Spectral Visualization of Alloy Reactions during Laser Melting
Abstract
1. Introduction
2. Materials and Methods
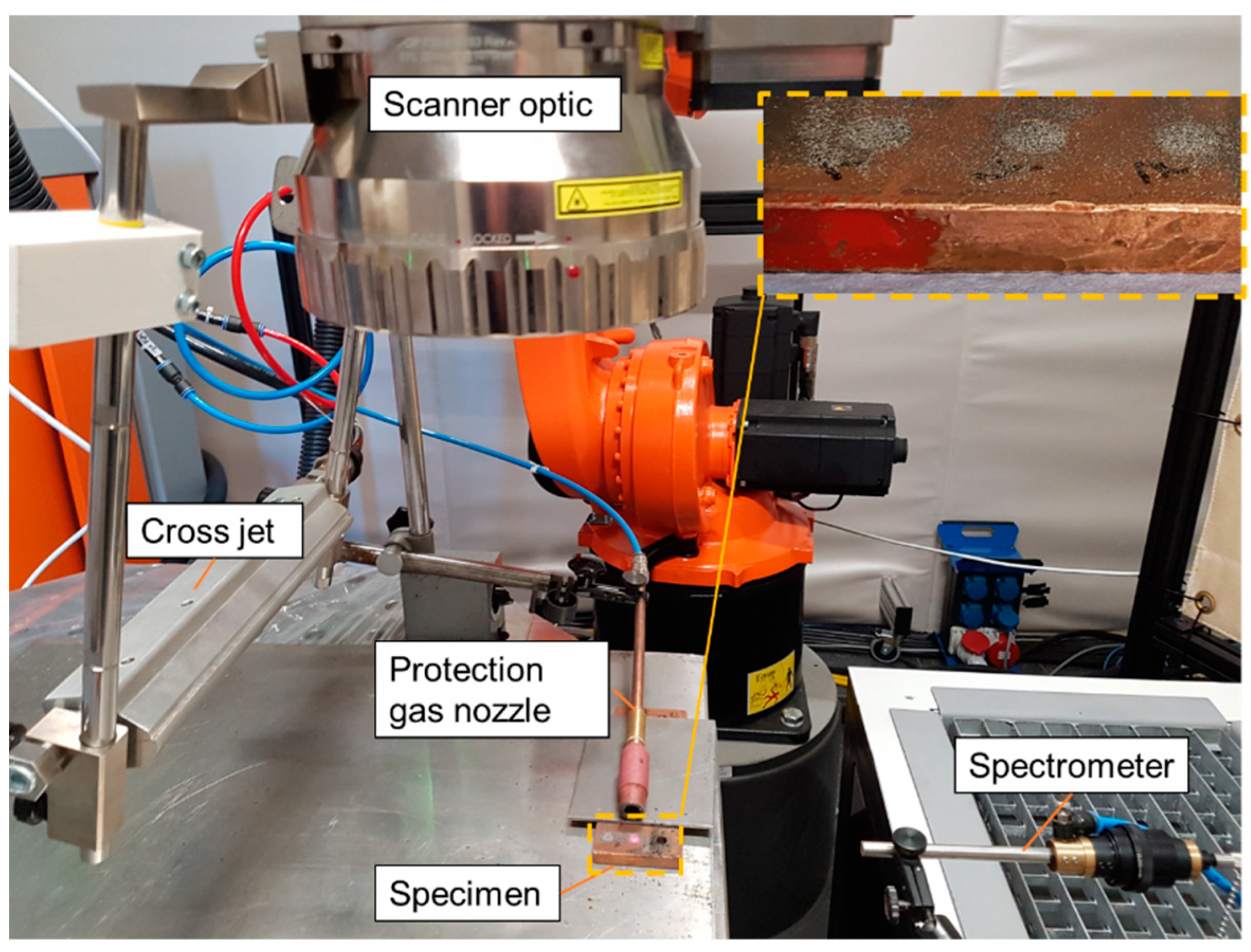
2.1. Experimental Setup
2.2. Spectral Analysis
