A Walk through Gumboro Disease
Abstract
1. Introduction
2. Etiology
3. Clinical Signs
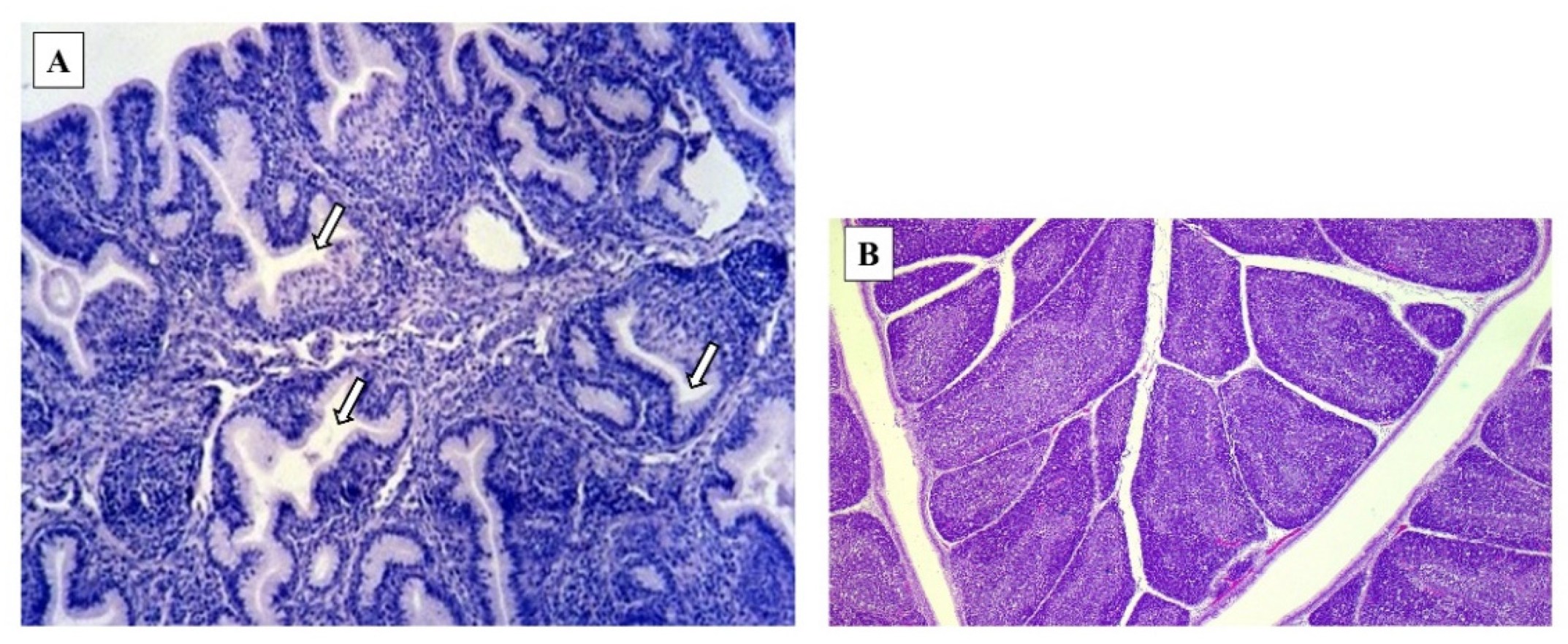
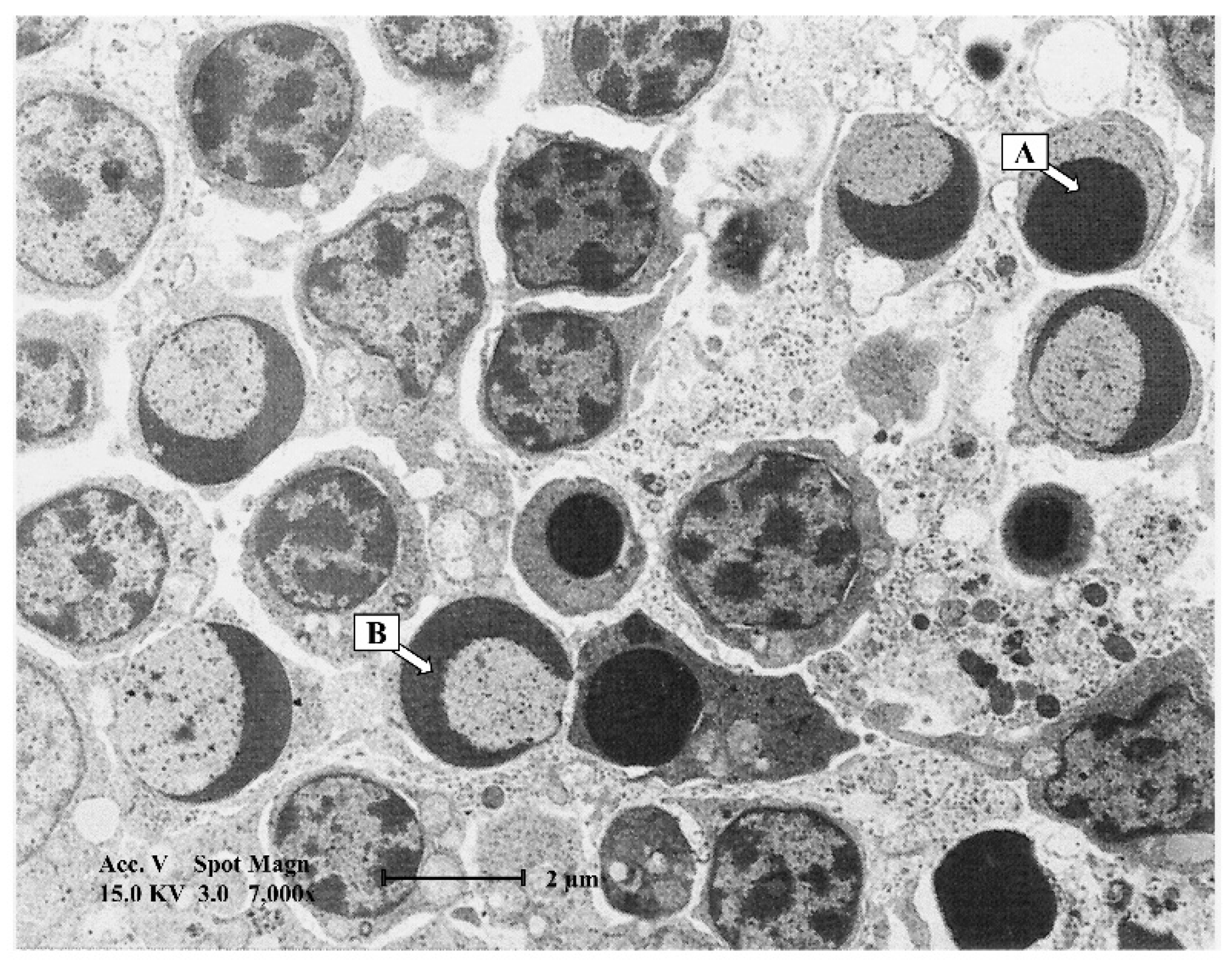
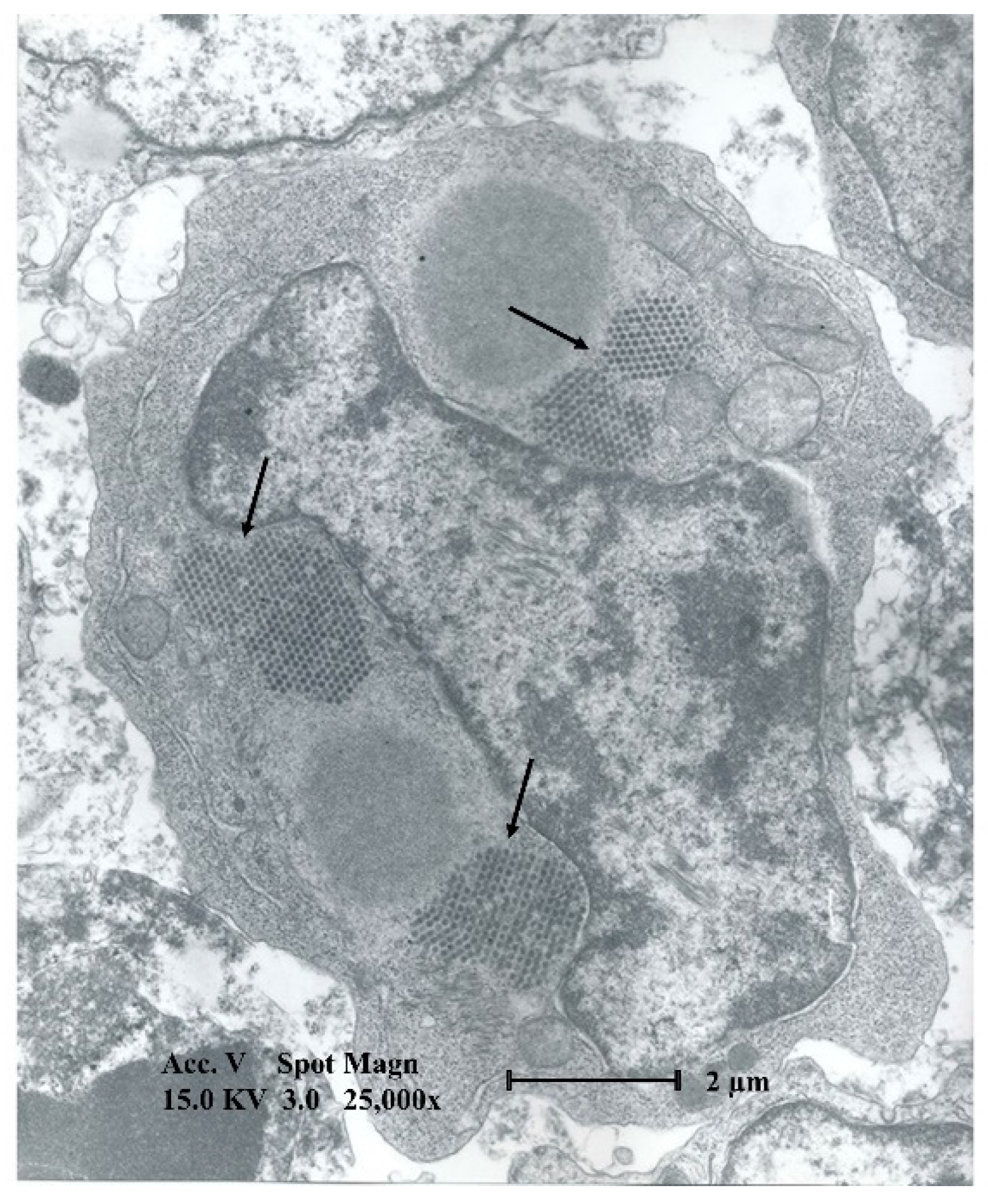
4. Macroscopic and Microscopic Lesions
5. Pathogenesis of IBDV
6. Immunosuppression
Acute and Sub-Acute Influences of IBDV Infections in Single- and in Multiple Virus Infections
7. Diagnosis
7.1. Virus Isolation
7.2. Detection of Viral Antigen
7.3. Molecular Methods
7.4. Serological Diagnosis
8. Vaccination Strategies
8.1. Subunit Vaccines
8.2. DNA Vaccines
8.3. Immune-Complex Vaccines
8.4. Live Viral Vector Vaccines
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, H.; Scholtissek, C.; Becht, H. The Genome of Infectious Bursal Disease Virus Consists of Two Segments of Double-Stranded RNA. J. Virol. 1979, 31, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Eterradossi, N.; Saif, Y.M. Infectious Bursal Disease. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 257–283. [Google Scholar] [CrossRef]
- Hoerr, F.J. Clinical Aspects of Immunosuppression in Poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef]
- Kabell, S.; Handberg, K.J.; Bisgaard, M. Impact of Coccidial Infection on Vaccine- and VvIBDV in Lymphoid Tissues of SPF Chickens as Detected by RT-PCR. Acta Vet. Scand. 2006, 48, 17. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, A.S. An Apparently New Disease of Chickens: Avian Nephrosis. Avian Dis. 1962, 6, 385. [Google Scholar] [CrossRef]
- Faragher, J.T. Infectious bursal disease of chickens. Vet Bull. 1972, 42, 361–369. [Google Scholar]
- Snyder, D.B. Changes in the Field Status of Infectious Bursal Disease Virus. Avian Pathol. 1990, 19, 419–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackwood, D.J.; Cookson, K.C.; Sommer-Wagner, S.E.; le Galludec, H.; de Wit, J.J. Molecular Characteristics of Infectious Bursal Disease Viruses from Asymptomatic Broiler Flocks in Europe. Avian Dis. 2006, 50, 532–536. [Google Scholar] [CrossRef]
- Chettle, N.; Stuart, J.C.; Wyeth, P.J. Outbreak of Virulent Infectious Bursal Disease in East Anglia. Vet. Rec. 1989, 125, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Pathak, D.C.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious Bursal Disease Virus in Chickens: Prevalence, Impact, and Management Strategies. Vet. Med. Res. Rep. 2019, 10, 85. [Google Scholar] [CrossRef]
- van den Berg, T.P.; Eterradossi, N.; Toquin, D.; Meulemans, G. La Bursite Infectieuse (Maladie de Gumboro). OIE Rev. Sci. Tech. 2000, 19, 509–526. [Google Scholar] [CrossRef]
- Jackwood, D.J.; Sommer-Wagner, S. Genetic Characteristics of Infectious Bursal Disease Viruses from Four Continents. Virology 2007, 365, 369–375. [Google Scholar] [CrossRef]
- Muniz, E.C.; Verdi, R.; Jackwood, D.J.; Kuchpel, D.; Resende, M.S.; Mattos, J.C.Q.; Cookson, K. Molecular Epidemiologic Survey of Infectious Bursal Disease Viruses in Broiler Farms Raised under Different Vaccination Programs. J. Appl. Poult. Res. 2018, 27, 253–261. [Google Scholar] [CrossRef]
- Ignjatovic, J.; Sapats, S.; Reece, R.; Gould, A.; Gould, G.; Selleck, P.; Lowther, S.; Boyle, D.; Westbury, H. Virus Strains from a Flock Exhibiting Unusually High Mortality Due to Infectious Bursal Disease. Aust. Vet. J. 2004, 82, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, N.; Cui, Y.; Hou, L.; Zhou, J.; Qiu, Y.; Yang, X.; Liu, C.; Wang, D.; Guo, J.; et al. Characterization and Pathogenicity of a Naturally Reassortant and Recombinant Infectious Bursal Disease Virus in China. Transbound. Emerg. Dis. 2022, 69, 746–758. [Google Scholar] [CrossRef]
- Kasanga, C.J.; Yamaguchi, T.; Wambura, P.N.; Maeda-Machang’u, A.D.; Ohya, K.; Fukushi, H. Molecular Characterization of Infectious Bursal Disease Virus (IBDV): Diversity of Very Virulent IBDV in Tanzania. Arch. Virol. 2007, 152, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Soubies, S.M.; Courtillon, C.; Briand, F.-X.; Queguiner-Leroux, M.; Courtois, D.; Amelot, M.; Grousson, K.; Morillon, P.; Herin, J.-B.; Eterradossi, N. Identification of a European Interserotypic Reassortant Strain of Infectious Bursal Disease. Avian Pathol. 2016, 46, 19–27. [Google Scholar] [CrossRef]
