A Review of MDA-5 Dermatomyositis and Associated Interstitial Lung Disease
Abstract
1. Introduction
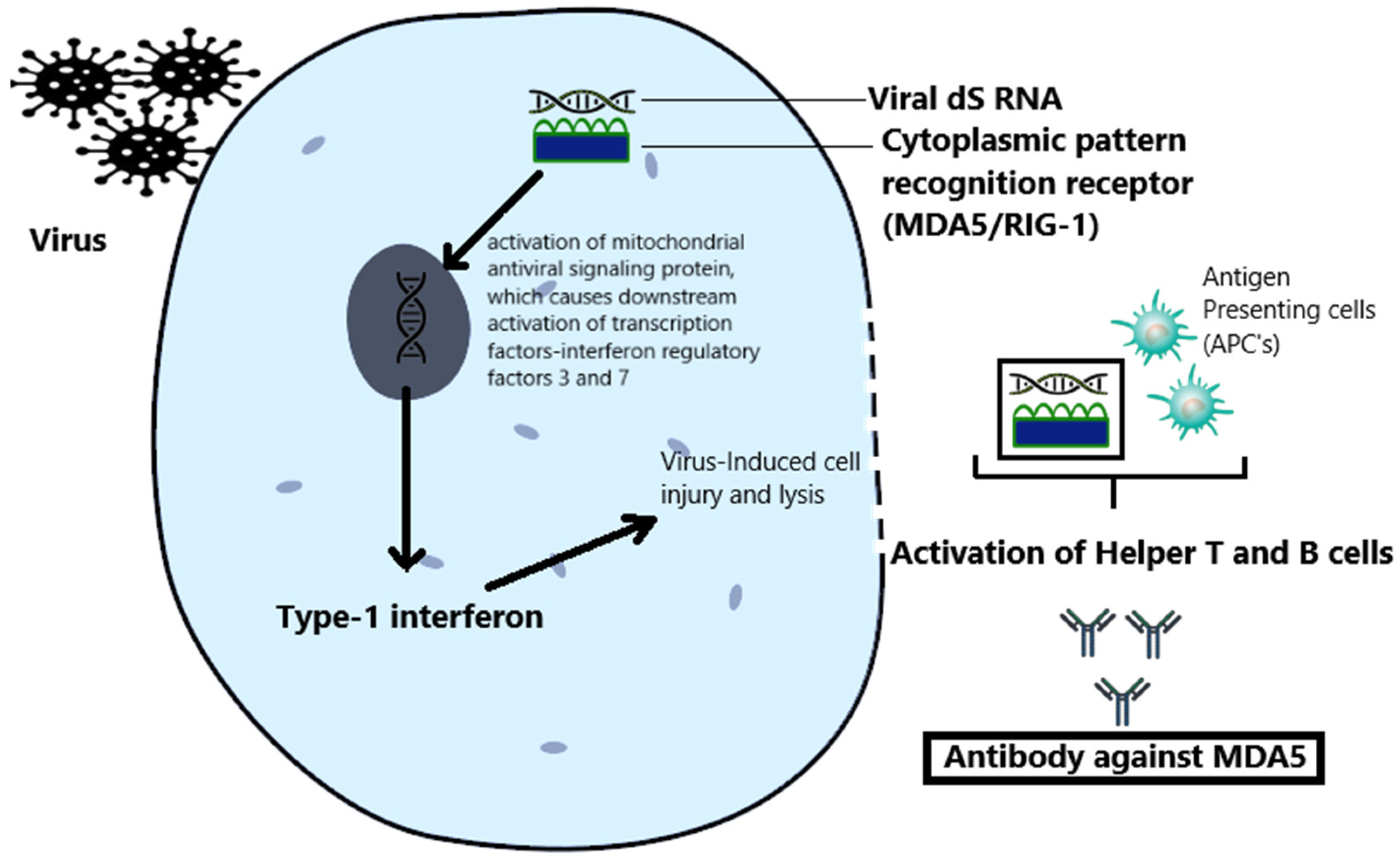
2. Pathogenesis
3. Clinical Manifestations
- Cluster 1, MDA-5 RP-ILD type, presents primarily with lung disease and mechanic’s hands. This phenotype has a poor prognosis and increased need for intensive care.
