Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives
Abstract
:1. Introduction
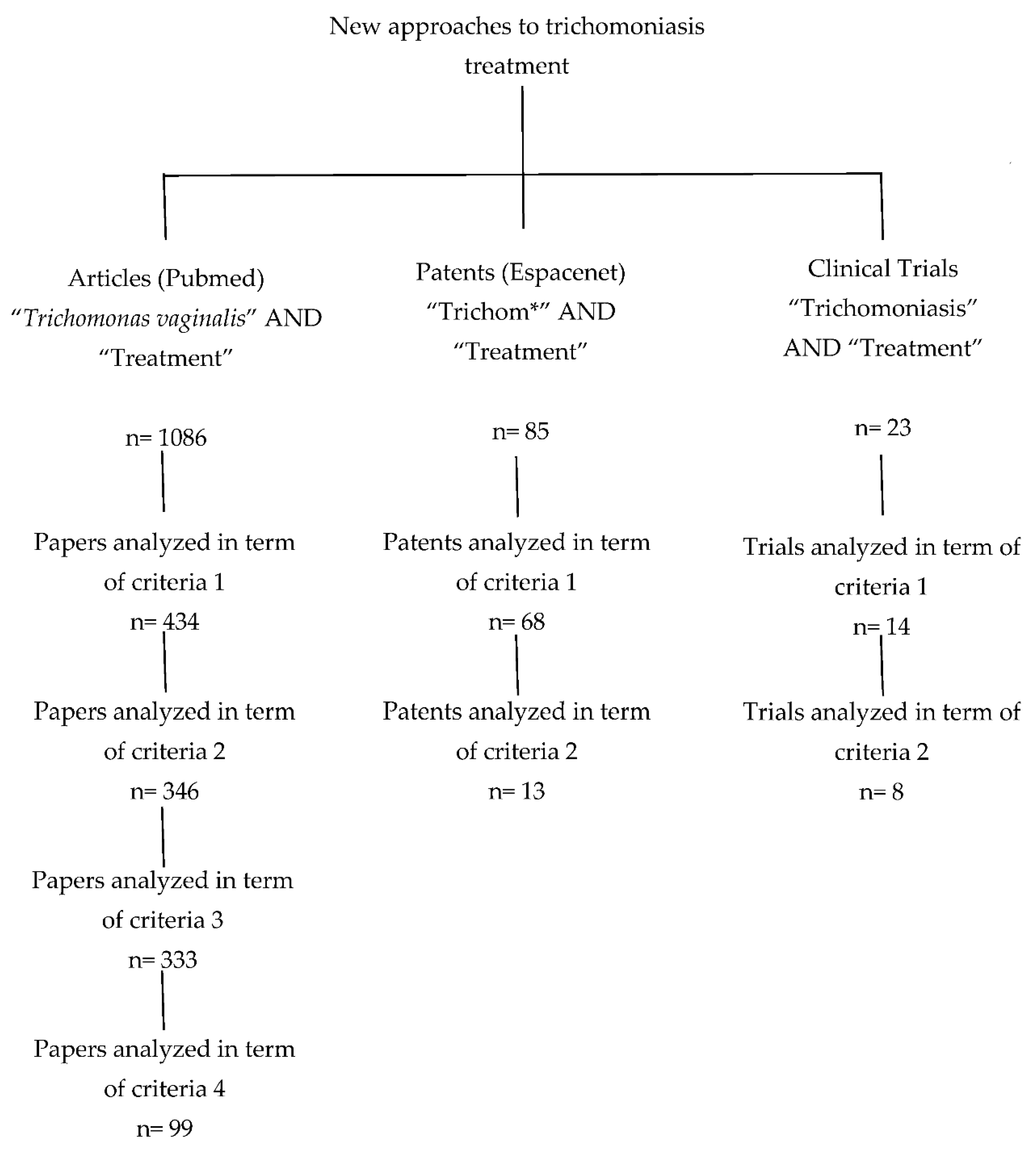
2. Methodology
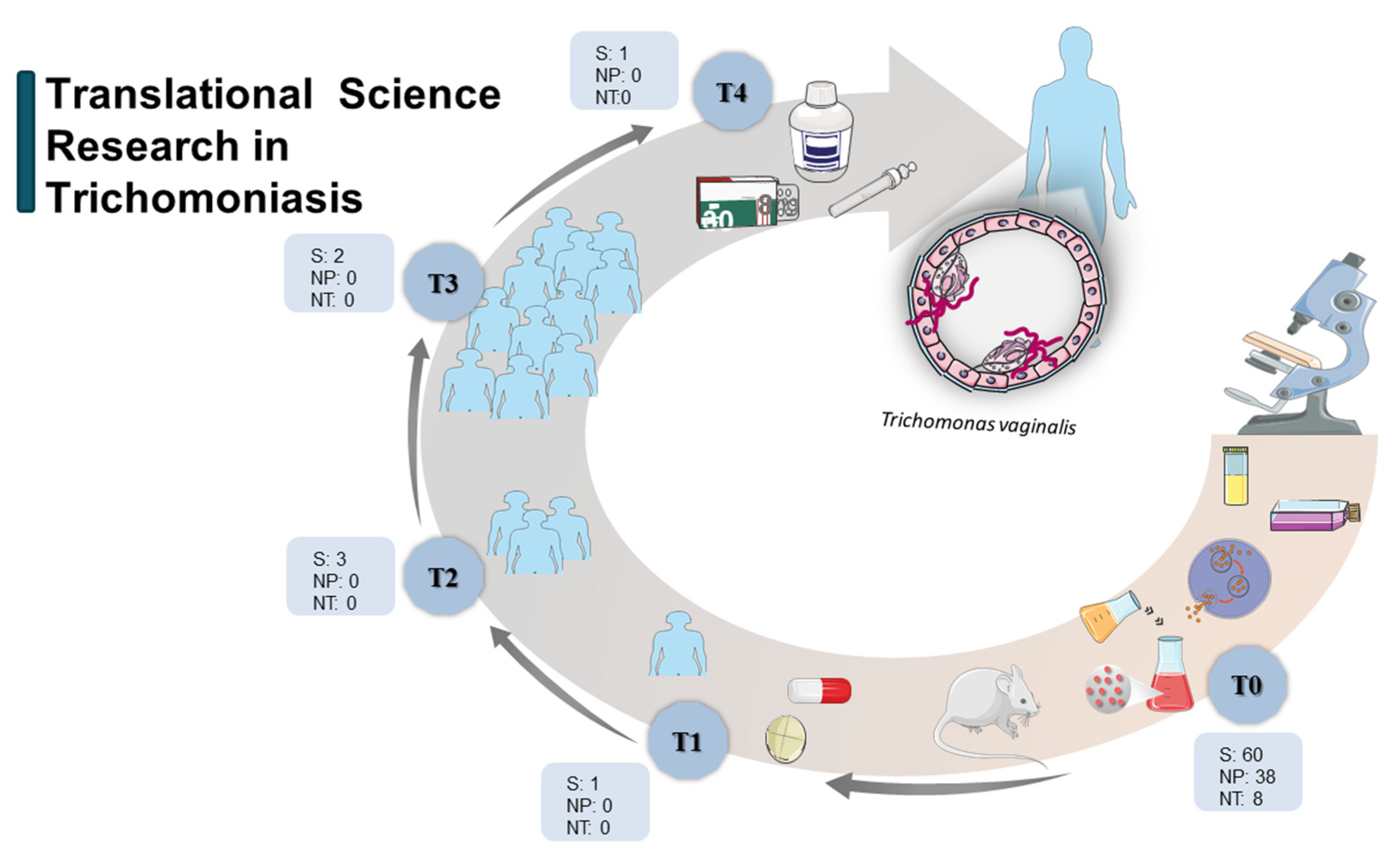
3. Results and Discussion
3.1. New Scientific Approaches from Basic Research (Articles)
3.1.1. Articles: Synthetic Compounds
3.1.2. Articles: Natural Products
3.1.3. Articles: Nanotechnology
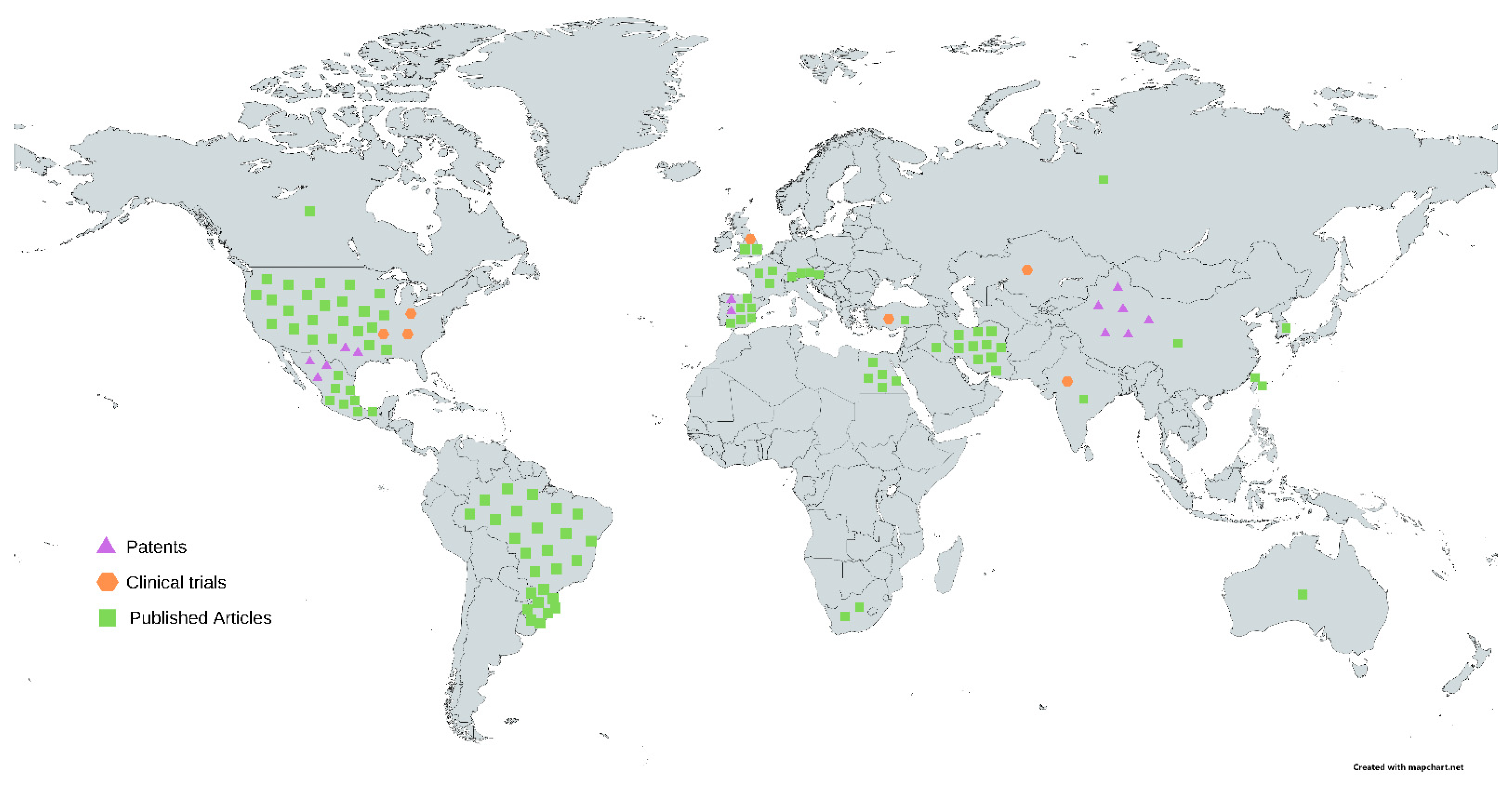
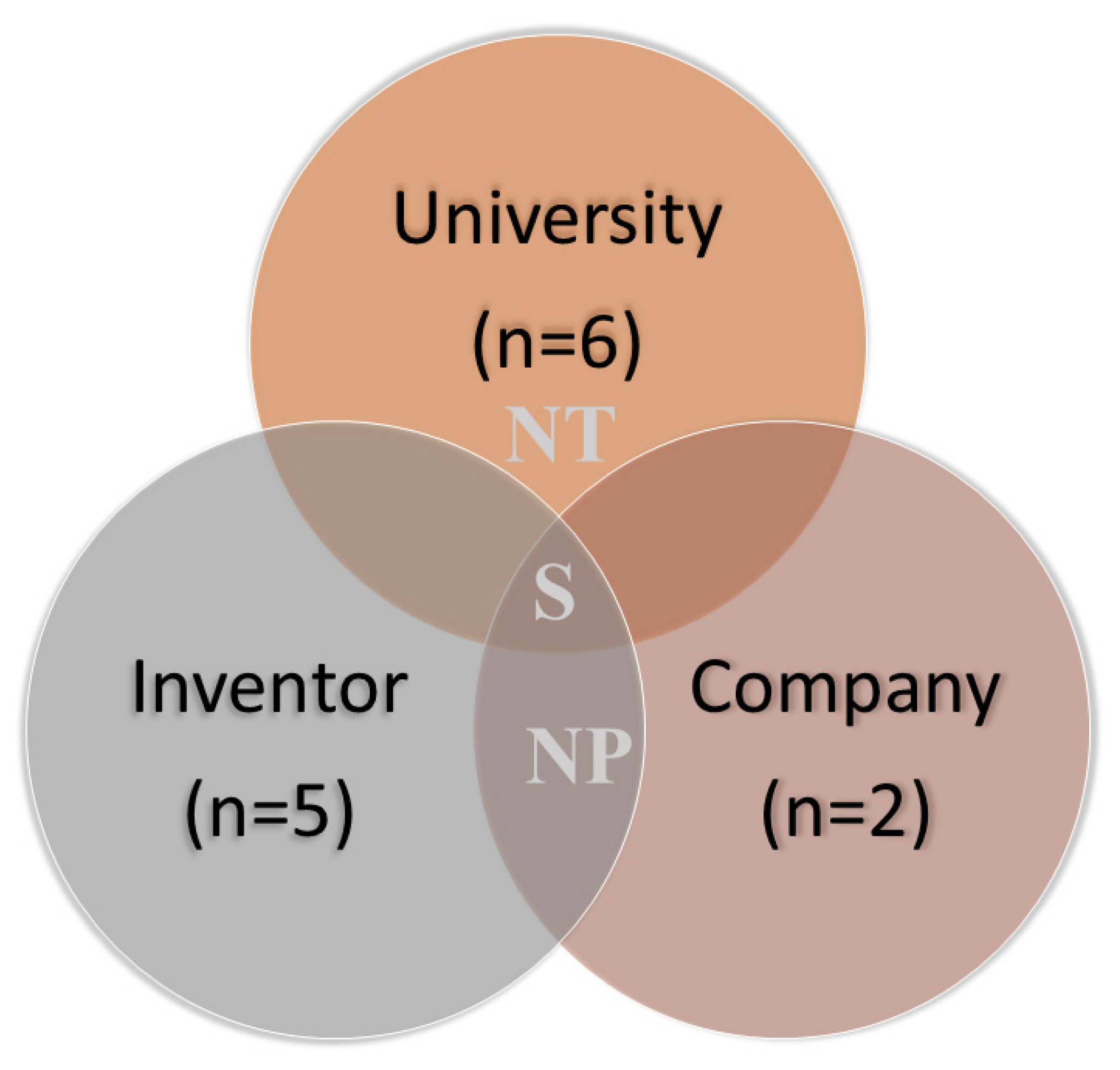
3.2. Technological Prospecting: Patent Searching and Screening
3.2.1. Patents: Synthetic Compounds
3.2.2. Patents: Natural Products
3.2.3. Patents: Nanotechnology
3.3. Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin, C. Translating translation. Nat. Rev. Drug Discov. 2018, 17, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.P. Opportunities and challenges in translational science. Clin. Transl. Sci. 2021, 14, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Hostiuc, S.; Moldoveanu, A.; Dascălu, M.I.; Unnthorsson, R.; Jóhannesson, Ó.I.; Marcus, I. Translational research-the need of a new bioethics approach. J. Transl. Med. 2016, 14, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linton, J.D.; Xu, W. Understanding and managing the biotechnology valley of death. Trends Biotechnol. 2021, 39, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Secor, W.E.; Meites, E.; Starr, M.C.; Workowski, K.A. Neglected parasitic infections in the United States: Trichomoniasis. Am. J. Trop. Med. Hyg. 2014, 90, 800–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.B.; Frasson, A.P.; Tasca, T. Trichomoniasis—Are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb. Cell 2016, 3, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, I.; Mandal, R.; Kundu, P.; Biswas, J. Association of Genital Infections Other Than Human Papillomavirus with Pre-Invasive and Invasive Cervical Neoplasia. J. Clin. Diagn. Res. 2016, 10, XE01–XE06. [Google Scholar] [CrossRef]
- Masha, S.C.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T. Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2019, 95, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Vieira, P.B.; Tasca, T.; Secor, W.E. Challenges and Persistent Questions in the Treatment of Trichomoniasis. Curr. Top. Med. Chem. 2017, 17, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Marques-Silva, M.; Lisboa, C.; Gomes, N.; Rodrigues, A.G. Trichomonas vaginalis and growing concern over drug resistance: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2007–2021. [Google Scholar] [CrossRef]
- Wagner, J.; Dahlem, A.M.; Hudson, L.D.; Terry, S.F.; Altman, R.B.; Gilliland, C.T.; DeFeo, C.; Austin, C.P. A dynamic map for learning, communicating, navigating and improving therapeutic development. Nat. Rev. Drug Discov. 2018, 17, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, J.A.; Dahlem, A.M.; Hudson, L.D.; Terry, S.F.; Altman, R.B.; Gilliland, C.T.; DeFeo, C.; Austin, C.P. Application of a Dynamic Map for Learning, Communicating, Navigating, and Improving Therapeutic Development. Clin. Transl. Sci. 2018, 11, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Aggarwal, S.; Celaje, J.; Ihara, S.; Ang, J.; Eremin, D.B.; Land, K.M.; Wrischnik, L.A.; Zhang, L.; Fokin, V.V.; et al. Gold(I) Phosphine Derivatives with Improved Selectivity as Topically Active Drug Leads to Overcome 5-Nitroheterocyclic Drug Resistance in Trichomonas vaginalis. J. Med. Chem. 2021, 64, 6608–6620. [Google Scholar] [CrossRef]
- Rigo, G.V.; Petro-Silveira, B.; Devereux, M.; McCann, M.; Souza Dos Santos, A.L.; Tasca, T. Anti-Trichomonas vaginalis activity of 1,10-phenanthroline-5,6-dione-based metallodrugs and synergistic effect with metronidazole. Parasitology 2019, 146, 1179–1183. [Google Scholar] [CrossRef]
- Sena-Lopes, Â.; Neves, R.N.; Bezerra, F.; Oliveira Silva, M.T.; Nobre, P.C.; Perin, G.; Alves, D.; Savegnago, L.; Begnini, K.R.; Seixas, F.K.; et al. Antiparasitic activity of 1,3-dioxolanes containing tellurium in Trichomonas vaginalis. Biomed. Pharmacother. 2017, 89, 284–287. [Google Scholar] [CrossRef]
- Silva, C.; Pacheco, B.S.; Neves, R.; Dié Alves, M.S.; Sena-Lopes, Â.; Moura, S.; Borsuk, S.; de Pereira, C. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibanez-Escribano, A.; Reviriego, F.; Cumella, J.; Morales, P.; Jagerovic, N.; Arán, V.J. Antichagasic and trichomonacidal activity of 1-substituted 2-benzyl-5-nitroindazolin-3-ones and 3-alkoxy-2-benzyl-5-nitro-2H-indazoles. Eur. J. Med. Chem. 2016, 115, 295–310. [Google Scholar] [CrossRef]
- Korosh, T.; Bujans, E.; Morada, M.; Karaalioglu, C.; Vanden, E.J.J.; Mayence, A.; Huang, T.L.; Yarlett, N. Potential of bisbenzimidazole-analogs toward metronidazole-resistant Trichomonas vaginalis isolates. Chem. Biol. Drug Des. 2017, 90, 489–495. [Google Scholar] [CrossRef]
- Weber, J.I.; Rigo, G.V.; Rocha, D.A.; Fortes, I.S.; Seixas, A.; de Andrade, S.F.; Tasca, T. Modulation of peptidases by 2,4-diamine-quinazoline derivative induces cell death in the amitochondriate parasite Trichomonas vaginalis. Biomed. Pharmacother. 2021, 139, 111611. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.N.; Sena-Lopes, Â.; Alves, M.; da Rocha Fonseca, B.; da Silva, C.C.; Casaril, A.M.; Savegnago, L.; de Pereira, C.; Ramos, D.F.; Borsuk, S. 2′-Hydroxychalcones as an alternative treatment for trichomoniasis in association with metronidazole. Parasitol. Res. 2020, 119, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Berzal, C.; Ibañez-Escribano, A.; Vela, N.; Cumella, J.; Nogal-Ruiz, J.J.; Escario, J.A.; Aran, V.J. Antichagasic, Leishmanicidal, and Trichomonacidal Activity of 2-Benzyl-5-nitroindazole-Derived Amines. Chem. Med. Chem. 2018, 13, 1246–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, D.A.; de Andrade Rosa, I.; Urbina, J.A.; de Souza, W.; Benchimol, M. The effect of 3-(biphenyl-4-yl)-3-hydoxyquinuclidine (BPQ-OH) and metronidazole on Trichomonas vaginalis: A comparative study. J. Parasitol. Res. 2014, 113, 2185–2197. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Reviriego, F.; Vela, N.; Fonseca-Berzal, C.; Nogal-Ruiz, J.J.; Arán, V.J.; Escario, J.A.; Gómez-Barrio, A. Promising hit compounds against resistant trichomoniasis: Synthesis and antiparasitic activity of 3-(omega-aminoalkoxy)-1-benzyl-5-nitroindazoles. Bioorg. Med. Chem. Lett. 2021, 37, 127843. [Google Scholar] [CrossRef]
