Effect of a Bacillus subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Diets
2.3. Study Design, Challenge, and Sampling
2.4. Environmental Swabs
2.5. Salmonella Enumeration
2.6. Data Analysis
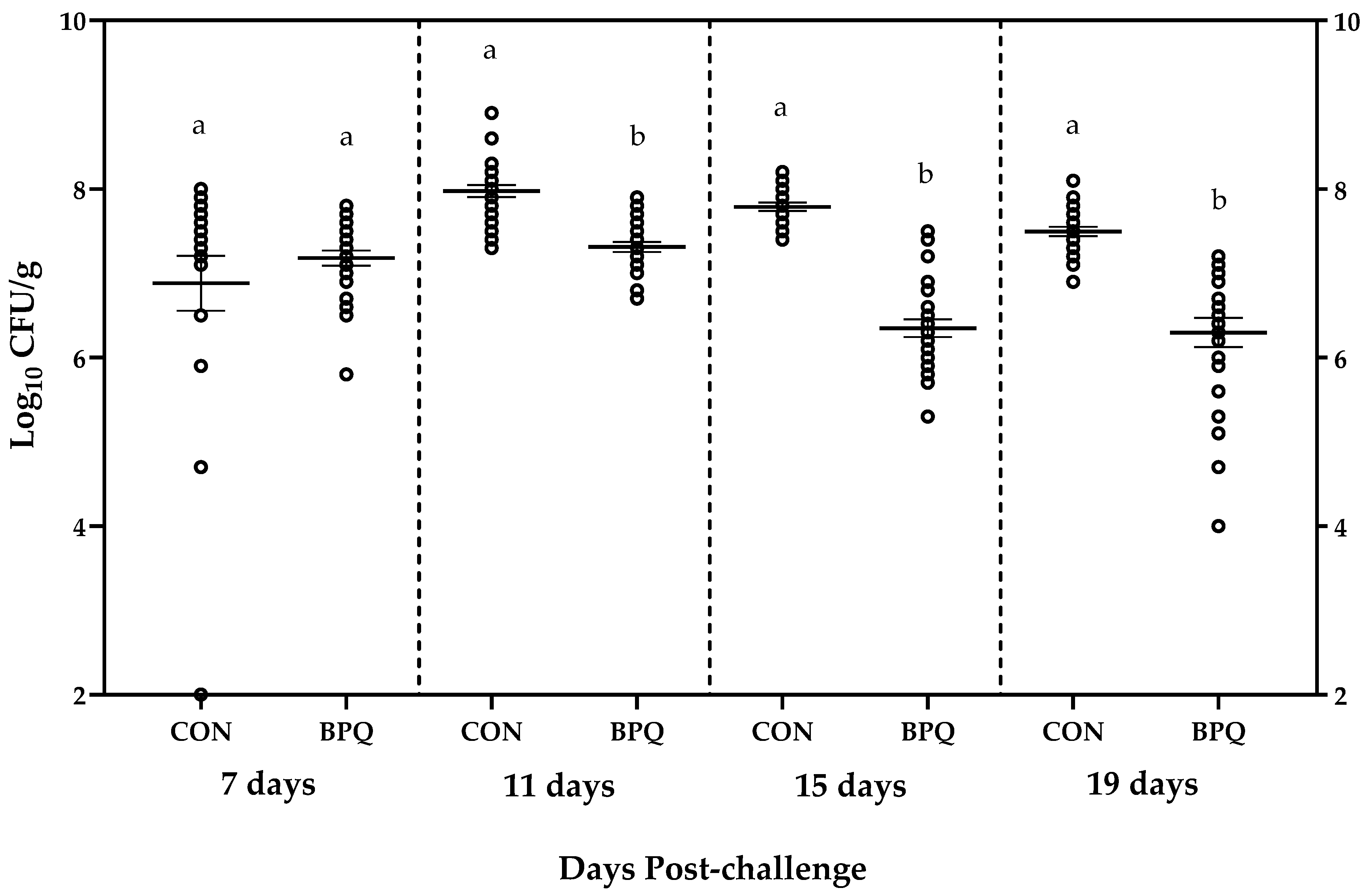
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.M.; Wang, Y.; Su, L.H.; Chiu, C.H. Nontyphoid Salmonella Infection: Microbiology, Clinical Features, and Antimicrobial Therapy. Pediatr. Neonatol. 2013, 54, 147–152. [Google Scholar] [CrossRef]
- GBD 2017 Non-Typhoid Salmonella Invasive Disease Collaborators. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- Center for Disease Control (CDC). Food Safety and Inspection Service; U.S. Department of Agriculture: Honolulu, HI, USA; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Ehuma, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Popa, G.L.; Popa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Juniora, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Vieira, A.R.; Hald, T.; Cole, D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014, 11, 667–676. [Google Scholar] [CrossRef]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Prevention of Salmonella Enteritidis in Shell Eggs During Production, Storage, and Transportation; Final Rule. Fed. Regist. 2009, 74, 33030–33100. [Google Scholar]
- EFSA Panel on Biological Hazards, European Commission. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, 5596. [Google Scholar] [CrossRef]
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.E.; Karcher, D.M. Contamination of eggs by Salmonella enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 5023–5027. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Al-Zenki, S.; Al-Nasser, A.; Al-Safar, A.; Alomirah, H.; Al-Haddad, A.; Hendriksen, R.S.; Aarestrup, F.M. Prevalence and antibiotic resistance of Salmonella isolated from a poultry farm and processing plant environment in the state of Kuwait. Foodborne Pathog. Dis. 2007, 4, 367–373. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Castro-Vargas, R.E.; Herrera-Sanchez, M.P.; Rodriguez-Hernandez, R.; Rondon-Barragan, I.S. Antibiotic resistance in Salmonella spp. Isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef]
- Sharma, M.; McDaniel, C.; Kiess, A.; Loar, R.; Adhikari, P. Effect of housing environment and hen strain on egg production and egg quality as well as cloacal and eggshell microbiology in laying hens. Poult. Sci. 2022, 101, 101595. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S. Impact of prebiotics on poultry production and food safety. Yale J. Biol. Med. 2018, 91, 151–159. [Google Scholar] [PubMed]
- Trampel, D.W.; Holder, T.D.; Gast, R.K. Integrated farm management to prevent Salmonella Enteritidis contamination in eggs. J. Appl. Poult. Res. 2014, 23, 353–365. [Google Scholar] [CrossRef]
