Abstract
Avian pathogenic Escherichia coli (APEC) causes colibacillosis in poultry, a leading cause of poultry mortality worldwide. It is crucial to control APEC in broiler breeders as it is vertically transferred to progeny via eggs. However, there is only limited knowledge on the current APEC population in breeders. This study characterized 28 APEC strains isolated from broiler breeders with colibacillosis. The genotypic-virulence characteristics as well as antimicrobial and heavy-metal resistance patterns of the isolates were determined. Results showed that O88 is the most prevalent serogroup and B2 is the predominant phylogenetic group. Among virulence genes, genes for iron acquisition (iroN and iutA), protectins (iss and ompT), and toxin production (hlyF) exhibited the highest prevalence. Further, 93% of the isolates carried at least one antimicrobial resistance gene with highest prevalence for tetracycline gene tetA. Among the isolates, 10.71% exhibited multidrug resistance. All isolates carried at least one heavy-metal resistance gene with the highest prevalence for arsenic gene arsC and the highest resistance towards silver. Our findings provide insight into the characteristics of current APEC populations in broiler breeders in Mississippi. This will help future research on the pathogenesis of APEC and the development of effective prevention and control strategies against APEC in broiler breeders.
1. Introduction
Escherichia coli is a ubiquitous bacterium that typically predominates the gut microflora of humans, animals, and birds [1,2]. Apart from commensal E. coli, there is a large variety of pathogenic strains that cause intestinal and/or extraintestinal infections. Among them, avian pathogenic E. coli (APEC) causes extraintestinal infections in poultry [3,4]. APEC infections in poultry cause the disease, colibacillosis, manifested as perihepatitis, pericarditis, airsacculitis, salpingitis, and peritonitis progressing to septicemia and death [5,6,7]. In fact, this is one of the leading causes of mortality and morbidity in poultry that affects all stages of production and is economically devastating to the industry [3,4,8]. It is estimated that at least 30% of the commercial flocks in the United States are affected by colibacillosis at any point of time, causing a multi-billion-dollar loss to the poultry industry annually [4,6,9].
APEC can infect poultry through different routes. Oral and respiratory routes enable the bacteria to colonize the gastrointestinal and respiratory tracts, and in the presence of stressors, they may migrate to different internal organs and cause infection. Further, the infected birds can horizontally transmit the bacteria to other birds by contaminating feed and water [3,4,5]. Another route of entry is the vaginal route leading to ascending infection of the reproductive tract, causing salpingitis [10]. This can lead to a contamination of the egg while it is formed in the oviduct, leading to vertical transmission from hens to chicks. Sometimes the bacteria may not be pathogenic to the embryo during egg incubation, but the chicks after hatching can act as a source of infection causing horizontal transmission to other chicks in the hatchery, leading to increased first-week mortality [11,12,13]. Previously, APEC was considered as a secondary pathogen causing disease outbreaks due to concurrent virus infections, inappropriate management practices, or insufficient egg hygiene. However, recent research has identified its potential role as a primary pathogen that causes severe disease and high mortality in the absence of stressors [14,15,16].
The pathogenic property of APEC is facilitated by multiple virulence factors such as adhesins, invasins, protectins, iron acquisition mechanisms, toxins, and plasmids [15]. These factors facilitate the attachment, invasion, colonization, replication, and damage of the host cells, as well as evasion from the host immune response [4,17,18]. These virulence factors are encoded by a large array of virulence-associated genes. For instance, papC (P-fimbriae) and tsh (temperature-sensitive hemagglutinin) code for adhesion, and ibeA (invasion of the brain endothelium protein A) code for the invasion of the host system. Genes like iutA (aerobactin siderophore receptor), along with iroN (salmochelin siderophore receptor), enable iron acquisition from the body fluids, while iss (increased serum survival) and ompT (outer membrane protease) provide protection against host immune response. Further, astA (heat-stable enterotoxin) and hlyF (putative avian hemolysin) enable APEC to produce toxins to damage the host’s tissues along with Colicin V (ColV) plasmid genes like cva/cvi [15,19,20]. Several studies have identified combinations of different virulence genes for predicting the disease-causing potential of APEC strains [6,21,22]. However, the high diversity of APEC strains is challenging for ensuring the accuracy of these predictors and, thus, hinders the effective diagnosis, treatment, and prevention of E. coli infections in poultry [23].
The high diversity of APEC strains is further evident in the number of serotypes established. Serotyping is vital for unraveling APEC-virulence mechanisms [5,24]. O (lipopolysaccharide) and H types (flagellar antigen) have been widely used to classify E. coli strains over decades. Currently, there are 188 O and 53 H groups of E. coli identified [25,26]. Some of the O serogroups associated with APEC strains causing disease in poultry are O1, O2, O21, O35, O36, and O78 [16,27,28], while some of the H serogroups are H1, H2, H4, H7, H23, and H10 [29,30,31]. Serotyping, along with phylogenetic grouping, is a better predictor of the virulence potential of E. coli [32]. Most of the extraintestinal pathogenic E. coli, including APEC, are typed under the phylogroups B2 and D, while most of the commensal ones fall under phylogroups A and B1 [33,34,35,36].
Antimicrobial resistance is globally observed in APEC strains [4]. They have been reported to exhibit resistance to a variety of antibiotics, such as tetracyclines, sulfonamides, and aminoglycosides, which are commonly used in the poultry industry to treat APEC infections [4]. The antimicrobial resistance genes, along with the virulence genes, are often associated with plasmids that aid in their transmission between bacteria and, thus, need to be continuously monitored [37]. Also, as the poultry industry is moving towards antibiotic-free production, new effective intervention strategies for APEC control need to be developed.
To develop alternative treatment strategies like vaccines, it is essential to understand the changing properties of APEC and the effects it can have on a bird. Additionally, there is only limited information available on APEC strains from broiler breeders. Over the years, the control of APEC in broiler breeders have been highly neglected; however, recent findings have emphasized the importance of its’ control in broiler breeders as they play a key role in spreading APEC through contaminated eggs to broilers [11,13]. Therefore, the objective of this study is to characterize the virulence properties as well as antimicrobial and heavy-metal resistance patterns of APEC strains isolated from broiler breeders with symptoms of colibacillosis in Mississippi.
2. Materials and Methods
2.1. E. coli Isolation and Identification
Twenty-eight APEC isolates were recovered from the lesions of twenty-eight broiler breeder hens of 1 day to 57 weeks of age diagnosed with colibacillosis. Isolates were recovered from different tissues including the ovary, oviduct, peritoneum, heart, liver, bone marrow, yolk sac, lung, air sac, and hock joint. The isolates were provided by Poultry Research and Diagnostic Laboratory/Mississippi Veterinary Research and Diagnostic Laboratory (PRDL/MVRDL) on blood-agar plates. Individual representative colonies were selected, streaked onto MacConkey agar (Becton, Dickinson and Company, Sparks, MD, USA), and incubated aerobically at 37 °C for 18 to 24 h. Pink lactose-positive colonies, suspected as E. coli strains, were selected and further confirmed positive by real-time PCR for the ybbW gene (Eurofins Genomics LLC, Louisville, KY), which is part of the E. coli core genome [38]. The positive control was ATCC 25922, while DNA from Campylobacter jejuni (ATCC 33560) was used as the negative control. The strains included in this study were collected between 2019 and 2021.
2.2. DNA Isolation
DNA used as a template for PCR amplifications was extracted by boiling-lysis method as described previously [39]. Briefly, an individual representative colony from MacConkey agar was inoculated into Luria–Bertani (LB) broth (Becton, Dickinson and Company, Sparks, MD, USA) and grown for 18 to 24 h at 37 °C with shaking. From this fresh overnight culture, 200 µL was centrifuged, the supernatant was discarded, and the bacterial pellet was resuspended in 150 µL of nuclease-free water (Thermo Fisher Scientific, Vilnius, Lithuania) and then boiled at 98 °C for 5 min. The resulting solution was allowed to cool, centrifuged, and the supernatant was transferred into a new tube to serve as the DNA template and was stored at −20 °C until further use.
2.3. Serotyping
All E. coli isolates were submitted to the College of Veterinary Medicine, University of Georgia for multiplex PCR-based O and H serotyping. A total of 27 O-serogroups and 10 H-serogroups were tested using the primers and PCR conditions as described by Iguchi et al. [40] for O-antigen encoding genes and Banjo et al. [41] for H-antigen encoding genes, respectively (Eurofins Genomics LLC, Louisville, KY, USA). For O-grouping, the reactions were carried out in a final volume of 25 μL under the PCR conditions: 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C for 2 min, and a final extension step of 72 °C for 10 min. For H-grouping, the PCR conditions were: 94 °C for 1 min, followed by 25 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, and then a final extension at 72 °C for 2 min. The positive control was E. coli ATCC 25922, while nuclease-free water was used as the negative control. PCR products were separated and visualized using the Kodak Gel Logic 200 imaging system (Eastman Kodak Co., Rochester, NY, USA) and SYBR safe DNA gel stain (Invitrogen, Carlsbad, CA, USA).