- Benítez-Cardoza, C.G.; Brieba, L.G.; Arroyo, R.; Vique-Sánchez, J. Triosephosphate isomerase as a therapeutic target against trichomoniasis. Mol. Biochem. Parasitol. 2021, 246, 111413. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Nogal-Ruiz, J.J.; Gómez-Barrio, A.; Arán, V.J.; Escario, J.A. In vitro trichomonacidal activity and preliminary in silico chemometric studies of 5-nitroindazolin-3-one and 3-alkoxy-5-nitroindazole derivatives. Parasitology 2016, 143, 34–40. [Google Scholar] [CrossRef]
- Trein, M.R.; Rodrigues EOliveira, L.; Rigo, G.V.; Garcia, M.; Petro-Silveira, B.; da Silva Trentin, D.; Macedo, A.J.; Regasini, L.O.; Tasca, T. Anti-Trichomonas vaginalis activity of chalcone and amino-analogues. Parasitol. Res. 2019, 118, 607–615. [Google Scholar] [CrossRef]
- Bitencourt, F.G.; de Brum Vieira, P.; Meirelles, L.C.; Rigo, G.V.; da Silva, E.F.; Gnoatto, S.; Tasca, T. Anti-Trichomonas vaginalis activity of ursolic acid derivative: A promising alternative. Parasitol. Res. 2018, 117, 1573–1580. [Google Scholar] [CrossRef]
- Singh, A.; Nisha; Bains, T.; Hahn, H.J.; Liu, N.; Tam, C.; Cheng, L.W.; Kim, J.; Debnath, A.; Land, K.M.; et al. Design, Synthesis and Preliminary Antimicrobial Evaluation of N-Alkyl Chain Tethered C-5 Functionalized Bis-Isatins. MedChemComm 2017, 8, 1982–1992. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 4221–4224. [Google Scholar] [CrossRef] [PubMed]
- Natto, M.J.; Hulpia, F.; Kalkman, E.R.; Baillie, S.; Alhejeli, A.; Miyamoto, Y.; Eckmann, L.; Van Calenbergh, S.; Koning, H.P. Deazapurine Nucleoside Analogues for the Treatment of Trichomonas vaginalis. ACS Infect. Dis. 2021, 7, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Escribano, A.; Reviriego, F.; Nogal-Ruiz, J.J.; Meneses-Marcel, A.; Gómez-Barrio, A.; Escario, J.A.; Arán, V.J. Synthesis and in vitro and in vivo biological evaluation of substituted nitroquinoxalin-2-ones and 2,3-diones as novel trichomonacidal agents. Eur. J. Med. Chem. 2015, 94, 276–283. [Google Scholar] [CrossRef]
- Shokar, A.; Au, A.; An, S.H.; Tong, E.; Garza, G.; Zayas, J.; Wnuk, S.F.; Land, K.M. S-Adenosylhomocysteine hydrolase of the protozoan parasite Trichomonas vaginalis: Potent inhibitory activity of 9-(2-deoxy-2-fluoro-β,D-arabinofuranosyl)adenine. Bioorg. Med. Chem. Lett. 2012, 22, 4203–4205. [Google Scholar] [CrossRef] [PubMed]
- Vique-Sánchez, J.L.; Caro-Gómez, L.A.; Brieba, L.G. Developing a new drug against trichomoniasis, new inhibitory compounds of the protein triosephosphate isomerase. Parasitol. Int. 2020, 76, 102086. [Google Scholar] [CrossRef] [PubMed]
- Hopper, M.; Yun, J.F.; Zhou, B.; Le, C.; Kehoe, K.; Le, R.; Hill, R.; Jongeward, G.; Debnath, A.; Zhang, L.; et al. Auranofin inactivates Trichomonas vaginalis thioredoxin reductase and is effective against trichomonads in vitro and in vivo. Antimicrob. Agents 2016, 48, 690–694. [Google Scholar] [CrossRef] [Green Version]
- Hübner, D.; de Brum Vieira, P.; Frasson, A.P.; Menezes, C.B.; Senger, F.R.; Santos da Silva, G.N.; Baggio Gnoatto, S.C.; Tasca, T. Anti-Trichomonas vaginalis activity of betulinic acid derivatives. Biomed. Pharmacother. 2016, 84, 476–484. [Google Scholar] [CrossRef]
- Brittingham, A.; Wilson, W.A. The antimicrobial effect of boric acid on Trichomonas vaginalis. Sex. Transm. Dis. 2014, 41, 718–722. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kalisiak, J.; Korthals, K.; Lauwaet, T.; Cheung, D.Y.; Lozano, R.; Cobo, E.R.; Upcroft, P.; Upcroft, J.A.; Berg, D.E.; et al. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc. Natl. Acad. Sci. USA 2013, 110, 17564–17569. [Google Scholar] [CrossRef] [Green Version]
- Chacon, M.O.; Fonseca, T.; Oliveira, S.; Alacoque, M.A.; Franco, L.L.; Tagliati, C.A.; Cassali, G.D.; Campos-Mota, G.P.; Alves, R.J.; Capettini, L.; et al. Chlorinated metronidazole as a promising alternative for treating trichomoniasis. Parasitol. Res. 2018, 117, 1333–1340. [Google Scholar] [CrossRef]
- Gumbo, M.; Beteck, R.M.; Mandizvo, T.; Seldon, R.; Warner, D.F.; Hoppe, H.C.; Isaacs, M.; Laming, D.; Tam, C.C.; Cheng, L.W.; et al. Cinnamoyl-Oxaborole Amides: Synthesis and Their in Vitro Biological Activity. Molecules 2018, 23, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigo, G.V.; Trein, M.R.; Trentin, D.S.; Macedo, A.J.; Oliveira, B.A.; de Almeida, A.M.; Giordani, R.B.; de Almeida, M.V.; Tasca, T. Diamine derivative anti-Trichomonas vaginalis and anti-Tritrichomonas foetus activities by effect on polyamine metabolism. Biomed. Pharmacother. 2017, 95, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Goodhew, E.B.; Secor, W.E. Drug library screening against metronidazole-sensitive and metronidazole-resistant Trichomonas vaginalis isolates. Sex. Transm. Infect. 2013, 89, 479–484. [Google Scholar] [CrossRef]
- Lam, A.Y.F.; Vuong, D.; Jex, A.R.; Piggott, A.M.; Lacey, E.; Emery-Corbin, S.J. TriTOX: A novel Trichomonas vaginalis assay platform for high-throughput screening of compound libraries. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; das Neves, R.N.; Sena-Lopes, Â.; Domingues, M.; Casaril, A.M.; Segatto, N.V.; Nogueira, T.; de Souza, M.; Savegnago, L.; Seixas, F.K.; et al. Antiparasitic activity of furanyl N-acylhydrazone derivatives against Trichomonas vaginalis: In vitro and in silico analyses. Parasit. Vectors 2020, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Villanueva, J.; Romo-Mancillas, A.; Hernández-Campos, A.; Yépez-Mulia, L.; Hernández-Luis, F.; Castillo, R. Antiprotozoal activity of proton-pump inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 7351–7354. [Google Scholar] [CrossRef]
- Malli, S.; Bories, C.; Pradines, B.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. In situ forming pluronic® F127/chitosan hydrogel limits metronidazole transmucosal absorption. Eur. J. Pharm. Biopharm. 2017, 112, 143–147. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Gilbert, J.; Mulcahy, L.J. Resistant Trichomoniasis: Successful Treatment with Combination Therapy. Sex. Transm. Dis. 2011, 38, 962–963. [Google Scholar] [CrossRef] [Green Version]
