Impact on Hatchability and Broiler Performance after Use of Hydrogen Peroxide Nebulization versus Formaldehyde Fumigation as Pre-Incubation Hatching Egg Disinfectants in Field Trial
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, R.; Kudirkiene, E.; Thofner, I.; Pors, S.; Karlskov-Mortensen, P.; Li, L.; Papasolomontos, S.; Angastiniotou, C.; Christensen, J. Impact of Egg Disinfection of Hatching Eggs on the Eggshell Microbiome and Bacterial Load. Poult. Sci. J. 2017, 96, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, G.; Calonico, C.; Runfola, M.; Lo Nostro, A. Free-range and Organic Farming. Eggshell Contamination by Mesophilic Bacteria and Unusual Pathogens. J. Appl. Poult. Res. 2017, 26, 509–517. [Google Scholar] [CrossRef]
- Sander, J.E.; Wilson, J.L.; Cheng, I.H.; Gibbs, P.S. Influence of Slat Material on Hatching Egg Sanitation and Slat Disinfection. J. Appl. Poult. Res. 2003, 12, 74–80. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, J.; Debevere, L.H. Eggshell Factors Influencing Eggshell Penetration and Whole Egg Contamination by Different Bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Ahmed, B.; de Boeck, C.; Dumont, A.; Cox, E.; de Reu, K.; Vanrompay, D. First Experimental Evidence for the Transmission of Chlamydia psittaci in Poultry through Eggshell Penetration. Transbound. Emerg. Dis. 2017, 64, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Berrang, M.E.; Cox, N.A.; Frank, J.F.; Buhr, R.J. Bacterial Penetration of the Eggshell and Shellmembranes of the Chicken Hatching Egg: A Review. J. Appl. Poult. Res. 1999, 8, 499–504. [Google Scholar] [CrossRef]
- Williams, J.E. Effect of High-Level Formaldehyde Fumigation on Bacterial Populations on the Surface of Chicken Hatching Eggs. Avian Dis. 1970, 14, 386–392. [Google Scholar] [CrossRef]
- Sander, J.E.; Wilson, J.L.; van Wiklen, G.L. Effect of Formaldehyde Exposure in the Hatcher and of Ventilation in Confinement Facilities on Broiler Performance. Avian Dis. 1995, 39, 420–424. [Google Scholar] [CrossRef]
