1. Introduction
With the increase in electricity consumption caused by the increasing use of automated equipment and electronic devices, including in everyday life, the need to increase the capacity of overhead power lines (overhead lines) is increasing. This can be achieved either by increasing the aluminum alloy content in the cross-section of the cable, consisting of aluminum wire and a steel (composite) core, or by increasing the operating temperature of the cable, which increases its heat resistance and allows the transmission of higher currents [
1]. In recent decades, there has been a significant expansion in the use of aluminum cable alloys with an iron content in the range of 0.3–1 wt. %. Studies [
2] have shown that the addition of up to 0.8 wt. % iron contributes to an increase in the strength and ductility of aluminum after deformation treatment, while practically not reducing its electrical conductivity. In other works [
3,
4], it was noted that the application of intensive plastic deformation to aluminum alloys with an iron content of up to 2 wt. % makes it possible to increase their plasticity and enable wire drawing up to 0.08 mm in diameter. These properties make Al–Fe alloys promising for industry, where stranded cables with low weight, strength over 600 MPa, and electrical conductivity of at least a standard value are in demand. However, this method is not yet applicable in industry and remains at the stage of laboratory research, demonstrating only the potential of these alloys. It is important to note that conductor alloys have strict requirements for the content of impurities, which limits the possibility of using scrap and waste (secondary raw materials) in their production. The exception is waste and scrap conforming to GOST R 54564-2011 (groups A1, A2, A3, A4), represented by building profiles and structures made of alloys 6063 and 6061 [
2,
3]. Since the use of secondary raw materials can significantly reduce the cost of production, the study of its possible use in conductive Al-Zr alloys becomes relevant. The key task in this case is the scientific substantiation of methods for binding impurities (in particular, Fe and Si) into phases with favorable morphology, avoiding needle structures, and minimizing their content in (Al). As for pure aluminum, it has reached the limit of possible improvement in physical and mechanical characteristics. According to [
5], its yield strength does not exceed 100 MPa, and its electrical conductivity is limited by the standard value. In this regard, Al–Fe aluminum alloys are widely used as conductors in power transmission lines, construction, and various engineering and transport applications. Their popularity is explained by their low cost and balanced set of physical and mechanical properties. Thus, studies [
6] show that the combination of rolling and heat treatment makes it possible to achieve the strength of Al–1 wt.% Fe wire at the level of 200 MPa [
7]. At the same time, the authors of [
8] found that drawing aluminum wire to a diameter of 0.15 mm is possible with the addition of no more than 0.8 wt.% Fe, which improves strength properties without significant loss of electrical conductivity. Despite a number of advantages, aluminum conductors are inferior to copper conductors in terms of mechanical strength, which limits their use.
Traditionally, the strength of aluminum is increased by creating aluminum alloys alloyed with manganese and silicon, which have a good balance of strength and electrical conductivity. It was noted in [
9] that after the heat treatment complex, the yield strength of these alloys reaches 150 MPa with good electrical conductivity. Studies [
10] confirm the relevance of aluminum alloys alloyed with manganese and silicon as conductors in transport systems, as well as their increased corrosion resistance after equal-channel angular pressing. However, despite the improved performance, they cannot completely replace copper conductors due to insufficient strength and electrical conductivity. In this regard, the development of conductive aluminum alloys combining high electrical conductivity, strength, and heat resistance is an urgent task. Recent studies show that Al–Fe alloys have significant potential for further improvement due to their low cost and the ability to optimize their physical and mechanical properties. Iron has very limited solubility in aluminum, minimizing the solid solution effect on electrical conductivity [
11,
12]. At the same time, traditional casting methods, such as coquille casting, can lead to the formation of large intermetallic particles that reduce ductility and strength during subsequent processing (drawing, rolling, etc.). A number of studies [
13,
14] have shown that the use of continuous casting in an electromagnetic mold makes it possible to form intermetallic compounds with nanometer dimensions and evenly distribute them in aluminum, due to the high cooling rate (10
3–10
4 K/s).
Unlike previous works that mainly focused on binary or ternary systems, this study investigates the quaternary Al–Fe–Si–Mn system both computationally and experimentally, providing practical insights into the design of conductive and corrosion-resistant aluminum alloys.
Aluminum alloys of the Al–Fe–Si–Mn system play an important role in industrial and engineering applications due to their high strength, good workability under pressure treatment, and enhanced corrosion resistance provided by the addition of manganese [
15]. Low-alloy alloys of this system can be considered for electrical engineering applications, in particular, for the production of conductive wire [
16], despite the presence of Mn in the alloy, which is one of the elements that most reduce the main property of the material of this type: specific electrical resistance [
16]. As a rule, the mechanical characteristics of the material, such as tensile strength, have an inverse relationship with the electrical resistance of the material, i.e., the electrical property. At the same time, achieving an optimal balance of electro-mechanical properties entails an increase in the throughput (current) of the material, which is an absolute advantage, since the current load on electrical networks increases from year to year. The optimization of chemical and phase compositions, on which the achievement of the balance of the properties described above largely depends, requires the use of modern methods, such as thermodynamic modeling. ThermoCalc software allows effectively predicting the phase equilibria, the phase composition, and changes in their properties depending on the concentration of components and temperature.
2. Materials and Methods
The TTAL8 database of the ThermoCalc software was used for the study. It includes information on the phases formed in aluminum alloys. Calculations were performed for the Al–Fe–Si–Mn system at various temperatures and component concentrations.