2.4. Phylogenetic Classification
The phylogenetic group of the isolates were determined according to the E. coli phylogenetic-typing method described by Clermont et al. [34] and based on the presence or absence of the genes chuA, yjaA, and DNA fragment TSPE4.C2 (Eurofins Genomics LLC, Louisville, KY). The E. coli strains were then assigned to the phylogenetic groups A, B1, B2, or D. The PCR conditions used were 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 59 °C for 30 s, extension at 72 °C for 45 s, and a final cycle of amplification at 72 °C for 10 min. The positive control was E. coli ATCC 25922, while nuclease-free water was used as the negative control. PCR products were separated and visualized using the Kodak Gel Logic 200 imaging system (Eastman Kodak Co., Rochester, NY, USA) and SYBR safe DNA gel stain (Invitrogen, Carlsbad, CA, USA).
2.5. Screening for Virulence Genes
All isolates were investigated for the presence of ten genes that encode for different virulence factors such as papC, tsh, ibeA, iutA, iroN, iss, ompT, astA, hlyF, and cva/cvi (Eurofins Genomics LLC, Louisville, KY, USA). Of these virulence encoding genes, iroN, ompT, hlyF, iss, and iutA are described as the minimal APEC predictors, and a pentaplex PCR was used for detecting them as described by Johnson et al. [6]. For amplifying the remaining virulence genes, conventional PCR was used. The positive control was E. coli ATCC 25922, while nuclease-free water was used as the negative control. The primer sequences and annealing temperatures used are shown in Table 1. All PCR products were analyzed by agarose-gel electrophoresis using 1% agarose gel (Bio-Rad Laboratories, Inc, Madrid, Spain), stained with SYBR Safe DNA Gel Stain (Invitrogen, Carlsbad, CA, USA), and visualized under UV light.

Table 1.
Primer sequences and annealing temperatures used for the PCR analysis of virulence-associated genes.
2.6. Antimicrobial Resistance (AMR) and Heavy-Metal Resistance Patterns
2.6.1. Screening for Antimicrobial and Heavy-Metal Resistance Genes
All isolates were screened by PCR for 11 AMR genes conferring resistance to β-lactamase inhibitors (blaTEM), cephalosporins (blaCTX-M), aminoglycosides (aac3Vla, aph3IA and aadA), tetracyclines (tetA), sulfonamides (dfr7, sul1), quinolones (qnr), phenicols (cat1), and quaternary ammonium compounds (QAC; qacEΔ); and for 11 heavy-metal resistance genes conferring resistance to arsenic (arsC), copper (pcoA, pcoD, and pcoE), silver (silE, silP), mercury (merA), and tellurite (terD, terF, terX, and terY3) (Eurofins Genomics LLC, Louisville, KY, USA). Primer sequences and annealing temperatures used for AMR genes and heavy-metal resistance genes are shown in Table 2 and the heavy-metal resistance genes were previously described by Li et al. [43]. The positive control was ATCC 25922, while nuclease-free water was used as the negative control.

Table 2.
Primer sequences and annealing temperatures used for PCR analysis of antimicrobial and heavy-metal resistance genes.
2.6.2. Antimicrobial and Heavy-Metal Susceptibility Testing
Antibiotic Susceptibility Testing
All isolates were tested for antibiotic susceptibility by the Kirby–Bauer disk-diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines at 37 °C for 18–24 h [47]. The antibiotics used extensively for treating E. coli infections in poultry, livestock, and humans were tested, including ampicillin/sulbactam (20/20 µg), cefotaxime (30 µg), gentamicin (10 µg), kanamycin (30 µg), streptomycin (10 µg), tetracycline (30 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), and chloramphenicol (30 µg) (Thermo Scientific, Waltham, MA, USA). E. coli ATCC 25922 was used as the reference strain.
QAC and Heavy-Metal Susceptibility Testing
The susceptibilities of the isolates to QAC and heavy metals were evaluated by determining their respective inhibitory concentrations by a broth-microdilution method [48]. The QAC benzalkonium chloride (2 to 256 μg/mL; Sigma Aldrich, Burlington, MA, USA) and the heavy metals, copper (CuSO4·5H2O, 32 to 8192 μg/mL; Sigma Aldrich, USA), silver (AgNO3, 0.5 to 64 μg/mL; Honeywell Fluka, Muskegon, MI, USA), mercury (HgCl2, 0.84 to 54.4 µg/mL; Labchem, Zelienople, PA, USA), and tellurite (K2TeO3, 0.025 to 512 μg/mL; Sigma Aldrich, USA), were tested [48,49,50]. A working solution was prepared in sterile, deionized water, and subsequent concentrations were achieved by two-fold serial dilutions. For all of the E. coli isolates, an inoculum was prepared from fresh overnight culture, adjusted to the turbidity standard of 0.5 McFarland, and diluted 1:200 with LB broth. A volume of 50 μL of the dilutions of QAC/metals and 50 μL of the diluted suspension of bacteria was added to each well of the microtiter plate and incubated for 18 to 24 h at 37 °C. A strain was considered tolerant when it was able to grow at a QAC/metal concentration that inhibits the growth of the E. coli reference strain ATCC 25922 [51].
3. Results
3.1. Serotyping
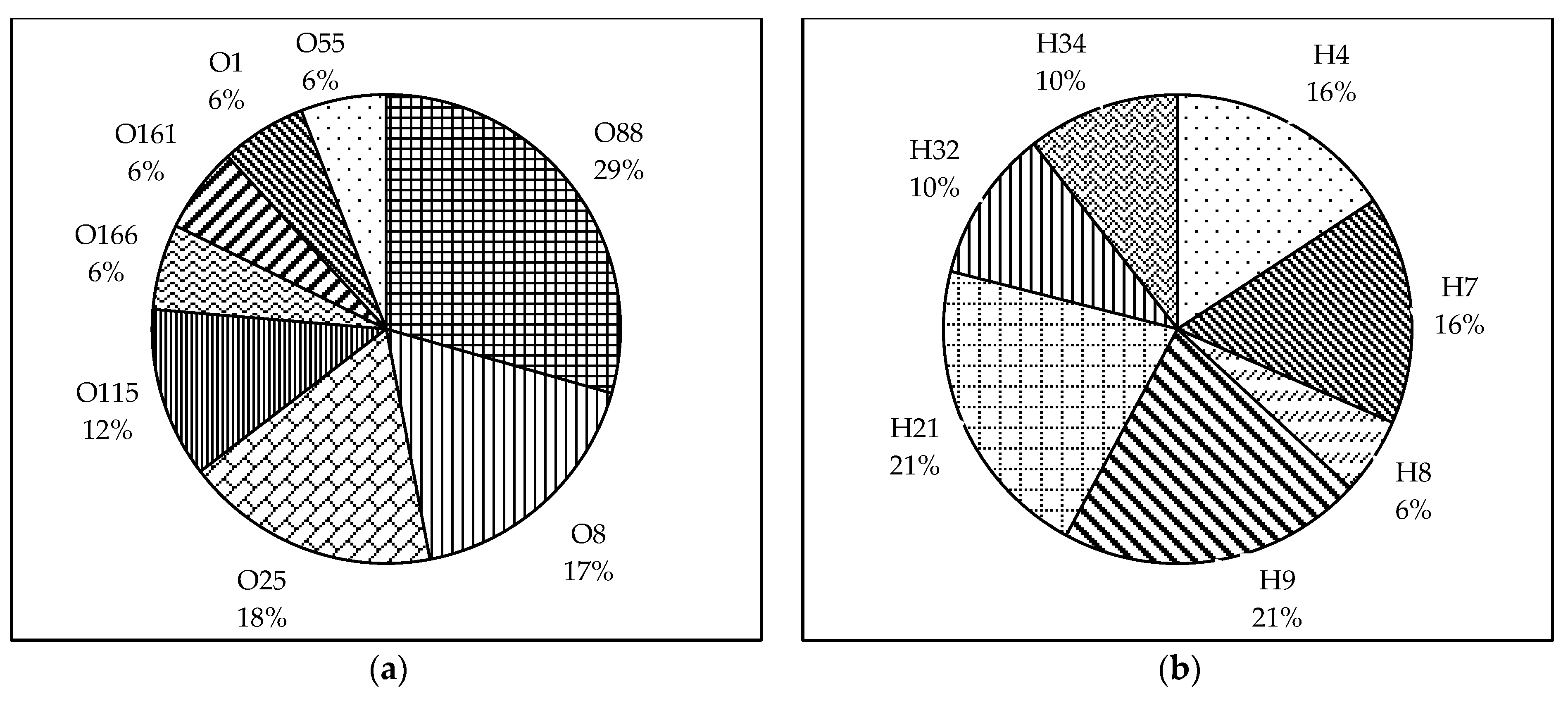
We were able to identify the O and H-types of 60.7% and 67.9% of the isolates, respectively, using the PCR–based method (Figure 1a,b). O88:H7 was the most prevalent serotype among the typed isolates (19%), followed by O8:H32 and O115:H34 (13%). The other types identified were O88:H21, O166:H21, O8:H9, O55:H9, O25:H4, and O25:H9.
