn Hens Fed Diet with and without Adequate Level of Calcium. Trop. Anim. Health Prod. 2021, 53, 444. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.H.; Ruszler, P.L. Effects of Boron on Growing Pullets. Biol. Trace Elem. Res. 1997, 56, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, V.; Kurtoğlu, F.; Coşkun, B. Effects of Boron Supplementation on Performance and Some Serum Biochemical Parameters in Laying Hens. Rev. De Médecine Vétérinaire 2006, 153, 823–828. [Google Scholar]
- Olgun, O.; Yazgan, O.; Cufadar, Y. Effects of Boron and Copper Dietary Supplementation in Laying Hens on Egg Shell Quality, Plasma and Tibia Mineral Concentrations and Bone Biomechanical Properties. Rev. Med. Vet. 2012, 163, 335–342. [Google Scholar]
- Mizrak, C.; Yenice, E.; Can, M.; Yildririm, U.; Atik, Z. Effects of Dietary Boron on Performance, Egg Production, Egg Quality, and Some Bone Parameters in Layer Hens. S. Afr. J. Anim. Sci. 2010, 40, 257–264. [Google Scholar] [CrossRef]
- Wilson, J.H.; Ruszler, P.L. Effects of Dietary Boron Supplementation on Laying Hens. Br. Poult. Sci. 1996, 37, 723–729. [Google Scholar] [CrossRef]
- Wilson, J.H.; Ruszler, P.L. Long Term Effects of Boron on Layer Bone Strength and Production Parameters. Br. Poult. Sci. 1998, 39, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hy-Line W36. Hy-Line Management Guide. Available online: https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf (accessed on 25 March 2024).
- Johnson, A.M.; Anderson, G.; Arguelles-Ramos, M.; Ali, A.A.B. Effect of Dietary Essential Oil of Oregano on Performance Parameters, Gastrointestinal Traits, Blood Lipid Profile, and Antioxidant Capacity of Laying Hens during the Pullet Phase. Front. Anim. Sci. 2022, 3, 1072712. [Google Scholar] [CrossRef]
- Harrison, C.; Jones, J.; Bridges, W.; Anderson, G.; Ali, A.; Mercuri, J. Associations among Computed Tomographic Measures of Bone and Muscle Quality and Biomechanical Measures of Tibiotarsal Bone Quality in Laying Hens. Am. J. Vet. Res. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.G.; Johnson, A.M.; Harrison, C.; Arguelles-Ramos, M.; Ali, A. Impact of Perch Provision Timing on Activity and Musculoskeletal Health of Laying Hens. Animals 2024, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Casey-Trott, T.M.; Korver, D.R.; Guerin, M.T.; Sandilands, V.; Torrey, S.; Widowski, T.M. Opportunities for Exercise during Pullet Rearing, Part I: Effect on the Musculoskeletal Characteristics of Pullets. Poult. Sci. 2017, 96, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- ANSI/ASAE. ANSI/ASAE S459 MAR1992 (R2017) Shear and Three-Point Bending Test of Animal Bone; St. Joseph: Lancaster, MA, USA, 2017. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Cheng, J.; Peng, K.; Jin, E.; Zhang, Y.; Liu, Y.; Zhang, N.; Song, H.; Liu, H.; Tang, Z. Effect of Additional Boron on Tibias of African Ostrich Chicks. Biol. Trace Elem. Res 2011, 144, 538–549. [Google Scholar] [CrossRef]
- Devirian, T.A.; Volpe, S.L. The Physiological Effects of Dietary Boron. Crit. Rev. Food Sci. Nutr. 2003, 43, 219–231. [Google Scholar] [CrossRef]
- Bai, Y.; Hunt, C.D. Dietary Boron Enhances Efficacy of Cholecalciferol in Broiler Chicks. J. Trace Elem. Exp. Med. 1996, 9, 117–132. [Google Scholar] [CrossRef]
- Nielsen, F.H. Dietary Fat Composition Modifies the Effect of Boron on Bone Characteristics and Plasma Lipids in Rats. BioFactors 2004, 20, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; Fleming, R.H.; Julian, R.J.; Sørensen, P. Skeletal Problems Associated with Selection for Increased Production. Poult. Genet. Breed. Biotechnol. 2003, 3, 29–52. [Google Scholar]