- Kalidari, G.A.; Moayyedian, H.; Eslamian, A.; Mohsenzadeh, M. Isolation and Identification of Non-coliform Gram-negative Bacteria in Hatching Eggs to Evaluate the Effect of Egg Fumigation by Formaldehyde. J. Poult. Sci. 2009, 46, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Hayretdag, S.; Kolankaya, D. Investigation of the Effects of Pre-incubation Formaldehyde Fumigation on the Tracheal Epithelium of Chicken Embryos and Chicks. Turk. J. Vet. Anim. Sci. 2008, 32, 263–267. [Google Scholar]
- Zulkifli, I.; Fauziah, O.; Omar, A.R.; Shaipullizan, S.; Siti Selena, A.H. Respiratory Epithelium, Production Performance and Behaviour of Formaldehyde-exposed Broiler Chicks. Vet. Res. Commun. 1999, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mut. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TRGS 522. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/TRGS-522.html (accessed on 27 August 2022).
- Yildirim, I.; Ozsan, M.; Yetisir, R. The Use of Oregano (Origanum vulgare L.) Essential Oil as Alternative Hatching Egg Disinfectant versus Formaldehyde Fumigation in Quails (Coturnix coturnix japonica) Eggs. Rev. Med. Vet. 2003, 154, 367–370. [Google Scholar]
- Chmielowiec-Korzeniowska, A.; Tymczyna, L.; Dobrowolska, M.; Banach, M.; Nowakowicz-Dębek, B.; Bryl, M.; Drabik, A.; Tymczyna-Sobotka, M.; Kolejko, M. Silver (Ag) in Tissues and Eggshells, Biochemical Parameters and Oxidative Stress in Chickens. Open Chem. 2015, 13, 1269–1274. [Google Scholar] [CrossRef]
- Batkowska, J.; Al-Shammari, K.I.A.; Gryzinska, M.M.; Brodacki, A.; Wlazlo, L.; Nowakowicz-Debek, B. Effect of Using Colloidal Silver in the Disinfection of Hatching Eggs on some Microbial, Hatchability and Performance Traits in Japanese Quail (Coturnix cot. japonica). Eur. Poult. Sci. 2017, 81, 211. [Google Scholar]
- Russell, S.M. The Effect of Electrolyzed Oxidative Water Applied Using Electrostatic Spraying on Pathogenic and Indicator Bacteria on the Surface of Eggs. Poult. Sci. 2003, 82, 158–162. [Google Scholar] [CrossRef]