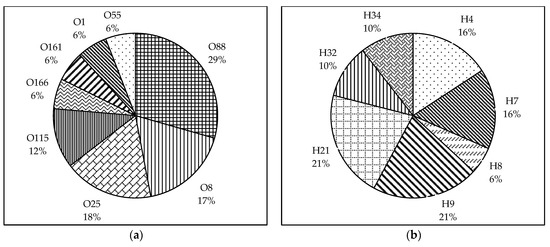
Figure 1.
Pie charts showing the prevalence of O and H serogroups among the broiler breeder APEC isolates. (a) The O-serogroups identified among the typed isolates were O88, O8, O25, O115, O166, O161, and O1. (b) The H serogroups identified among the typed isolates were H4, H7, H8, H9, H21, H32, and H34. Data represented as the percentage of prevalence.
Regarding the O serogroups, the most prevalent among the typed isolates were O88 (31%), followed by O8 and O25 (19%), and O115 (13%). The serogroups O166, O161, O1, and O55 were distributed equally (6%) among the typed isolates. Following H-typing, H9 and H21 (21%) were found to be the most prevalent among the isolates. Other H serogroups found were H4 and H7 (16%), H32 and H34 (10%), and H8 (5%).
3.2. Phylogenetic Classification
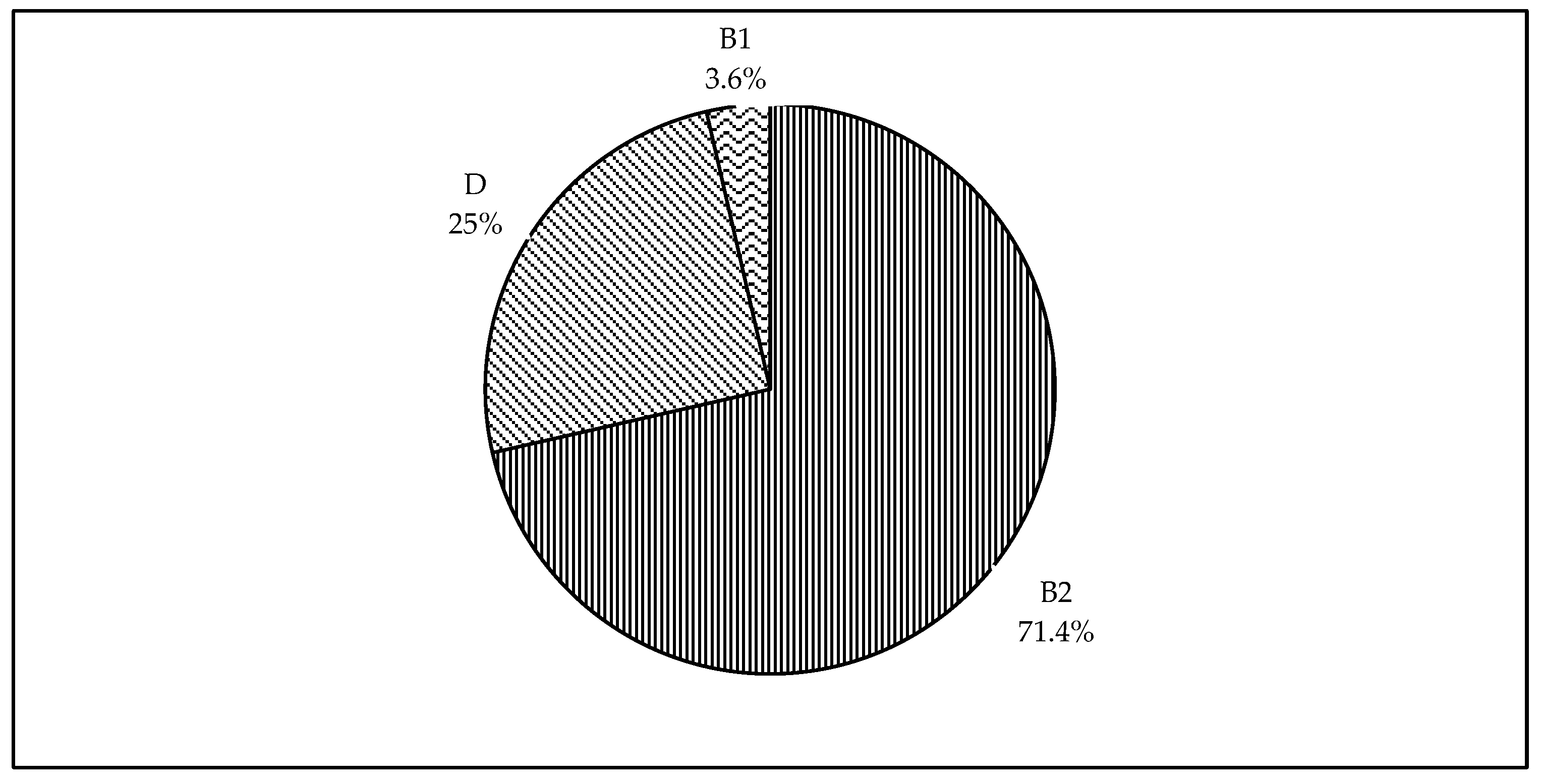
Figure 2 shows the distribution of phylogenetic groups identified among the isolates of this study. We were able to type all 28 isolates into different phylogroups. The majority of the isolates were of the phylogenetic group B2 (71.4%). Lower prevalence was observed for the phylogenetic groups D and B1 with 25% and 3.6%, respectively. Of interest, none of the isolates were classified under the phylogenetic group A.
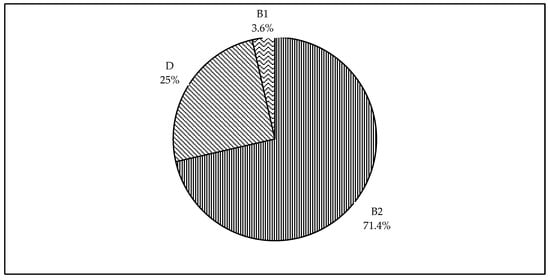
Figure 2.
Pie chart showing phylogenetic classification of broiler breeder APEC isolates. The phylogenetic groups identified among the 28 isolates were B2, D, and B1; and each segment represent the percentage prevalence.