- Cluster 2, MDA-5 rheumatic DM type, is more common in women, presents with inflammatory arthralgias and arthritis, and has a positive prognosis. This phenotype has a lower incidence of skin lesions, myositis, and RP-ILD.
- Cluster 3, MDA-5 vasculopathy DM type, carries an intermediate prognosis and is associated with the cutaneous vasculopathy findings of Raynaud’s phenomenon, digital necrosis, and calcinosis alongside an increased incidence of myositis [10].
4. Lung Involvement
5. Skin Manifestations
6. Muscle Involvement
7. Inflammatory Arthritis
8. Others
9. Similarity to COVID-19
10. Association with Cancer
11. Diagnosis
12. Prognosis
13. Biomarkers
- (a)
- (b)
- Anti-MDA-5 Ab: Studies have shown that serum levels of anti-MDA-5 Abs are significantly higher in people who develop RP-ILD than in those who do not [8]. A decrease in anti-MDA-5 Ab levels has been associated with longer remission in some studies [38]. Another Japanese study suggests that monitoring anti-MDA-5 Ab levels could be useful in predicting the risk of relapse during the remission maintenance phase [35]. However, two US studies found no correlation between MDA-5 Ab titers and the disease course [5,6]. More studies are necessary to determine the clinical relevance of this potential association.
- (c)
- Krebs von den Lungen-6 (KL-6): Elevated serum KL-6 levels are produced by regenerating alveolar type II pneumocytes and are thought to be associated with impaired alveolar–capillary barriers. Reflecting the severity of ILD and its progression, they are associated with increased mortality in people with MDA-5 DM ILD [39]. This test is commercially available but not widely used in clinical practice.
- (d)
- Type 1 interferon: Studies have shown that the absolute type 1 interferon score is directly proportional to ILD, muscle inflammation, and skin disease activity among people with MDA-5 DM [40].
- (e)
- Peripheral lymphocyte counts: A decline in peripheral lymphocyte counts among individuals in MDA-5 DM ILD was associated with poor prognosis [41].
- (f)
- Ro52 Ab: A study revealed an inverse correlation between the levels of Ro52 Abs and survival time, underscoring the potential prognostic importance of these antibodies [28].
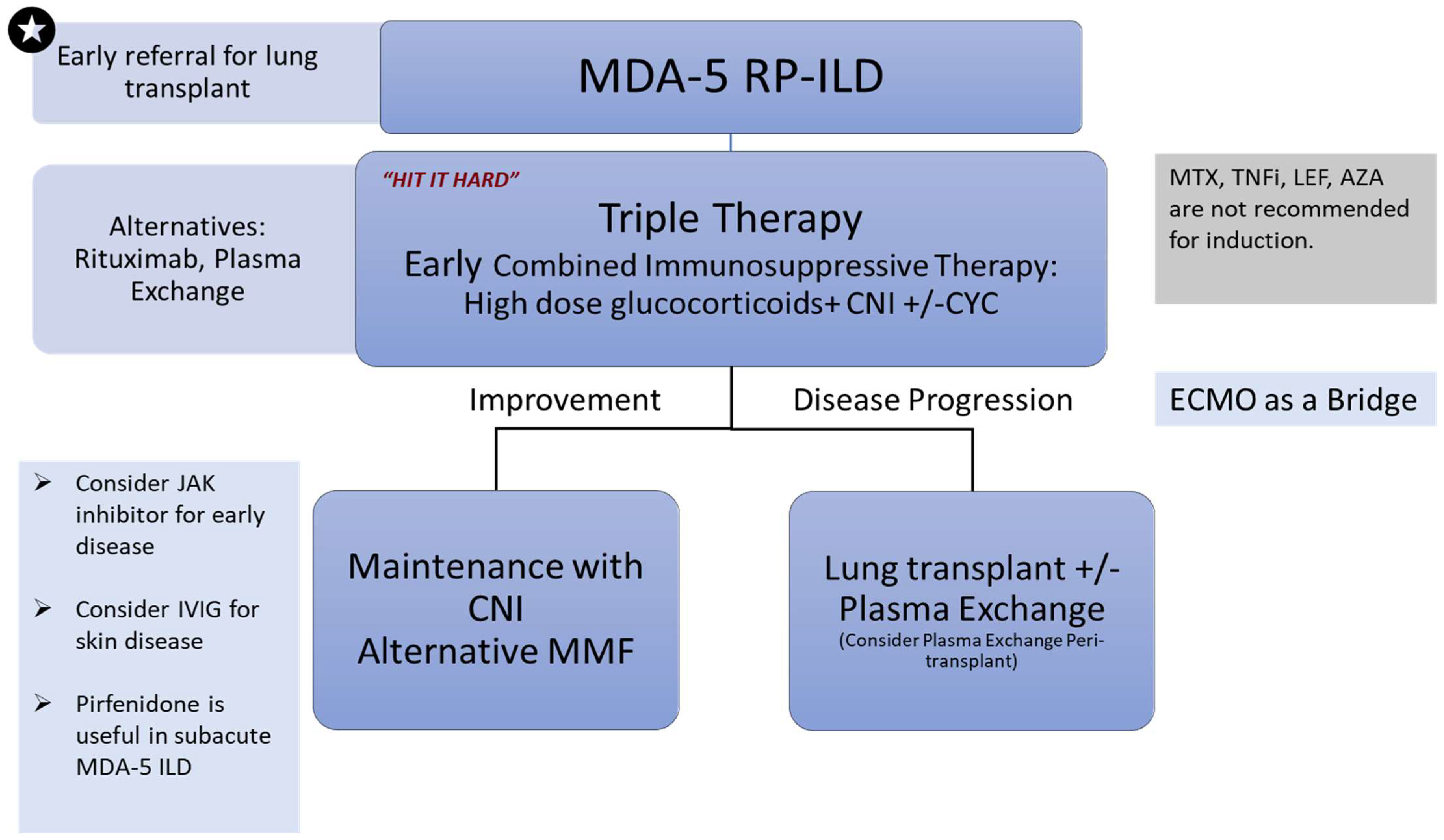
14. Management
14.1. “Hit it Hard”/Triple Therapy
14.2. Other Therapies Described in the Literature
15. Summary of Treatment Guidelines
16. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Dermatomyositis |
| ILD | Interstitial lung disease |
| RP-ILD | Rapidly progressive interstitial lung disease |
| IIMs | Idiopathic inflammatory myopathies |
| MDA-5 | Anti-melanoma differentiation-associated gene 5 |
| Ab | antibody |
| RIG-1 | Retinoic acid-inducible gene-1 |
| IFIH1 | Interferon-induced helicase C domain-containing protein 1 |
| ds RNA | double-stranded ribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| HRCT | High resolution computed tomography |
| CNI | Calcineurin inhibitor |