- Bourassa, D.V.; Buhr, R.J.; Wilson, J.L. Hatchability of Eggs Sanitized with Increasing Concentrations of BioSentry 904 or Bio-Phene. J. Appl. Poult. Res. 2002, 11, 397–401. [Google Scholar] [CrossRef]
- Rehkopf, A.C.; Byrd, J.A.; Coufal, C.D.; Duong, T. Advanced Oxidation Process Sanitization of Hatching Eggs Reduces Salmonella in Broiler Chicks. Poult. Sci. 2017, 96, 3709–3716. [Google Scholar] [CrossRef]
- Whistler, P.E.; Sheldon, B.W. Bactericidal Activity, Eggshell Conductance, and Hatchability Effects of Ozone versus Formaldehyde Disinfection. Poult. Sci. 1989, 68, 1074–1077. [Google Scholar] [CrossRef]
- Gerrits, A.R.; Dijk, D.J. Effect of X-ray Irradiation of Hatching Eggs on Hatching Time, Hatchability and Broiler Weight. Arch. Gefluglk. 1992, 56, 179–181. [Google Scholar]
- Castaneda, S.M.P.; Tellez, I.G.; Bustos, R.E.; Quintana, L.J.A.; Sanchez, R.E.; Hargis, M.B. Effect of Electron Irradiation on Hatchability and Broiler Performance of Hatching Eggs. Act. Aliment. 1996, 25, 305–310. [Google Scholar] [CrossRef]
- Zakaria, A.H. Effect of Low Doses of Gamma Irradiation Prior to Egg Incubation on Hatchability and Body Weight of Broiler Chickens. Br. Poult. Sci. 1991, 32, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.A.; Bailey, J.S.; Berrang, M.E. Bactericidal Treatment of Hatching Eggs I. Chemical Immersion Treatments and Salmonella. J. Appl. Poult. Res. 1998, 7, 347–350. [Google Scholar] [CrossRef]
- Cox, N.A.; Berrang, M.E.; Bailey, J.S.; Stern, N.J. Bactericidal Treatment of Hatching Eggs V Efficiency of Repetitive Immersions in Hydrogen Peroxide or Phenol to Eliminate Salmonella from Hatching Eggs. J. Appl. Poult. Res. 2002, 11, 328–331. [Google Scholar] [CrossRef]
- Hafez, H.M.; Jodas, S. Behandlungsverfahren mit verschiedenen Desinfektionsmitteln zur Bekämpfung von Salmonella enteritidis bei künstlich infizierten Bruteiern (Legehybriden). Tierarztl. Umsch. 1993, 48, 517–521. [Google Scholar]
- Padron, M. Egg Dipping in Hydrogen-Peroxide Solution to Eliminate Salmonella-Typhimurium from Eggshell Membranes. Avian Dis. 1995, 39, 627–630. [Google Scholar] [CrossRef]