3.3. Screening for Virulence Genes
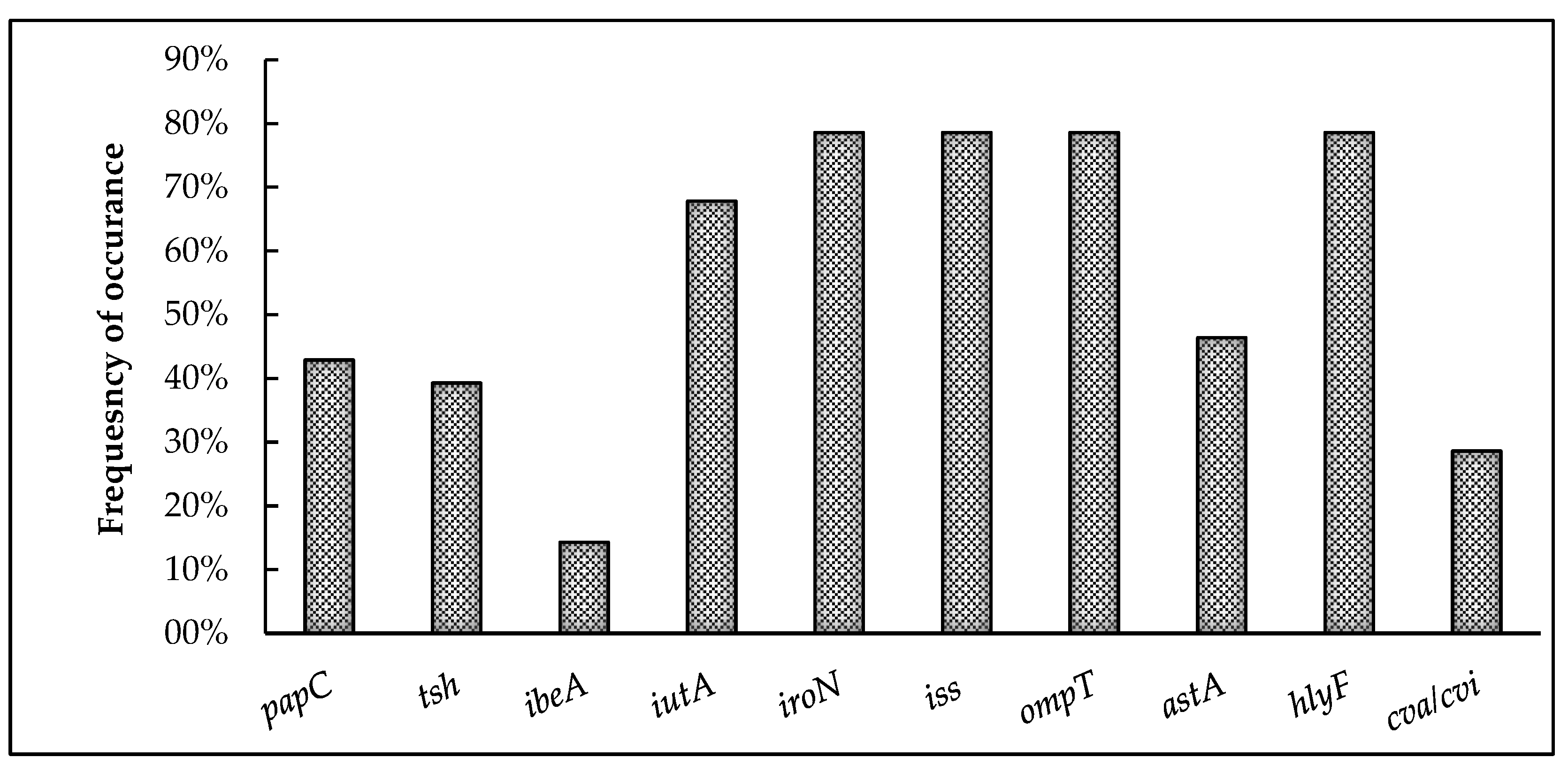
Figure 3 represents the overall prevalence of virulence-associated genes detected among the 28 APEC isolates. Interestingly, 96.4% of the isolates harbored at least one virulence-associated gene. The genes encoding for iron acquisition (iroN and iutA), protectins (iss and ompT), and toxin production (hlyF) exhibited the highest prevalence, with iroN, ompT, hylF, and iss present in 78.6% and iutA in 68% of the isolates. This is followed by the genes coding for adhesins papC (42.9%) and tsh (39.3%) as well as the colicin V plasmid-operon gene cva/cvi (28.6%). The gene encoding for invasin, ibeA (14.3%), showed the least prevalence among the virulence-associated genes tested.
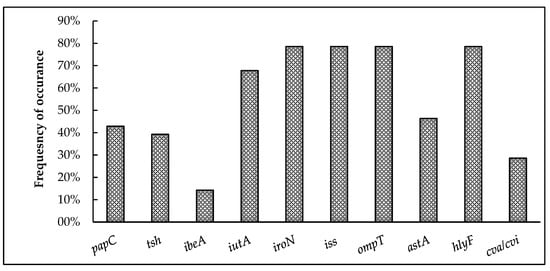
Figure 3.
Prevalence of virulence-associated genes among broiler breeder APEC isolates. papC and tsh code for adhesins; ibeA for invasin; iutA and iroN indicate iron acquisition systems; iss and ompT for protectins; astA and hylF indicate toxins; and cva/cvi is part of the colicin V plasmid.
3.4. AMR and Heavy-Metal Resistance Patterns
3.4.1. Screening for Antimicrobial and Heavy-Metal Resistance Genes
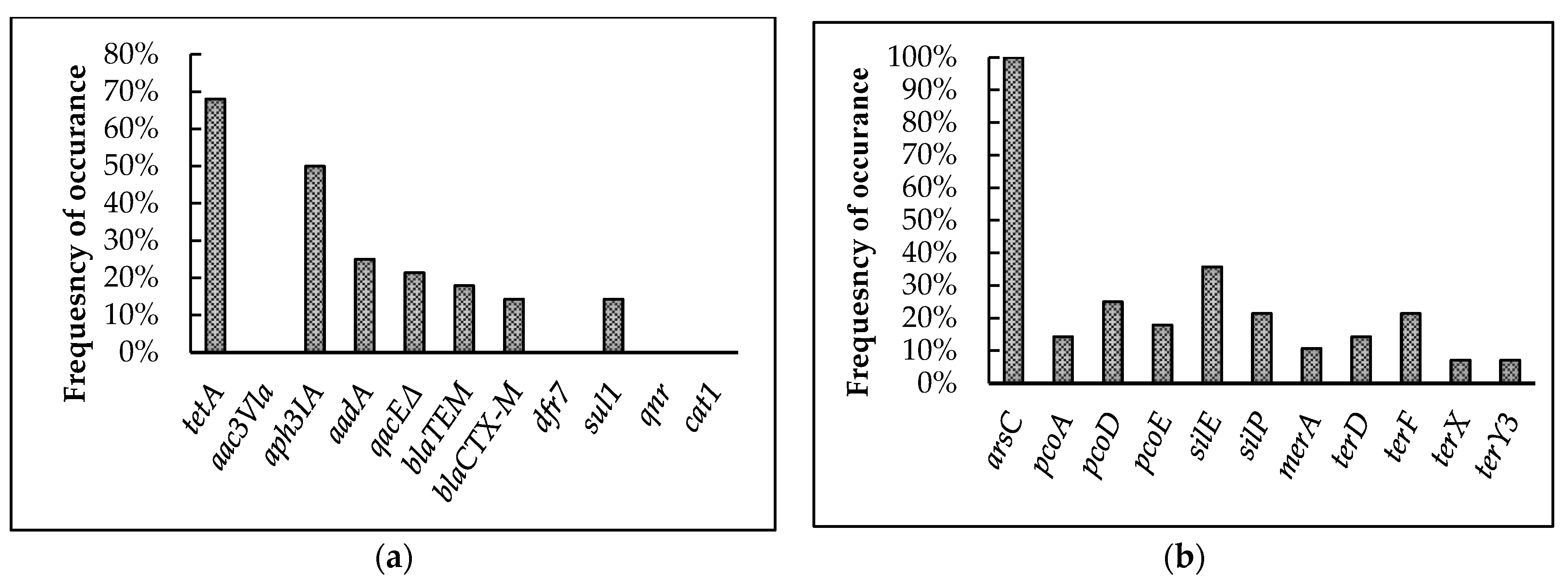
The presence of antimicrobial and heavy-metal resistance genes identified in the 28 isolates are represented in Figure 4a,b. Screening for AMR genes showed the highest prevalence for tetA (68%) followed by aph3IA (50%), aadA (25%), qacEΔ (21.4%), and blaTEM (17.9%). Further, blaCTX-M and sul1 exhibited an equal prevalence of 14.3% among the isolates. On the other hand, qnr, aac3Vla, cat1, and dfr7 were absent among the isolates analyzed.
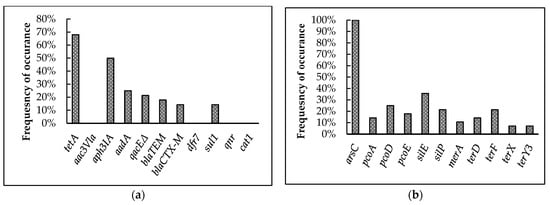
Figure 4.
Prevalence of antimicrobial and heavy-metal resistance genes among broiler breeder APEC isolates. (a) Antimicrobial resistance genes tested in the 28 isolates. tetA confers resistance to tetracycline; aac3Vla, aph3IA, and aadA to aminoglycosides; qacEΔ to quaternary ammonium compounds; blaTEM to β-lactamase; blaCTX-M to cephalosporins; dfr7 and sul1 to sulfonamides; qnr to quinolones; and cat1 to phenicols. (b) arsC confers resistance to arsenic; pcoA, pcoD, pcoE to copper; silE, silP to silver; merA to mercury; and terD, terF, terX, and terY3 to tellurium.
Among the heavy-metal resistance genes, arsC (100%) was present in all the isolates. The genes silE and silP were present among 35.7 and 21.4% of the isolates, respectively. Further, pcoA, pcoD, and pcoE were present in 14.3, 25, and 17.9% of the isolates, respectively. The mercury-resistance gene, merA, showed 10.7% prevalence among the isolates. In the case of tellurite-resistance genes, terF showed 21.4% and terD showed 14.3% prevalence among the isolates, while terX and terY3 showed the least prevalence among all heavy metal resistance genes, at 7.1%.
Pearson correlation analysis using SAS 9.4 was performed to identify the relationship of AMR genes with virulence and heavy-metal resistance genes as well as serotypes and phylogenetic groups, but no significant correlations were observed.
