Fungal Necrotrophic Interaction: A Case Study of Seed Immune Response to a Seed-Borne Pathogen
Abstract
1. Introduction
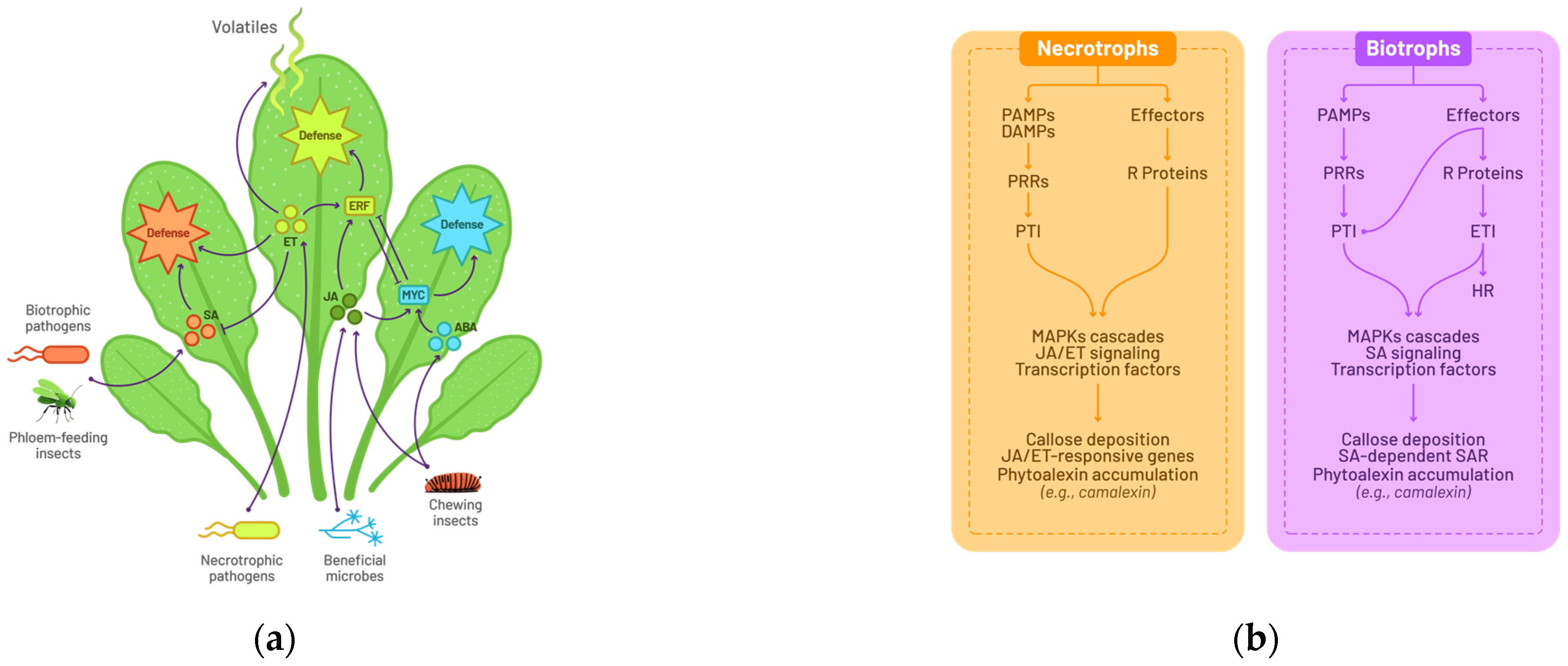
2. Plant Immune Response
2.1. A General Model for Plant–Pathogen Interactions
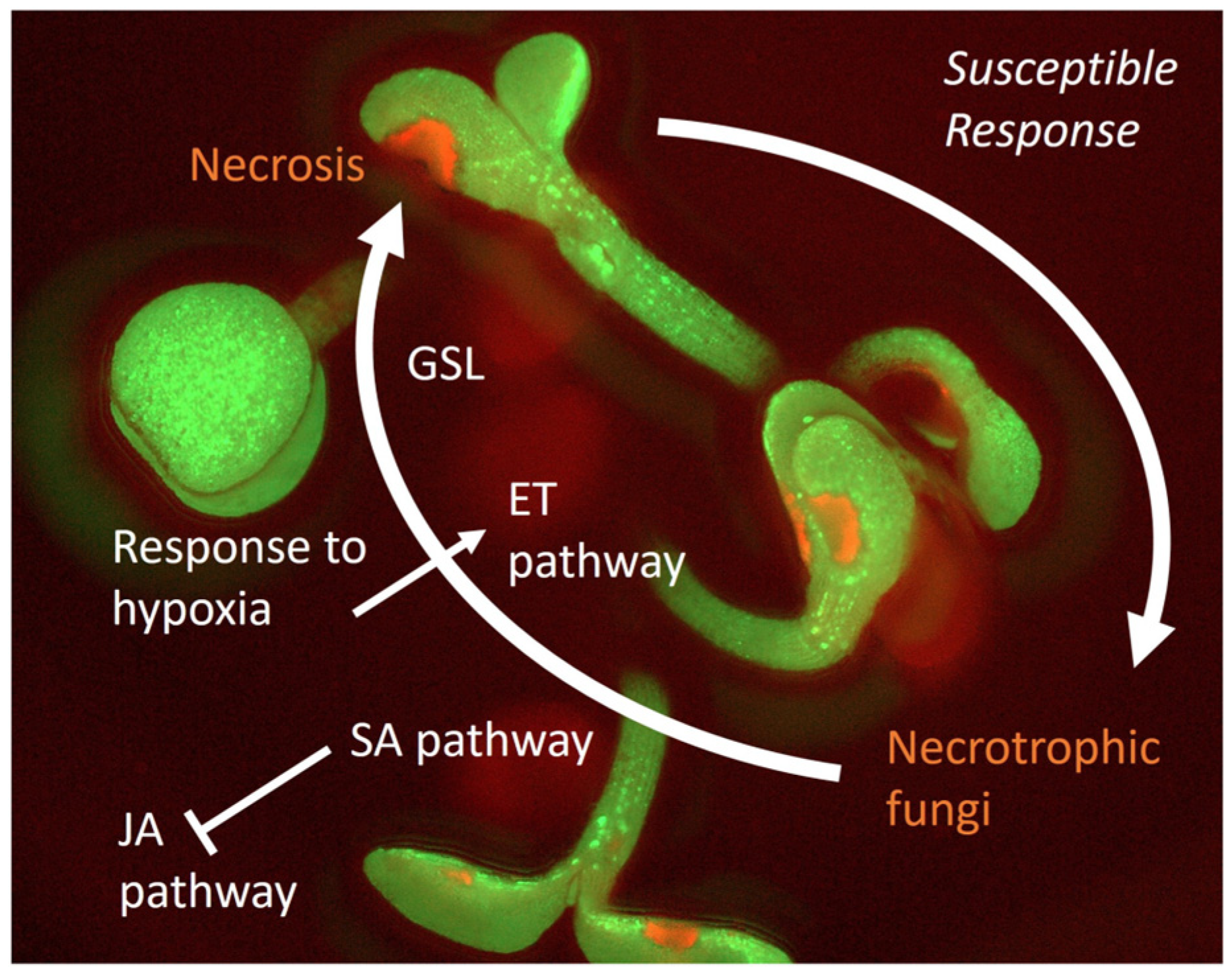
2.2. Susceptible Response and Plant Tolerance
3. Alternaria brassicicola: An Important Seed-Borne Pathogen in Brassicaceae and a Model for Studying Pathogen Interactions with Seeds
4. Seed Defense Mechanisms and Interactions with the Necrotrophic Seed-Borne Fungi Alternaria brassicicola
4.1. A silent Interaction with the Seed
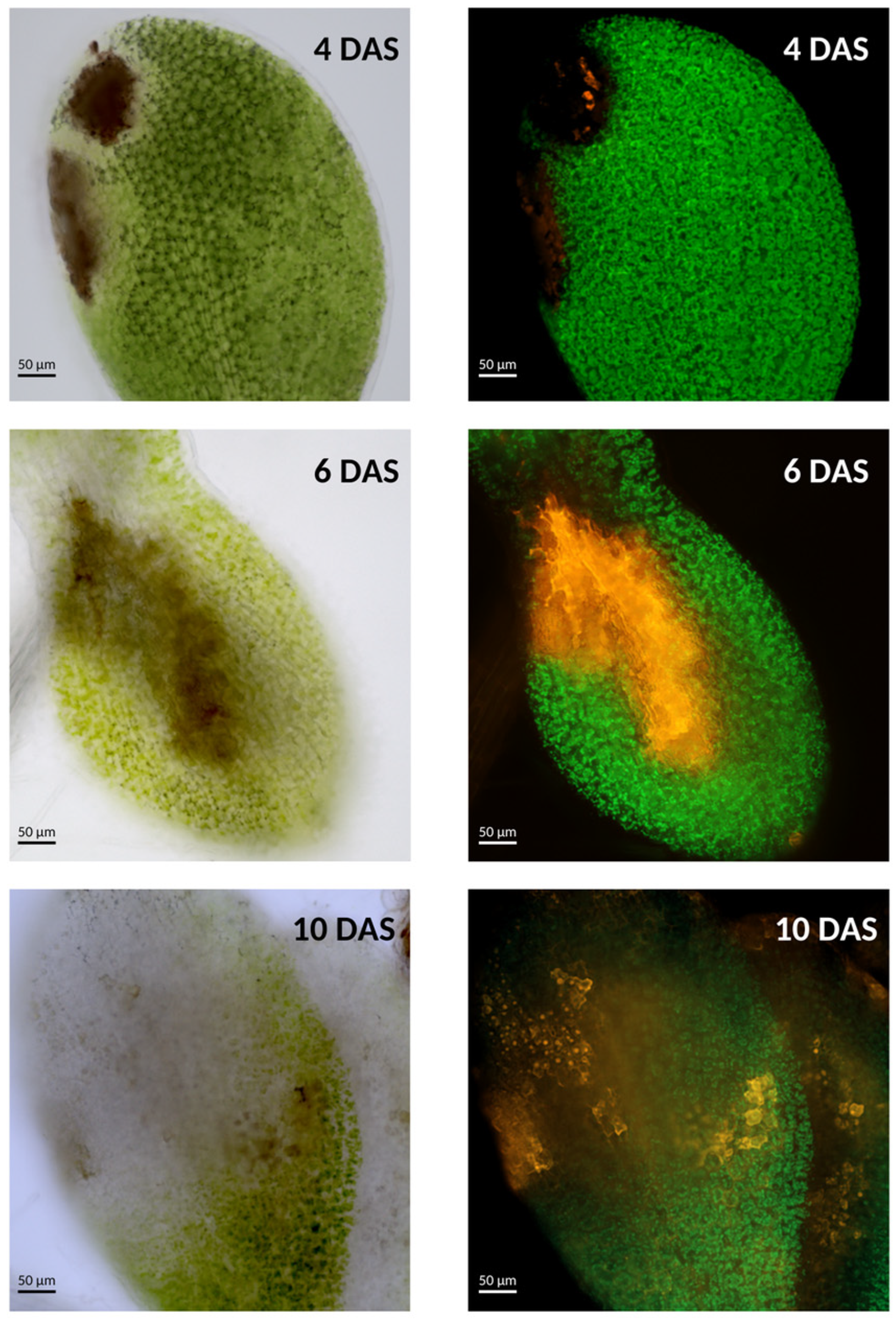
4.2. Interaction with the Necrotrophic Seed-Borne Fungi Alternaria brassicicola