- Musgrove, M.T.; Cox, N.A.; Berrang, M.E.; Buhr, R.J.; Richardson, L.J.; Mauldin, J.M. Effect of Inoculation and Application Methods on the Performance of Chemicals used to Disinfect Salmonella-contaminated Broiler Hatching Eggs. J. Appl. Poult. Res. 2010, 19, 387–392. [Google Scholar] [CrossRef]
- Sheldon, B.W.; Brake, J. Hydrogen-Peroxide as an Alternative Hatching Egg Disinfectant. Poult. Sci. 1991, 70, 1092–1098. [Google Scholar] [CrossRef]
- Sander, J.E.; Wilson, J.L. Effect of Hydrogen Peroxide Disinfection During Incubation of Chicken Eggs on Microbial Levels and Productivity. Avian Dis. 1999, 43, 27–233. [Google Scholar] [CrossRef]
- Keita, A.; Huneau-Salaun, A.; Guillot, A.; Galliot, P.; Tavares, M.; Puterflam, J. A Multi-pronged Approach to the Search for an Alternative to Formaldehyde as an Egg Disinfectant without Affecting Worker Health, Hatching, or Broiler Production Parameters. Poult. Sci. 2016, 95, 1609–1616. [Google Scholar] [CrossRef]
- Melo, E.F.; Climaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; de Souza, M.R.; Baiao, N.C.; Pompeu, M.A.; Lara, L.J.C. An Evaluation of Alternative Methods for Sanitizing Hatching Eggs. Poult. Sci. 2019, 98, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Alasri, A.; Roques, C.; Michel, G.; Cabassud, C.; Aptel, P. Bactericidal Properties of Peracetic-Acid and Hydrogen-Peroxide, Alone and in Combination, and Chlorine and Formaldehyde Against Bacterial Water Strains. Can. J. Microbiol. 1992, 38, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.B.; Coufal, C.D.; Parker, H.M.; McDaniel, C.D. Disinfection of Eggshells Using Ultraviolet Light and Hydrogen Peroxide Independently and in Combination. Poult. Sci. 2010, 89, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Tebrün, W.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Schmidt, V.; Renfert, K.; Reichelt, C.; Brüggemann-Schwarze, S.; Pees, M. Preliminary Study: Health and Performance Assessment in Broiler Chicks Following Application of Six Different Hatching Egg Disinfection Protocols. PLoS ONE 2020, 15, e0232825. [Google Scholar] [CrossRef]