- Bozkurt, M.; Küçükyilmaz, K.; Çath, A.U.; Çinar, M.; Çabuk, M.; Bintaş, E. Effects of Boron Supplementation to Diets Deficient in Calcium and Phosphorus on Performance with Some Serum, Bone and Fecal Characteristics of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2012, 25, 248. [Google Scholar] [CrossRef]
- Magnusson, P.; Arlestig, L.; Paus, E.; Di Mauro, S.; Testa, M.P.; Stigbrand, T.; Farley, J.R.; Nustad, K.; Millan, J.L. Monoclonal Antibodies against Tissue-Nonspecific Alkaline Phosphatase. Tumor Biol. 2002, 23, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Portero-Muzy, N.; Roux, J.-P.; Garnero, P.; Chapurlat, R. Are Biochemical Markers of Bone Turnover Representative of Bone Histomorphometry in 370 Postmenopausal Women? J. Clin. Endocrinol. Metab. 2015, 100, 4662–4668. [Google Scholar] [CrossRef] [PubMed]
- Shipov, A.; Sharir, A.; Zelzer, E.; Milgram, J.; Monsonego-Ornan, E.; Shahar, R. The Influence of Severe Prolonged Exercise Restriction on the Mechanical and Structural Properties of Bone in an Avian Model. Vet. J. 2010, 183, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, M.; Flavel, R.J.; Sibanda, T.Z.; Schneider, D.; Welch, M.C.; Ruhnke, I. Various Bone Parameters Are Positively Correlated with Hen Body Weight While Range Access Has No Beneficial Effect on Tibia Health of Free-Range Layers. Poult. Sci. 2019, 98, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Manet, M.W.E.; Kliphuis, S.; Nordquist, R.E.; Goerlich, V.C.; Tuyttens, F.A.M.; Rodenburg, T.B. Brown and White Layer Pullet Hybrids Show Different Fear Responses towards Humans, but What Role Does Light during Incubation Play in That? Appl. Anim. Behav. Sci. 2023, 267, 106056. [Google Scholar] [CrossRef]
- De Haas, E.N.; Bolhuis, J.E.; de Jong, I.C.; Kemp, B.; Janczak, A.M.; Rodenburg, T.B. Predicting Feather Damage in Laying Hens during the Laying Period. Is It the Past or Is It the Present? Appl. Anim. Behav. Sci. 2014, 160, 75–85. [Google Scholar] [CrossRef]
- Moinard, C.; Statham, P.; Green, P.R. Control of Landing Flight by Laying Hens: Implications for the Design of Extensive Housing Systems. Br. Poult. Sci. 2004, 45, 578–584. [Google Scholar] [CrossRef]
- Harlander-Matauschek, A.; Rodenburg, T.B.; Sandilands, V.; Tobalske, B.W.; Toscano, M.J. Causes of Keel Bone Damage and Their Solutions in Laying Hens. Worlds Poult. Sci. J. 2015, 71, 461–472. [Google Scholar] [CrossRef]
- Jackson, B.E.; Tobalske, B.W.; Dial, K.P. The Broad Range of Contractile Behaviour of the Avian Pectoralis: Functional and Evolutionary Implications. J. Exp. Biol. 2011, 214, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Pufall, A.; Harlander-Matauschek, A.; Hunniford, M.; Widowski, T.M. Effects of Rearing Aviary Style and Genetic Strain on the Locomotion and Musculoskeletal Characteristics of Layer Pullets. Animals 2021, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Donalson, L.M.; Herrera, P.; Woodward, C.L.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Research Note: Effects of Different Bone Preparation Methods (Fresh, Dry, and Fat-Free Dry) on Bone Parameters and the Correlations Between Bone Breaking Strength and the Other Bone Parameters. Poult. Sci. 2004, 83, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J. Bone Mechanical Testing by Three-Point Bending. Washington University Musculoskeletal Structure and Strength Core. 2016. Available online: https://cpb-us-w2.wpmucdn.com/sites.wustl.edu/dist/f/1982/files/2019/05/Understanding-3pt-Bending-outcomes.pdf (accessed on 25 March 2024).
- McCoy, H.; Kenney, M.A.; Montgomery, C.; Irwin, A.; Williams, L.; Orrell, R. Relation of Boron to the Composition and Mechanical Properties of Bone. Environ. Health Perspect. 1994, 102, 49–53. [Google Scholar] [CrossRef]
- Wilson, J.H.; Ruszler, P.L. Effects of Dietary Boron on Poultry Bone Strength. Trans. ASAE 1995, 38, 167–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).