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Gupta, S.; Van Staden, J.; Doležal, K. An Understanding of the Role of Seed Physiology for Better Crop Productivity and Food Security. Plant Growth Regul. 2022, 97, 171–173. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Biology Updates–Highlights and New Discoveries in Seed Dormancy and Germination Research. Front. Plant Sci. 2017, 8, 524. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Germination and Dormancy: The Classic Story, New Puzzles, and Evolution. J. Integr. Plant Biol. 2019, 61, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed dormancy and germination—Emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil. 2018, 422, 7–34. [Google Scholar] [CrossRef]
- War, A.F.; Bashir, I.; Reshi, Z.A.; Kardol, P.; Rashid, I. Insights into the Seed Microbiome and Its Ecological Significance in Plant Life. Microbiol. Res. 2023, 269, 127318. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Advances in Seed Science and Technology for More Sustainable Crop Production, 1st ed.; Buitink, J., Leprince, O., Eds.; Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Dell’Olmo, E.; Tiberini, A.; Sigillo, L. Leguminous Seedborne Pathogens: Seed Health and Sustainable Crop Management. Plants 2023, 12, 2040. [Google Scholar] [CrossRef]
- Cram, M.M.; Fraedrich, S.W. Seed Diseases and Seedborne Pathogens of North America. Tree Plant. Notes 2010, 53, 35–44. [Google Scholar]
- Kumar, R.; Gupta, A. Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R., Gupta, A., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Roberts Steven, J.; Aoife, O.; John, W. Brassica Diseases. Agriculture and Horticulture Development Board. Available online: https://bit.ly/3uOmXi9 (accessed on 26 February 2024).