- Lupini, C.; Giovanardi, D.; Pesente, P.; Bonci, M.; Felice, V.; Rossi, G.; Morandini, E.; Cecchinato, M.; Catelli, E. A Molecular Epidemiology Study Based on VP2 Gene Sequences Reveals That a New Genotype of Infectious Bursal Disease Virus Is Dominantly Prevalent in Italy. Avian Pathol. 2016, 45, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lupini, C.; Quaglia, G.; Mescolini, G.; Russo, E.; Salaroli, R.; Forni, M.; Boldini, S.; Catelli, E. Alteration of Immunological Parameters in Infectious Bronchitis Vaccinated-Specific Pathogen-Free Broilers after the Use of Different Infectious Bursal Disease Vaccines. Poult. Sci. 2020, 99, 4351–4359. [Google Scholar] [CrossRef]
- Felice, V.; Franzo, G.; Catelli, E.; Francesco, A.D.; Bonci, M.; Cecchinato, M.; Mescolini, G.; Giovanardi, D.; Pesente, P.; Lupini, C.; et al. Molecular And Cellular Biology Genome Sequence Analysis of a Distinctive Italian Infectious Bursal Disease Virus. Poult. Sci. 2017, 96, 4370–4377. [Google Scholar] [CrossRef]
- Delmas, B.; Attoui, H.; Ghosh, S.; Malik, Y.S.; Mundt, E.; Vakharia, V.N. ICTV Virus Taxonomy Profile: Birnaviridae. J. Gen. Virol. 2019, 100, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, V.N.; He, J.; Ahamed, B.; Snyder, D.B. Molecular Basis of Antigenic Variation in Infectious Bursal Disease Virus. Virus Res. 1994, 31, 265–273. [Google Scholar] [CrossRef]
- Letzel, T.; Coulibaly, F.; Rey, F.A.; Delmas, B.; Jagt, E.; van Loon, A.A.M.W.; Mundt, E. Molecular and Structural Bases for the Antigenicity of VP2 of Infectious Bursal Disease Virus. J. Virol. 2007, 81, 12827–12835. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gao, X.; Lu, Z.; Zhang, L.; Wang, Y.; Gao, L.; Gao, Y.; Li, K.; Gao, H.; Liu, C.; et al. A Single Mutation in the PBC Loop of VP2 Is Involved in the in Vitro Replication of Infectious Bursal Disease Virus. Sci. China Life Sci. 2016, 59, 717–723. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, T.P.; Morales, D.; Eterradossi, N.; Rivallan, G.; Toquin, D.; Raue, R.; Zierenberg, K.; Zhang, M.F.; Zhu, Y.P.; Wang, C.Q.; et al. Assessment of Genetic, Antigenic and Pathotypic Criteria for the Characterization of IBDV Strains. Avian Pathol. 2004, 33, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.O.; Jackwood, D.J. Classification of Infectious Bursal Disease Virus into Genogroups. Arch. Virol. 2017, 162, 3661–3670. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Fan, L.-J.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.-L.; Liu, C.-J.; Cui, H.-Y.; Pan, Q.; Zhang, Y.-P.; et al. An Improved Scheme for Infectious Bursal Disease Virus Genotype Classification Based on Both Genome-Segments A and B. J. Integr. Agric. 2021, 20, 1372–1381. [Google Scholar] [CrossRef]
- van den Berg, T.P.; Gonze, M.; Morales, D.; Meulemans, G. Acute Infectious Bursal Disease in Poultry: Immunological and Molecular Basis of Antigenicity of a Highly Virulent Strain. Avian Pathol. 1996, 25, 751–768. [Google Scholar] [CrossRef]
- Ogbe, A.O.; Audu, Z.; Kwaia, E.Z. Pathological Diagnosis of Infectious Bursal Disease in 24 weeks old vaccinated commercial layng hens in Kakargo, Nigeria a case report. J. Dairy Vet. Anim. Res. 2020, 172–176. [Google Scholar] [CrossRef]
- Mcilroy, S.G.; Goodall, E.A.; Bruce, D.W.; Mccracken, R.M.; Mcnulty, M.S. The Cost Benefit of Vaccinating Broiler Flocks against Subclinical Infectious Bursal Disease. Avian Pathol. 2007, 21, 65–76. [Google Scholar] [CrossRef]
- Homer, B.L.; Butcher, G.D.; Miles, R.D.; Rossi, A.F. Subclinical Infectious Bursal Disease in an Integrated Broiler Production Operation. J. Vet. Diagn. Investig. 1992, 4, 406–411. [Google Scholar] [CrossRef]
- Zeryehun, T.; Hair-Bejo, M.; Rasedee, A. Hemorrhagic and Clotting Abnormalities in Infectious Bursal Disease in Specific-Pathogen-Free Chicks. World Appl. Sci. J. 2012, 16, 1123–1130. [Google Scholar]