3.4.2. Antimicrobial and Heavy-Metal Susceptibility Testing
Antibiotic-Susceptibility Testing
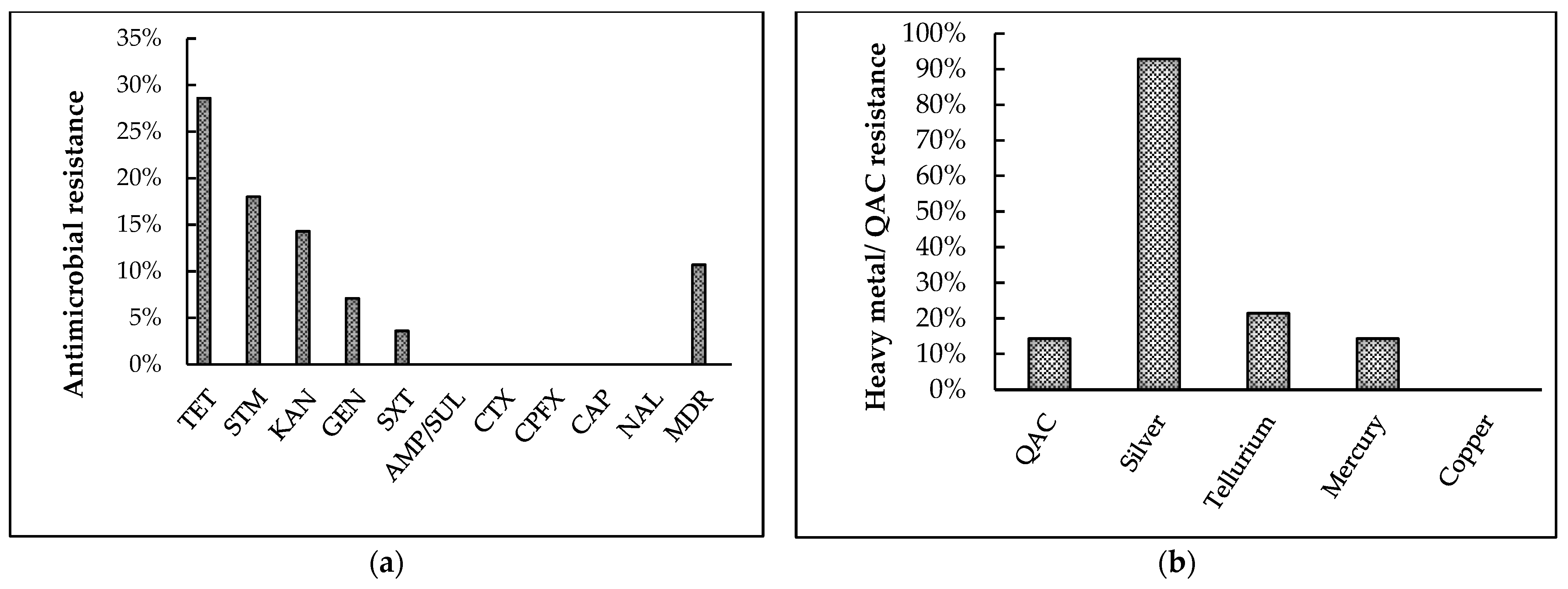
Of the 28 isolates tested (Figure 5a), the highest resistance was exhibited towards tetracycline (28.6%) followed by streptomycin (18%), kanamycin (14.3%), and gentamicin (7.1%), with the least resistance exhibited towards sulfamethoxazole-trimethoprim (3.6%). Interestingly, 10.7% of the isolates were multidrug-resistant (resistant to three or more antibiotic classes), and 42.9% of isolates demonstrated intermediate or complete resistance to at least one antibiotic tested. However, 100% isolates were susceptible to ampicillin/ sulbactam, cefotaxime, ciprofloxacin, chloramphenicol, and nalidixic acid.
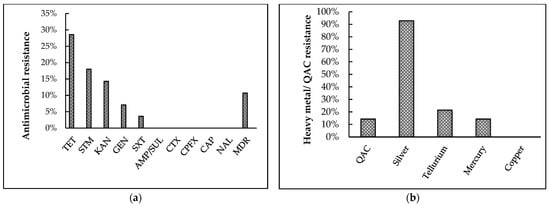
Figure 5.
Results of phenotypic antimicrobial and heavy-metal resistance pattern of broiler breeder APEC isolates. (a) Antibiotic-resistance patterns towards ten antibiotics were tested using Kirby–Bauer disk diffusion assay. TET: tetracycline, STM: streptomycin, KAN: kanamycin, GEN: gentamicin, SXT: sulphamethoxazole-trimethoprim, AMP/SUL: ampicillin/ sulbactam, CTX: cefotaxime, CPFX: ciprofloxacin, CAP: chloramphenicol, NAL: nalidixic acid, MDR: multi- drug resistance (b) Quaternary ammonium compound (QAC) and heavy-metal resistance patterns were tested using a broth microdilution assay.
QAC and Heavy-Metal Susceptibility Testing
Following the broth-microdilution assay (Figure 5b), 14.3% of the isolates were found to be resistant towards QAC. Among the five metals tested, the isolates demonstrated highest resistance towards silver (92.8%), followed by tellurium (21.4%), and mercury (14.3%). Furthermore, 96.4% of the isolates were resistant to at least one of the five metals tested. In contrast, all isolates were susceptible to copper, and all isolates were susceptible to at least two metals tested.
4. Discussion
There is a multitude of data on the characteristics of APEC isolates from broilers and layers worldwide [52,53,54]. However, there is only limited information about the isolates from broiler breeders. Recent studies have emphasized the importance of broiler breeders as reservoirs for APEC infection through vertical transmission to chicks and subsequent horizontal transmission between chicks [11,13,55]. Moreover, the constantly evolving genetic diversity of APEC strains requires their continuous monitoring in all species of poultry [23,56]. To our knowledge, this is the first study to characterize APEC isolates from broiler breeders with colibacillosis in Mississippi, and it provides information on their genotypic-virulence properties as well as antimicrobial and heavy-metal resistance patterns.
Serotyping revealed that O88:H7 was the most prevalent serotype among the APEC isolates typed in this study. Among the O serogroups, O88 was most prevalent, followed by O8, O25, O115, O166, O161, O1, and O55. Historically, in poultry, O1, O2, and O78 were most often correlated with disease-causing APEC worldwide [7]. Specifically, in broiler breeders, O2, O78, and O5 have been reported to be the most prevalent among APEC population [57]. Whereas, in the current study, all of the dominant serogroups detected were new serogroups. A recent study on layers with colibacillosis reported higher prevalence of the O88 serogroup [58]. Furthermore, the new serogroups, O25 and O8, were also observed to be dominant among E. coli isolated from turkeys with cellulitis [4] and poultry with colibacillosis in the United States [16]. Awad et al. [59] found O115 was the most prevalent serogroup among broilers, and none of the historic serogroups were detected. Among the H serogroups, H9 and H21 were highly prevalent among our isolates. The same pattern of prevalence for H9 was found in a study conducted among broilers with colibacillosis in Spain [53]. Previously, Nolan et al. [60] emphasized the diverse nature of APEC possible according to geographical location and disease; thus, it is not surprising to see newly evolving serogroups. Current data, along with findings from recent studies across globe, indicate the changing trend among APEC serotypes causing disease, and this warrants continuous monitoring.
Results of phylogenetic classification revealed a higher prevalence of phylogroup B2, followed by D and B1. Many studies have reported that extraintestinal pathogenic E. coli belongs to phylogroups B2 and D [27,44]. Also, this is consistent with APEC strains isolated from broiler breeders with salpingitis and peritonitis [35]. Similarly, a study on free-range chickens further supports our findings, with majority of APEC isolates belonging to phylogroups B2 and D [61]. Interestingly, in commercial broiler breeders vaccinated using the autogenous E. coli vaccine, the repression of most phylogroups present in the vaccine and a phylogenetic shift to B2 group have been observed among APEC isolates [62]. This, in fact, indicates the opportunistic nature of APEC and its ability to evolve.
The prevalence of virulence genes tested in the present study are highly variable, ranging from 14.3% to 78.6%. The genes encoding for iron acquisition (iroN and iutA), protectins (iss and ompT), and toxin production (hlyF) were found to exhibit the highest prevalence among the isolates. In fact, these five genes are associated with the ColV plasmid [63] and have been identified as genes more predominantly associated with highly pathogenic APEC (APEC minimal predictors) by Johnson et al. [6]. A similarly high prevalence of these five genes was observed in E. coli isolated from broilers and broiler breeders with colibacillosis from different geographical locations, such as Canada [64], Brazil [65,66], Egypt [67], Korea [37], and the United States [16]. On the other hand, the gene cva/cvi, which is also part of the ColV plasmid, was found to have a lower prevalence compared to these five ColV plasmid genes. This was unexpected but was also reported by de Oliveira et al. [39] in turkeys with cellulitis and warrants further investigation. Similarly, the occurrence of other virulence genes analyzed, papC, astA, and tsh, was also less compared to the minimal predictors and was similar to that reported in APEC from broilers in Nepal [42]. The gene encoding the mechanism for invasion, ibeA, was the lowest among the isolates and was comparable to that observed in E. coli isolated from cellulitis lesions in turkeys from Iowa, United States [39]. In general, the current results indicate the virulence-defining nature of the APEC minimal-predictor genes in E. coli isolated from broiler breeders with colibacillosis.