- Gullino, M.L.; Munkvold, G. (Eds.) Global Perspectives on the Health of Seeds and Plant Propagation Material; Springer: Dordrecht, The Netherlands, 2014; Volume 6. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Divon, H.H.; Fluhr, R. Nutrition Acquisition Strategies during Fungal Infection of Plants. FEMS Microbiol. Lett. 2007, 266, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ghozlan, M.H.; EL-Argawy, E.; Tokgöz, S.; Lakshman, D.K.; Mitra, A. Plant Defense against Necrotrophic Pathogens. Am. J. Plant Sci. 2020, 11, 2122–2138. [Google Scholar] [CrossRef]
- Bolton, M.D. Primary Metabolism and Plant Defense—Fuel for the Fire. Mol. Plant-Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Łaźniewska, J.; Macioszek, V.K.; Kononowicz, A.K. Plant-Fungus Interface: The Role of Surface Structures in Plant Resistance and Susceptibility to Pathogenic Fungi. Physiol. Mol. Plant Pathol. 2012, 78, 24–30. [Google Scholar] [CrossRef]
- van Wees, S.C.M.; Chang, H.-S.; Zhu, T.; Glazebrook, J. Characterization of the Early Response of Arabidopsis to Alternaria Brassicicola Infection Using Expression Profiling. Plant Physiol. 2003, 132, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Pochon, S.; Terrasson, E.; Guillemette, T.; Iacomi-Vasilescu, B.; Georgeault, S.; Juchaux, M.; Berruyer, R.; Debeaujon, I.; Simoneau, P.; Campion, C. The Arabidopsis Thaliana-Alternaria Brassicicola Pathosystem: A Model Interaction for Investigating Seed Transmission of Necrotrophic Fungi. Plant Methods 2012, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Cuadros, M.; De Souza, T.L.; Berruyer, R.; Aligon, S.; Pelletier, S.; Renou, J.-P.; Arias, T.; Campion, C.; Guillemette, T.; Verdier, J.; et al. Seed Transmission of Pathogens: Non-Canonical Immune Response in Arabidopsis Germinating Seeds Compared to Early Seedlings against the Necrotrophic Fungus Alternaria Brassicicola. Plants 2022, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Łaźniewska, J.; Macioszek, V.K.; Lawrence, C.B.; Kononowicz, A.K. Fight to the Death: Arabidopsis Thaliana Defense Response to Fungal Necrotrophic Pathogens. Acta Physiol. Plant 2010, 32, 1–10. [Google Scholar] [CrossRef]
- Dalling, J.W.; Davis, A.S.; Arnold, A.E.; Sarmiento, C.; Zalamea, P.-C. Extending Plant Defense Theory to Seeds. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 123–141. [Google Scholar] [CrossRef]
- Fuerst, E.P.; Okubara, P.A.; Anderson, J.V.; Morris, C.F. Polyphenol Oxidase as a Biochemical Seed Defense Mechanism. Front. Plant Sci. 2014, 5, 117840. [Google Scholar] [CrossRef]
- Panstruga, R.; Moscou, M.J. What Is the Molecular Basis of Nonhost Resistance? Mol. Plant-Microbe Interact. 2020, 33, 1253–1264. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Jęcz, T.; Ciereszko, I.; Kononowicz, A.K. Jasmonic Acid as a Mediator in Plant Response to Necrotrophic Fungi. Cells 2023, 12, 1027. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant Antimicrobial Peptides: Structures, Functions, and Applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef]
- Stotz, H.U.; Sawada, Y.; Shimada, Y.; Hirai, M.Y.; Sasaki, E.; Krischke, M.; Brown, P.D.; Saito, K.; Kamiya, Y. Role of Camalexin, Indole Glucosinolates, and Side Chain Modification of Glucosinolate-Derived Isothiocyanates in Defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J. 2011, 67, 81–93. [Google Scholar] [CrossRef]