- El Jeni, R.; Dittoe, D.K.; Olson, E.G.; Lourenco, J.; Corcionivoschi, N.; Ricke, S.C.; Callaway, T.R. Probiotics, and potential applications for alternative poultry production systems. Poult. Sc. 2021, 100, 101156. [Google Scholar] [CrossRef] [PubMed]
- Morikoshi, K.; Yokomizo, F. Method of Inhibiting Salmonella in Livestock and Poultry. U.S. Patent 8,999,374B2, 7 April 2015. [Google Scholar]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis reduction in layer ceca with a Bacillus probiotic. Vet. World 2020, 13, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Padgett, J.C.; Price, P.T.; Byrd, J.A.; Bailey, C.A. Salmonella Enteritidis control in mature laying hens through dry fed parietal yeast fraction or Bacillus blend probiotic. Int. J. Anim. Sci. Technol. 2021, 5, 1–6. [Google Scholar] [CrossRef]
- Price, P.T.; Gaydos, T.; Legendre, H.; Krehling, J.; Macklin, K.; Padgett, J.C. Production Layer Salmonella Enteritidis Control through Dry Fed Pre & Probiotic Products. Braz. J. Poult. Sci. 2021, 23, 1–6. [Google Scholar]
- Hofacre, C.L.; Berghaus, R.D.; Jalukar, S.; Mathis, G.F.; Smith, J.A. Effect of a yeast cell wall preparation on cecal and ovarian colonization with Salmonella Enteritidis in commercial layers. J. Appl. Poult. Res. 2018, 27, 453–460. [Google Scholar] [CrossRef]
- Girgis, G.; Powell, M.; Youssef, M.; Graugnard, D.E.; King, W.D.; Dawson, K.A. Effects of a mannan-rich yeast cell wall-derived preparation on cecal concentrations and tissue prevalence of Salmonella Enteritidis in layer chickens. PLoS ONE 2020, 15, e0232088. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.O.; Chaney, W.E. Effect of feeding a postbiotic derived from Saccharomyces cerevisiae fermentation as a preharvest food safety hurdle for reducing Salmonella Enteritidis in the ceca of layer pullets. J. Food Prot. 2021, 84, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chaney, W.E.; McBride, H.; Girgis, G. Effect of a Saccharomyces cerevisiae Postbiotic Feed Additive on Salmonella Enteritidis Colonization of Cecal and Ovarian Tissues in Directly Challenged and Horizontally Exposed Layer Pullets. Animals 2023, 13, 1186. [Google Scholar] [CrossRef] [PubMed]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of probiotic and prebiotic impacts Salmonella Enteritidis infection in layer hens. J. Appl. Poult. Res. 2021, 30, 100200. [Google Scholar] [CrossRef]
- Suganuma, K.; Hamasaki, T.; Hamaoka, T. Effect of dietary direct-fed microbial and yeast cell walls on cecal digesta microbiota of layer chicks inoculated with nalidixic acid resistant Salmonella Enteritidis. Poult. Sci. 2021, 100, 101385. [Google Scholar] [CrossRef] [PubMed]