The widespread use of antimicrobials in poultry over the years against multiple pathogens has resulted in antimicrobial resistance [68]. Moreover, the extensive use of antibiotics as growth-promoters in feed over the past 60 years made this a big issue for the poultry industry worldwide [68]. Furthermore, the spread of AMR genes has affected the genetics and mechanisms of resistance of bacteria to combat other antimicrobial agents [69]. In this study, tetA (68%), which encodes for tetracycline resistance, showed the highest prevalence among all of the antibiotic resistance genes tested. This genotypic characteristic was expressed phenotypically following disk-diffusion assay, where the highest resistance was noted towards tetracycline among the isolates. Tetracycline is one of the most widely used antibiotics in poultry and animals [70] and that might be the reason for the high resistance towards this antibiotic among the isolates. Furthermore, our results were consistent with that reported in E. coli isolated from broiler breeders from different parts of the world [64,71,72,73]. Also, similar findings were reported among APEC isolates from broilers in Mississippi [43], Jordan [74], and Egypt [67]. Besides tetA, our isolates showed a high prevalence of genes encoding for aminoglycoside resistance, aph3IA and aadA, and sulfonamide resistance, sul1, and this might be the reason for high kanamycin, streptomycin, and sulfamethoxazole-trimethoprim resistance among the isolates, respectively. On the other hand, the gene coding for resistance to gentamicin, aac3Vla, was absent, but resistance towards this antibiotic was noted among the isolates. There are six clusters of genes that code for gentamicin resistance [75] and some of these genes that we have not tested might be present in our isolates and could be the plausible reason for this resistance among the isolates. A similar resistance pattern towards these antibiotics was previously reported in APEC isolated from broilers and broiler breeders in Canada [64].
Our study also identified the genes encoding for β-lactamase inhibitor blaTEM (Ampicillin and Sulbactam) and third generation cephalosporin blaCTX-M (Cefotaxime) resistance among our isolates. However, no isolates were completely resistant to these antibiotics following disk-diffusion assay. A recent study from Algeria reported the occurrence of blaTEM in E. coli isolated from the ovaries of healthy broiler breeders and that it co-harbored with blaCTX-M. This in fact indicates the vertical-transmission potential of extended-spectrum β-lactamases in the poultry-production pyramid [76]. Additionally, vertical transmission of extended-spectrum β-lactamases-producing E. coli from broiler breeders down the production pyramid was previously reported in Switzerland [77], Finland [78], and Sweden [79]. This is definitely a rising concern that seeks our attention because controlling APEC colonization and infections in broiler breeders might be one of the ways to control the increasing antibiotic resistance in poultry.
Of interest, 10.7% of our isolates were multidrug-resistant, which is a concern considering the control of various bacterial diseases in broiler breeders. A majority of the isolates exhibited multidrug resistance towards tetracycline, kanamycin, streptomycin, and gentamicin. A similar pattern of multidrug resistance was previously reported in broiler breeder APEC isolates from Algeria [76]. Also, several recent studies globally from different poultry species have reported multidrug resistance of APEC to different combinations of antibiotics [43,80,81]. The potential reason for this multidrug resistance is the spread of mobile genetic elements or plasmids carrying genes encoding the antibiotic resistance phenotype and promoting their co-selection, co-resistance, and co-expression [76,82].
The antimicrobial-resistance gene, qacEΔ (QAC), was also identified in our isolates (21.4%). Further, following broth microdilution assay, 14.3% of the isolates exhibited resistance to QAC benzalkonium chloride. Similar results on the prevalence of genes encoding for QAC resistance were found in E. coli collected from omphalitis-affected baby chicks [83] and broilers [43,74]. QAC-based disinfectants are widely used in poultry facilities for cleaning and sanitation. Their inappropriate use, such as long-term exposure of bacteria to subinhibitory concentrations, may lead to the development of resistance [84].
Among 11 heavy-metal resistance genes tested, arsC (100%) was present in all isolates. Even though FDA has currently banned the use of arsenic-based products in poultry feed, this could be the effect of the long-term use of poultry feed supplemented with arsenicals-based antimicrobials for disease control and growth promotion [85]. A similarly high prevalence was also reported in E. coli from broilers in Mississippi [43]. The prevalence of arsC was followed by silver resistance genes, silE (35.7%) and silP (21.4%), in our isolates. Phenotypically, 92.8% of the isolates showed resistance towards silver, which was the highest among all of the heavy metals tested. One possible reason for this difference is that, apart from the two genes tested, there are other genes responsible for silver resistance in E. coli [86]. This is the first report of such high resistance towards silver in broiler breeder APEC isolates. Besides, the genes for tellurite (terF, terD, terY3, and terX) and mercury (merA) resistance were identified in our isolates. These genes were also reported in poultry from China [86], broilers from Mississippi [43], and turkeys from Iowa [39]. Further, genes for copper resistance (pcoA, pcoD, and pcoE) were also identified in the isolates; however, all of the isolates were susceptible to copper. At the moment, copper might be a possible solution for controlling APEC in broiler breeders in Mississippi, however, the presence of these genes is quite concerning, and further studies on the extent of their vertical and horizontal transmission potentials are required.
5. Conclusions
Broiler breeders are critical in the vertical transmission of APEC to progeny and, thus, controlling the disease in breeder flock is crucial to lowering the occurrence of colibacillosis in broilers [11]. Hence, continuous monitoring of APEC isolates from broiler breeders is imperative. To our knowledge, this is the first time APEC isolates were specifically collected and characterized from broiler breeders with colibacillosis in Mississippi. In this study, we identified the serogroup pattern, phylogenetic groups, and the presence of virulence genes, as well as the antimicrobial and metal resistance patterns among the APEC isolates. Results showed that O88 is the most prevalent serogroup, and B2 is the predominant phylogenetic group. Among virulence genes, iroN, iutA, iss, ompT, and hlyF exhibited the highest prevalence. Multidrug resistance and resistance towards heavy metals were also observed among the isolates. These findings provide a reference basis for future research on the pathogenesis of APEC as well as to develop effective intervention strategies in the prevention and control of APEC in broiler breeders.
Author Contributions
Conceptualization, R.R.; project administration, methodology, investigation, and data curation, J.J., M.J., N.B., L.Z. and R.R.; writing—original draft preparation, J.J.; writing—review and editing, J.J., R.R., N.B., P.A. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by USDA-ARS SCA grant 58-6064-2-014. This work is a contribution of the Mississippi Agricultural and Forestry Experiment Station under CRIS project# MIS-329350.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Martha Pulido-Landínez, PRDL/MVRDL, Mississippi State University for providing the E. coli strains used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sannes, M.R.; Kuskowski, M.A.; Owens, K.; Gajewski, A.; Johnson, J.R. Virulence factor profiles and phylogenetic background of Escherichia coli isolates from veterans with bacteremia and uninfected control subjects. J. Infect. Dis. 2004, 190, 2121–2128. [Google Scholar] [CrossRef]
- Vila, J.; Aez-L’Opez, E.S.; Johnson, J.R.; Omling, U.R.; Dobrindt, U.; Cantón, R.; Cantón, C.; Giske, C.G.; Naas, T.; Carattoli, A.; et al. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 5, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. 1999, 30, 299–316. [Google Scholar] [PubMed]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Dziva, F.; Stevens, M. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.N.; Sukumaran, A.T. Avian Pathogenic Escherichia coli and Clostridium perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef] [PubMed]