- Henien, M.; Nyirjesy, P.; Smith, K. Metronidazole-Resistant Trichomoniasis: Beneficial Pharmacodynamic Relationship with High-Dose Oral Tinidazole and Vaginal Paromomycin Combination Therapy. Sex. Transm. Dis. 2019, 46, e1–e2. [Google Scholar] [CrossRef]
- Kissinger, P.; Muzny, C.A.; Mena, L.A.; Lillis, R.A.; Schwebke, J.R.; Beauchamps, L.; Taylor, S.N.; Schmidt, N.; Myers, L.; Augostini, P.; et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 1251–1259. [Google Scholar] [CrossRef]
- Muzny, C.A.; Mena, L.A.; Lillis, R.A.; Schmidt, N.; Martin, D.H.; Kissinger, P. A Comparison of Single versus Multi-Dose Metronidazole by Select Clinical Factors for the Treatment of Trichomonas vaginalis in Women. Sex. Transm. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Lensing, S.Y.; Sobel, J. Intravaginal metronidazole/miconazole for the treatment of vaginal trichomoniasis. Sex. Transm. Dis. 2013, 40, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Malli, S.; Bories, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Phase solubility studies and anti-Trichomonas vaginalis activity evaluations of metronidazole and methylated β-cyclodextrin complexes: Comparison of CRYSMEB and RAMEB. Exp. Parasitol. 2018, 189, 72–75. [Google Scholar] [CrossRef]
- Rocha, D.A.; de Andrade Rosa, I.; de Souza, W.; Benchimol, M. Evaluation of the effect of miltefosine on Trichomonas vaginalis. J. Parasitol. Res. 2014, 113, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, A.A.; Hammouda, M.M.; Mosbah, A.; El-Henawy, A.A. In vitro activity of nitazoxanide against some metronidazole-resistant and susceptible Trichomonas vaginalis isolates. J. Infect. Chemother. 2017, 23, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Jarrad, A.M.; Debnath, A.; Miyamoto, Y.; Hansford, K.A.; Pelingon, R.; Butler, M.S.; Bains, T.; Karoli, T.; Blaskovich, M.A.; Eckmann, L.; et al. Nitroimidazole carboxamides as antiparasitic agents targeting Giardia lamblia, Entamoeba histolytica and Trichomonas vaginalis. Eur. J. Med. Chem. 2016, 120, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Fürnkranz, U.; Nagl, M.; Gottardi, W.; Duchêne, M.; Aspöck, H.; Walochnik, J. In vitro activity of N-chlorotaurine (NCT) in combination with NH4Cl against Trichomonas vaginalis. Int. J. Antimicrob. Agents 2011, 37, 171–173. [Google Scholar] [CrossRef]
- Küng, E.; Pietrzak, J.; Klaus, C.; Walochnik, J. In vitro effect of octenidine dihydrochloride against Trichomonas vaginalis. Int. J. Antimicrob. Agents 2016, 47, 232–234. [Google Scholar] [CrossRef]
- Fonseca, T.H.; Gomes, J.M.; Alacoque, M.; Vannier-Santos, M.A.; Gomes, M.A.; Busatti, H.G. Transmission electron microscopy revealing the mechanism of action of photodynamic therapy on Trichomonas vaginalis. Acta Trop. 2019, 190, 112–118. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R.; Nyirjesy, P.; Kaufman, G.; Mena, L.A.; Lazenby, G.B.; Van Gerwen, O.T.; Graves, K.J.; Arbuckle, J.; Carter, B.A.; et al. Efficacy and Safety of Single Oral Dosing of Secnidazole for Trichomoniasis in Women: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled, Delayed-Treatment Study. Clin. Infect. Dis. 2021, 73, e1282–e1289. [Google Scholar] [CrossRef]
- Ghosh, A.P.; Aycock, C.; Schwebke, J.R. In Vitro Study of the Susceptibility of Clinical Isolates of Trichomonas vaginalis to Metronidazole and Secnidazole. Antimicrob. Agents Chemother. 2018, 62, e02329-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.Y.; Ku, F.M.; Cheng, W.H.; Lee, C.C.; Huang, P.J.; Chu, L.J.; Cheng, C.C.; Fang, Y.K.; Wu, H.H.; Tang, P. Novel insights into the molecular events linking to cell death induced by tetracycline in the amitochondriate protozoan Trichomonas vaginalis. Antimicrob. Agents Chemother. 2015, 59, 6891–6903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, I.; Carne, C.; Sonnex, C.; Carmichael, A. Successful treatment of refractory Trichomonas vaginalis infection using intravenous metronidazole. Int. J. STD AIDS 2015, 26, 676–678. [Google Scholar] [CrossRef]
- Butt, S.; Tirmizi, A. Intravenous metronidazole, liquid tinidazole, and intra-vaginal boric acid to cure trichomonas in a patient with gastric bypass surgery. Int. J. STD AIDS 2018, 29, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Midlej, V.; Rubim, F.; Villarreal, W.; Martins-Duarte, É.S.; Navarro, M.; de Souza, W.; Benchimol, M. Zinc-clotrimazole complexes are effective against Trichomonas vaginalis. Parasitology 2019, 146, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Jeong, D.H.; Kim, Y.N.; Lee, K.B.; Sung, M.S.; Kim, K.T. Experience of successful treatment of patients with metronidazole-resistant Trichomonas vaginalis with zinc sulfate: A case series. Taiwan. J. Obstet. Gynecol. 2015, 54, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.; Peng, C.; Peng, F.; Xie, C.; Wang, P.; Sun, F. Anti-Trichomonas vaginalis properties of the oil of Amomum tsao-ko and its major component, geraniol. Pharm. Biol. 2016, 54, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Castro, A.J.; Arana-Argáez, V.; Yáñeez-Barrientos, E.; Torres-Romero, J.C.; Chable-Cetz, R.J.; Worbel, K.; Euan-Canto, A.J.; Wrobel, K.; González-Ibarra, A.; Solorio-Alvarado, C.R.; et al. Pharmacological activities of Asclepias curassavica L. (Apocynaceae) aerial parts. J. Ethnopharmacol. 2021, 281, 114554. [Google Scholar] [CrossRef]
- Duarte, M.; Seixas, A.; Peres de Carvalho, M.; Tasca, T.; Macedo, A.J. Amaurocine: Anti-Trichomonas vaginalis protein produced by the basidiomycete Amauroderma camerarium. Exp. Parasitol. 2016, 161, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, D.; Van-Vuuren, S.F.; Van-Zyl, R.L.; Wet, H. Plants traditionally used individually and in combination to treat sexually transmitted infections in northern Maputaland, South Africa: Antimicrobial activity and cytotoxicity. J. Ethnopharmacol. 2013, 149, 656–667. [Google Scholar] [CrossRef]
- Fakhrieh-Kashan, Z.; Arbabi, M.; Delavari, M.; Mohebali, M.; Hooshyar, H. Induction of Apoptosis by Alcoholic Extract of Combination Verbascum thapsus and Ginger officinale on Iranian Isolate of Trichomonas vaginalis. Iran. J. Parasitol. 2018, 13, 72. [Google Scholar] [PubMed]
- El-Sherbiny, G.M.; El Sherbiny, E.T. The Effect of Commiphora molmol (Myrrh) in Treatment of Trichomoniasis vaginalis infection. Iran. Red Crescent Med. J. 2011, 13, 480–486. [Google Scholar] [PubMed]