- L Schlaich, N. Arabidopsis Thaliana-the Model Plant to Study Host-Pathogen Interactions. Curr. Drug Targets 2011, 12, 955–966. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The Plant Hypersensitive Response: Concepts, Control and Consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef]
- Ádám, A.; Nagy, Z.; Kátay, G.; Mergenthaler, E.; Viczián, O. Signals of Systemic Immunity in Plants: Progress and Open Questions. Int. J. Mol. Sci. 2018, 19, 1146. [Google Scholar] [CrossRef]
- Conrath, U. Systemic Acquired Resistance. Plant Signal Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef]
- Sellam, A.; Iacomi-Vasilescu, B.; Hudhomme, P.; Simoneau, P. In Vitro Antifungal Activity of Brassinin, Camalexin and Two Isothiocyanates against the Crucifer Pathogens Alternaria Brassicicola and Alternaria Brassicae. Plant Pathol. 2007, 56, 296–301. [Google Scholar] [CrossRef]
- Gill, U.S.; Lee, S.; Mysore, K.S. Host Versus Nonhost Resistance: Distinct Wars with Similar Arsenals. Phytopathology 2015, 105, 580–587. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Panstruga, R. A Molecular Evolutionary Concept Connecting Nonhost Resistance, Pathogen Host Range, and Pathogen Speciation. Trends Plant Sci. 2011, 16, 117–125. [Google Scholar] [CrossRef]
- Nürnberger, T.; Lipka, V. Non-host Resistance in Plants: New Insights into an Old Phenomenon. Mol. Plant Pathol. 2005, 6, 335–345. [Google Scholar] [CrossRef]
- Patkar, R.N.; Naqvi, N.I. Fungal Manipulation of Hormone-Regulated Plant Defense. PLoS Pathog. 2017, 13, e1006334. [Google Scholar] [CrossRef]
- Shao, D.; Smith, D.L.; Kabbage, M.; Roth, M.G. Effectors of Plant Necrotrophic Fungi. Front. Plant Sci. 2021, 12, 995. [Google Scholar] [CrossRef]
- Frías, M.; González, C.; Brito, N. BcSpl1, a Cerato-Platanin Family Protein, Contributes to Botrytis Cinerea Virulence and Elicits the Hypersensitive Response in the Host. New Phytol. 2011, 192, 483–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a Glycoprotein from Botrytis Cinerea, Elicits Defence Response and Improves Disease Resistance in Host Plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef]
- El Oirdi, M.; El Rahman, T.A.; Rigano, L.; El Hadrami, A.; Rodriguez, M.C.; Daayf, F.; Vojnov, A.; Bouarab, K. Botrytis Cinerea Manipulates the Antagonistic Effects between Immune Pathways to Promote Disease Development in Tomato. Plant Cell 2011, 23, 2405–2421. [Google Scholar] [CrossRef]
- González-Lamothe, R.; El Oirdi, M.; Brisson, N.; Bouarab, K. The Conjugated Auxin Indole-3-Acetic Acid–Aspartic Acid Promotes Plant Disease Development. Plant Cell 2012, 24, 762–777. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tsers, I. Plant Susceptible Responses: The Underestimated Side of Plant–Pathogen Interactions. Biol. Rev. 2022, 97, 45–66. [Google Scholar] [CrossRef]
- German, D.A.; Hendriks, K.P.; Koch, M.A.; Lens, F.; Lysak, M.A.; Bailey, C.D.; Mummenhoff, K.; Al-Shehbaz, I.A. An Updated Classification of the Brassicaceae (Cruciferae). PhytoKeys 2023, 220, 127. [Google Scholar] [CrossRef]
- Rakow, G. Species Origin and Economic Importance of Brassica; Springer: Berlin/Heidelberg, Germany, 2004; Volume 54, pp. 3–11. [Google Scholar] [CrossRef]