- Mahgoub, H.; Mahgoub, H.A. An Overview of Infectious Bursal Disease. Arch. Virol. 2012, 157, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Banga, H.S.; Brar, R.S.; Singh, N.D.; Sodhi, S.; Leishangthem, G.D. Histopathological and Immunohistochemical Diagnosis of Infectious Bursal Disease in Poultry Birds. Vet. World 2015, 8, 1331. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, M.P.; Coletti, M. Serological, Histological and Immuno-Histochemistry Studies on Infectious Bursal Disease Vaccine Strain with Residual Pathogenicity. In Proceedings of the COST ACTION 839, Belfast, Ireland, 18–19 June 1999; pp. 199–206. [Google Scholar]
- Vasconcelos, A.C.; Lain, K.M. Apoptosis Induced by Infectious Bursal Disease Virus. J. Gen. Virol. 1994, 75, 1803–1806. [Google Scholar] [CrossRef]
- Franciosini, M.P.; Asdrubali, G.; Coletti, M.; Mughetti, L. Alterazioni al Timo in Polli Affetti Da Malattia Di Gumboro. Proceeding of the XXXIV Italian Avian Pathology Conference, Forlì, Italy, 6–7 October 1995; pp. 76–79. [Google Scholar]
- Inoue, M.; Fukuda, M.; Miyano, K. Thymic Lesions in Chicken Infected with Infectious Bursal Disease Virus. Avian Dis. 1994, 38, 839–846. [Google Scholar] [CrossRef]
- Laster, S.M.; Wood, J.G.; Gooding, L.R. Tumor Necrosis Factor Can Induce Both Apoptic and Necrotic Forms of Cell Lysis. J. Immunol. 1988, 141, 2629–2634. [Google Scholar]
- Asdrubali, G.; Mughetti, L. Contributo Alla Conoscenza Degli Aspetti Ultrastrutturali Del La Borsa Di Fabrizio Nella Malattia Di Gumboro Sperimentale. La Nuova Vet. 1972, 48, 71–87. [Google Scholar]
- Kaufer, I.; Weiss, E. Significance of Bursa of Fabricius as Target Organ in Infectious Bursal Disease of Chickens. Infect. Immun. 1980, 27, 364–367. [Google Scholar] [CrossRef]
- Ogawa, M.; Yamaguchi, T.; Setiyono, A.; Ho, T.; Matsuda, H.; Furusawa, S.; Fukushi, H.; Hirai, K. Some Characteristics of a Cellular Receptor for Virulent Infectious Bursal Disease Virus by Using Flow Cytometry. Arch. Virol. 1998, 143, 2327–2341. [Google Scholar] [CrossRef]
- Moyer, C.L.; Nemerow, G.R. Viral Weapons of Membrane Destruction: Variable Modes of Membrane Penetration by Non-Enveloped Viruses. Curr. Opin. Virol. 2011, 1, 44–49. [Google Scholar] [CrossRef]
- Galloux, M.; Libersou, S.; Morellet, N.; Bouaziz, S.; da Costa, B.; Ouldali, M.; Lepault, J.; Delmas, B. Infectious Bursal Disease Virus, a Non-Enveloped Virus, Possesses a Capsid-Associated Peptide That Deforms and Perforates Biological Membranes. J. Biol. Chem. 2007, 282, 20774–20784. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zheng, S.J. Infectious Bursal Disease Virus-Host Interactions: Multifunctional Viral Proteins That Perform Multiple and Differing Jobs. Int. J. Mol. Sci. 2017, 18, 161. [Google Scholar] [CrossRef]
- Williams, A.E.; Davison, T.F. Avian Pathology Enhanced Immunopathology Induced by Very Virulent Infectious Bursal Disease Virus Enhanced Immunopathology Induced by Very Virulent Infectious Bursal Disease Virus. Avian Pathol. 2010, 34, 4–14. [Google Scholar] [CrossRef]
- Hiraga, M.; Nunoya, T.; Otaki, Y.; Tajima, M.; Saito, T.; Nakamura, T. Pathogenesis of Highly Virulent Infectious Bursal Disease Virus Infection in Intact and Bursectomized Chickens. J. Vet. Med. Sci. 1994, 56, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khatri, M.; Murgia, M.V.; Jung, K.; Saif, Y.M. Differential Modulation of Cytokine, Chemokine and Toll like Receptor Expression in Chickens Infected with Classical and Variant Infectious Bursal Disease Virus. Vet. Res. 2011, 42, 85. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Wang, Y.; Guo, Y.; Xiong, Y.; Chen, M.; Zhao, A.; Liu, H. T Cell Subset Profile and Inflammatory Cytokine Properties in the Gut-Associated Lymphoid Tissues of Chickens during Infectious Bursal Disease Virus (IBDV) Infection. Arch. Virol. 2020, 165, 2249–2258. [Google Scholar] [CrossRef]