3. Results and Discussion
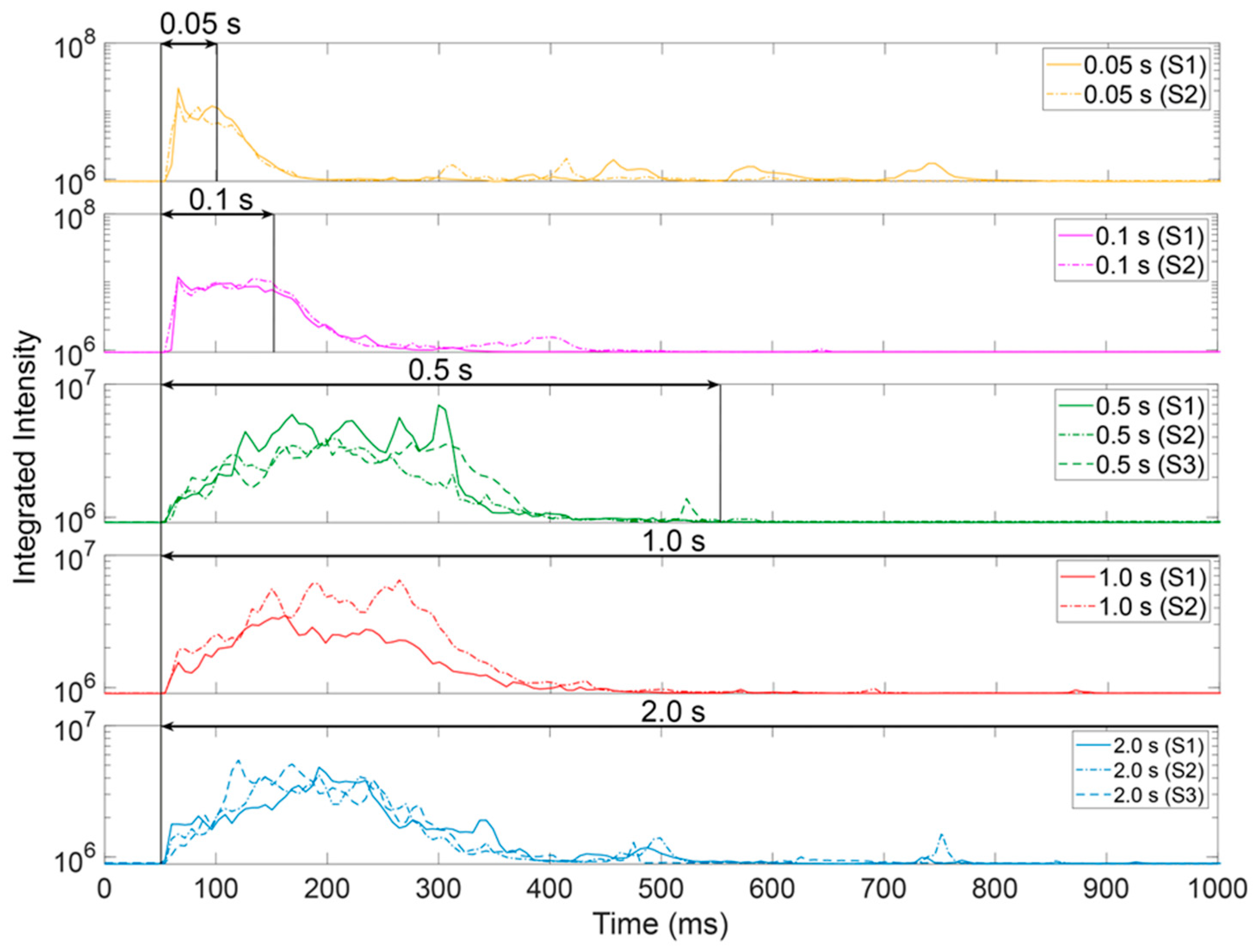
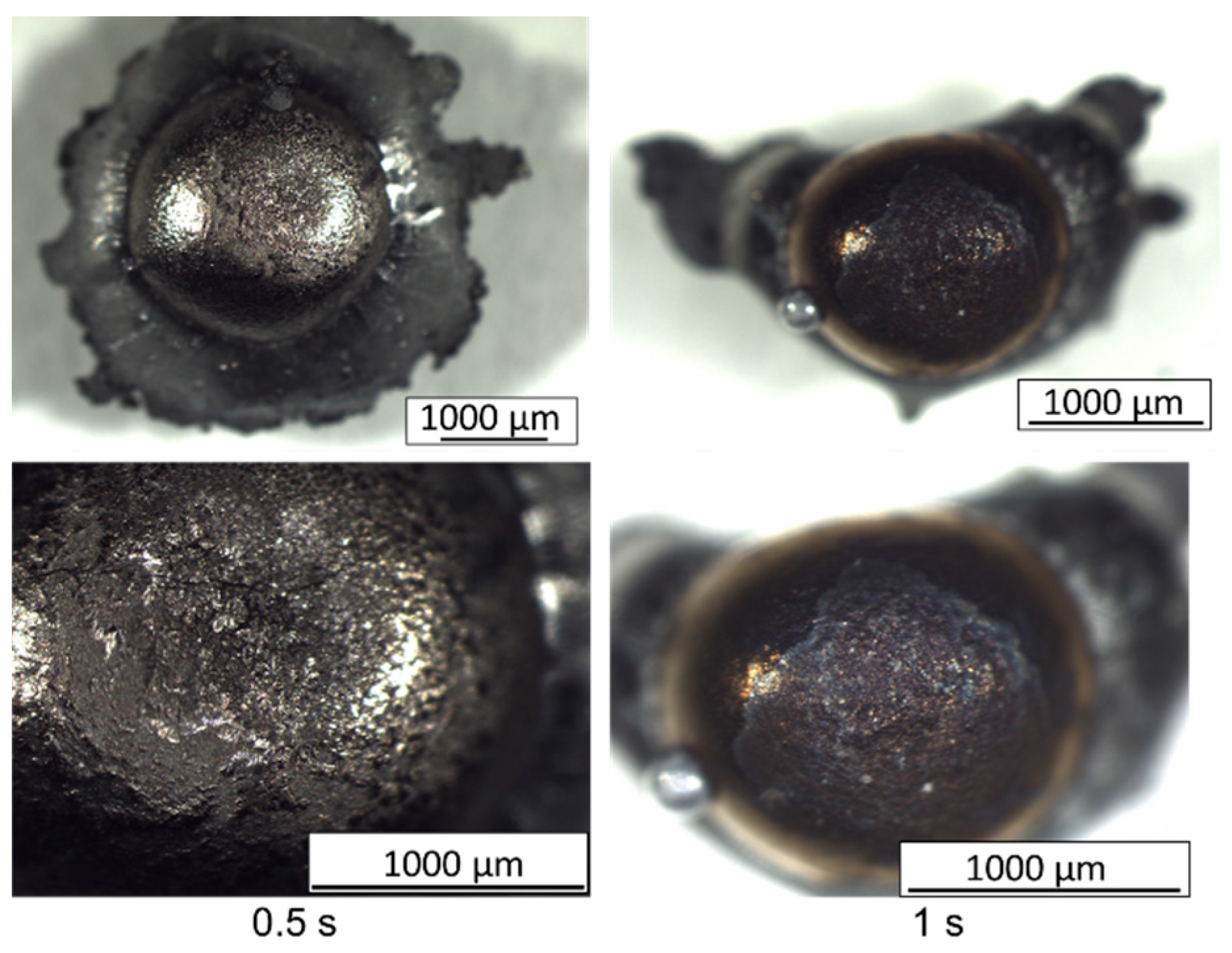
3.1. Variation of Laser Illumination Duration
3.2. Material Impact
4. Conclusions
- The spectral data of metal vapor melt ejections are an indicator of chemical reactions in the melt. The emitted vapor during laser processing of reacting alloy components contains information about the reaction time and intensity.