- Ghunaim, H.; Abu-Madi, M.A.; Kariyawasam, S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: Potentials and limitations. Vet. Microbiol. 2014, 172, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef]
- Ozaki, H.; Yonehara, K.; Murase, T. Virulence of Escherichia coli Isolates Obtained from Layer Chickens with Colibacillosis Associated with Pericarditis, Perihepatitis, and Salpingitis in Experimentally Infected Chicks and Embryonated Eggs. Avian Dis. 2018, 62, 233–236. [Google Scholar] [CrossRef]
- Giovanardi, D.; Campagnari, E.; Sperati Ruffoni, L.; Pesente, P.; Ortali, G.; Furlattini, V. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol. 2005, 34, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yassin, H.; Velthuis, A.G.J.; Boerjan, M.; van Riel, J.V. Field study on broilers’ first-week mortality. Poult. Sci. J. 2009, 88, 798–804. [Google Scholar] [CrossRef]
- Poulsen, L.L.; Thøfner, I.; Bisgaard, M.; Christensen, J.P.; Olsen, R.H.; Christensen, H. Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Vet. Microbiol. 2017, 207, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Collingwood, C.; Kemmett, K.; Williams, N.; Wigley, P.; Tellez, G. Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Front Vet. Sci. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.K.; Barnes, H.; Jean Pirre, V.; Tahseen, A.A.; Catherine, M.L. Diseases of Poultry, 13th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 751–785. [Google Scholar]
- Newman, D.M.; Barbieri, N.L.; de Oliveira, A.L.; Willis, D.; Nolan, L.K.; Logue, C.M. Characterizing avian pathogenic Escherichia coli (APEC) from colibacillosis cases. PeerJ. 2021, 9, e11025. [Google Scholar] [CrossRef]
- Jiang, F.; An, Y.; Bao, X.; Zhao, R.; Jernigan, L.; Lithio, A.; Nettleton, D.; Li, L.; Wurtele, E.S.; Nolan, L.K.; et al. ArcA Controls Metabolism, Chemotaxis, and Motility Contributing to the Pathogenicity of Avian Pathogenic Escherichia coli. Infect. Immun. 2015, 83, 3545. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-PLoSkonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Kariyawasam, S.; Johnson, J.R.; Logue, C.M.; Nolan, L.K. Prevalence of avian-pathogenic Escherichia coli strain O1 Genomic islands among extraintestinal and commensal E. Coli isolates. J. Bacteriol. 2012, 194, 2846–2853. [Google Scholar] [CrossRef]
- Barbieri, N.L.; De Oliveira, A.L.; Tejkowski, T.M.; Pavanelo, D.B.; Rocha, D.A.; Matter, L.B.; Callegari-Jacques, S.M.; De Brito, B.G.; Horn, F. Genotypes and pathogenicity of cellulitis isolates reveal traits that modulate APEC virulence. PLoS ONE 2013, 8, e72322. [Google Scholar] [CrossRef]
- Schouler, C.; Schaeffer, B.; Brée, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef]
- Dissanayake, D.R.A.; Octavia, S.; Lan, R. Population structure and virulence content of avian pathogenic Escherichia coli isolated from outbreaks in Sri Lanka. Vet. Microbiol. 2014, 168, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, 118. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.E.; Blanco, M.; Mora, A.; Jansen, W.H.; García, V.; Vázquez, M.L.; Blanco, J. Serotypes of Escherichia coli isolated from septicaemic chickens in Galicia (northwest Spain). Vet. Microbiol. 1998, 61, 229–235. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed]
- Lía Padola, N.; Martin Dozois, C.; Delannoy, S.; Fleiss, A.; Beutin, L.; Mariani-Kurkdjian, P.; Bonacorsi, S.; Fach, P. The Escherichia coli Serogroup O1 and O2 Lipopolysaccharides Are Encoded by Multiple O-antigen Gene Clusters. Front Cell Infect. Microbiol. 2017, 7, 30. [Google Scholar]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Nolan, L.K. Characterizing the APEC pathotype. Vet. Res. 2005, 36, 241–256. [Google Scholar] [CrossRef]
- Knöbl, T.; Moreno, A.M.; Paixão, R.; Gomes, T.A.; Vieira, M.A.; da Silva Leite, D.; Blanco, J.E.; Ferreira, A.J. Prevalence of avian pathogenic Escherichia coli (APEC) clone harboring sfa gene in Brazil. Sci. World J. 2012, 2012, 437342. [Google Scholar] [CrossRef]
- Ronco, T.; Stegger, M.; Olsen, R.H. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genom. 2017, 18, 13. [Google Scholar] [CrossRef]
- Doyle, M.P.; Schoeni, J.L. Isolation of Escherichia coli O157: H7 from retail fresh meats and poultry. Appl. Environ. Microbiol. 1987, 53, 2394–2396. [Google Scholar] [CrossRef]
- Cortés, P.; Blanc, V.; Mora, A.; Dahbi, G.; Blanco, J.E.; Blanco, M.; López, C.; Andreu, A.; Navarro, F.; Alonso, M.P.; et al. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol. 2010, 76, 2799–2805. [Google Scholar] [CrossRef]
- Ishii, N.; Nakahigashi, K.; Baba, T.; Robert, M.; Soga, T.; Kanai, A.; Hirasawa, T.; Naba, M.; Hirai, K.; Hoque, A.; et al. Multiple high-throughput analyses monitor the response of E. coli to perturbations. Science 2007, 316, 593–597. [Google Scholar] [CrossRef]
- EscobarPáramo, P.; LeMenac’h, A.; LeGall, T.; Amorin, C.; Gouriou, S.; Picard, B.; Skurnik, D.; Denamur, E. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 2006, 8, 1975–1984. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef]
- Pires-dos-Santos, T.; Bisgaard, M.; Christensen, H. Genetic diversity and virulence profiles of Escherichia coli causing salpingitis and peritonitis in broiler breeders. Vet. Microbiol. 2013, 162, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Cordoni, G.; Woodward, M.J.; Wu, H.; Alanazi, M.; Wallis, T.; La Ragione, R.M. Comparative genomics of European avian pathogenic E. coli. (APEC). BMC Genom. 2016, 17, 960. [Google Scholar] [CrossRef]
- Kim, Y.B.; Yoon, M.Y.; Ha, J.S.; Seo, K.W.; Noh, E.B.; Son, S.H.; Lee, Y.J. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poult. Sci. J. 2020, 99, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.I.; McQuillan, J.; Taiwo, M.; Parks, R.; Stenton, C.A.; Morgan, H.; Mowlem, M.C.; Lees, D.N. A highly specific Escherichia coli qPCR and its comparison with existing methods for environmental waters. Water Res. 2017, 126, 101–110. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.; Newman, D.M.; Sato, Y.; Noel, A.; Rauk, B.; Nolan, L.K.; Barbieri, N.L.; Logue, C.M. Characterization of Avian Pathogenic Escherichia coli (APEC) Associated with Turkey Cellulitis in Iowa. Front Vet. Sci. 2020, 7, 380. [Google Scholar] [CrossRef]
- Iguchi, A.; Iyoda, S.; Seto, K.; Morita-Ishihara, T.; Scheutz, F.; Ohnishi, M. Escherichia coli O-Genotyping PCR: A Comprehensive and Practical Platform for Molecular O Serogrouping. J. Clin. Microbiol. 2015, 53, 2427–2432. [Google Scholar] [CrossRef]
- Banjo, M.; Iguchi, A.; Seto, K.; Kikuchi, T.; Harada, T.; Scheutz, F.; Iyoda, S. Escherichia coli H-Genotyping PCR: A complete and practical platform for molecular h typing. J. Clin. Microbiol. 2018, 56, 190–208. [Google Scholar] [CrossRef]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef]
- Li, T.; Castañeda, C.D.; Miotto, J.; McDaniel, C.; Kiess, A.S.; Zhang, L. Effects of in ovo probiotic administration on the incidence of avian pathogenic Escherichia coli in broilers and an evaluation on its virulence and antimicrobial resistance properties. Poult. Sci. J. 2021, 100, 100903. [Google Scholar] [CrossRef]
- Logue, C.M.; Wannemuehler, Y.; Nicholson, B.A.; Doetkott, C.; Barbieri, N.L.; Nolan, L.K. Comparative analysis of phylogenetic assignment of human and avian ExPEC and fecal commensal Escherichia coli using the (previous and revised) clermont phylogenetic typing methods and its impact on avian pathogenic Escherichia coli (APEC) classification. Front Microbiol. 2017, 8, 283. [Google Scholar] [CrossRef]
- Batchelor, M.; Clifton-Hadley, F.A.; Stallwood, A.D.; Paiba, G.A.; Davies, R.H.; Liebana, E. Detection of multiple cephalosporin-resistant Escherichia coli from a cattle fecal sample in Great Britain. Microb. Drug Resist. 2005, 1, 58–61. [Google Scholar] [CrossRef]
- Van, T.T.; Chin, J.; Chapman, T.; Tran, L.T.; Coloe, P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008, 10, 217–223. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI: Wayne, PA, USA, 2022; p. 34. [Google Scholar]
- Deus, D.; Krischek, C.; Pfeifer, Y.; Sharifi, A.R.; Fiegen, U.; Reich, F.; Klein, G.; Kehrenberg, C. Comparative analysis of the susceptibility to biocides and heavy metals of extended-spectrum β-lactamase-producing Escherichia coli isolates of human and avian origin, Germany. Diagn. Microbiol. Infect. Dis. 2017, 88, 88–92. [Google Scholar] [CrossRef]
- Vajiheh, K.; Naser, B.; Giti, E. Antimicrobial, heavy metal resistance and plasmid profile of coliforms isolated from nosocomial infections in a hospital in Isfahan, Iran. Afr. J. Biotechnol. 2003, 2, 379–383. [Google Scholar] [CrossRef]