- Girgis, G.; McBride, H.; Boyle, B.; Araba, M.; Bodle, B.C.; Lohrmann, T. Effects of a synbiotic combination of Bacillus subtilis and yeast cell wall-derived glucomannan on cecal colonization of Salmonella Enteritidis in layer chickens. J. Appl. Poult. Res. 2022, 31, 100240. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Agricultural Animals in Research and Teaching. 4th ed Federation of Animal Science Societies. 2020. Available online: https://poultryscience.org/files/galleries/AG_Guide_4th_Ed_2020.pdf (accessed on 10 April 2023).
- Lohmann LSL Lite Management Guide, North America Edition. Available online: https://hylinena.com/wp-content/uploads/2019/10/Lohmann_LSL-Lite16-2.pdf (accessed on 10 April 2023).
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994.
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Turnbull, P.C.; Snoeyenbos, G.H. Experimental Salmonellosis in the chicken. 1. Fate and host response in alimentary canal, liver, and spleen. Avian Dis. 1974, 18, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Krueger, L.A.; Gaydos, T.A.; Sims, M.D.; Spangler, D.A. Avi-Lution supplemented at 250 or 500 mg per kg in feed decreases the abundance of Salmonella Enteritidis in ceca of layer pullets. J. Appl. Poult. Res. 2020, 29, 995–1003. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef]
- Chaney, W.E.; Naqvi, S.A.; Gutierrez, M.; Gernat, A.; Johnson, T.J.; Petry, D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms 2022, 10, 544. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Dawson, K.A.; Newman, K.E. The Effects of Dietary Mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000, 79, 205–211. [Google Scholar] [CrossRef]
- Lowry, V.K.; Farnell, M.B.; Ferro, P.J.; Swaggerty, C.L.; Bahl, A.; Kogut, M.H. Purified beta-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Woodward, M.J. Competitive exclusion by Bacillus subtilis of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003, 94, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Ashida, N.; Nakagawa, K.; Iwatani, S.; Yamamoto, N. Dietary Bacillus subtilis C-3102 Supplementation Enhances the Exclusion of Salmonella enterica from Chickens. J. Poult. Sci. 2021, 58, 138–145. [Google Scholar] [CrossRef]
- Shaji, S.; Selvaraj, K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- Ricke, S.; Dittoe, D.; Olson, E. Microbiome applications for laying hen performance and egg production. Poult. Sci. 2022, 101, 101784. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T. Salmonella prevalence alone is not a good indicator of poultry food safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Holt, P.S. Persistence of Salmonella Enteritidis from one day of age until maturity in experimentally infected layer chickens. Poult. Sci. 1998, 77, 1759–1762. [Google Scholar] [CrossRef]