- Wachter, B.; Syrowatka, M.; Obwaller, A.; Walochnik, J. In vitro efficacy of curcumin on Trichomonas vaginalis. Wien. Klin. Wochenschr. 2014, 126, S32–S36. [Google Scholar] [CrossRef]
- Mallo, N.; Lamas, J.; Sueiro, R.A.; Leiro, J.M. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules 2020, 25, 5321. [Google Scholar] [CrossRef] [PubMed]
- Korosh, T.; Jordan, K.D.; Wu, J.S.; Yarlett, N.; Upmacis, R.K. Eicosapentaenoic Acid Modulates Trichomonas vaginalis Activity. J. Eukaryot. Microbiol. 2016, 63, 153–161. [Google Scholar] [CrossRef]
- Huang, H.N.; Chuang, C.M.; Chen, J.Y.; Chieh-Yu, P. Epinecidin-1: A marine fish antimicrobial peptide with therapeutic potential against Trichomonas vaginalis infection in mice. Peptides 2019, 112, 139–148. [Google Scholar] [CrossRef]
- Youse, H.A.; Kazemian, A.; Sereshti, M.; Rahmanikhoh, E.; Ahmadinia, E.; Rafaian, M.; Maghsoodi, R.; Darani, H.Y. Effect of Echinophora platyloba, Stachys lavandulifolia, and Eucalyptus camaldulensis plants on Trichomonas vaginalis growth in vitro. Adv. Biomed. Res. 2012, 1, 79. [Google Scholar]
- Hassani, S.; Asghari, G.; Yousefi, H.; Kazemian, A.; Rafieiean, M.; Darani, H.Y. Effects of different extracts of Eucalyptus camaldulensis on Trichomonas vaginalis parasite in culture medium. Adv. Biomed. Res. 2013, 2, 47. [Google Scholar]
- Aslani, A.; Asghari, G.; Darani, H.Y.; Ghanadian, M.; Hosseini, F. Design, Formulation, and Physicochemical Evaluation of Vaginal Cream Containing Eucalyptus camaldulensis, Viola odorata, and Mentha piperita extracts for Prevention and Treatment of Trichomoniasis. Int. J. Prev. Med. 2019, 10, 179. [Google Scholar] [CrossRef]
- Ibrahim, A.N. Comparison of in vitro activity of metronidazole and garlic-based product (Tomex®) on Trichomonas vaginalis. Parasitol Res. 2013, 112, 2063–2067. [Google Scholar] [CrossRef]
- Gokmen, A.A.; Can, H.; Kayalar, H.; Pektaş, B.; Kaya, S. In vitro anti-Trichomonas vaginalis activity of Haplophyllum myrtifolium. J. Infect. Dev. Ctries. 2019, 13, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.B.; Rigo, G.V.; Bridi, H.; Trentin, D.; Macedo, A.J.; von Poser, G.L.; Tasca, T. The anti-Trichomonas vaginalis phloroglucinol derivative isoaustrobrasilol B modulates extracellular nucleotide hydrolysis. Chem. Biol. Drug Des. 2017, 90, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Scopel, M.; dos Santos, O.; Frasson, A.P.; Abraham, W.R.; Tasca, T.; Henriques, A.T.; Macedo, A.J. Anti-Trichomonas vaginalis activity of marine-associated fungi from the South Brazilian Coast. Exp. Parasitol. 2013, 133, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elizondo-Luévano, J.H.; Pérez-Narváez, O.A.; Sánchez-García, E.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In-Vitro Effect of Kalanchoe daigremontiana and Its Main Component, Quercetin against Entamoeba histolytica and Trichomonas vaginalis. Iran. J. Parasitol. 2021, 16, 394–401. [Google Scholar]
- Vieira, P.B.; Silva, N.; Menezes, C.B.; da Silva, M.V.; Silva, D.B.; Lopes, N.P.; Macedo, A.J.; Bastida, J.; Tasca, T. Trichomonicidal and parasite membrane damaging activity of bidesmosic saponins from Manilkara rufula. PLoS ONE 2017, 12, e0188531. [Google Scholar]
- Moraes, M.E.; Cunha, G.H.; Bezerra, M.M.; Fechine, F.V.; Pontes, A.V.; Andrade, W.S.; Frota Bezerra, F.A.; Moraes, M.O.; Cavalcanti, P.P. Efficacy of the Mentha crispa in the treatment of women with Trichomonas vaginalis infection. Arch. Gynecol. Obstet. 2012, 286, 125–130. [Google Scholar] [CrossRef]
- Cáceres-Castillo, D.; Pérez-Navarro, Y.; Torres-Romero, J.C.; Mirón-López, G.; Ceballos-Cruz, J.; Arana-Argáez, V.; Vázquez-Carrillo, L.; Fernández-Sánchez, J.M.; Alvarez-Sánchez, M.E. Trichomonicidal activity of a new anthraquinone isolated from the roots of Morinda panamensis Seem. Drug Dev. Res. 2019, 80, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Kranzler, M.; Syrowatka, M.; Leitsch, D.; Winnips, C.; Walochnik, J. Pentamycin shows high efficacy against Trichomonas vaginalis. Int. J. Antimicrob. Agents 2015, 45, 434–437. [Google Scholar] [CrossRef]
- Aminou, H.A.; Alam-Eldin, Y.H.; Hashem, H.A. Effect of Nigella sativa alcoholic extract and oil, as well as Phaseolus vulgaris (kidney bean) lectin on the ultrastructure of Trichomonas vaginalis trophozoites. J. Parasit. Dis. 2016, 40, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Ezz Eldin, H.M.; Badawy, A.F. In vitro anti-Trichomonas vaginalis activity of Pistacia lentiscus mastic and Ocimum basilicum essential oil. J. Parasit. Dis. 2015, 39, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Frasson, A.P.; dos Santos, O.; Duarte, M.; da Silva Trentin, D.; Giordani, R.B.; da Silva, A.G.; da Silva, M.V.; Tasca, T.; Macedo, A.J. First report of anti-Trichomonas vaginalis activity of the medicinal plant Polygala decumbens from the Brazilian semi-arid region, Caatinga. J. Parasitol. Res. 2012, 110, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Sgibnev, A.; Elena, K. Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: A randomized, placebo-controlled, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Flores, J.L.; Rodriguez, M.C.; Gastelum Arellanez, A.; Alvarez-Morales, A.; Avila, E.E. Effect of recombinant prophenin 2 on the integrity and viability of Trichomonas vaginalis. Biomed Res. Int. 2015, 2015, 430436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazil, N.T.; Medeiros-Neves, B.; Fachel, F.; Pittol, V.; Schuh, R.S.; Rigo, G.V.; Tasca, T.; von Poser, G.L.; Teixeira, H.F. Optimization of Coumarins Extraction from Pterocaulon balansae by Box-Behnken Design and Anti-Trichomonas vaginalis Activity. Planta Med. 2021, 87, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.D.; de Brum Vieira, P.; Gnoatto, S.C.; Tasca, T.; Gosmann, G. Anti-Trichomonas vaginalis activity of saponins from Quillaja, Passiflora, and Ilex species. J. Parasitol. Res. 2012, 110, 2551–2556. [Google Scholar] [CrossRef]
- Saghafi, F.; Mirzaie, F.; Gorji, E.; Nabimeybodi, R.; Fattahi, M.; Mahmoodian, H.; Zareshahi, R. Antibacterial and anti-Trichomonas vaginalis effects of Rosa Damascena mill petal oil (a persian medicine product), aqueous and hydroalcoholic extracts. BMC Complement. Med. Ther. 2021, 21, 265. [Google Scholar] [CrossRef]