- The chemical reaction between hematite and aluminum seems to require the illumination of the laser to maintain, which is likely to provide the mixing for enabling the reaction partner connection.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frerichs, F.; Lu, Y.; Lübben, T.; Radel, T. Process Signature for Laser Hardening. Metals 2021, 11, 465. [Google Scholar] [CrossRef]
- Dausinger, F.; Shen, J. Energy Coupling Efficiency in Laser Surface Treatment. ISIJ Int. 1993, 33, 925–933. [Google Scholar] [CrossRef]
- Watkins, K.; McMahon, M.; Steen, W. Microstructure and corrosion properties of laser surface processed aluminium alloys: A review. Mater. Sci. Eng. A 1997, 231, 55–61. [Google Scholar] [CrossRef]
- Powell, J.; Kaplan, A. Laser cutting: From first principles to the state of the art. In Proceedings of the 1st Pacific International Conference on Application of Lasers and Optics 2004, Melbourne, Australia, 19–21 April 2004; Laser Institute of America: Scottsdale, AZ, USA; Volume 1, p. 101. [Google Scholar]
- Volpp, J. Keyhole stability during laser welding—Part II: Process pores and spatters. Prod. Eng. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kawaguchi, I.; Arakane, G.; Honda, H. Keyhole behavior in high power laser welding. In Proceedings of the International Congress on Laser Advanced Materials Processing (LAMP), Kharagpur, India, 29–31 August 2022; International Society for Optics and Photonics: Bellingham, WA, USA, 2022. [Google Scholar]
- Koch, H.; Gómez Vázquez, R.; Otto, A. Analysis of Distortion Effects on Weld Pool Dynamics in Laser Beam Welding. In Proceedings of the IWOTE 2014: International Workshop on Thermal Forming and Welding Distortion, Bremen, Germany, 9–10 April 2014; Strahltechnik/BIAS Verlag: Bremen, Germany; pp. 165–172. [Google Scholar]
- Sing, S.L.; Yeong, W.Y. Laser powder bed fusion for metal additive manufacturing: Perspectives on recent de-velopments. Virtual Phys. Prototyp. 2020, 15, 359–370. [Google Scholar] [CrossRef]
- Li, Z.; Sui, S.; Ma, X.; Tan, H.; Zhong, C.; Bi, G.; Clare, A.T.; Gasser, A.; Chen, J. High deposition rate powder- and wire-based laser directed energy deposition of metallic materials: A review. Int. J. Mach. Tools Manuf. 2022, 181, 103942. [Google Scholar] [CrossRef]
- Kumar, K.S. Analytical Modeling of Temperature Distribution, Peak Temperature, Cooling Rate and Thermal Cycles in a Solid Work Piece Welded by Laser Welding Process. Procedia Mater. Sci. 2014, 6, 821–834. [Google Scholar] [CrossRef]
- Poorhaydari, K.; Patchett, B.M.; Ivey, D.G. Estimation of cooling rate in the welding of plates with intermediate thickness. Weld. J. 2005, 84, 149s–155s. [Google Scholar]
- Dewi, H.S.; Volpp, J.; Kaplan, A.F. Short thermal cycle treatment with laser of vanadium microalloyed steels. J. Manuf. Process. 2020, 57, 543–551. [Google Scholar] [CrossRef]
- LDurães, í.; Costa, B.F.; Santos, R.; Correia, A.; Campos, J.; Portugal, A. Fe2O3/aluminum thermite reaction intermediate and final products characterization. Mater. Sci. Eng. A 2007, 465, 199–210. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.; Maximov, Y. Thermite reactions: Their utilization in the synthesis and processing of materials; Department of Mechanical Aeronautical, and Materials Engineering, University of Cafifornia. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Xie, J.; Kar, A. Laser welding of thin sheet steel with surface oxidation. Weld. J. 1999, 78, 343-s. [Google Scholar]
- Saravanan, R.; Molina, J.; Narciso, J.; García-Cordovilla, C.; Louis, E. Effects of nitrogen on the surface tension of pure aluminium at high temperatures. Scr. Mater. 2001, 44, 965–970. [Google Scholar] [CrossRef]
- Fedina, T.; Brueckner, F.; Kaplan, A.F.H.; Wilsnack, C. Laser-assisted reduction of iron ore using aluminum powder. J. Laser Appl. 2023, 35, 022007. [Google Scholar] [CrossRef]
- Kaplan, A.F.; Fedina, T.; Brueckner, F. Study of Si-domains enabling local reduction of laser-melted iron ore for iron-making during 3D-printing. Procedia CIRP 2022, 111, 377–380. [Google Scholar] [CrossRef]
- Chmielewska, A.; Wysocki, B.; Buhagiar, J.; Michalski, B.; Adamczyk-Cieślak, B.; Gloc, M.; Święszkowski, W. In situ alloying of NiTi: Influence of laser powder bed fusion (LPBF) scanning strategy on chemical composition. Mater. Today Commun. 2022, 30, 103007. [Google Scholar] [CrossRef]
- Mosallanejad, M.H.; Niroumand, B.; Aversa, A.; Saboori, A. In-situ alloying in laser-based additive manufacturing processes: A critical review. J. Alloy. Compd. 2021, 872, 159567. [Google Scholar] [CrossRef]
- Fuhrich, T.; Berger, P.; Hügel, H. Marangoni effect in laser deep penetration welding of steel. J. Laser Appl. 2001, 13, 178–186. [Google Scholar] [CrossRef]
- Robertson, S.M.; Frostevarg, J.; Kaplan, A.F.H.; Hong, S.M.; Kim, J.-H.; Bang, H.-S. Tailored laser pulse method to manipulate filler wire melt metallurgy from thermal cycles. J. Laser Appl. 2019, 31, 022605. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database, Version 5.10; National Institute of Standards and Technology: Gaithersburg, MD, USA. Available online: https://physics.nist.gov/asd (accessed on 15 May 2023).