- Chen, R.; Chen, J.; Xiang, Y.; Chen, Y.; Shen, W.; Wang, W.; Li, Y.; Wei, P.; He, X. Differential Modulation of Innate Antiviral Profiles in the Intestinal Lamina Propria Cells of Chickens Infected with Infectious Bursal Disease Viruses of Different Virulence. Viruses 2022, 14, 393. [Google Scholar] [CrossRef]
- Eldaghayes, I.; Rothwell, L.; Williams, A.; Withers, D.; Balu, S.; Davison, F.; Kaiser, P. Infectious Bursal Disease Virus: Strains That Differ in Virulence Differentially Modulate the Innate Immune Response to Infection in the Chicken Bursa. Viral Immunol. 2006, 19, 83–91. [Google Scholar] [CrossRef]
- Lam, K.M. Morphological Evidence of Apoptosis in Chickens Infected with Infectious Bursal Disease Virus. J. Comp. Pathol. 1997, 116, 367–377. [Google Scholar] [CrossRef]
- Tanimura, N.; Sharma, J.M. In-Situ Apoptosis in Chickens Infected with Infectious Bursal Disease Virus. J. Comp. Pathol. 1998, 118, 15–27. [Google Scholar] [CrossRef]
- Elankumaran, S.; Heckert, R.A.; Moura, L. Pathogenesis and Tissue Distribution of a Variant Strain of Infectious Bursal Disease Virus in Commercial Broiler Chickens. Avian Dis. 2002, 46, 169–176. [Google Scholar] [CrossRef]
- Abdel-Alim, G.A.; Saif, Y.M. Detection and Persistence of Infectious Bursal Disease Virus in Specific-Pathogen-Free and Commercial Broiler Chickens. Avian Dis. 2001, 45, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, N.A.; Smith, L.P.; Mwangi, W.; Boyd, A.; Broadbent, A.J.; Smith, A.L.; Nair, V. Early Pathogenesis during Infectious Bursal Disease in Susceptible Chickens Is Associated with Changes in B Cell Genomic Methylation and Loss of Genome Integrity. Dev. Comp. Immunol. 2017, 73, 169–174. [Google Scholar] [CrossRef]
- Yasmin, A.R.; Yeap, S.K.; Tan, S.W.; Hair-Bejo, M.; Fakurazi, S.; Kaiser, P.; Omar, A.R. In Vitro Characterization of Chicken Bone Marrow-Derived Dendritic Cells Following Infection with Very Virulent Infectious Bursal Disease Virus. Avian Pathol. 2015, 44, 452–462. [Google Scholar] [CrossRef]
- Allan, W.H.; Faragher, J.T.; Cullen, G.A. Immunosuppression by the Infectious Bursal Agent in Chickens Immunised against Newcastle Disease. Vet. Rec. 1972, 90, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Faragher, J.T.; Allan, W.H.; Wyeth, P.J. Immunosuppressive Effect of Infectious Bursal Agent on Vaccination against Newcastle Disease. Vet. Rec. 1974, 95, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J.K.; Gelb, J. Response to Several Avian Respiratory Viruses as Affected by Infectious Bursal Disease Virus. Avian Dis. 1978, 22, 95–105. [Google Scholar] [CrossRef]
- Phillips, R.A.; Opitz, H.M. Pathogenicity and Persistence of Salmonella Enteritidis and Egg Contamination in Normal and Infectious Bursal Disease Virus-Infected Leghorn Chicks. Avian Dis. 1995, 39, 778–787. [Google Scholar] [CrossRef]
- Anderson, W.I.; Reid, W.M.; Lukert, P.D.; Fletcher, J.O., Jr. Influence of Infectious Bursal Disease on the development of immunity to Eimeria tenella. Avian Dis. 1977, 21, 637–641. [Google Scholar]
- Schat, K.A.; Skinner, M.A. Avian Immunosuppressive Diseases and Immunoevasion. In Avian Immunology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 275–297. [Google Scholar] [CrossRef]
- Khatri, M.; Sharma, J.M. Modulation of Macrophages by Infectious Bursal Disease Virus. Cytogenet. Genome Res. 2007, 117, 388–393. [Google Scholar] [CrossRef]
- Wang, S.; Teng, Q.; Jia, L.; Sun, X.; Wu, Y.; Zhou, J. Infectious Bursal Disease Virus Influences the Transcription of Chicken Γc and Γc Family Cytokines during Infection. PLoS ONE 2014, 9, e84503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Sun, H.; Shen, P.; Zhang, X.; Xia, X. Effective Inhibition of Infectious Bursal Disease Virus Replication by Recombinant Avian Adeno-Associated Virus-Delivered MicroRNAs. J. Gen. Virol. 2009, 90, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Schat, K.A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018, 62, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I. Avian Oncogenic and Immunosuppressive Viruses. In Encyclopedia of Sustainability Science and Technology, Meyers ed.; Springer: New York, NY, USA, 2020; Invited Chapter ; pp. 1–20. [Google Scholar] [CrossRef]