| JAK | Janus kinase |
| IVIG | Intravenous immunoglobulin |
| ECMO | Extracorporeal membrane oxygenation |
| RCT | Randomized controlled trial |
| PFT | Pulmonary function test |
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primer. 2021, 7, 86. [Google Scholar] [CrossRef]
- Mehta, P.; Machado, P.M.; Gupta, L. Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol. Int. 2021, 41, 1021–1036. [Google Scholar] [CrossRef]
- McPherson, M.; Economidou, S.; Liampas, A.; Zis, P.; Parperis, K. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: A systematic review. Semin. Arthritis Rheum. 2022, 53, 151959. [Google Scholar] [CrossRef] [PubMed]
- Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005, 52, 1571–1576. [CrossRef]
- Hall, J.C.; Casciola-Rosen, L.; Samedy, L.; Werner, J.; Owoyemi, K.; Danoff, S.K.; Christopher-Stine, L. Anti-Mda5-Associated Dermatomyositis: Expanding the Clinical Spectrum. Arthritis Care Res. 2013, 65, 1307–1315. [Google Scholar] [CrossRef]
- Tiniakou, E.; Mecoli, C.A.; Kelly, W.; Albayda, J.; Paik, J.; Adler, B.; Lin, C.T.; Mammen, A.L.; Danoff, S.K.; Casciola-Rosen, L.; et al. Anti-MDA5-positive dermatomyositis and remission in a single referral centre population. Clin. Exp. Rheumatol. 2023, 41, 309–315. Available online: https://www.clinexprheumatol.org/abstract.asp?a=19287 (accessed on 23 January 2024). [CrossRef]
- Cheng, L.; Xu, L.; Xu, Y.; Yuan, F.; Li, J.; Wu, M.; Da, Z.; Wei, H.; Zhou, L.; Yin, S.; et al. Gender differences in patients with anti-MDA5-positive dermatomyositis: A cohort study of 251 cases. Clin. Rheumatol. 2024, 43, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Oddis, C.V.; Sato, S.; Kuwana, M.; Aggarwal, R. Antimelanoma Differentiation-associated Gene 5 Antibody: Expanding the Clinical Spectrum in North American Patients with Dermatomyositis. J. Rheumatol. 2017, 44, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Nombel, A.; Fabien, N.; Coutant, F. Dermatomyositis with Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front. Immunol. 2021, 12, 773352. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.773352 (accessed on 7 January 2024). [CrossRef]
- Allenbach, Y.; Uzunhan, Y.; Toquet, S.; Leroux, G.; Gallay, L.; Marquet, A.; Meyer, A.; Guillaud, C.; Limal, N.; Gagnadoux, F.; et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody. Neurology 2020, 95, e70–e78. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Fujikawa, K.; Horai, Y.; Okada, A.; Kawashiri, S.-Y.; Iwamoto, N.; Suzuki, T.; Nakashima, Y.; Tamai, M.; Arima, K.; et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 2012, 51, 1278–1284. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Sato, S.; Kuwana, M.; Aggarwal, R. Anti–Melanoma Differentiation–Associated Gene 5 Is Associated with Rapidly Progressive Lung Disease and Poor Survival in US Patients with Amyopathic and Myopathic Dermatomyositis. Arthritis Care Res. 2015, 68, 689–694. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Jin, Q.; Peng, Q.; Zhang, L.; Lin, S.; Lu, X.; Liu, M.; Wang, Y.; Song, A.; et al. Clinical, radiological and pathological features of anti-MDA5 antibody-associated interstitial lung disease. RMD Open 2023, 9, e003150. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, N.; Aleissa, M. Cutaneous Features of Anti-MDA-5 Antibody-Positive Amyopathic Dermatomyositis in a Sudanese Patient. Case Rep. Dermatol. 2021, 13, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Suda, T. Inverse Gottron’s sign in anti-MDA5 dermatomyositis. QJM Int. J. Med. 2023, hcad250. [Google Scholar] [CrossRef] [PubMed]
- Charbit, L.; Bursztejn, A.-C.; Mohamed, S.; Kaminsky, P.; Lerondeau, B.; Barbaud, A.; Deibener-Kaminsky, J.; Schmutz, J.-L. Nécroses digitales étendues au cours d’une dermatomyosite avec anticorps anti-MDA-5. Ann. Dermatol. Vénéréologie 2016, 143, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Chung, L.; Casciola-Rosen, L.; Fiorentino, D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014, 150, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Vincent, T.; Bessis, D. Dermatomyositis and acute interstitial lung disease associated with MDA-5 antibodies: An atypical case. Ann. Dermatol. Venereol. 2013, 140, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Christopher-Stine, L.; Albayda, J. On the Nose: Anti-MDA-5 Dermatomyositis Manifesting as Perinasal Swelling. Case Rep. Dermatol. 2022, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, N.C.C.; Hoff, L.S.; Borges, I.B.P.; de Souza, F.H.C.; Shinjo, S.K. Clinical manifestations, outcomes, and antibody profile of Brazilian adult patients with dermatomyositis: A single-center longitudinal study. Adv. Rheumatol. Lond. Engl. 2022, 62, 41. [Google Scholar] [CrossRef]
- Mangal, V.; Hegde, A.; Hasvi, J.; Harikrishnan, P.; Kumar, A.; Goel, N.; Menon, A.S. Fever of Unknown Origin and Hepatitis as the Initial Presentation of Anti-MDA-5 Positive Dermatomyositis: A Case Report. Mediterr. J. Rheumatol. 2022, 33, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, G.; Zhang, G.; Matucci-Cerinic, M.; Furst, D.E. Similarities and differences between severe COVID-19 pneumonia and anti-MDA-5-positive dermatomyositis-associated rapidly progressive interstitial lung diseases: A challenge for the future. Ann. Rheum. Dis. 2022, 81, e192. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Noumi, B.; Malik, F.; Wang, S. A Rare Case of MDA-5-Positive Amyopathic Dermatomyositis with Rapidly Progressive Interstitial Lung Disease Following COVID-19 mRNA Vaccination—A Case Report. Sn Compr. Clin. Med. 2023, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Anderle, K.; Machold, K.; Kiener, H.P.; Bormann, D.; Hoetzenecker, K.; Geleff, S.; Prosch, H.; Laccone, F.; Heil, P.M.; Petzelbauer, P.; et al. COVID-19 as a putative trigger of anti-MDA5-associated dermatomyositis with acute respiratory distress syndrome (ARDS) requiring lung transplantation, a case report. BMC Rheumatol. 