- West, J.B.; Broida, H.P. Chemiluminescence and photoluminescence of diatomic iron oxide. J. Chem. Phys. 1975, 62, 2566–2574. [Google Scholar] [CrossRef]
- Toro, C.; Torres, S.; Parra, V.; Fuentes, R.; Castillo, R.; Díaz, W.; Reyes, G.; Balladares, E.; Parra, R. On the Detection of Spectral Emissions of Iron Oxides in Combustion Experiments of Pyrite Concentrates. Sensors 2020, 20, 1284. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sanned, D.; Huang, J.; Berrocal, E.; Cai, W.; Aldén, M.; Richter, M.; Li, Z. Stereoscopic high-speed imaging of iron microexplosions and nanoparticle-release. Opt. Express 2021, 29, 34465. [Google Scholar] [CrossRef] [PubMed]
- Keene, B.J. Review of data for the surface tension of pure metals. Int. Mater. Rev. 1993, 38, 157–192. [Google Scholar] [CrossRef]
- Keene, B.J. Review of data for the surface tension of iron and its binary alloys. Int. Mater. Rev. 1988, 33, 1–37. [Google Scholar] [CrossRef]
- Yousefi, E.; Sun, Y.; Kunwar, A.; Guo, M.; Moelans, N.; Seveno, D. Surface Tension of Aluminum Oxide: A Molecular Dynamics Study. AMI Acta Materialia 2021. [Google Scholar] [CrossRef]
- Fiori, L.; Ricci, E.; Arato, E. Dynamic surface tension measurements on molten metal-oxygen systems: Model validation on molten tin. Acta Mater. 2003, 51, 2873–2890. [Google Scholar] [CrossRef]
- Ricci, E.; Arato, E.; Passerone, A.; Costa, P. Oxygen tensioactivity on liquid-metal drops. Adv. Colloid Interface Sci. 2005, 117, 15–32. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpp, J.; Naesstroem, H.; Wockenfuss, L.; Schmidt, M.; Partes, K. Spectral Visualization of Alloy Reactions during Laser Melting. Alloys 2023, 2, 140-147. https://doi.org/10.3390/alloys2030010
Volpp J, Naesstroem H, Wockenfuss L, Schmidt M, Partes K. Spectral Visualization of Alloy Reactions during Laser Melting. Alloys. 2023; 2(3):140-147. https://doi.org/10.3390/alloys2030010
Chicago/Turabian StyleVolpp, Joerg, Himani Naesstroem, Lisanne Wockenfuss, Malte Schmidt, and Knut Partes. 2023. "Spectral Visualization of Alloy Reactions during Laser Melting" Alloys 2, no. 3: 140-147. https://doi.org/10.3390/alloys2030010
APA StyleVolpp, J., Naesstroem, H., Wockenfuss, L., Schmidt, M., & Partes, K. (2023). Spectral Visualization of Alloy Reactions during Laser Melting. Alloys, 2(3), 140-147. https://doi.org/10.3390/alloys2030010