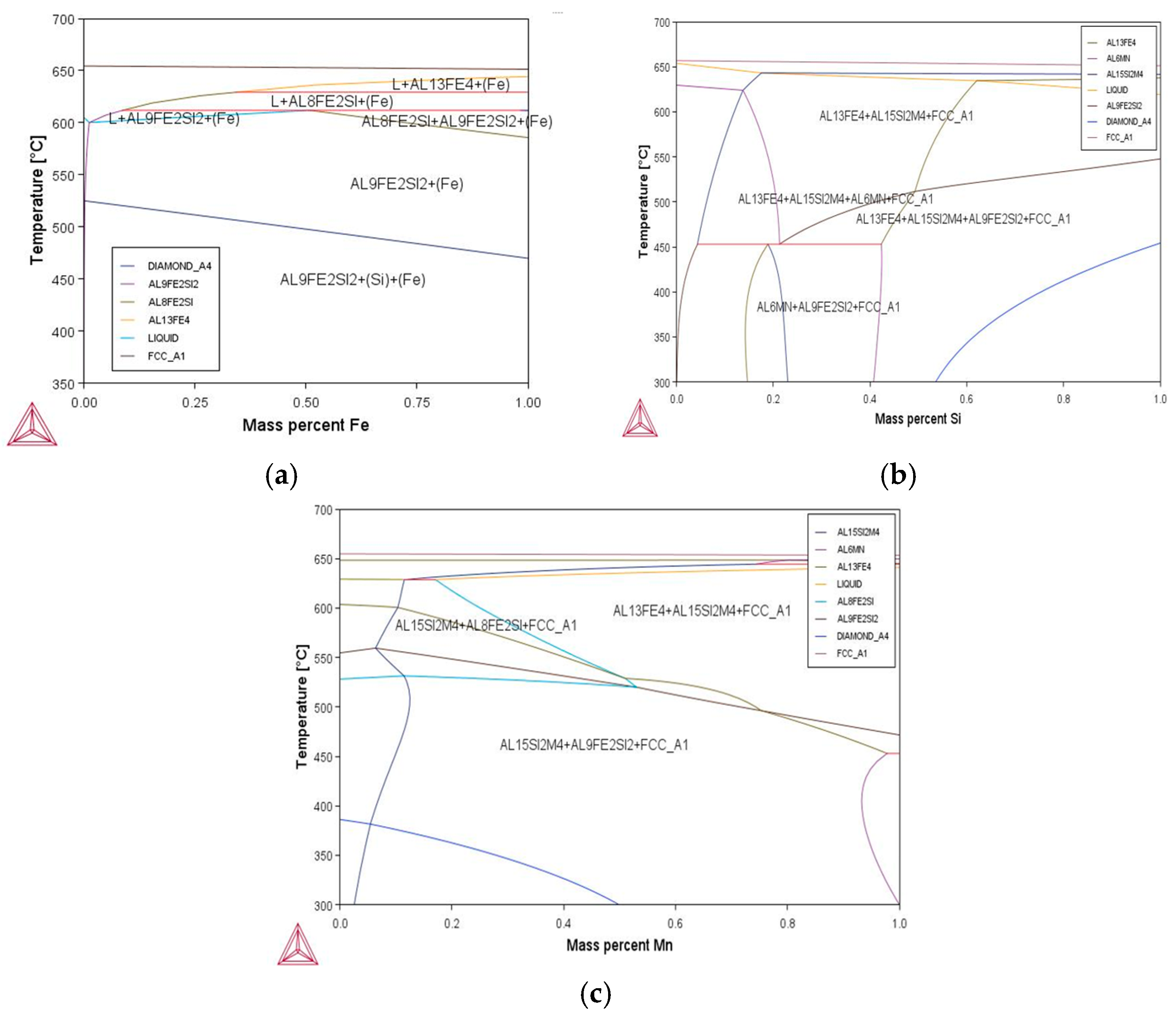
The construction of fragments of phase diagrams of the Al–Fe–Si–Mn system is a key step in establishing and interpreting phase transformations and equilibrium states in alloys of this system. Accurate diagram construction was based on thermodynamic data from the TTAL8 database [
15,
16].
As part of the study, phase diagrams were built for various concentrations of iron and silicon in temperature ranges characteristic of the melting and heat treatment of aluminum alloys. Modeling showed that with an increase in iron and silicon content in the alloy, the phase composition changed as follows significantly.
According to the literature data [
15] and according to the simulation results in the ThermoCalc program, the Al–Fe–Si–Mn diagram has a complex structure, and the following phases can be in equilibrium with (Al):
Al3Fe—the phase that is known for its high strength but also prone to decreasing ductility with increasing the Fe content.
AlFeSi—the phase that improves mechanical properties and corrosion resistance. This compound stabilizes the alloy structure and promotes a uniform distribution of secondary phases, enhancing strength and stabilizing electrical behavior.
Al6Mn—the phase that can also affect the mechanical properties of the alloy, but its formation depends on the presence of manganese in the system.
The considered calculations (
Figure 1) made it possible to determine the phase composition of the alloys depending on the concentration of iron and silicon, as well as on the temperature, which made it possible to predict the formation of various phases and their distribution in the alloy. These data can be used to optimize the composition and conditions of heat treatment to improve the mechanical and operational characteristics of the alloys, such as strength, ductility, and corrosion resistance. Modeling phase diagrams showed that increasing the iron content contributes to the formation of more stable Al
3Fe phases, which increases the strength characteristics but also reduces the material ductility. The addition of silicon promotes the formation of AlFeSi phases, which improve the distribution of secondary phases and also increase corrosion resistance.
Increasing conductivity in the aluminum–iron–silicon–manganese system can be caused by the formation of certain intermetallics that have high conductivity compared to pure metals or alloys. This improvement in conductivity can be caused by a specific electronic structure or bonding features of the intermetallics.