- Vrionis, H.A.; Wang, S.; Haslam, B.; Turner, R.J. Selenite protection of tellurite toxicity toward Escherichia coli. Front Mol. Biosci. 2015, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Herisse, M.; Duverger, Y.; Martin-Verstraete, I.; Barras, F.; Ezraty, B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol. Microbiol. 2017, 105, 115–126. [Google Scholar] [CrossRef] [PubMed]
- McPeake, S.J.; Smyth, J.A.; Ball, H.J. Characterisation of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Vet. Microbiol. 2005, 110, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Solà-Ginés, M.; Cameron-Veas, K.; Badiola, I.; Dolz, R.; Majó, N.; Dahbi, G. Diversity of Multi-Drug Resistant Avian Pathogenic Escherichia coli (APEC) Causing Outbreaks of Colibacillosis in Broilers during 2012 in Spain. PLoS ONE. 2015, 10, e0143191. [Google Scholar] [CrossRef]
- Someya, A.; Otsuki, K.; Murase, T. Characterization of Escherichia coli strains obtained from layer chickens affected with colibacillosis in a commercial egg-producing farm. J. Vet. Med. Sci. 2007, 69, 1009–1014. [Google Scholar] [CrossRef]
- Naundrup, T.I.C.; Poulsen, L.L.; Bisgaard, M.; Christensen, H.; Olsen, R.H.; Christensen, J.P. Longitudinal study on causes of mortality in Danish broiler breeders. Avian Dis. 2019, 63, 400–410. [Google Scholar] [CrossRef]
- Christensen, H.; Bachmeier, J.; Bisgaard, M. New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathol. 2021, 50, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Monroy, M.A.R.; Knöbl, T.; Bottino, J.A.; Astolfi Ferreira, C.S.; Ferreira, A.J.P. Virulence characteristics of Escherichia coli isolates obtained from broiler breeders with salpingitis. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Koutsianos, D.; Athanasiou, L.V.; Mossialos, D.; Franzo, G.; Cecchinato, M.; Koutoulis, K.C. Investigation of Serotype Prevalence of Escherichia coli Strains Isolated from Layer Poultry in Greece and Interactions with Other Infectious Agents. Vet. Sci. 2022, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; El-shall, N.A.; Khalil, D.S.; El-hack, M.E.A.; Swelum, A.A.; Mahmoud, A.H.; Ebaid, H.; Komany, A.; Sammour, R.H.; Sedeik, M.E. Incidence, Pathotyping, and Antibiotic Susceptibility of Avian Pathogenic Escherichia coli among Diseased Broiler Chicks. Pathogens 2020, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.K.; Vaillancourt, J.P.; Barbieri, N.L.; Logue, C.M. Diseases of Poultry, 14th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 770–830. [Google Scholar]
- Oliveira, E.S.; Cardozo, M.V.; Borzi, M.M.; Borges, C.A.; Guastalli, E.A.L.; Ávila, F.A. Highly Pathogenic and Multidrug Resistant Avian Pathogenic Escherichia Coli in Free-Range Chickens from Brazil. Braz. J. Poult. Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Lozica, L.; Kabalin, A.E.; Dolenčić, N.; Vlahek, M.; Gottstein, Ž. Phylogenetic characterization of avian pathogenic Escherichia coli strains longitudinally isolated from broiler breeder flocks vaccinated with autogenous vaccine. Poult. Sci. J. 2021, 100, e101079. [Google Scholar] [CrossRef]
- Johnson, T.J.; Johnson, S.J.; Nolan, L.K. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J. Bacteriol. 2006, 188, 5975–5983. [Google Scholar] [CrossRef]
- Varga, C.; Brash, M.L.; Slavic, D.; Boerlin, P.; Ouckama, R.; Weis, A.; Petrik, M.; Philippe, C.; Barham, M.; Guerin, M.T. Evaluating Virulence-Associated Genes and Antimicrobial Resistance of Avian Pathogenic Escherichia coli Isolates from Broiler and Broiler Breeder Chickens in Ontario, Canada. Avian Dis. 2018, 62, 291–299. [Google Scholar] [CrossRef] [PubMed]
- De Carli, S.; Ikuta, N.; Lehmann, F.K.M.; da Silveira, V.P.; de Melo Predebon, G.; Fonseca, A.S.K.; Lunge, V.R. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult. Sci. J. 2015, 94, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.; Rocha, D.A.; Finkler, F.; de Moraes, L.B.; Barbieri, N.L.; Pavanelo, D.B.; Winkler, C.; Grassotti, T.T.; de Brito, K.C.; de Brito, B.G.; et al. Prevalence of ColV plasmid-linked genes and in vivo pathogenicity of avian strains of Escherichia coli. Foodborne Pathog. Dis. 2015, 12, 679–685. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 2013, 303, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. Veterinary Drug Usage and Antimicrobial Resistance in Bacteria of Animal Origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef]
- Mazel, D. Integrons: Agents of bacterial evolution. Nat. Rev. Microbiol. 2006, 4, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antimicrobial Resistance—A Global Threat, 1st ed.; Intechopen Limited: London, UK, 2018; pp. 33–51. [Google Scholar]
- Indrawati, A.; Khoirani, K.; Setiyaningsih, S.; Affif, U.; Safika; Ningrum, S.G. Detection of Tetracycline Resistance Genes among Escherichia coli Isolated from Layer and Broiler Breeders in West Java, Indonesia. Trop. J. Anim. Sci. 2021, 44, 267–272. [Google Scholar] [CrossRef]
- Thomrongsuwannakij, T.; Blackall, P.J.; Djordjevic, S.P.; Cummins, M.L.; Chansiripornchai, N. A comparison of virulence genes, antimicrobial resistance profiles and genetic diversity of avian pathogenic Escherichia coli (APEC) isolates from broilers and broiler breeders in Thailand and Australia. Avian Pathol. 2020, 49, 457–466. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, K.; JuLee, Y. Comparative analysis of antimicrobial resistance and genetic characteristics of Escherichia coli from broiler breeder farms in Korea. Can. J. Anim. Sci. 2022, 102, 342–351. [Google Scholar] [CrossRef]
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.Q.; Basha, E.A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Vet. Res. 2019, 15, 159. [Google Scholar] [CrossRef]
- Heuer, H.; Krögerrecklenfort, E.; Wellington, E.M.H.; Egan, S.; van Elsas, J.D.; van Overbeek, L.; Collard, J.M.; Karagouni, G.A.D.; Nikolakopoulou, T.L.; Smalla, K. Gentamicin resistance genes in environmental bacteria: Prevalence and transfer. FEMS Microbiol. Ecol. 2002, 42, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Benameur, Q.; Tali-Maamar, H.; Assaous, F.; Guettou, B.; Rahal, K.; Ben-Mahdi, M.H. Detection of multidrug resistant Escherichia coli in the ovaries of healthy broiler breeders with emphasis on extended-spectrum β-lactamases producers. Comp. Immunol. Micrbiol. Infect. Dis. 2019, 64, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Wang, J.; Klumpp, J.; Nüesch-Inderbinen, M.; Fanning, S.; Stephan, R. Vertical transmission of highly similar blaCTX-M-1-harbouring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front. Microbiol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Oikarainen, P.E.; Pohjola, L.K.; Pietola, E.S.; Heikinheimo, A. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet. Microbiol 2019, 231, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Börjesson, S.; Landén, A.; Bengtsson, B. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 2014, 69, 1497–1500. [Google Scholar] [CrossRef]
- Cyoia, P.S.; Koga, V.L.; Nishio, E.K.; Houle, S.; Dozois, C.M.; de Brito, K.C.T.; de Brito, B.G.; Nakazato, G.; Kobayashi, R.K.T. Distribution of ExPEC Virulence Factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli Isolated from Commercialized Chicken Carcasses. Front. Microbiol. 2019, 14, 3254. [Google Scholar] [CrossRef] [PubMed]
- Afayibo, D.J.A.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 25, 319. [Google Scholar] [CrossRef]
- Neil, W.; Jane, F.T.; David, M.L. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar]
- Ashraf, A.A.T.; Soad, A.N.; Fatma, I.E.H.; Ola, A.I. Prevalence of eaeA and qacEΔ1 genes in Escherichia coli isolated from omphalitis in baby chicks. Benha. Vet. Med. J. 2017, 32, 184–192. [Google Scholar]
- Maertens, H.; Demeyere, K.; De Reu, K. Effect of subinhibitory exposure to quaternary ammonium compounds on the ciprofloxacin susceptibility of Escherichia coli strains in animal husbandry. BMC Microbiol. 2020, 20, 155. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, H.; Lu, X.; Zuidhof, M.J.; Li, X.F.; Le, X.C. Arsenic Species in Chicken Breast: Temporal Variations of Metabolites, Elimination Kinetics, and Residual Concentrations. Environ. Health Perspect. 2016, 124, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Deng, W.; Liu, S.; Yu, X.; Mustafa, G.R.; Chen, S.; He, L.; Ao, X.; Yang, Y.; Zhou, K.; et al. Presence of heavy metal resistance genes in Escherichia coli and Salmonella isolates and analysis of resistance gene structure in E. coli E308. J. Glob. Antimicrob. Resist. 2020, 21, 420–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).