- Motola, G.; Hafez, H.M.; Brüggemann- Schwarze, S. Efficacy of Six Disinfection Methods Against Extended-spectrum Beta- lactamase (ESBL) Producing E. coli on Eggshells in Vitro. PLoS ONE 2020, 15, e0238860. [Google Scholar] [CrossRef]
- Ulmer-Franco, A.M.; Fasenko, G.M.; O’Dea Christopher, E.E. Hatching Egg Characteristics, Chick Quality, and Broiler Performance at 2 Breeder Flock Ages and from 3 Egg Weights. Poult. Sci. 2010, 89, 2735–2742. [Google Scholar] [CrossRef]
- Elibol, O.; Peak, S.D.; and Brake, J. Effect of Flock Age, Length of Egg Storage, and Frequency of Turning During Storage on Hatchability of Broiler Hatching Eggs. Poult. Sci. 2002, 81, 945–950. [Google Scholar] [CrossRef]
- Gumułka, M.; Kapkowska, E. Age Effect of Broiler Breeders on Fertility and Sperm Penetration of the Perivitelline Layer of the Ovum. Anim. Reprod. Sci. 2005, 90, 135–148. [Google Scholar] [CrossRef]
- Musgrove, M.T.; Stephens, C.B.; Bourassa, D.V.; Cox, N.A.; Mauldin, J.M.; Berrang, M.E.; Buhr, R.J. Enterobacteriaceae and Salmonella Recovered from Non-Sanitized and Sanitized Broiler Hatching Eggs. J. Appl. Poult. Res. 2014, 23, 516–522. [Google Scholar] [CrossRef]
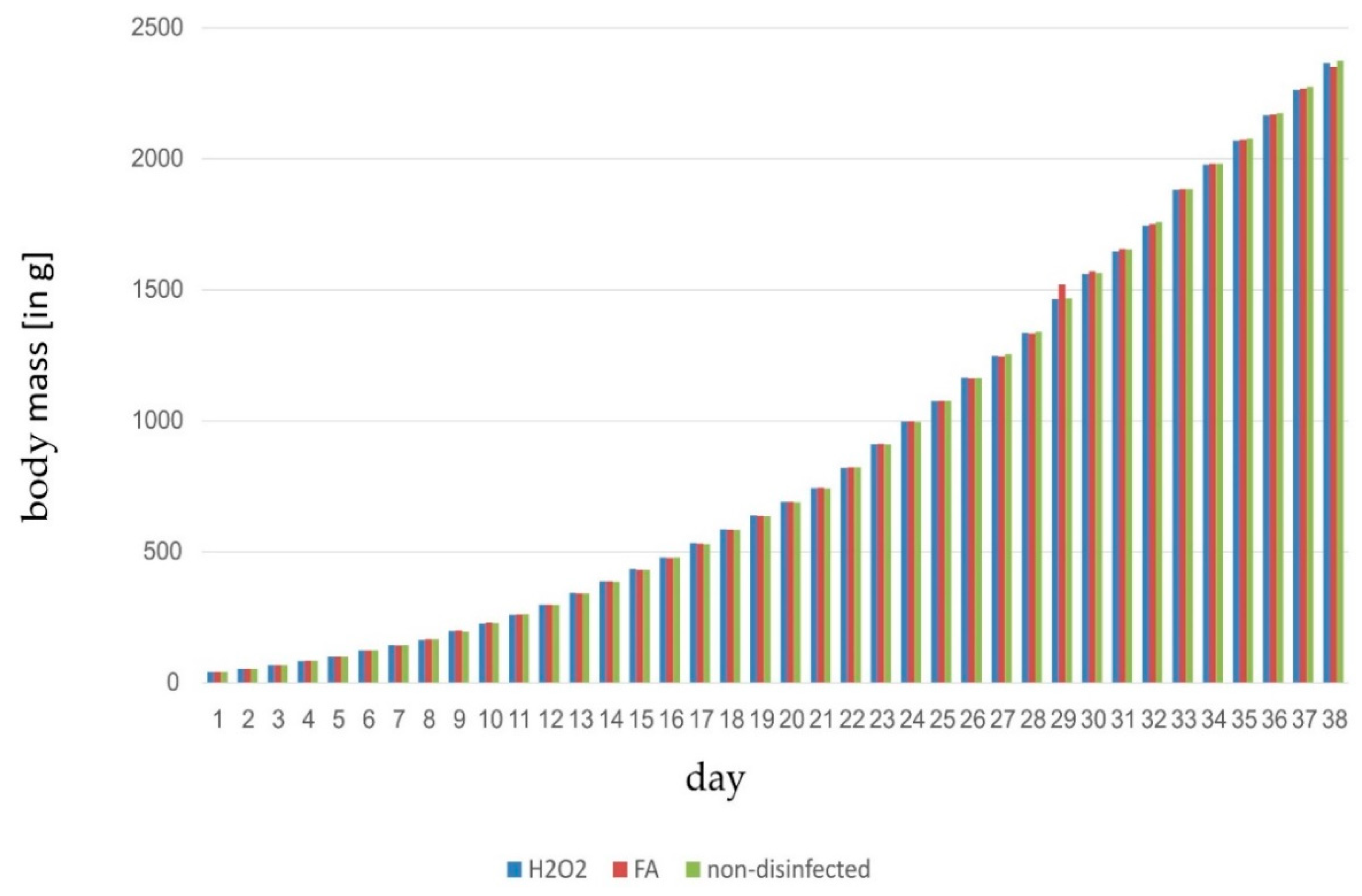
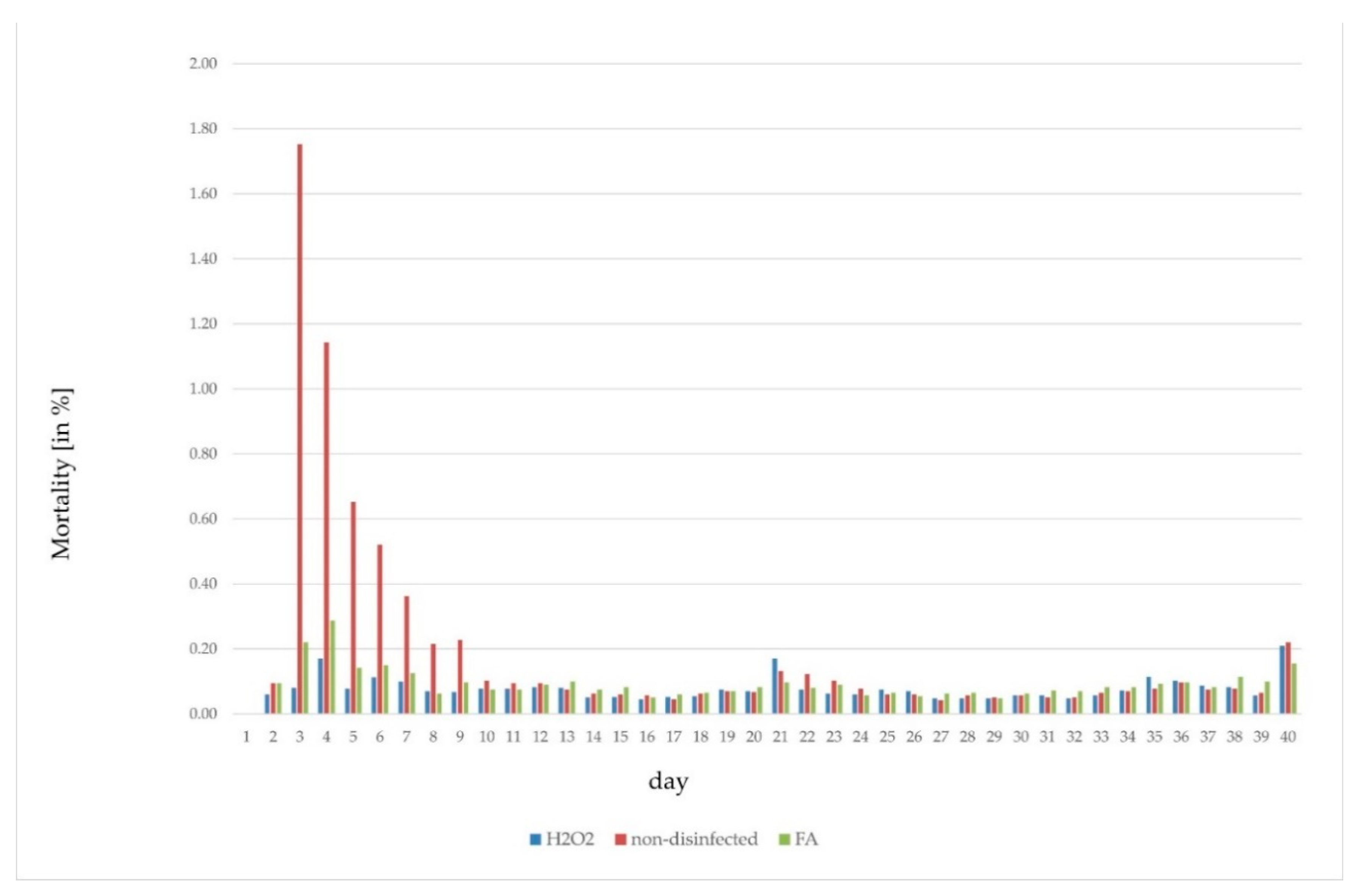
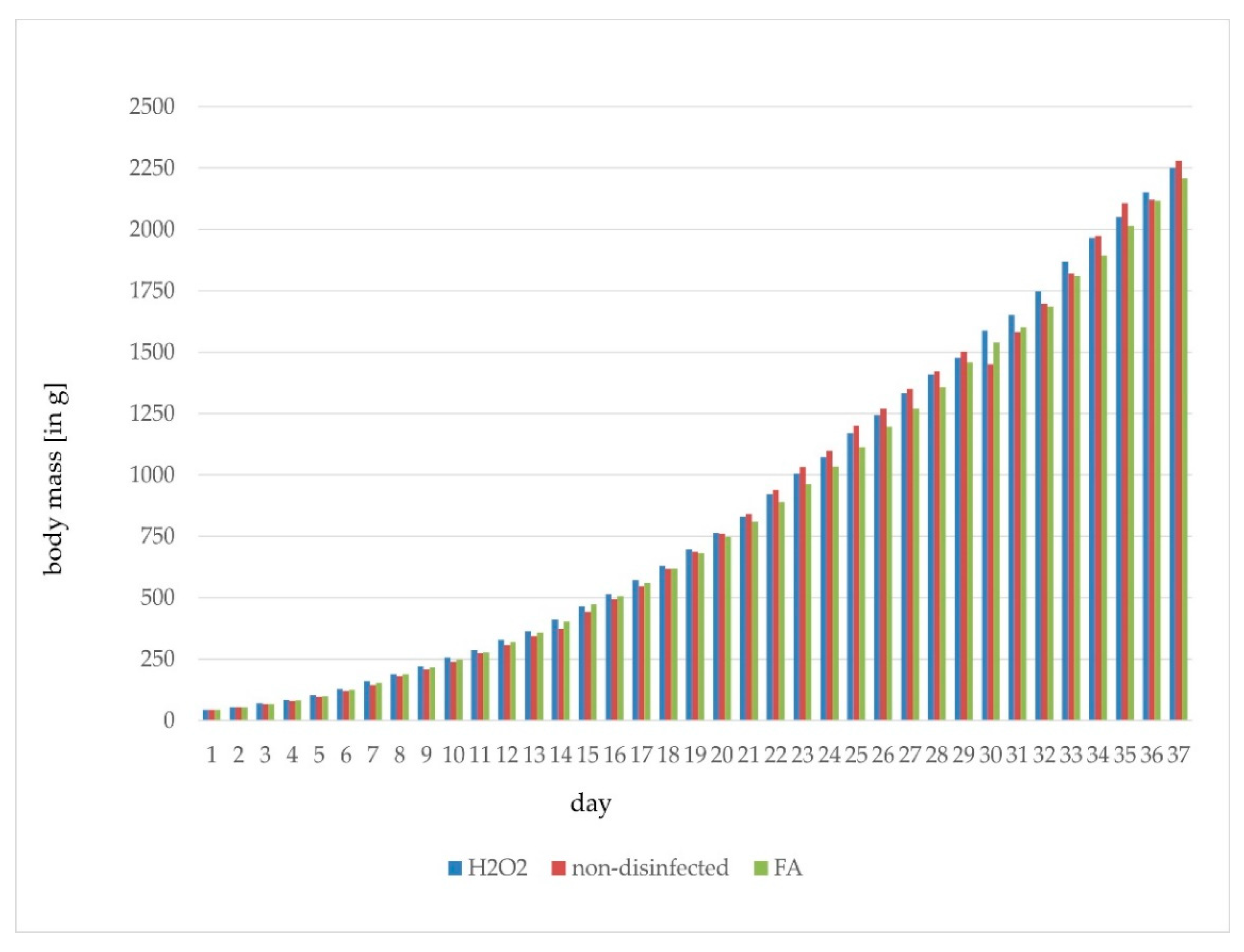
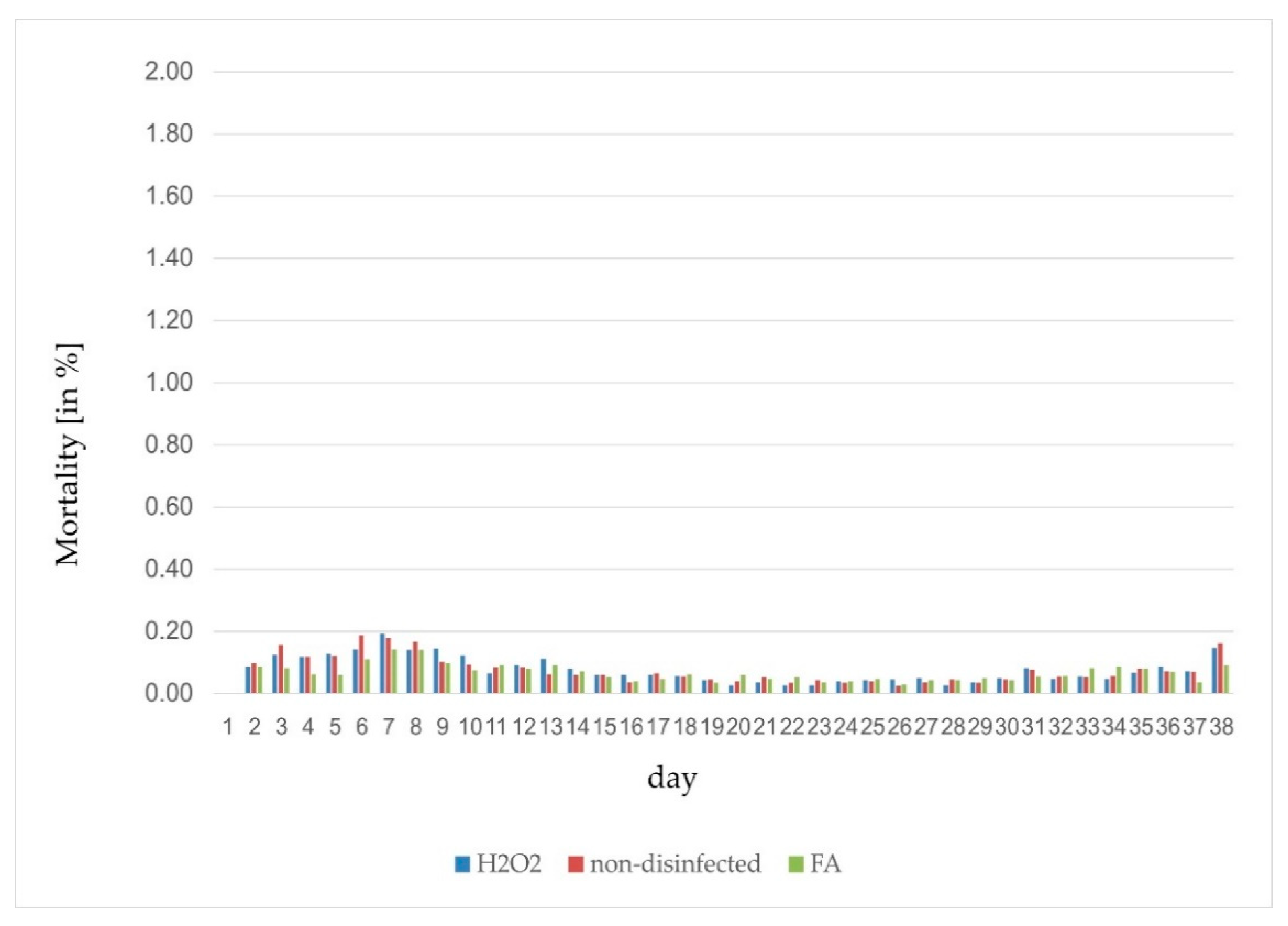
| Disinfection | Trial | Flock | Fertile [%] | Late-Dead [%] | Early-Dead [%] | Infertile [%] | Hatch [%] |
|---|---|---|---|---|---|---|---|
| H2O2 | 1 | A | 94.14 | 0.77 | 0.42 | 4.67 | 90.52 |
| H2O2 | 1 | B | 93.23 | 0.78 | 0.36 | 5.63 | 90.46 |
| Non-disinfected | 1 | A | 94.36 | 0.66 | 0.47 | 4.51 | 90.87 |
| Non-disinfected | 1 | B | 93.16 | 0.74 | 0.56 | 5.54 | 89.66 |
| FA | 1 | A | 93.70 | 0.63 | 0.66 | 5.01 | 90.11 |
| FA | 1 | B | 93.51 | 0.57 | 0.55 | 5.36 | 89.59 |
| H2O2 | 2 | A | 92.87 | 0.69 | 0.58 | 5.86 | 88.56 |
| H2O2 | 2 | B | 92.62 | 0.67 | 0.53 | 6.18 | 87.94 |