- Price, P.T.; Gaydos, T.; Berghaus, R.; Hofacre, C. Reduction of Salmonella Enteritidis colonization in production layers fed high levels of mannan and beta-glucan. Asian J. Poult. Sci. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Samper, J.; Araba, M.; Ruano, M.; Lohrmann, T.; Persia, M. Effects of a Direct Fed Microbial Fed from 0 to 70 Weeks of Age on Laying Hen Performance and Egg Quality from 18–70 Weeks of Age. J. Poult. Sci. 2022, 101 (Suppl. S1), 64. [Google Scholar]
| Ingredient | Diet, as-Fed Basis |
|---|---|
| Corn, % | 56.9 |
| Soybean meal, % | 33.5 |
| Calcium carbonate, % | 1.95 |
| Monocalcium phosphate (21% p), % | 1.79 |
| Vegetable oil, % | 3.91 |
| Salt, % | 0.50 |
| Choline chloride, % | 0.06 |
| L-lysine, % | 0.68 |
| DL methionine, % | 0.47 |
| Vitamin and mineral premix, % | 0.10 |
| Nutrient | |
| Metabolizable energy, Kcal/Kg | 3054 |
| Crude protein, % | 22.0 |
| Crude fat, % | 5.47 |
| Crude fiber, % | 2.57 |
| Calcium, % | 1.19 |
| Available phosphorus, % | 0.51 |
| Lysine, % | 1.54 |
| Methionine, % | 0.78 |
| Methionine + cysteine, % | 1.16 |
| Sodium, % | 0.25 |
| Days Post-Challenge | 7 | 11 | 15 | 19 | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment | CON | BPQ | CON | BPQ | CON | BPQ | CON | BPQ |
| Number of pullets | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Minimum (log10 CFU/g) | 2.0 | 5.8 | 7.3 | 6.7 | 7.4 | 5.3 | 6.9 | 4.0 |
| Maximum (log10 CFU/g) | 8.0 | 7.8 | 8.9 | 7.9 | 8.2 | 7.5 | 8.1 | 7.2 |
| Mean (log10 CFU/g) | 6.88 | 7.18 | 7.98 | 7.31 | 7.79 | 6.35 | 7.50 | 6.30 |
| STD (log10 CFU/g) | 1.63 | 0.45 | 0.363 | 0.293 | 0.246 | 0.521 | 0.276 | 0.869 |
| SEM (log10 CFU/g) | 0.326 | 0.090 | 0.073 | 0.059 | 0.049 | 0.104 | 0.055 | 0.174 |
| BPQ mean—CON mean (log10 CFU/g) | +0.30 | −0.67 | −1.45 | −1.20 | ||||
| p-value | 0.379 | <0.0001 | <0.0001 | <0.0001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araba, M.; Girgis, G.; McBride, H.; Lohrmann, T. Effect of a Bacillus subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets. Poultry 2024, 3, 26-35. https://doi.org/10.3390/poultry3010003
Araba M, Girgis G, McBride H, Lohrmann T. Effect of a Bacillus subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets. Poultry. 2024; 3(1):26-35. https://doi.org/10.3390/poultry3010003
Chicago/Turabian StyleAraba, Miloud, George Girgis, Hannah McBride, and Troy Lohrmann. 2024. "Effect of a Bacillus subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets" Poultry 3, no. 1: 26-35. https://doi.org/10.3390/poultry3010003
APA StyleAraba, M., Girgis, G., McBride, H., & Lohrmann, T. (2024). Effect of a Bacillus subtilis plus Yeast Cell Wall Synbiotic on Salmonella Enteritidis Colonization in Ceca of Layer Pullets. Poultry, 3(1), 26-35. https://doi.org/10.3390/poultry3010003