- Cho, Y. How the Necrotrophic Fungus Alternaria Brassicicola Kills Plant Cells Remains an Enigma. Eukaryot. Cell 2015, 14, 335–344. [Google Scholar] [CrossRef]
- Dharmendra, K.; Neelam, M.; Yashwant, K.B.; Ajay, K.; Kamlesh, K.; Kalpana, S.; Gireesh, C.; Chanda, K.; Sushil, K.S.; Raj, K.M.; et al. Alternaria Blight of Oilseed Brassicas: A Comprehensive Review. Afr. J. Microbiol. Res. 2014, 8, 2816–2829. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-Host Interactions: New Insights on Its Pathogenesis, Defense Mechanisms and Control Strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria Pathogenicity and Its Strategic Controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Nowicki, M.; Nowakowska, M.; Niezgoda, A.; Kozik, E. Alternaria Black Spot of Crucifers: Symptoms, Importance of Disease, and Perspectives of Resistance Breeding. J. Fruit. Ornam. Plant Res. 2012, 76, 5–19. [Google Scholar] [CrossRef]
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria Toxins: Potential Virulence Factors and Genes Related to Pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Chumala, P.B.; Jin, W.; Islam, M.S.; Hauck, D.W. The Phytopathogenic Fungus Alternaria Brassicicola: Phytotoxin Production and Phytoalexin Elicitation. Phytochemistry 2009, 70, 394–402. [Google Scholar] [CrossRef]
- Lawrence, C.B.; Mitchell, T.K.; Craven, K.D.; Cho, Y.-R.; Cramer, R.A.; Kim, K.-H. At Death’s Door: Alternaria Pathogenicity Mechanisms. Plant Pathol. J. 2008, 24, 101–111. [Google Scholar] [CrossRef]
- Darrasse, A.; Darsonval, A.; Boureau, T.; Brisset, M.-N.; Durand, K.; Jacques, M.-A. Transmission of Plant-Pathogenic Bacteria by Nonhost Seeds without Induction of an Associated Defense Reaction at Emergence. Appl. Environ. Microbiol. 2010, 76, 6787–6796. [Google Scholar] [CrossRef]
- Darsonval, A.; Darrasse, A.; Meyer, D.; Demarty, M.; Durand, K.; Bureau, C.; Manceau, C.; Jacques, M.-A. The Type III Secretion System of Xanthomonas Fuscans Subsp. Fuscans Is Involved in the Phyllosphere Colonization Process and in Transmission to Seeds of Susceptible Beans. Appl. Environ. Microbiol. 2008, 74, 2669–2678. [Google Scholar] [CrossRef]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the Testa on Seed Dormancy, Germination, and Longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The Role of the Testa during Development and in Establishment of Dormancy of the Legume Seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Endo, A.; Tatematsu, K.; Hanada, K.; Duermeyer, L.; Okamoto, M.; Yonekura-Sakakibara, K.; Saito, K.; Toyoda, T.; Kawakami, N.; Kamiya, Y.; et al. Tissue-Specific Transcriptome Analysis Reveals Cell Wall Metabolism, Flavonol Biosynthesis and Defense Responses Are Activated in the Endosperm of Germinating Arabidopsis Thaliana Seeds. Plant Cell Physiol. 2012, 53, 16–27. [Google Scholar] [CrossRef]
- Ortega-Cuadros, M.; Chir, L.; Aligon, S.; Arias, T.; Verdier, J.; Grappin, P. Dual-Transcriptomic Datasets Evaluating the Effect of the Necrotrophic Fungus Alternaria Brassicicola on Arabidopsis Germinating Seeds. Data Brief. 2022, 44, 108530. [Google Scholar] [CrossRef]