- Davidson, I. Out of Sight, but Not Out of Mind: Aspects of the Avian Oncogenic Herpesvirus, Marek’s Disease Virus. Animals 2020, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, V.R.; Mohammadi, A.; Dadras, H. Infectious Bursal Disease Virus Suppresses H9N2 Avian Influenza Viral Shedding in Broiler Chickens. Br. Poult. Sci. 2019, 60, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Winterfield, R.W.; Thacker, H.L.; Feldman, D.S. Lesions Induced in the Respiratory Tract of Chickens by Serologically Different Adenoviruses. Avian Dis. 1982, 26, 478–486. [Google Scholar] [CrossRef]
- Xu, A.H.; Sun, L.; Tu, K.H.; Teng, Q.Y.; Xue, J.; Zhang, G.Z. Experimental Co-Infection of Variant Infectious Bursal Disease Virus and Fowl Adenovirus Serotype 4 Increases Mortality and Reduces Immune Response in Chickens. Vet. Res. 2021, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arafat, N.; Eladl, A.H.; Mahgoub, H.; El-shafei, R.A. Effect of Infectious Bursal Disease (IBD) Vaccine on Salmonella Enteritidis Infected Chickens. Vaccine 2017, 35, 3682–3689. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; van Santen, V.L.; Hoerr, F.J.; Breedlove, C. Effects of Chicken Anemia Virus and Infectious Bursal Disease Virus in Commercial Chickens. Avian Dis. 2009, 53, 94–102. [Google Scholar] [CrossRef]
- Rosenberger, J.K.; Cloud, S.S. The Effects of Age, Route of Exposure, and Coinfection with Infectious Bursal Disease Virus on the Pathogenicity and Transmissibility of Chicken Anemia Agent (CAA). Avian Dis. 1989, 33, 753–759. [Google Scholar] [CrossRef]
- Haridy, M.; Goryo, M.; Sasaki, J.; Okada, K. Pathological and Immunohistochemical Study of Chickens with Co-Infection of Marek’s Disease Virus and Chicken Anaemia Virus. Avian Pathol. 2009, 38, 469–483. [Google Scholar] [CrossRef]
- Eregae, M.E.; Dewey, C.E.; McEwen, S.A.; Ouckama, R.; Ojkić, D.; Guerin, M.T. Flock Prevalence of Exposure to Avian Adeno-Associated Virus, Chicken Anemia Virus, Fowl Adenovirus, and Infectious Bursal Disease Virus Among Ontario Broiler Chicken Flocks. Avian Dis. 2014, 58, 71–77. [Google Scholar] [CrossRef]
- Cong, F.; Zhu, Y.; Wang, J.; Lian, Y.; Liu, X.; Xiao, L.; Huang, R.; Zhang, Y.; Chen, M.; Guo, P. A Multiplex XTAG Assay for the Simultaneous Detection of Five Chicken Immunosuppressive Viruses. BMC Vet. Res. 2018, 14, 347. [Google Scholar] [CrossRef]
- Brandt, M.; Yao, K.; Liu, M.; Heckert, R.A.; Vakharia, V.N. Molecular Determinants of Virulence, Cell Tropism, and Pathogenic Phenotype of Infectious Bursal Disease Virus. J. Virol. 2001, 75, 11974. [Google Scholar] [CrossRef]
- Zhang, G.P.; Li, Q.M.; Yang, Y.Y.; Guo, J.Q.; Li, X.W.; Deng, R.G.; Xiao, Z.J.; Xing, G.X.; Yang, J.F.; Zhao, D.; et al. Development of a One-Step Strip Test for the Diagnosis of Chicken Infectious Bursal Disease. Avian Dis. 2005, 49, 177–181. [Google Scholar] [CrossRef]
- Zierenberg, K.; Raue, R.; Müller, H. Rapid Identification of “Very Virulent” Strains of Infectious Bursal Disease Virus by Reverse Transcription-Polymerase Chain Reaction Combined with Restriction Enzyme Analysis. Avian Pathol. 2001, 30, 55–62. [Google Scholar] [CrossRef]
- Peters, M.A.; Tsang, L.L.; Ching, C.W. Real-Time RT-PCR Differentiation and Quantitation of Infectious Bursal Disease Virus Strains Using Dual-Labeled Fluorescent Probes. J. Virol. Methods 2005, 127, 87–95. [Google Scholar] [CrossRef]
- Rubinelli, P.; Long Lin, T. Molecular Detection and Differentiation of Infectious Bursal Disease Virus (Detección y Diferenciación Molecular Del Virus de La Enfermedad Infecciosa de La Bolsa). Avian Dis. 2007, 51, 515–526. [Google Scholar]
- Freimanis, G.L.; Oade, M.S. Whole-Genome Sequencing Protocols for IBV and Other Coronaviruses Using High-Throughput Sequencing. Methods Mol. Biol. 2020, 2203, 67–74. [Google Scholar] [CrossRef]