| Non-disinfected | 2 | A | 93.77 | 0.66 | 0.59 | 4.98 | 89.95 |
| Non-disinfected | 2 | B | 92.88 | 0.81 | 0.62 | 5.67 | 88.67 |
| FA | 2 | A | 92.02 | 0.92 | 0.65 | 6.41 | 88.59 |
| FA | 2 | B | 91.46 | 0.84 | 0.75 | 6.95 | 87.93 |
| H2O2 | 3 | A | 92.20 | 1.24 | 0.88 | 5.67 | 87.42 |
| H2O2 | 3 | B | 93.01 | 1.05 | 0.94 | 5.01 | 89.82 |
| Non-disinfected | 3 | A | 92.91 | 1.04 | 0.51 | 5.54 | 86.03 |
| Non-disinfected | 3 | B | 91.39 | 0.97 | 0.78 | 6.86 | 88.75 |
| FA | 3 | A | 91.83 | 1.27 | 0.96 | 5.94 | 87.26 |
| FA | 3 | B | 93.17 | 1.01 | 0.79 | 5.03 | 89.48 |
| H2O2 | 4 | A | 92.03 | 0.95 | 0.72 | 6.30 | 88.77 |
| H2O2 | 4 | B | 93.60 | 0.79 | 0.52 | 5.09 | 89.81 |
| Non-disinfected | 4 | A | 91.35 | 0.90 | 0.77 | 6.98 | 86.61 |
| Non-disinfected | 4 | B | 93.09 | 0.84 | 0.59 | 3.28 | 89.66 |
| FA | 4 | A | 91.50 | 1.05 | 0.91 | 6.54 | 86.73 |
| FA | 4 | B | 93.52 | 0.98 | 0.71 | 4.79 | 89.67 |
| Trial | Method | Flock | Age | Rej [%] | BM [g] | AB | Runts [%] | Ascites [%] | Ema [%] | Derm [%] | Gen Inf [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FA | A | 32 | 0.83 | 1931 | 0 | 0 | 0.18 | 0 | 0.12 | 0.42 |
| 1 | FA | B | 32 | 1.88 | 2188 | 1 | 0.39 | 0.27 | 0.09 | 0.03 | 1.04 |
| 1 | H2O2 | A | 32 | 1.41 | 1924 | 0 | 0.15 | 0.43 | 0.03 | 0.24 | 0.52 |
| 1 | H2O2 | B | 32 | 1.07 | 1863 | 0 | 0.3 | 0.27 | 0.12 | 0.09 | 0.48 |
| 1 | Non-disinfected | A | 32 | 1.39 | 1911 | 1 | 0.06 | 0.26 | 0 | 0.32 | 0.68 |
| 1 | Non-disinfected | B | 32 | 1.7 | 2219 | 1 | 0.04 | 0.63 | 0.04 | 0.26 | 0.7 |
| 1 | FA | B | 37 | 1.92 | 2418 | 1 | 0.08 | 0.47 | 0 | 0.35 | 0.55 |
| 1 | H2O2 | B | 37 | 2.15 | 2448 | 0 | 0.51 | 0.64 | 0.02 | 1.06 | 0.22 |
| 1 | Non-disinfected | B | 37 | 2.22 | 2403 | 1 | 0.25 | 0.55 | 0.01 | 0.83 | 0.41 |
| 1 | FA | A | 38 | 1.92 | 2640 | 1 | 0.07 | 0.49 | 0 | 0.99 | 0.27 |
| 1 | H2O2 | A | 38 | 2.44 | 2605 | 0 | 0.2 | 0.36 | 0 | 1.28 | 0.49 |
| 1 | Non-disinfected | A | 38 | 2 | 2583 | 1 | 0.61 | 0.82 | 0 | 0.87 | 0.2 |
| 2 | FA | A | 33 | 1.34 | 2053 | 0 | 0.05 | 0.09 | 0.09 | 0.47 | 1.14 |