- Friedman, M.; Tam, C.C.; Kim, J.H.; Escobar, S.; Gong, S.; Liu, M.; Mao, X.Y.; Do, C.; Kuang, I.; Boateng, K.; et al. Anti-Parasitic Activity of Cherry Tomato Peel Powders. Foods. 2021, 10, 230. [Google Scholar] [CrossRef]
- Noritake, S.M.; Liu, J.; Kanetake, S.; Levin, C.E.; Tam, C.; Cheng, L.W.; Land, K.M.; Friedman, M. Phytochemical-rich foods inhibit the growth of pathogenic trichomonads. BMC Complement. Altern. Med. 2017, 17, 461. [Google Scholar] [CrossRef]
- Vieira, P.B.; Silva, N.L.; da Silva, G.N.; Silva, D.B.; Lopes, N.P.; Gnoatto, S.C.; da Silva, M.V.; Macedo, A.J.; Bastida, J.; Tasca, T. Caatinga plants: Natural and semi-synthetic compounds potentially active against Trichomonas vaginalis. Bioorg. Med. Chem. Lett. 2016, 26, 2229–2236. [Google Scholar] [CrossRef]
- Brandelli, C.L.; Vieira, P.; Macedo, A.J.; Tasca, T. Remarkable anti-Trichomonas vaginalis activity of plants traditionally used by the Mbyá-Guarani indigenous group in Brazil. Biomed Res. Int. 2013, 2013, 826370. [Google Scholar] [CrossRef] [Green Version]
- Abdali, K.; Jahed, L.; Amooee, S.; Zarshenas, M.; Tabatabaee, H.; Bekhradi, R. Comparison of the Effect of Vaginal Zataria multiflora Cream and Oral Metronidazole Pill on Results of Treatments for Vaginal Infections including Trichomoniasis and Bacterial Vaginosis in Women of Reproductive Age. Biomed Res. Int. 2015, 2015, 683640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbabi, M.; Devalari, M.; Fakhrieh, K.Z.; Taghizadeh, M.; Hooshyar, H. Ginger (Zingiber officinale) induces apoptosis in Trichomonas vaginalis in vitro. Int. J. Reprod. Biomed. 2016, 14, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Miyamoto, Y.; Ihara, S.; Yang, J.Z.; Zuill, D.E.; Angsantikul, P.; Zhang, Q.; Gao, W.; Zhang, L.; Eckmann, L. Composite thermoresponsive hydrogel with auranofin-loaded nanoparticles for topical treatment of vaginal trichomonad infection. Adv. Ther. 2019, 2, 1900157. [Google Scholar] [CrossRef] [PubMed]
- Pradines, B.; Bories, C.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Drug-free chitosan coated poly(isobutylcyanoacrylate) nanoparticles are active against Trichomonas vaginalis and non-toxic towards pig vaginal mucosa. Pharm. Res. 2015, 32, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Osmari, B.F.; Giuliani, L.M.; Reolon, J.B.; Rigo, G.V.; Tasca, T.; Cruz, L. Gellan gum-based hydrogel containing nanocapsules for vaginal indole-3-carbinol delivery in trichomoniasis treatment. Eur. J. Pharm. Sci. 2020, 151, 105379. [Google Scholar] [CrossRef] [PubMed]
- Elmi, T.; Rahimi Esboei, B.; Sadeghi, F.; Zamani, Z.; Didehdar, M.; Fakhar, M.; Chabra, A.; Hajialiani, F.; Namazi, M.J.; Tabatabaie, F. In Vitro Antiprotozoal Effects of Nano-chitosan on Plasmodium falciparum, Giardia lamblia and Trichomonas vaginalis. Acta Parasitol. 2021, 66, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Ardi, M.H. Anti-parasitic activity of nano Citrullus colocynthis and nano Capparis spinose against Trichomonas vaginalis in vitro. J. Parasit. Dis. 2021, 45, 845–850. [Google Scholar] [CrossRef]
- Vazini, H. Anti-Trichomonas vaginalis activity of nano Micana cordifolia and Metronidazole: An in vitro study. J. Parasit. Dis. 2017, 41, 1034–1039. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Montazeri, M.; Ahmadi, A.; Nami, S.; Hamishehkar, H.; Shahrivar, F.; Bakhtiar, N.M.; Nissapatorn, V.; Spotin, A.; Ahmadpour, E. Nanoliposomes increases Anti-Trichomonas vaginalis and apoptotic activities of metronidazole. Acta Trop. 2021, 224, 106156. [Google Scholar] [CrossRef]
- Patton, D.L.; Sweeney, Y.T.; Agnew, K.J.; Balkus, J.E.; Rabe, L.K.; Hillier, S.L. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex. Transm. Dis. 2006, 33, 743–746. [Google Scholar] [CrossRef]
- Biagi, M.; Slipke, W.; Smalley, A.; Tsaras, G. Successful treatment of trichomoniasis with tinidazole following desensitization in a patient allergic to metronidazole. Int. J. STD AIDS 2021, 32, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.M. Designing a research project: Randomised controlled trials and their principles. Emerg. Med. J. 2003, 20, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClelland, R.S.; Balkus, J.E.; Lee, J.; Anzala, O.; Kimani, J.; Schwebke, J.; Bragg, V.; Lensing, S.; Kavak, L. Randomized Trial of Periodic Presumptive Treatment With High-Dose Intravaginal Metronidazole and Miconazole to Prevent Vaginal Infections in HIV-negative Women. J. Infect. Dis. 2015, 211, 1875–1882. [Google Scholar] [CrossRef]
- Harvey, A.; Edrada-Ebel, R.; Quinn, R. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Senyigit, Z.A.; Karavana, S.Y.; Bernkop-Schnürch, A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Contri, R.V.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 623–639. [Google Scholar] [CrossRef]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.A.; Gazzi, R.P.; de Andrade Mello, P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Imiquimod-loaded nanocapsules improve cytotoxicity in cervical cancer cell line. Eur. J. Pharm. Biopharm. 2019, 136, 9–17. [Google Scholar] [CrossRef]
- Treatment for Trichomonas Inflammation. Available online: https://worldwide.espacenet.com/patent/search/family/058865157/publication/CN106667983A?q=CN106667983A (accessed on 28 November 2021).
- Derivatives of 5-Nitroindazol and Its Use as Antiprotozoal Agents. Available online: https://worldwide.espacenet.com/patent/search/family/058629836/publication/ES2614131B2?q=ES2614131B2 (accessed on 5 January 2022).
- Use of Trichomonacidal Molecules. Available online: https://worldwide.espacenet.com/patent/search/family/061832068/publication/WO2018065807A1?q=WO2018065807A1 (accessed on 28 November 2021).
- New Composition for the Treatment of Trichomoniasis. Available online: https://worldwide.espacenet.com/patent/search/family/061832083/publication/WO2018065809A1?q=WO2018065809A1&queryLang=en%3Ade%3Afr (accessed on 28 November 2021).
- Use of Novel Trichomonacidal Molecules. Available online: https://worldwide.espacenet.com/patent/search/family/061831389/publication/WO2018065808A1?q=WO2018065808A1 (accessed on 28 November 2021).