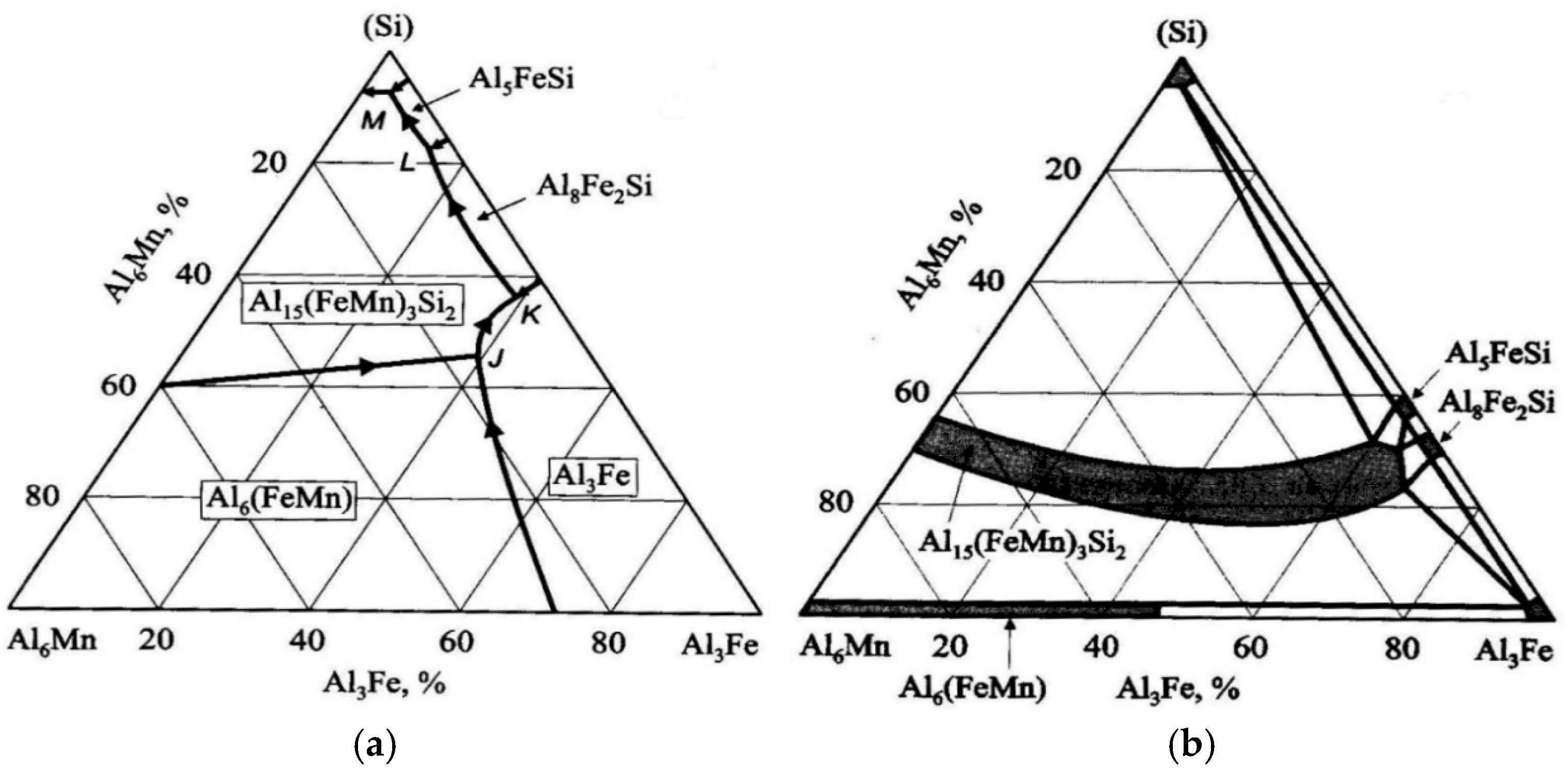
The structure of the Al–Fe–Mn–Si system is still a subject of debate. The phase diagram of this system is shown in
Figure 2. The basis of the discussion is the presence or absence of a four-fold phase. Previously, it was believed that there was a continuous series of solid solutions between the Al
8Fe
2Si and Al
15Mn
3Si
2 compounds [
17]. Later, this concept was reduced to the fact that those compounds had different crystal structures: hexagonal and cubic, respectively.
The Al
15Mn
3Si
2 compound is based on a large solid solution region extending to the Al–Fe–Si boundary. In this diagram variant, manganese is replaced by iron in a ternary compound of up to 31% Fe, 1.5% Mn, 8% Si, and in a wider homogeneity region, Al
15(FeMn)
3Si
2 is interpreted as a four-fold phase assembly. The distribution of these phase regions and the possible transformation of Al
15(FeMn)
3Si
2 into an invariant form at concentrations of up to 12% Si, 1% Fe, and 2% Mn are given in
Table 1.
It was found that alloys containing 10–14% Si, 0–1% Fe, and 0–4% Mn contain a four-fold compound Al
16(FeMn)
4Si
3-Si, which leads to the formation of a quasitron section of Al
16(FeMn)
4Si
3 [
17]. On both sides of this section, two second-order systems are formed: Al-Al
16(FeMn)
4Si
3-Al
5FeSi-Si (adjacent to the Al–Fe–Si boundary). In addition, one more second-order system can be distinguished, which is below 596 °C: Al
5FeSi-Al
4FeSi
2-Al
16(FeMn)
4Si
3-Si.
According to [
18], in alloys containing 10–14% Si, non-variant reactions occur, as shown in
Table 2.