| 2 | FA | B | 33 | 2.17 | 2021 | 0 | 0 | 0.22 | 0.05 | 0.16 | 1.14 |
| 2 | H2O2 | A | 33 | 2.63 | 2005 | 0 | 0.1 | 0.62 | 0.1 | 0.58 | 1.01 |
| 2 | H2O2 | B | 33 | 2.33 | 2030 | 0 | 0.06 | 0.3 | 0.03 | 0.27 | 1.2 |
| 2 | Non-disinfected | A | 33 | 1.99 | 2042 | 2 | 0.08 | 0.33 | 0.04 | 0.17 | 0.83 |
| 2 | Non-disinfected | B | 33 | 2.89 | 2072 | 2 | 0 | 0.5 | 0.04 | 0.79 | 1.21 |
| 2 | FA | A | 38 | 2.17 | 2520 | 0 | 0.09 | 0.27 | 0.06 | 0.75 | 0.75 |
| 2 | H2O2 | A | 38 | 1.94 | 2582 | 0 | 0.05 | 0.42 | 0 | 0.72 | 0.69 |
| 2 | H2O2 | B | 38 | 2.06 | 2438 | 0 | 0.01 | 0.26 | 0.02 | 0.5 | 0.99 |
| 2 | Non-disinfected | A | 38 | 2.17 | 2569 | 2 | 0.04 | 0.13 | 0.02 | 0.48 | 1.26 |
| 2 | Non-disinfected | B | 38 | 2.63 | 2532 | 2 | 0.02 | 0.38 | 0.01 | 0.53 | 1.5 |
| 2 | FA | B | 39 | 2.41 | 2639 | 0 | 0.01 | 0.63 | 0.01 | 0.62 | 0.59 |
| 3 | FA | A | 33 | 0.01 | 2031 | 0 | 0 | 0.33 | 0 | 0.87 | 0.33 |
| 3 | FA | B | 33 | 0.08 | 2007 | 0 | 6.47 | 0.43 | 0 | 1.83 | 0.22 |
| 3 | H2O2 | A | 33 | 0 | 2049 | 0 | 0.06 | 0.26 | 0 | 0.19 | 0.19 |
| 3 | H2O2 | B | 33 | 0.01 | 2042 | 0 | 0 | 0.26 | 0 | 1.03 | 0.13 |
| 3 | Non-disinfected | A | 33 | 0.02 | 2081 | 0 | 0.11 | 1.12 | 0 | 0.56 | 0.56 |
| 3 | Non-disinfected | B | 33 | 0.02 | 2070 | 0 | 0.08 | 1.17 | 0 | 0.5 | 0.5 |
| 3 | FA | B | 36 | 0.03 | 2285 | 0 | 0.04 | 0.33 | 0.02 | 2.01 | 0.58 |
| 3 | Non-disinfected | A | 36 | 0.01 | 2320 | 0 | 0.09 | 0.13 | 0.05 | 1.04 | 0.53 |
| 3 | FA | A | 37 | 0.02 | 2397 | 0 | 0.55 | 0.37 | 0.02 | 0.92 | 0.66 |
| 3 | H2O2 | A | 37 | 0.01 | 2457 | 0 | 0.11 | 0.29 | 0.01 | 0.64 | 0.42 |
| 3 | H2O2 | B | 37 | 0.03 | 2429 | 0 | 0.06 | 0.53 | 0.05 | 1.67 | 0.86 |
| 3 | Non-disinfected | B | 37 | 0.02 | 2455 | 0 | 0.25 | 0.8 | 0.01 | 0.92 | 0.28 |
| 4 | FA | A | 33 | 2.77 | 2165 | 0 | 0.28 | 2.71 | 0 | 0.06 | 0.17 |
| 4 | FA | B | 33 | 0.27 | 2117 | 0 | 0.22 | 0.32 | 0 | 0.11 | 0.11 |
| 4 | H2O2 | A | 33 | 0.28 | 2162 | 0 | 0.22 | 0.11 | 0 | 0.28 | 0.22 |
| 4 | H2O2 | B | 33 | 1.06 | 2106 | 0 | 0.42 | 0.65 | 0 | 0.37 | 0.23 |