- Lachheb, J.; Jbenyeni, A.; Nsiri, J.; Larbi, I.; Ammouna, F.; El Behi, I.; Ghram, A. Full-Length Genome Sequencing of a Very Virulent Infectious Bursal Disease Virus Isolated in Tunisia. Poult. Sci. 2021, 100, 496–506. [Google Scholar] [CrossRef]
- Jackwood, D.H.; Saif, Y.M. Antigenic Diversity of Infectious Bursal Disease Viruses. Avian Dis. 1987, 31, 766–770. [Google Scholar] [CrossRef]
- Gómez, E.; Cassani, M.F.; Lucero, M.S.; Parreño, V.; Chimeno Zoth, S.; Berinstein, A. Development of diagnostic tools for IBDV detection using plants as bioreactors. AMB Express 2020, 10, 95. [Google Scholar] [CrossRef]
- Muller, H.; Mundt, E.; Eteradossi, N.; Islam, M.R. Current status of vaccines against Infectious Bursal Diseas. Avian Pathol. 2012, 41, 133–139. [Google Scholar] [CrossRef]
- Fahey, K.J.; Crooks, J.K.; Fraser, R.A. Assessment by ELISA of Passively Acquired Protection against Infectious Bursal Disease Virus in Chickens. Aust. Vet. J. 1987, 64, 203–207. [Google Scholar] [CrossRef]
- Block, H.; Meyer-Block, K.; Rebeski, D.E.; Scharr, H.; de Wit, S.; Rohn, K.; Rautenschlein, S. A Field Study on the Significance of Vaccination against Infectious Bursal Disease Virus (IBDV) at the Optimal Time Point in Broiler Flocks with Maternally Derived IBDV Antibodies. Avian Pathol. 2010, 36, 401–409. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Sharma, J.; Tarpey, I. Practical Aspects of Poultry Vaccination. In Avian Immunology, 2nd ed.; Davison, F., Kaspers, B., Schat, K.A., Eds.; Academic Press: Amsterdam, The Netherlands; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; Chapter 20; pp. 373–393. [Google Scholar] [CrossRef]
- Coletti, M.; del Rossi, E.; Franciosini, M.P.; Passamonti, F.; Tacconi, G.; Marini, C. Efficacy and Safety of an Infectious Bursal Disease Virus Intermediate Vaccine in Ovo. Avian Dis. 2001, 45, 1036–1043. [Google Scholar] [CrossRef]
- Rautenschlein, S.; Kraemer, C.; Vanmarcke, J.; Montiel, E. Protective Efficacy of Intermediate and Intermediate plus Infectious Bursal Disease Virus (IBDV) Vaccines against Very Virulent IBDV in Commercial Broilers. Avian Dis. 2005, 49, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.M.; Ashash, U.; Muthukumar, S. A Field Study on the Evaluation of Day-of-Hatch and in Grow-out Application of Live Infectious Bursal Disease Virus Vaccine in Broiler Chickens. Poult. Sci. 2021, 100, 101252. [Google Scholar] [CrossRef]
- Müller, H.; Schnitzler, D.; Bernstein, F.; Becht, H.; Cornelissen, D.; Lütticken, D.H. Infectious Bursal Disease of Poultry: Antigenic Structure of the Virus and Control. Vet. Microbiol. 1992, 33, 175–183. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Dormitorio, T.; Brown, T.; Takeshita, K. Monitoringthe immune status of broiler breeders against Infectious Burdal Disease Virus using progeny challenge and serological data. J. Appl. Poult. Res. 1999, 8, 362–367. [Google Scholar] [CrossRef]
- Snyder, D.B.; Vakharia, V.N.; Mengel-Whereat, S.A.; Edwards, G.H.; Savage, P.K.; Lutticken, D.; Goodwin, M.A. Active Cross-Protection Induced by a Recombinant Baculovirus Expressing Chimeric Infectious Bursal Disease Virus Structural Proteins. Avian Dis. 1994, 38, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, H.; Goldway, M.; Gutter, B.; Michael, A.; Godfried, Y.; Shaaltiel, Y.; Levi, B.Z.; Pitcovski, J. Transfer of Antibodies Elicited by Baculovirus-Derived VP2 of a Very Virulent Bursal Disease Virus Strain to Progeny of Commercial Breeder Chickens. Avian Pathol. 2000, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Upadhyay, C.; Madhan Mohan, C.; Kataria, J.M.; Vakharia, V.N. Formation of Subviral Particles of the Capsid Protein VP2 of Infectious Bursal Disease Virus and Its Application in Serological Diagnosis. J. Virol. Methods 2009, 157, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Jiang, T.; Cheng, T.; Shen, M.; Du, Y.; Li, S.; Wang, S.; Xu, B.; Fan, G. Large-Scale Manufacture and Use of Recombinant VP2 Vaccine against Infectious Bursal Disease in Chickens. Vaccine 2007, 25, 7900–7908. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Song, Y.; Wang, W.; Zhang, Y.; Wang, T.; Li, K.; Pan, Q.; Qi, X.; Gao, Y.; et al. Recombinant Lactococcus Lactis Co-Expressing OmpH of an M Cell-Targeting Ligand and IBDV-VP2 Protein Provide Immunological Protection in Chickens. Vaccine 2018, 36, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Pitcovski, J.; Gutter, B.; Gallili, G.; Goldway, M.; Perelman, B.; Gross, G.; Krispel, S.; Barbakov, M.; Michael, A. Development and Large-Scale Use of Recombinant VP2 Vaccine for the Prevention of Infectious Bursal Disease of Chickens. Vaccine 2003, 21, 4736–4743. [Google Scholar] [CrossRef]
- Heine, H.G.; Boyle, D.B. Infectious Bursal Disease Virus Structural Protein VP2 Expressed by a Fowlpox Virus Recombinant Confers Protection against Disease in Chickens. Arch. Virol. 1993, 131, 277–292. [Google Scholar] [CrossRef]
- Martinez-Torrecuadrada, J.L.; Saubi, N.; Pagès-Manté, A.; Castón, J.R.; Espuña, E.; Casal, J.I. Structure-Dependent Efficacy of Infectious Bursal Disease Virus (IBDV) Recombinant Vaccines. Vaccine 2003, 21, 3342–3350. [Google Scholar] [CrossRef]
- Fodor, I.; Horváth, E.; Fodor, N.; Nagy, E.; Rencendorsh, A.; Vakharia, V.N.; Dube, S.K. Induction of Protective Immunity in Chickens Immunised with Plasmid DNA Encoding Infectious Bursal Disease Virus Antigens. Acta Vet. Hung. 1999, 47, 481–492. [Google Scholar] [CrossRef]
- Chang, H.C.; Lin, T.L.; Wu, C.C. DNA-Mediated Vaccination against Infectious Bursal Disease in Chickens. Vaccine 2001, 20, 328–335. [Google Scholar] [CrossRef]
- Pradhan, S.N.; Prince, P.R.; Madhumathi, J.; Arunkumar, C.; Roy, P.; Narayanan, R.B.; Antony, U. DNA Vaccination with VP2 Gene Fragment Confers Protection against Infectious Bursal Disease Virus in Chickens. Vet. Microbiol. 2014, 171, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Haygreen, E.A.; Kaiser, P.; Burgess, S.C.; Davison, T.F. In Ovo DNA Immunisation Followed by a Recombinant Fowlpox Boost Is Fully Protective to Challenge with Virulent IBDV. Vaccine 2006, 24, 4951–4961. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, H.; Gao, L.; Qi, X.; Gao, Y.; Qin, L.; Wang, Y.; Wang, X. Adjuvant Effects of Interleukin-18 in DNA Vaccination against Infectious Bursal Disease Virus in Chickens. Vaccine 2013, 31, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Zhang, J.; Fan, J.; Wang, X.; Wu, F.; Zuo, Y.; Zhong, F. Co-Expression of Chicken IL-2 and IL-7 Enhances the Immunogenicity and Protective Efficacy of a VP2-Expressing DNA Vaccine against IBDV in Chickens. Viruses 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Whitfill, C.E.; Haddad, E.E.; Ricks, C.A.; Skeeles, J.K.; Newberry, L.A.; Beasley, J.N.; Andrews, P.D.; Thoma, J.A.; Wakenell, P.S. Determination of Optimum Formulation of a Novel Infectious Bursal Disease Virus (IBDV) Vaccine Constructed by Mixing Bursal Disease Antibody with IBDV. Avian Dis. 1995, 39, 687–699. [Google Scholar] [CrossRef]
- Haddad, E.E.; Whitfill, C.E.; Avakian, A.P.; Ricks, C.A.; Andrews, P.D.; Thoma, J.A.; Wakenell, P.S. Efficacy of a Novel Infectious Bursal Disease Virus Immune Complex Vaccine in Broiler Chickens. Avian Dis. 1997, 41, 882–889. [Google Scholar] [CrossRef]
- Garcia, C.; Soriano, J.M.; Cortés, V.; Sevilla-Navarro, S.; Marin, C.; Balaguer, J.L.; Català-Gregori, P. Monitoring serologic response to single in ovo vaccination with an immune complex against infectious bursal disease in broiler. Poult. Sci. 2021, 100, 4. [Google Scholar] [CrossRef]
- Dertzbaugh, M.T. Genetically Engineered Vaccines: An Overview. Plasmid 1998, 39, 100–113. [Google Scholar] [CrossRef]
- Francois, A.; Chevalier, C.; Delmas, B.; Eterradossi, N.; Toquin, D.; Rivallan, G.; Langlois, P. Avian Adenovirus CELO Recombinants Expressing VP2 of Infectious Bursal Disease Virus Induce Protection against Bursal Disease in Chickens. Vaccine 2004, 22, 2351–2360. [Google Scholar] [CrossRef]