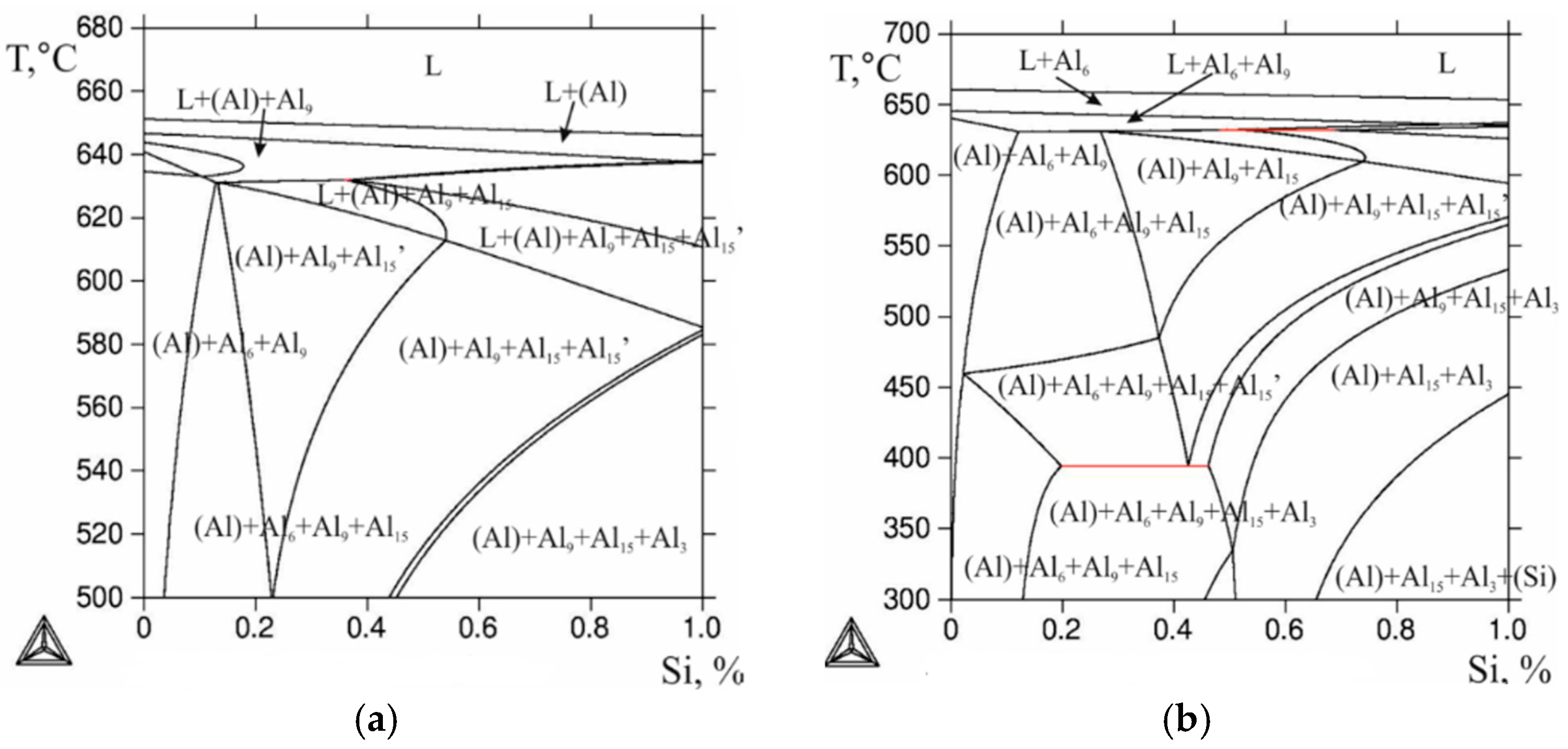
The combined effect of iron and silicon at 0.6% Mn shows that at 0.5% Si with 0.5% Fe and at 0.5% Si with 0.8% Fe, primary crystals of the Al
6(FeMn) phase must inevitably form, but the iron content of less than ~0.03% leads to the formation of the Al
9Fe phase (
Figure 3). Due to the low solubility of Fe in (Al), the Al
6(FeMn) phase is mainly of crystallization origin. The consequence of this is decreasing the Mn concentration in (Al) in the cast state and decreasing the amount of Al
6Mn dispersoids formed during annealing [
18,
19,
20,
21,
22,
23].
At a sufficient silicon concentration (over 0.5%), at 0.8% Fe and 0.6% Mn, primary crystals of the Al
15(Fe,Mn)
2Si
3 phase can form, and this can be seen in
Figure 3.
These intermetallic compounds contribute to improved electrical performance due to their specific crystal lattice and strong metallic bonding between aluminum and iron atoms. Their structure facilitates efficient electron transport, which enhances the overall conductivity of the alloy.
Phases such as AlSi (e.g., AlSi2) may enhance electrical behavior due to their characteristic electronic structure. Aluminum silicides can facilitate more efficient electron transport, thereby contributing to favorable conductivity in the alloy.
Iron silicides such as FeSi may contribute to electrical performance in high-silicon alloys. The presence of silicon in these compounds influences the electronic structure, as iron silicides typically exhibit metallic-type conductivity.
Intermetallics such as FeMn3 and other Fe–Mn compounds can also influence electrical behavior. Manganese, in turn, may enhance charge transport in iron-rich alloys by modifying the electronic structure and increasing the electron density.
There are also intermetallics such as AlMn2 that can affect conductivity. These phases can have their own characteristics that contribute to improved conductivity due to the features of their crystal structure and composition.
Thus, increasing conductivity in this system can be associated with the formation of intermetallics such as AlFe, AlSi, FeSi, AlMn2 and others, which have a certain structure and contribute to better electron movement through the material. It is important to note that this improvement in conductivity depends on the content of each element in the alloy and on the temperature, since phase transformations and the degree of alloying can significantly change the electrophysical properties of the material.
Calculations indicate that achieving optimal mechanical characteristics requires taking into account the percentage content of iron, silicon, and manganese in order to avoid the formation of unfavorable phases such as Al3Fe and AlFeSi in excessive quantities, which can lead to deterioration in ductility and decreasing impact toughness. The balanced content of these elements promotes the formation of stable and uniformly distributed phases, which is expected to improve structural stability and corrosion resistance, making the alloy potentially more suitable for industrial applications.