- Anti-parasitic Methods and Compositions Utilizing Diindolylmethane-Related Indoles. Available online: https://worldwide.espacenet.com/patent/search/family/039364993/publication/US2010055201A1?q=US2010055201A1 (accessed on 28 November 2021).
- Amines Derived from 2-Benzyl-5-nitroindazole with Antiprotozoal Properties against Trypanosoma, Leishmania and Trichomonas. Available online: https://worldwide.espacenet.com/patent/search/family/061094649/publication/WO2019077174A1?q=WO2019077174A1 (accessed on 28 November 2021).
- Drug for Treating Trichomonal vaginitis and Preparation Method Thereof. Available online: https://worldwide.espacenet.com/patent/search/family/058826282/publication/CN106668673A?q=CN106668673A (accessed on 28 November 2021).
- Traditional Chinese Medicine Bags for Fumigating Treatment of Trichomonas vaginitis. Available online: https://worldwide.espacenet.com/patent/search/family/055319876/publication/CN105343717A?q=CN105343717A (accessed on 28 November 2021).
- Traditional Chinese Medicine Composition for Treating Trichomonal vaginitis and Preparation Method of Composition Pills. Available online: https://worldwide.espacenet.com/patent/search/family/045100272/publication/CN102274327A?q=CN102274327A (accessed on 28 November 2021).
- Chinese Medicament for Treating Trichomonas vaginitis and Preparation Method. Available online: https://worldwide.espacenet.com/patent/search/family/053580735/publication/CN104740113A?q=CN104740113A (accessed on 28 November 2021).
- Compound Oil-in-Water Phellodendron Oil Nanoemulsion Composition. Available online: https://worldwide.espacenet.com/patent/search/family/045880277/publication/CN102397379A?q=CN102397379A (accessed on 28 November 2021).
- Secnidazole for Use in the Treatment of Trichomoniasis. Available online: https://worldwide.espacenet.com/patent/search/family/055436475/publication/US2020289470A1?q=US20200289470A1 (accessed on 28 November 2021).
- Nascimento Junior, J.A.C.; Santos, A.M.; Cavalcante, R.C.M.; Quintans-junior, L.J.; Walker, C.I.B.; Borges, L.; Frank, L.A.; Serafini, M.R. Mapping the technological landscape of SARS, MERS, and SARS-CoV-2 vaccines. Drug Dev. Ind. Pharm. 2021, 47, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.; Nascimento Junior, J.A.C.; Santos, A.M.; Borges, L.P.; Quintans-Júnior, L.J.; Walker, C.I.B.; Frank, L.A.; Serafini, M.R. Technological scenario for masks in patent database during Covid-19 pandemic. AAPS PharmSciTech 2021, 22, 71–94. [Google Scholar]
- Serafini, M.R.; Santos, V.V.; Torres, B.G.S.; Johansson Azeredo, F.; Savi, F.M.; Alves, I.A. A patent review of antibiofilm fungal drugs (2002-present). Crit. Rev. Biotechnol. 2021, 41, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Fialkoski, D.; Malfatti, C.R.M. Nanotecnologia: Uma prospecção tecnológica no âmbito nacional e internacional. Cad. De Prospecção 2019, 12, 3. [Google Scholar]
- A Phase 3 Study of Solosec for the Treatment of Trichomoniasis. Available online: https://clinicaltrials.gov/ct2/show/NCT03935217?cond=NCT03935217&draw=2&rank=1 (accessed on 28 November 2021).
- Trichomonas Vaginalis Repeat Infections among HIV Negative Women. Available online: https://clinicaltrials.gov/ct2/show/NCT01832480?cond=NCT01832480&draw=2&rank=1 (accessed on 28 November 2021).
- Comparison of Two Topical Formulations Containing Clindamycin and Clotrimazole in Patients with Vaginal Infections. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01697826?cond=NCT01697826&draw=2&rank=1 (accessed on 28 November 2021).
- The ASPIRE Trial—Aiming for Safe Pregnancies by Reducing Malaria and Infections of the Reproductive Tract. Available online: https://clinicaltrials.gov/ct2/show/NCT04189744?cond=NCT04189744&draw=2&rank=1 (accessed on 28 November 2021).
- Evaluation of Efficacy and Safety of Gynomax® XL Ovule (Gyno-Türk). Available online: https://clinicaltrials.gov/ct2/show/record/NCT03839875?cond=NCT03839875&draw=2&rank=1 (accessed on 28 November 2021).
- Neo-Penotran Forte Vaginal Suppository for Vaginal Trichomoniasis. Available online: https://clinicaltrials.gov/ct2/show/NCT01361048?cond=NCT01361048&draw=2&rank=1 (accessed on 28 November 2021).
- Observational Program Neo-Penotran® Forte. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01335373?cond=NCT01335373&draw=2&rank=1 (accessed on 28 November 2021).
- Solosec (Secnidazole) Oral Granules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209363Orig1s000Approv.pdf (accessed on 10 January 2022).
- Solosec (Secnidazole) Oral Granules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/209363s012lbl.pdf (accessed on 10 January 2022).
| Most active Compounds | Dose | Testing Method | Pharmaceutical Form | Reference |
|---|---|---|---|---|
| Synthetic Compounds | ||||
| (Tri-n-ethylphosphine)gold(I) chloride (4) | pEC50: 6.06 μM (24 h) | in vitro (T. vaginalis), in vivo (T. foetus) | Solution | [15] |
| (Tri-n-methylphosphine)-gold(I) chloride (10) | pEC50: 5.84 μM (24 h) | in vitro (T. vaginalis), in vivo (T. foetus) | Solution | [15] |
| 1,10-phenanthroline-5,6-dione-based metallodrugs (Copper-phendione) | MIC: 8.84 μM (24 h) IC50: 0.87 μM (24 h) | in vitro | Solution | [16] |
| 1,3-dioxolanes that contain tellurium (PTeDOX 01) | MIC: 90 μM and IC50: 60 μM (24 h) | in vitro | Solution | [17] |
| 1,5-bis(2-chlorophenyl)penta-1,4-dien-3-one (3e) | MIC/IC50: 90 μM/50 μM (24 h) | in vitro | Solution | [18] |
| 1,5-diphenylpenta-1,4-dien-3-one (3a) | MIC/IC50: 80 μM/50 μM (24 h) | in vitro | Solution | [18] |
| 2-Benzyl-3-(3-hydroxypropoxy)-5-nitro-2H-indazole | IC50: 7.25 and 9.11 μM (24 h) (sensitive and resistant strains) | in vitro | Solution | [19] |
| 2,2′-[α,ω-propanediylbis(oxy-1,3-phenylene)]bis-1H-benzimidazole | MIC: 9.0 μM (48 h) | in vitro, in vivo | Solution | [20] |
| 2,4-diamine-quinazoline derivative (PH100) | Clinical isolate: MIC/IC50 80 μM/14.8 μM. (24 h) long-term-grown: MIC/IC50 90 μM/50 μM (24 h) | in vitro | Solution | [21] |
| 2,6-bis(2-chlorobenzylidene)cyclohexanone (5e) | MIC/IC50: 200 μM/70 μM (24 h) | in vitro | Solution | [18] |
| 2′-Hydroxychalcones (3c) | MIC: 100 μM (24 h) IC50: 50.64 μM (24 h) | in silico, in vitro | Solution | [22] |
| 3-(aminoalkoxy)indazoles (27) | IC50: 5.6 and 8.5 μM (24 h) (sensitive and resistant strains) | in vitro | Solution | [23] |
| 3-(biphenyl-4-yl)-3-hydroxyquinuclidine (BPQ-OH) | IC50: 46 μM (24 h) | in vitro | Solution | [24] |
| 3-(ω-aminoalkoxy)-1-benzyl-5-nitroindazoles (6) | IC50: 19.2 and 1.3 μM (sensitive and resistant strains) | in vitro | Solution | [25] |
| 3-(ω-aminoalkoxy)-1-benzyl-5-nitroindazoles (10) | IC50: 2.5 and 0.5 μM (sensitive and resistant strains) | in vitro | Solution | [25] |
| 3,3′-{[4-(4-morpholinyl)phenyl] methylene} bis (4-hydroxy-2H-chromen-2-one) (A4) | IC50: 47 μM (24 h) | in silico, in vitro | Solution | [26] |
| 3-alkoxy-5-nitroindazoles derivatives | GI: 40% (1.0 μg/mL) (24 h) | in silico, in vitro | Solution | [27] |
| 3′-aminochalcone (3) | IC50: 29 μM (24 h) | in vitro | Solution | [28] |
| 3-oxime-urs-12-en-28-oic-ursolic acid (9) | MIC: 25 μM (24 h) | in vitro | Solution | [29] |
| 5-Bromo-1-[3-(2,3-dioxo-2,3-dihydro-indol-1-yl)propyl]-1H-indole-2,3-dione (4t) | IC50: 3.72 μM (24 h) | in vitro | Solution | [30] |
| 5-Chloro-6-ethoxy-2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1-methyl-1H-benzimidazole (51) | IC50: 0.0698 μM | in vitro | Solution | [31] |
| 7-deaza,7-(3,4-dichlorophenyl)adenosine (FH3147) | EC50: 0.029 μM (24 h) | in vitro (T. vaginalis), in vivo (T. foetus) | Solution | [32] |
| 7-Nitro-4-(3-piperidinopropyl)quinoxalin-2-one | IC50: 18.26 μM (24 h) | in vitro, in vivo | Solution | [33] |
| 9-(2-deoxy-2-fluoro-β,d-arabinofuranosyl)adenine | IC50: 0.09 μM (24 h) | in vitro | Solution | [34] |
| A5 (C22H26N4O4S2) | IC50: 105.2 μM (24 h) | in silico, in vitro | Solution | [35] |
| Auranofin | IC50: 0.7–2.5 µM and MLC: 2.0–6.0 µM (24 h) | in vitro (T. vaginalis), in vivo (T. foetus) | Solution | [36] |