- Bublot, M.; Pritchard, N.; le Gros, F.X.; Goutebroze, S. Use of a Vectored Vaccine against Infectious Bursal Disease of Chickens in the Face of High-Titred Maternally Derived Antibody. J. Comp. Pathol. 2007, 137, S81–S84. [Google Scholar] [CrossRef]
- Darteil, R.; Bublot, M.; Laplace, E.; Bouquet, J.F.; Audonnet, J.C.; Riviè, M. Herpesvirus of Turkey Recombinant Viruses Expressing Infectious Bursal Disease Virus (IBDV) VP2 Immunogen Induce Protection against an IBDV Virulent Challenge in Chickens. Virology 1995, 211, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Prandini, F.; Simon, B.; Jung, A.; Pöppel, M.; Lemiere, S.; Rautenschlein, S. Comparison of Infectious Bursal Disease Live Vaccines and a HVT-IBD Vector Vaccine and Their Effects on the Immune System of Commercial Layer Pullets. Avian Pathol. 2016, 45, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ramon, G.; Legnardi, M.; Cecchinato, M.; Cazaban, C.; Tucciarone, C.M.; Fiorentini, L.; Gambi, L.; Mato, T.; Berto, G.; Koutoulis, K.; et al. Efficacy of live attenuated, vector and immune complex infectious bursal disease virus (IBDV) vaccines in preventing field strain bursa colonization: A European multicentric study. Front. Vet. Sci. 2022, 9, 978901. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Otomo, H.; Suzuki, S.; Ono, Y.; Taneno, A.; Oishi, E. Efficacy of a Novel in Ovo-Attenuated Live Vaccine and Recombinant Vaccine against a Very Virulent Infectious Bursal Disease Virus in Chickens. J. Vet. Med. Sci. 2021, 83, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- le Gros, F.X.; Dancer, A.; Giacomini, C.; Pizzoni, L.; Bublot, M.; Graziani, M.; Prandini, F. Field Efficacy Trial of a Novel HVT-IBD Vector Vaccine for 1-Day-Old Broilers. Vaccine 2009, 27, 592–596. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Kojima, C.; Komori, Y.; Tanimura, N.; Mase, M.; Yamaguchi, S. Protection of Chickens against Very Virulent Infectious Bursal Disease Virus (IBDV) and Marek’s Disease Virus (MDV) with a Recombinant MDV Expressing IBDV VP2. Virology 1999, 257, 352–362. [Google Scholar] [CrossRef]
- Perozo, F.; Villegas, P.; Estevez, C.; Alvarado, I.R.; Purvis, L.B.; Williams, S. Protection against Infectious Bursal Disease Virulent Challenge Conferred by a Recombinant Avian Adeno-Associated Virus Vaccine. Avian Dis. 2008, 52, 315–319. [Google Scholar] [CrossRef]
- Mató, T.; Tatár-Kis, T.; Felföldi, B.; Jansson, D.S.; Homonnay, Z.; Bányai, K.; Palya, V. Occurrence and Spread of a Reassortant Very Virulent Genotype of Infectious Bursal Disease Virus with Altered VP2 Amino Acid Profile and Pathogenicity in Some European Countries. Vet. Microbiol. 2020, 245, 108663. [Google Scholar] [CrossRef]
- Mató, T.; Medveczki, A.; Kiss, I. Research Note: “Hidden” Infectious Bursal Disease Virus Infections in Central Europe. Poult. Sci. 2022, 101, 101958. [Google Scholar] [CrossRef]
- Pagès-Manté, A.; Torrents, D.; Maldonado, J.; Saubi, N. Dogs as Potential Carriers of Infectious Bursal Disease Virus. Avian Pathol. 2004, 33, 205–209. [Google Scholar] [CrossRef]
- Graziosi, G.; Catelli, E.; Fanelli, A.; Lupini, C. Infectious Bursal Disease Virus in Free-Living Wild Birds: A Systematic Review and Meta-Analysis of Its Sero-Viroprevalence on a Global Scale. Transbound. Emerg. Dis. 2021, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franciosini, M.P.; Davidson, I. A Walk through Gumboro Disease. Poultry 2022, 1, 229-242. https://doi.org/10.3390/poultry1040020
Franciosini MP, Davidson I. A Walk through Gumboro Disease. Poultry. 2022; 1(4):229-242. https://doi.org/10.3390/poultry1040020
Chicago/Turabian StyleFranciosini, Maria Pia, and Irit Davidson. 2022. "A Walk through Gumboro Disease" Poultry 1, no. 4: 229-242. https://doi.org/10.3390/poultry1040020
APA StyleFranciosini, M. P., & Davidson, I. (2022). A Walk through Gumboro Disease. Poultry, 1(4), 229-242. https://doi.org/10.3390/poultry1040020