In addition, changes in phase boundaries with changing cooling conditions were studied, which is important for controlling the alloy structure after heat treatment. Modeling phase diagrams taking into account the cooling rate made it possible to predict how the phases will be distributed in the alloy, as well as to indicate the optimal conditions for the formation of the desired structural elements.
The evaluation of phase transformations in aluminum Al–Fe–Si–Mn alloys is an important step in predicting their structure and properties. The ThermoCalc software package enables the detailed modeling of these processes, accounting for changes in temperature and composition, which makes it possible to analyze in detail the behavior of the phases under various process conditions.
Modeling phase transformations with changes in temperature showed that with increasing the temperature, significant changes in the composition and morphology of the phases occur. In the Al–Fe–Si–Mn system, such phases as Al3Fe, AlFeSi, Al6Mn, and AlMn2 have different temperatures of formation and solubility, which must be taken into account when developing alloys with the required properties.
At temperatures below 400 °C, such primary phases as Al3Fe and AlFeSi begin to form in the alloy, which are stable at low temperatures.
At approximately 600 °C, the Al3Fe phase begins to decompose into smaller crystals, which changes the mechanical properties of the alloy. At this time, the AlFeSi phase also forms, which stabilizes at high temperatures, improving corrosion resistance.
At temperatures above 700 °C, such phases as Al3Fe completely dissolve in the liquid metal, which makes the alloys easier to cast and process but can also lead to a loss of the original strength characteristics.
Changing the content of Fe, Si, and Mn components also affects phase transformations. Modeling has shown the following:
Increasing the iron content promotes the early formation of the Al3Fe phase, which increases the strength of the alloy but reduces its ductility. In this case, the phase boundary between Al3Fe and the liquid solution shifts towards lower temperatures.
Increasing the silicon content leads to a more significant formation of the AlFeSi phase, which stabilizes the alloy structure and promotes a more uniform distribution of secondary phases, which reduces the risk of forming large brittle intermetallics.
The balanced silicon and iron content avoids the excessive formation of brittle phases, maintaining optimal mechanical properties at higher temperatures.
The results of phase transformation modeling can be used to optimize heat treatment, including the selection of temperature conditions for melting, annealing, quenching and subsequent cooling. This allows minimizing the formation of undesirable phases, such as large intermetallic compounds, and ensuring a structure with the required mechanical properties, such as high strength, ductility, and corrosion resistance. This approach helps improve the technology of alloy production and their adaptation to specific operating conditions. Thus, the assessment of phase transformations with changes in temperature and composition is the key to managing the properties of Al–Fe–Si–Mn aluminum alloys. The obtained results made it possible to develop recommendations for controlling the composition and thermal conditions to achieve optimal mechanical properties of the alloys.
To confirm the obtained results using ThermoCalc software, the main objects of the study were aluminum alloy sheets containing up to 1% Fe 0.5% Si 0.6% Mn. Experimental alloys (
Table 3) were prepared in an electric resistance furnace in a graphite–chamotte crucible made of primary aluminum of grade A7E (SSThe c11069-2001). Flat ingots with dimensions of 40 mm × 120 mm × 200 mm were obtained by casting into a graphite mold.
Annealing was carried out in an SNOL electric furnace in stepwise modes at 400–650 °C with a 50 °C step and a holding time of 3 h, as summarized in
Table 4.
Designations such as T400–T650 correspond to stepwise annealing temperature ranges (e.g., T500 indicates annealing from 450 to 500 °C).
HP refers to cold-rolled sheets, S refers to hot-rolled sheets, and L indicates as-cast ingots.
Numbers following the letters indicate the final annealing temperature or processing condition. For example, HP450 refers to a cold-rolled sheet annealed at 450 °C.
After casting, the ingots were subjected to two types of deformation: hot rolling and cold rolling. Hot rolling was performed at a temperature of approximately 450–500 °C to reduce the thickness of the ingots. Cold rolling was carried out at room temperature after intermediate annealing, achieving a final thickness reduction of approximately 80%. Intermediate annealing steps were performed between passes to relieve internal stresses and refine the microstructure.
The microstructure of the samples was studied using a Magus Metal VD700 BD LCD optical microscope (Guangzhou Liss Optical Instrument Co., Ltd., Guangzhou, China) and a TESCAN VEGA 3 scanning electron microscope (TESCAN GROUP, Brno, Czech Republic). Both mechanical and electrolytic polishing were used to prepare the sections, which were carried out at the voltage of 12 V in an electrolyte containing 6 parts C2H5OH, 1 part HClO4 and 1 part glycerol.