| B3 (C16H15N5O4S2) | IC50: 66.6 μM (24 h) | in silico, in vitro | Solution | [35] |
| Betulinic acid derivative (4) | MIC: 25–50 μM (24 h) | in vitro | Solution | [37] |
| Boric acid | MLC: 0.3–0.6% | in vitro | Solution | [38] |
| C-131 | IC50: 0.033 µM | in vitro | Solution | [39] |
| C-120 | IC50: 0.173 µM | in vitro | Solution | [39] |
| C4 (C14H28N6O2S2) | IC50: 98.3 μM (24 h) | in silico, in vitro | Solution | [35] |
| Chlorinated metronidazole | IC50: 0.006 and 0.24 μM (48 h) (sensitive and resistant strains) | in vitro | Solution | [40] |
| Cinnamoyl-Oxaborole Amides: (E)-N-(1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-3-(4-nitrophenyl)acrylamide (5c) | IC50: 10.2 μM (24 h) | in vitro | Solution | [41] |
| Diamine derivative (4): | MIC: 70 μM (24 h) | in vitro | Solution | [42] |
| Disulfiram | IC50 (µM) value (aerobic/anaerobic): 0.06/0.09 for MTZ-sensitive and 0.10/1.52 MTZ-resistant (48 h) | in vitro | Solution | [43] |
| Fumagillin | IC50: 0.26 μM (48 h) | in silico, in vitro | Solution | [44] |
| Furanyl N-acylhydrazone derivatives (PFUR 4a) | IC50: 1.69 µM (24 h) | in silico, in vitro | Solution | [45] |
| Furanyl N-acylhydrazone derivatives (PFUR 4b) | IC50: 1.98 µM (24 h) | in silico, in vitro | Solution | [45] |
| Lansoprazole | IC50: 0.12 μM | in vitro | Solution | [46] |
| Metronidazole | MTZ (0.7 wt. %) combined with pluronic® F127 (20 wt. %) and chitosan (1 wt. %) | in vitro | Hydrogel | [47] |
| Metronidazole, tinidazole and boric acid | 500 mg MTZ every 8 h/7 day + tinidazole 2 g + 600 mg boric acid | case reports | Intravenous (MTZ), liquid (tinidazole), and intra-vaginal (boric acid) | [48] |
| Metronidazole | 500 mg MTZ (one week) | case report | Intravenous and vaginal ge | [49] |
| Metronidazole | 2 g (single-dose group) or 500 mg twice daily for 7 days (7-day-dose group). | randomized controlled trial | Oral | [50] |
| Metronidazole | 2 g (single-dose) versus 500 mg twice daily for 7-days (multi-dose) | clinical trial | Oral | [51] |
| Metronidazole and Miconazole | MTZ 750 mg plus miconazole 200 mg (5 consecutive nights each month for 12 months) | Randomized Controlled Trial | Vaginal suppositories | [50] |
| Metronidazole/miconazole | MTZ 750 mg/miconazole nitrate 200 mg (once or twice a day) | randomized controlled trial | vaginal suppository | [52] |
| Metronidazol/RAMEB and Metronidazol/CRYSMEB | 0.01 to 10 μg/mL (24 h) | in vitro | Solution | [53] |
| Miltefosine | IC50: 14.5 μM (24 h) | in vitro | Solution | [54] |
| Nitazoxanide | MLC: 50 μg/mL (MTZ-resistant) and 6.0 (MTZ-sensitive) (24 h) | in vitro | Dilution | [55] |
| Nithiamide | IC50 (µM) value (aerobic|anaerobic) of 1.33|0.78 for MTZ-sensitive and 5.88|1.51 MTZ-resistant (48 h) | in vitro | Solution | [43] |
| Nitroimidazole carboxamides | EC50 = 0.6–1.4 μM | in vitro | Solution | [56] |
| N-chlorotaurine (NCT) in combination with NH4Cl | 5.5 mM (0.1%) NCT plus 19 mM (0.1%) NH4Cl (5 min) | in vitro | Solution | [57] |
| Octenidine dihydrochloride with phe-noxyethanol | EC50: 0.68–2.11 µg/mL (30 min) | in vitro | Dilution | [58] |
| Omeprazole | IC50: 0.1216 µM | in vitro | Solution | [46] |
| Pantoprazole | IC50: 0.0756 µM | in vitro | Solution | [46] |
| Paromomycin and tinidazole | 5.0 g of a 5.0% (paromomycin) with concomitant oral tinidazole 1.0 g 3 times daily for 14 days | case reports | intravaginal cream (paromomycin) and tablet (tinidazole) | [20] |
| Photodynamic therapy: methylene blue and light-emitting diode | 68.1 J/cm2 (35.6 s.) | in vivo | fiber-optic tip 2 mm in diameter to the LED device | [59] |
| Rabeprazole | IC50: 0.1057 µM | in vitro | Solution | [46] |
| Secnidazole | 2 g | clinical trial | Oral Granules | [60] |
| Secnidazole | MLC: 1.6 µg/mL | in vitro | Solution | [61] |
| Tetracycline | Cytotoxic effect: 700 µg/mL (4 h) | in vitro | Dilution | [62] |
| Tinidazole | 3.3–1000 mg | case report | Oral | [63] |
| Tinidazole and Paromomycin Combination | oral tinidazole (1 g, 3 times daily) and 4 g of 6.25% intravaginal paromomycin | case report | Cream (paromomycin) and tablet (Tinidazole) | [64] |
| Zinc–clotrimazole complex (Zn(CTZ)2(Ac)2) | IC50: 4.9 μM (48 h) | in vitro | Solution | [65] |
| Zinc sulfate | 1% (14–28 days) | case report | Douche | [66] |
| Natural Products | ||||
| Amomum tsao-ko Crevost and Lemarié (essential oil and geraniol) | MLC/IC50 (µg/mL) of 44.97/22.49 and 342.96/171.48 (48 h) | in vitro | Solution | [67] |
| Asclepias curassavica L. (Apocynaceae) (ethanol extract) | IC50: 302 μg/mL (24 h) | in vitro | Solution | [68] |
| Basidiomycete Amauroderma camerarium (Amaurocine) | MIC: 4.56 μM (24 h) | in vitro | Solution | [69] |
| Bidens Pilosa L | MIC: 1.0 mg/mL (24 h) | in vitro | Solution | [70] |
| Combination Verbascum thapsus L. and Zingiber officinale Roscoe (erroneously cited as Ginger officinale) (alcoholic extract) | IC50: 73.80 μg/mL | in vitro | Solution | [71] |
| Commiphora molmol Engl. ex Tschirch (Mirazid) | two capsules (600 mg) for 6 to 8 consecutive days | humans | Capsules | [72] |
| Curcuma longa L. (Curcumin) | EC50: 73.0–105.8 µg/mL | in vitro | Solution | [73] |
| Curcumin | IC50: 117 ± 7 μM (24 h) and 173 ± 15 μM (48 h) | in vitro | Solution | [74] |
| Eicosapentaenoic Acid | 100 μM (48 h) | in vitro | Solution | [75] |
| Epinecidin-1 (synthetic fish antimicrobial peptide) | Growth inhibition: 62.5 μg/mL (180 min) | in vivo and in vitro | Solution | [76] |
| Eucalyptus camaldulensis Dehnh. | 60 μg (72 h) | in vitro | Solution | [77] |
| Eucalyptus camaldulensis Dehnh. (Ethyl acetate fraction) | GI: 12.5 mg/mL (24 h) | in vitro | Solution | [78] |
| Eucalyptus camaldulensis Dehnh. (phenolic extract), Viola odorata L. (phenolic extract), and Mentha piperita L. (hydroalcoholic extracts) | 100% T. vaginalis growth inhibition (24 h): 2.5 mg E. camaldulensis, 0.06 mg V. odorata, and 1.0 mg M. piperita/1.0 g of cream | in vitro | Vaginal creams | [79] |
| garlic-based product (Tomex®) | MIC: 100 μg/mL (24 h), 50 μg/mL (48 h), 25 μg/mL (72 h), and 12.5 μg/mL (96 h) | in vitro | Solution | [80] |
| Haplophyllum myrtifolium Boiss. (ethanol extract, alkaloid extract, and skimmianine) | MIC/MLC (μg/mL): 200/400, 400/800, and 50/150 (48 h) | in vitro | Solution | [81] |
| Hypericum L. spp. (phloroglucinol derivative isoaustrobrasilol B) | IC50: 38 μM (24 h) | in vitro | Solution | [82] |
| Hypocrea lixii (F02) and Penicillium citrinum (F40) | MIC: 2.5 mg/mL (24 h) | in vitro | Solution | [83] |
| Kalanchoe daigremontiana Raym.-Hamet and H. Perrier (flavonoid quercetin and methanol extract) | IC50: 21.17 μg/mL and 105.27 μg/mL, respectively | in vitro | Solution | [84] |
| Manilkara rufula (Miq.) H.J.Lam (H100: enriched saponin fraction) | MIC: 0.5–1.0 mg/mL (24 h) | in vitro | Solution | [85] |
| Mentha crispa L. (Giamebil®, Hebron Pharmaceutical Industry, Brazil) | 24 mg | randomized controlled trial | Tablets | [86] |
| Morinda panamensis Seem. (anthraquinone lucidin-ω-isopropyl ether) | IC50: 1.32 μg/mL (48 h) | in vitro | Solution | [87] |
| Ozoroa engleri R. Fern. and A. Fern | MIC: 1 mg/mL (24 h) | in vitro | Solution | [70] |
| Pentamycin | EC50: 2.36– 3.62 g/mL (6 h) | in vitro | Solution | [88] |
| Phaseolus vulgaris L. (lecitin) and Nigella sativa L. (oil) | 500 µg/mL for both | in vitro | Solution | [89] |
| Pistacia lentiscus L. mastic and Ocimum basilicum L. oil | MIC: 15 mg/mL and 30 μg/mL (24 h) | in vitro | Solution | [90] |
| Polygala decumbens A.W. Benn. | MIC: 1.56 mg/mL (24 h) | in vitro | Solution | [91] |
| Probiotic Gynophilus® and metronidazole | MTZ at 500 mg twice a day and 1 capsule of probiotic twice a day | randomized, placebo-controlled, double-blind study | Vaginal capsule (probiotic) and oral (MTZ) | [92] |
| ProProphenin 2 peptide | LD50: 47.66 μM (24 h) | in vitro | Solution | [93] |
| Pterocaulon balansae Chodat (Coumarins from dry hydroethanolic extract) | MIC: 30 μg/mL and IC50: 3.2 μg/mL (24 h) | in vitro | Solution | [94] |
| Quillaja saponaria Molina (saponins) | MIC: 0.025% | in vitro | Solution | [95] |