- Chung, H.; Lee, Y.-H. Hypoxia: A Double-Edged Sword During Fungal Pathogenesis? Front. Microbiol. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Tao, H.; Miao, H.; Chen, L.; Wang, M.; Xia, C.; Zeng, W.; Sun, B.; Zhang, F.; Zhang, S.; Li, C.; et al. WRKY33-mediated Indolic Glucosinolate Metabolic Pathway Confers Resistance against Alternaria Brassicicola in Arabidopsis and Brassica Crops. J. Integr. Plant Biol. 2022, 64, 1007–1019. [Google Scholar] [CrossRef]
- Horton, M.W.; Hancock, A.M.; Huang, Y.S.; Toomajian, C.; Atwell, S.; Auton, A.; Muliyati, N.W.; Platt, A.; Sperone, F.G.; Vilhjálmsson, B.J.; et al. Genome-Wide Patterns of Genetic Variation in Worldwide Arabidopsis Thaliana Accessions from the RegMap Panel. Nat. Genet. 2012, 44, 212–216. [Google Scholar] [CrossRef]
- Koornneef, M.; Alonso-Blanco, C.; Vreugdenhil, D. Naturally Occurring Genetic Variation in Arabidopsis Thaliana. Annu. Rev. Plant Biol. 2004, 55, 141–172. [Google Scholar] [CrossRef]
- Somerville, C.; Koornneef, M. A Fortunate Choice: The History of Arabidopsis as a Model Plant. Nat. Rev. Genet. 2002, 3, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, L.A.; Shushkov, P.; Nevado, B.; Gan, X.; Al-Shehbaz, I.A.; Filatov, D.; Bailey, C.D.; Tsiantis, M. Resolving the Backbone of the Brassicaceae Phylogeny for Investigating Trait Diversity. New Phytol. 2019, 222, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
| Host | Pathogen-Induced Plant Response Pathway | Reference |
|---|---|---|
| Solanum lycopersicum (tomato), Nicotiana tabacum (tobacco), and Arabidopsis thaliana (Arabidopsis) | Programmed cell death (PCD) | Frías et al. [41] Zhang et al. [42] |
| Solanum lycopersicum (tomato) | SA pathway | El Oirdi M et al. [43] |
| Arabidopsis thaliana (Arabidopsis) | Auxin pathway | González-Lamothe R et al. [44] |
| Solanum lycopersicum (tomato) and Arabidopsis thaliana (Arabidopsis) | Peroxiredoxin (oxidative stress-related gene), mitogen-activated protein kinases (MPK1, MPK2, MPKKK4), and a cell wall-associated kinase (WAK) | Weiberg et al. [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Cuadros, M.; Aligon, S.; Arias, T.; Vasco-Palacios, A.M.; Rosier--Pennevert, C.; Guschinskaya, N.; Rolland, A.; Grappin, P. Fungal Necrotrophic Interaction: A Case Study of Seed Immune Response to a Seed-Borne Pathogen. Seeds 2024, 3, 216-227. https://doi.org/10.3390/seeds3020017
Ortega-Cuadros M, Aligon S, Arias T, Vasco-Palacios AM, Rosier--Pennevert C, Guschinskaya N, Rolland A, Grappin P. Fungal Necrotrophic Interaction: A Case Study of Seed Immune Response to a Seed-Borne Pathogen. Seeds. 2024; 3(2):216-227. https://doi.org/10.3390/seeds3020017
Chicago/Turabian StyleOrtega-Cuadros, Mailen, Sophie Aligon, Tatiana Arias, Aída M. Vasco-Palacios, Cassandre Rosier--Pennevert, Natalia Guschinskaya, Aurélia Rolland, and Philippe Grappin. 2024. "Fungal Necrotrophic Interaction: A Case Study of Seed Immune Response to a Seed-Borne Pathogen" Seeds 3, no. 2: 216-227. https://doi.org/10.3390/seeds3020017
APA StyleOrtega-Cuadros, M., Aligon, S., Arias, T., Vasco-Palacios, A. M., Rosier--Pennevert, C., Guschinskaya, N., Rolland, A., & Grappin, P. (2024). Fungal Necrotrophic Interaction: A Case Study of Seed Immune Response to a Seed-Borne Pathogen. Seeds, 3(2), 216-227. https://doi.org/10.3390/seeds3020017