3. Results and Discussion
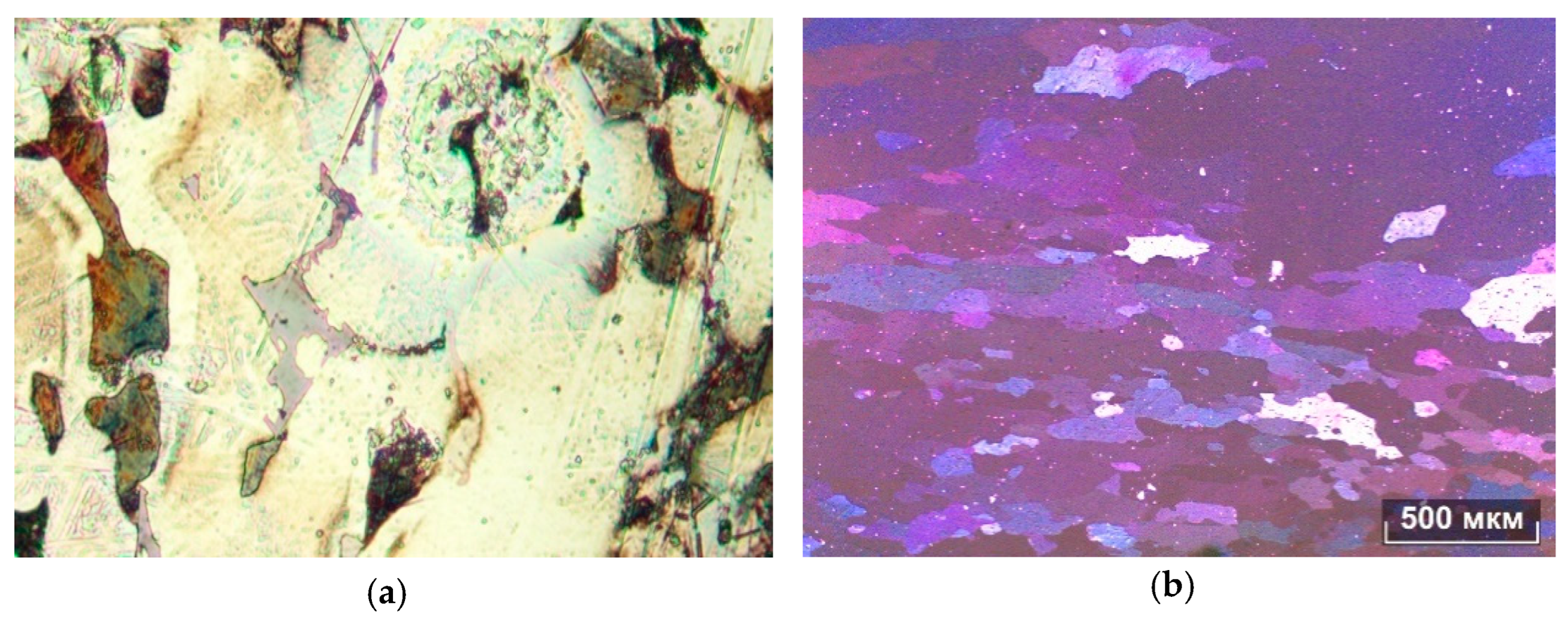
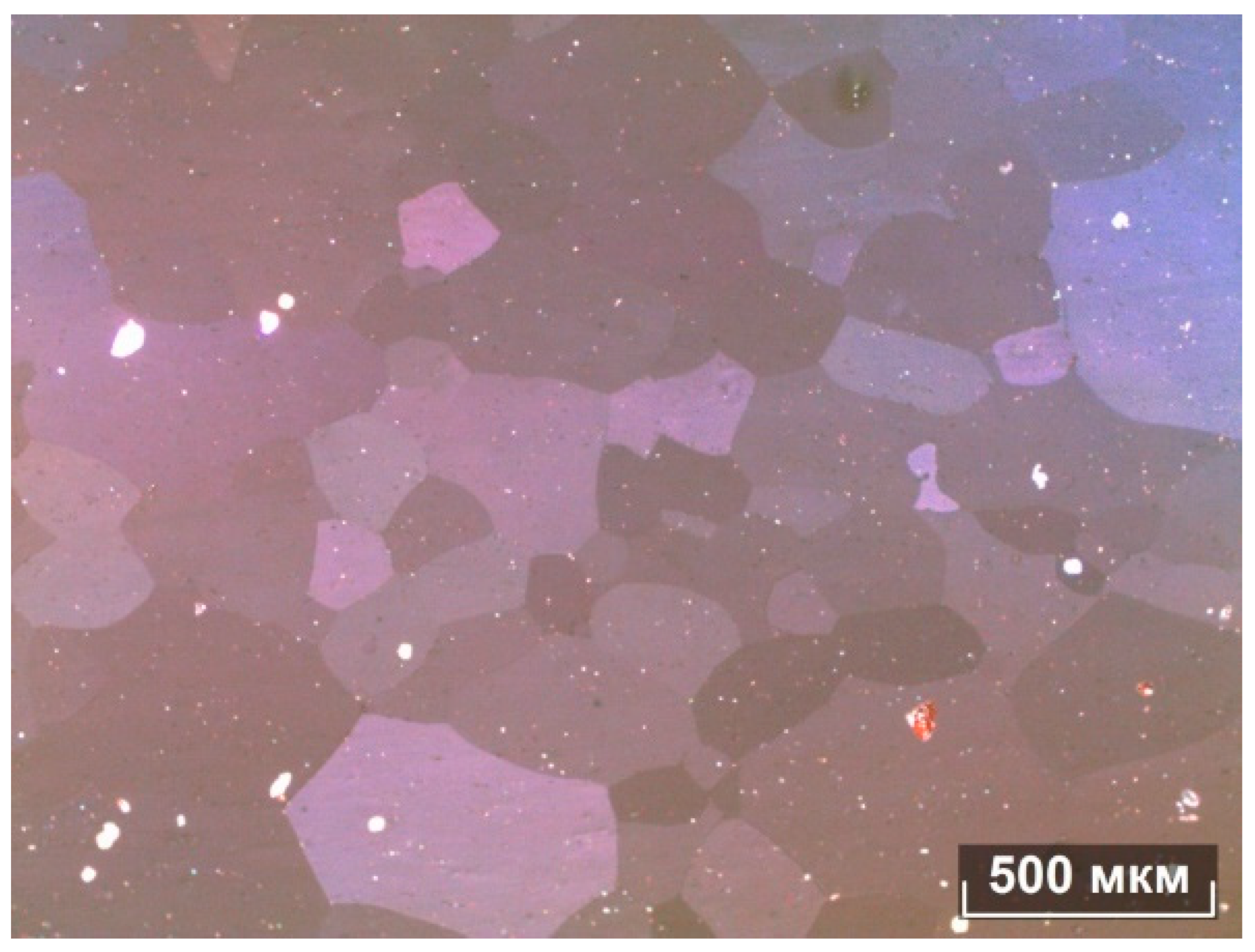
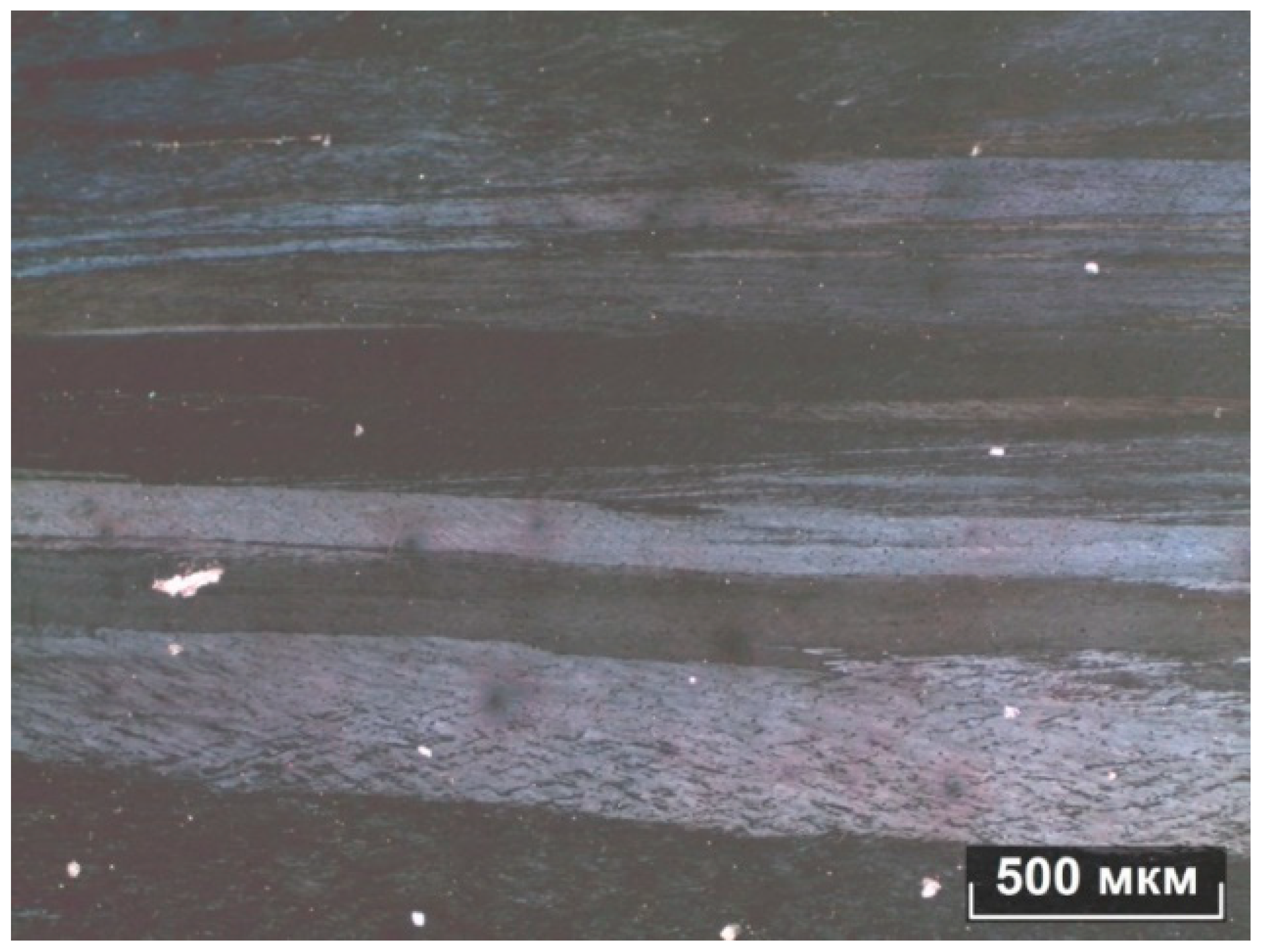
It can be seen from the metallographic analysis of the samples that the assumption about the formation of the FeSi, FeMn3, and Al15Mn3Si2 phases was confirmed. There are inclusions of the phase (Al, Fe, Si) in the form of skeletal fragments or veins along the boundaries of dendritic cells (Al). During the rolling process, the equiaxed grain structure was transformed into a fibrous one, and the inclusions of the iron-containing phase also stretched out.
Annealing did not cause significant microstructural changes observable via optical microscopy; therefore, structural and phase transformations during the annealing process were assessed by changes in resistivity and hardness, as well as by the calculation results.
For each mode, specific electrical conductivity was measured, which was determined by the eddy current method on a VE-26NP device, and then they were converted into specific electrical resistance. As follows from the dependences shown in
Figure 4a, when using multi-stage annealing, the minimum values of resistivity of the experimental alloys are achieved at 450–500 °C, which can be explained by the maximum decrease in the concentration of iron in the aluminum solid solution. It is important to note that in this state, the experimental alloys of 0.5% Fe and 0.8% Fe have the same resistivity values. It follows that the effect of L
12 phase nanoparticles on electrical resistance is much less.
With increasing temperature, increasing resistivity is observed for both ingots (
Figure 4a) and sheets (
Figure 4b,c), which is obviously caused by increasing the concentration value according to the solvus of the Al–Fe diagram. The difference in resistivity of the alloys of 0.5% Fe and 0.8% Fe again begins to increase, reaching the maximum at 650 °C.
Analyzing the obtained results, it can be concluded that the decomposition of the (Al) solid solution occurs at the slowest rate in the ingots and at the fastest rate in the cold-rolled sheets. In particular, for the alloy containing 0.5 wt.% Fe, the minimum resistivity values are observed to be 29.7 × 10−9 Ω·m for the ingot annealed under the T500 condition and 28.7 × 10−9 Ω·m for the cold-rolled sheet in the HP450 condition.