| 4 | Non-disinfected | A | 33 | 1.8 | 2104 | 0 | 0.18 | 1.02 | 0.05 | 0.46 | 0.55 |
| 4 | Non-disinfected | B | 33 | 1.48 | 2066 | 0 | 0.32 | 0.79 | 0 | 0.8 | 0.37 |
| 4 | FA | B | 36 | 0.59 | 2382 | 0 | 0.16 | 0.43 | 0 | 0.42 | 0.11 |
| 4 | H2O2 | A | 36 | 1.32 | 2405 | 0 | 0.14 | 0.7 | 0.01 | 0.65 | 0.3 |
| 4 | H2O2 | B | 36 | 1.43 | 2406 | 0 | 0.26 | 0.87 | 0 | 0.54 | 0.22 |
| 4 | Non-disinfected | A | 36 | 1.32 | 2392 | 0 | 0.28 | 0.76 | 0.01 | 0.7 | 0.09 |
| 4 | Non-disinfected | B | 36 | 1.68 | 2371 | 0 | 0.21 | 0.89 | 0 | 0.88 | 0.18 |
| 4 | FA | A | 37 | 1.34 | 2514 | 0 | 0.24 | 0.95 | 0 | 0.44 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pees, M.; Motola, G.; Brüggemann-Schwarze, S.; Bachmeier, J.; Hafez, H.M.; Tebrün, W. Impact on Hatchability and Broiler Performance after Use of Hydrogen Peroxide Nebulization versus Formaldehyde Fumigation as Pre-Incubation Hatching Egg Disinfectants in Field Trial. Poultry 2023, 2, 1-11. https://doi.org/10.3390/poultry2010001
Pees M, Motola G, Brüggemann-Schwarze S, Bachmeier J, Hafez HM, Tebrün W. Impact on Hatchability and Broiler Performance after Use of Hydrogen Peroxide Nebulization versus Formaldehyde Fumigation as Pre-Incubation Hatching Egg Disinfectants in Field Trial. Poultry. 2023; 2(1):1-11. https://doi.org/10.3390/poultry2010001
Chicago/Turabian StylePees, Michael, Gerzon Motola, Sarah Brüggemann-Schwarze, Josef Bachmeier, Hafez Mohamed Hafez, and Wiebke Tebrün. 2023. "Impact on Hatchability and Broiler Performance after Use of Hydrogen Peroxide Nebulization versus Formaldehyde Fumigation as Pre-Incubation Hatching Egg Disinfectants in Field Trial" Poultry 2, no. 1: 1-11. https://doi.org/10.3390/poultry2010001
APA StylePees, M., Motola, G., Brüggemann-Schwarze, S., Bachmeier, J., Hafez, H. M., & Tebrün, W. (2023). Impact on Hatchability and Broiler Performance after Use of Hydrogen Peroxide Nebulization versus Formaldehyde Fumigation as Pre-Incubation Hatching Egg Disinfectants in Field Trial. Poultry, 2(1), 1-11. https://doi.org/10.3390/poultry2010001