| Rosa damascena Mill. (Oil and Hydroalcoholic extract) | IC50: 1.79 and 1.41 mg/mL respectively (24 h) | in vitro | Solution | [96] |
| Sarcophyte sanguinea Sparrm. | MIC: 1 mg/mL (24 h) | in vitro | Solution | [70] |
| Solanum lycopersicum var. cerasiforme (Dunal) D.M. Spooner, G.J. Anderson and R.K. Jansen | GI: 0.02% (24 h) | in vitro | Solution | [97] |
| Syzygium cordatum Hochst. ex Krauss | MIC: 1 mg/mL (24 h) | in vitro | Solution | [70] |
| Tabernaemontana elegans Stapf | MIC: 1 mg/mL (24 h) | in vitro | Solution | [70] |
| Theaflavin-rich black tea extract | IC50: 0.0118–0.0173% w/w (24 h) | in vitro | Solution | [98] |
| Ursolic acid | MIC: 50–12.5 μM (24 h) | in vitro | Solution | [99] |
| Verbena L. sp. and Campomanesia xanthocarpa O. Berg | MIC value of 4.0 mg/mL | in vitro | Solution | [100] |
| Zataria multiflora Boiss. | 0.1%/7 days | randomized controlled trial | Vaginal creams | [101] |
| Zingiber officinale Roscoe (Ginger-alcoholic extract) | IC50: 93.8 μg/mL (24 h) GI: 800 μg/mL (48 h) | in vitro | Solution | [102] |
| Nanotechnology | ||||
| Auranofin-loaded nanoparticles | EC50 = 22 μM (24 h) | in vitro (T. vag) and in vivo (T. foetus) | Hydrogel | [103] |
| Drug-free chitosan coated poly(isobutylcyanoacrylate) nanoparticles | 100 μg/mL (24 h) | in vitro | Hydrogel | [104] |
| Nanocapsules containg indole-3-carbinol | IC50 = 2.09 µg/mL (24 h) | in vitro | Gellan gum-based hydrogel | [105] |
| Nano-chitosan | IC50: 11 μg/mL | in vitro | Suspension | [106] |
| Nano-emulsion of Capparis spinosa L. | GI: 500 ppm (72 h) | in vitro | Suspension | [107] |
| Nano-emulsion of Citrullus colocynthis (L.) Schrad. | GI: 500 ppm (72 h) | in vitro | Suspension | [107] |
| Nano-emulsion of Micana Mikania cordifolia (L.f.) Willd. (erroneously cited as Micana cordifolia) | 1000 ppm (72 h) | in vitro | Suspension | [108] |
| Nano-liposomal metronidazole | IC50: 15.90 μg/mL (6 h) | in vitro | Suspension | [109] |
| Active | Dose | Testing Method | Pharmaceutical Form | Inventor (Patent Applicants) | Identification | Reference |
|---|---|---|---|---|---|---|
| Synthetic Drugs | ||||||
| 1,6-bis (N1-p-chlorophenyl-N5-biguanidino) hexane | Aqueous acetate solution: 1%, 0.1% and 0.01% (m/v) Purified aqueous gluconate: 1%, 0.1% and 0.05% (m/v). | Patient | Lotion | DUAN JINGCHAO | CN106667983A | [121] |
| 3 amine derivatives (1-aminoalquil)indazolinonas, 3-(aminoalcoxi)indazoles and 3-(alquilamino)indazoles]). | DMSO Solution: 300 µM (maximum dose) | In vitro | Solution | ESCARIO GARCIA-TREVIJANO, JOSÉ ANTONIO; GOMEZ BARRIO, ALICIA; NOGAL RUIZ, JUAN JOSÉ; FONSECA BERZAL, CRISTINA ROSA; IBANEZ ESCRIBANO, ALEXANDRA; ARAN REDO, VICENTE JESÚS; DARDONVILLE, CHRISTOPHE; VELA ORTEGA, NEREA; SIFONTES RODRIGUEZ, SERGIO; MENESES MARCEL, ALFREDO IRENALDO | ES2653674B2 | [122] |
| 3,3′-{[4-(4-morpholinyl) phenyl] methylene} bis (4-hydroxy-2H-chromen-2-one) or hereafter referred to indistinctly as compound A4, and derivatives | 100 μM | In vitro | Solution | BENITEZ CARDOZA CLAUDIA GUADALUPE | WO2018065807A1 | [123] |
| 3,3′-{[4-(4-morpholinyl) phenyl] methylene} bis (4-hydroxy-2H-chromen-2-one) or hereafter referred to indistinctly as compound A4 and 5,5′-[(4-nitrophenyl) methylene] bis (6-hydroxy-2-mercapto-3-methyl-4 (3H) -pyrimidinone or hereafter referred to indistinctly as compound D4 | IC50 (1:3 ratio of A4 + D4 respectively): 48 μΜ (12 μΜ A4 + 36 μΜ D4). | In vitro | Solution | BENITEZ CARDOZA CLAUDIA GUADALUPE | WO2018065809A1 | [124] |
| 5,5′-[(4-nitrophenyl) methylene] bis (6-hydroxy-2-mercapto-3-methyl-4 (3H)-pyrimidinone or hereafter referred to indistinctly as compound D4 | Cl50: 153 μΜ. | In vitro | Solution | VIQUE SÁNCHEZ JOSÉ LUIS-BENITEZ CARDOZA CLAUDIA | WO2018065808A1 | [125] |
| Diindolylmethane compounds-related indoles | 100–200 mg orally once or twice a day for 1–2 weeks | Oral | ZELIGS MICHAEL | US2010055201A1 | [126] | |
| Secnidazole | 2 g as a single dose | Patient | Microgranule | PENTIKIS HELEN S | US2020289470A1 | [121] |
| three families of amines derived from 5-nitroindazole [1-(aminoalkyl)indazolinones, 3-(aminoalkoxy) indazoles and 3-(alkylamino)indazoles] | IC50 less than 50 µM | In vitro | Solution | ESCARIO GARCÍA-TREVIJANO JOSÉ ANTONIO | WO2019077174A1 | [127] |
| Natural Products | ||||||
| Coix seed, jade grass, gentian, gorgon, purslane, hundreds skin, gardenia, anemarrhena, white fresh leather, phellodendron, cnidium, guanzhong, tactylodes, chrysanthemum, lotus seed, plant grass, licorice, peony skin, rehmannia, bai wei, sophora | Multidose | Human | Complex mixture | LI SHAOLUN | CN106668673A | [128] |
| Earthworms, cnidium, Sophora flavescens aiton, white fresh skin, berberine, 100 parts, phellodendron, chuanjiao, chuanpi | Multidose | NM | Herbal mix | THE INVENTOR HAS WAIVED THE RIGHT TO BE MENTIONED | CN105343717A | [129] |
| Snake bed, Wuyu, Honey, realgar | Snake bed 35–55%, wuyu 10–15%, honey 31.85–49.7%, realgar 0.15–0.3% | NM | Nanopill | CHANG HAOLIANG; FENG TIANBAO | CN102274327A | [130] |
| Water spinach, ampelopsin grossedentata, Haloragis micrantha (Thunb.) R.Br. ex Sieb. and Zucc, malabar spinach, Silene gallica L., root of pilular adina, Ajuga taiwanensis nakai ex murata, herb of prostrate euphorbia, willow root, common nandina leaf, wing nut leaf, David’s buddleia, Chenopodium album L., sensitive joint vetch wood, Hedyotis diffusa Willd, Adiantum davidii Franch. and Pteridium revolutum (Blume) Nakai | Multidose | In vivo | Herbal mix | XUE JIANFANG; ZONG XIUHONG; FENG ZUOJI; YANG HAIXIA; CHU JINGPING | CN104740113A | [131] |
| Nanotechnology | ||||||
| Oil-in-water phellodendron oil nanoemulsion | Phellodendron oil 5.8% | In vivo | Nanoemulsion | WUQING OUYANG | CN102397379A | [132] |
| Active/Formulation | Dose | Phase | Pharmaceutical Form | Identification | Ref |
|---|---|---|---|---|---|
| Clinsupv | Clindamycin 100 mg and clotrimazole 200 mg (both administered per vaginally for 3 consecutive days) | 4 | Soft gelatin capsule versus extended release tablet | NCT01697826 | [140] |
| Drug: iptp-sulphadoxine-pyrimethamine plus metronidazole Drug: iptp-dihydroartemisinin-piperaquine plus metronidazole drug: iptp-sulphadoxine-pyrimethamine | SP = 3 tablets each containing 500 mg sulphadoxine and 25 mg pyrimethamine (Day 0) MTZ = 4 tablets each containing 500 mg as directly observed therapy (Day 0) DP = 3 tablets of 40 mg of dihydroartemisinin and 320 mg of piperaquine (Days 0, 1, 2) | 3 | Tablets | NCT04189744 | [141] |
| Gynomax® XL | Lidocaine 100 mg, thioconazole 200 mg, tinidazole 300 mg | 4 | Vaginal ovule | NCT03839875 | [142] |
| Metronidazole | 500 mg twice daily for 7 days or 2 g single dose | 3 | Oral | NCT01832480 | [139] |
| Neo-Penotran Forte | Metronidazole 750 mg and miconazole nitrate 200 mg | 2 | Vaginal suppository | NCT01361048 | [143] |
| Neo-Penotran® Forte | Metronidazole 750 mg and miconazole nitrate 200 mg | Observational | Vaginal suppository | NCT01335373 | [144] |
| Solosec (Secnidazole) or placebo | 2 g | 3 | Oral granules | NCT03935217 | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigo, G.V.; Frank, L.A.; Galego, G.B.; Santos, A.L.S.d.; Tasca, T. Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives. Venereology 2022, 1, 47-80. https://doi.org/10.3390/venereology1010005
Rigo GV, Frank LA, Galego GB, Santos ALSd, Tasca T. Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives. Venereology. 2022; 1(1):47-80. https://doi.org/10.3390/venereology1010005
Chicago/Turabian StyleRigo, Graziela Vargas, Luiza Abrahão Frank, Giulia Bongiorni Galego, André Luis Souza dos Santos, and Tiana Tasca. 2022. "Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives" Venereology 1, no. 1: 47-80. https://doi.org/10.3390/venereology1010005
APA StyleRigo, G. V., Frank, L. A., Galego, G. B., Santos, A. L. S. d., & Tasca, T. (2022). Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives. Venereology, 1(1), 47-80. https://doi.org/10.3390/venereology1010005