Obviously, as the annealing holding time increases, the difference between these values is expected to decrease.
In addition to the detailed results for 0.5Fe and 0.8Fe alloys, the alloys with lower iron contents—0.2Fe, 0.3Fe, and 0.4Fe—were also investigated. These compositions demonstrated expected trends, with slightly lower hardness and electrical resistivity compared to the higher-iron alloys. The 0.2Fe and 0.3Fe alloys, in particular, showed reduced volume fractions of intermetallic phases such as Al3Fe, resulting in more ductile microstructures. While the structural differences were less pronounced under light microscopy, measurements confirmed that electrical resistivity decreased gradually from 0.2Fe to 0.4Fe before reaching minimum values in the 0.5Fe alloy. These results confirm the transitional behavior across the series and highlight the continuous effect of iron content on physical properties.
The microstructures of the alloys are shown in
Figure 5,
Figure 6,
Figure 7 and
Figure 8. In the 0.5Fe alloy, veins of the Al
3Fe phase are visible, and in the 0.8Fe alloy, particles of the Al
15(Fe,Mn)
3Si
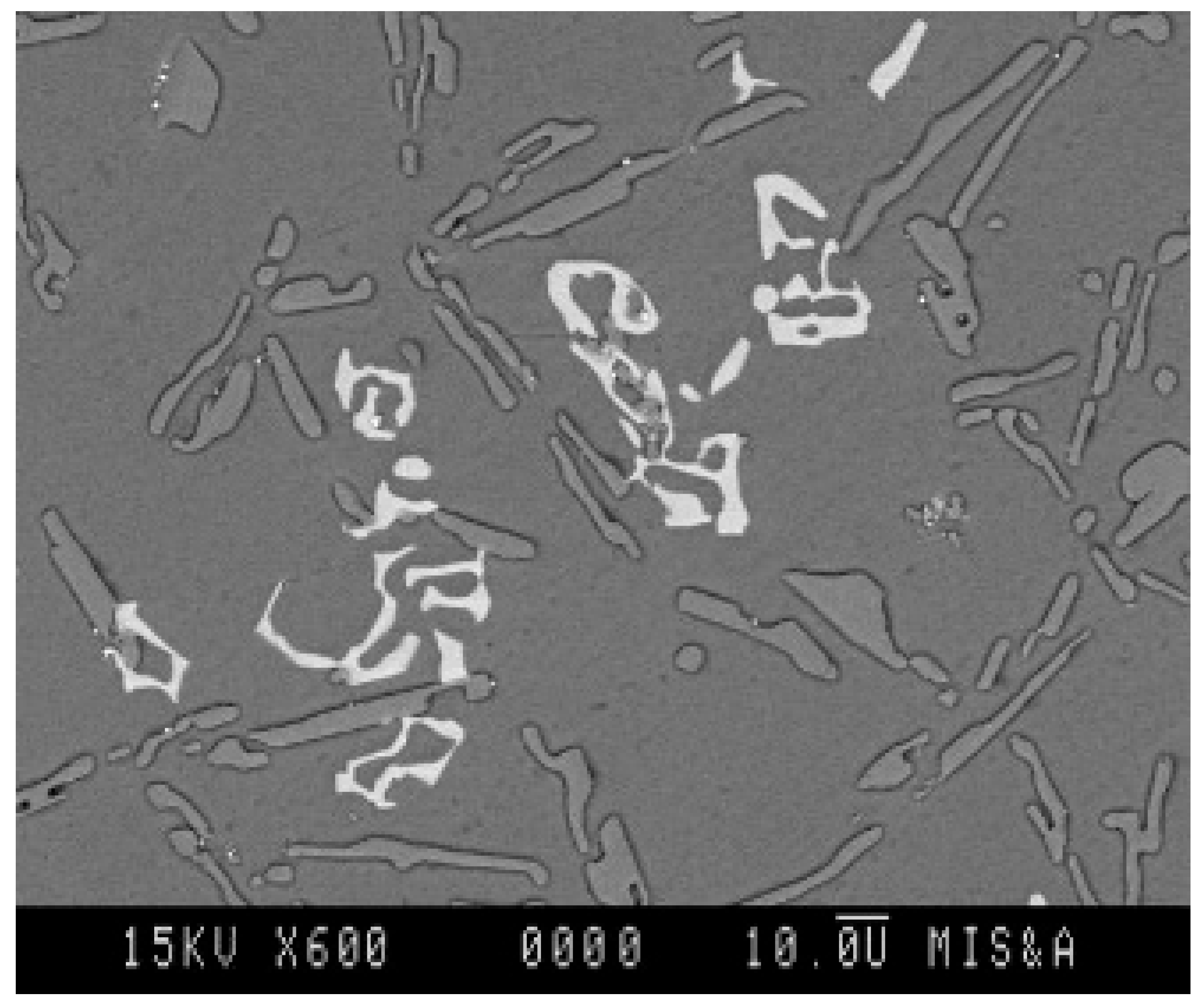
2 phase are visible. The confirmation was obtained from microstructural analysis, and the morphology indicates the presence of eutectic structures. During intermediate annealing, phase separation and microparticles can be clearly seen. There is an increase in dendritic cells (Al).
The addition of manganese is believed to reduce the detrimental effects of iron by promoting the formation of complex intermetallics such as the Al
15(Fe,Mn)
3Si
2 phase, which exhibits skeletal morphology, as observed in
Figure 8. While the morphology is consistent with literature reports, further confirmation using EDS or XRD would be necessary to verify the exact phase composition (
Figure 8) [
24].
The results obtained can be used to optimize the composition of aluminum alloys, especially in cases where a combination of high strength and resistance to external influences is required. Thermodynamic modeling with ThermoCalc can significantly reduce the development time of new alloys and improve their manufacturability, which is especially important for high-performance engineering applications where precise material matching is essential.