2022, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ichiyasu, H.; Sakamoto, Y.; Yoshida, C.; Sakamoto, K.; Fujita, R.; Nakayama, G.; Okabayashi, H.; Saeki, S.; Okamoto, S.; Kohrogi, H. Rapidly progressive interstitial lung disease due to anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis complicated with cervical cancer: Successful treatment with direct hemoperfusion using polymyxin B-immobilized fiber column therapy. Respir. Med. Case Rep. 2016, 20, 51–54. [Google Scholar] [CrossRef]
- Ge, Y.; Li, S.; Tian, X.; He, L.; Lu, X.; Wang, G. Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin. Rheumatol. 2021, 40, 2311–2317. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Du, Y.; Wang, L.; Wang, Q.; Wu, H.; Liu, L.; Xue, J. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res. Ther. 2023, 25, 127. [Google Scholar] [CrossRef]
- Interstitial Lung Disease Clinical Practice Guidelines. Available online: https://rheumatology.org/interstitial-lung-disease-guideline (accessed on 17 January 2024).
- Nguyen, M.; Do, V.; Yell, P.C.; Jo, C.; Liu, J.; Burns, D.K.; Wright, T.; Cai, C. Distinct tissue injury patterns in juvenile dermatomyositis auto-antibody subgroups. Acta Neuropathol. Commun. 2020, 8, 125. [Google Scholar] [CrossRef]
- Yasin, S.A.; Schutz, P.W.; Deakin, C.T.; Sag, E.; Varsani, H.; Simou, S.; Marshall, L.R.; Tansley, S.L.; McHugh, N.J.; Holton, J.L.; et al. Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: Analysis by myositis autoantibody and pathological features. Neuropathol. Appl. Neurobiol. 2019, 45, 495–512. [Google Scholar] [CrossRef]
- Allenbach, Y.; Leroux, G.; Suárez-Calvet, X.; Preusse, C.; Gallardo, E.; Hervier, B.; Rigolet, A.; Hie, M.; Pehl, D.; Limal, N.; et al. Dermatomyositis With or Without Anti-Melanoma Differentiation-Associated Gene 5 Antibodies: Common Interferon Signature but Distinct NOS2 Expression. Am. J. Pathol. 2016, 186, 691–700. [Google Scholar] [CrossRef]
- Liu, C.H.; Kor, C.T.; Hung, M.H.; Hsiao, K.H.; Cheng, Y.H.; Tien, Y.C. Differences in the Clinical Characteristics and 1-Year Mortality Rates of Patients with Dermatomyositis with anti-Jo-1 and anti-MDA5 Antibodies. J. Immunol. Res. 2023, 2023, 2988422. [Google Scholar] [CrossRef]
- He, S.; Zhou, Y.; Fan, C.; Ma, J.; Chen, Y.; Wu, W.; Zhang, X. Differences in sex- and age-associated mortality in patients with anti-MDA5-positive dermatomyositis. Mod. Rheumatol. 2023, 33, 975–981. [Google Scholar] [CrossRef]
- Ida Ida, T.; Furuta, S.; Fujiwara, M.; Hiraguri, M.; Hirose, K.; Ikeda, K.; Iwamoto, T.; Kagami, S.-I.; Kobayashi, Y.; Kurasawa, K.; et al. Short-term and long-term outcomes of patients with anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. Rheumatology 2024, keae011. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lyu, K.; Li, J.; Zhang, P.; Guan, W.; Zhang, L.; Han, L.; Liu, S.; Li, T. Anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis exhibit three clinical phenotypes with different prognoses. Clin. Exp. Rheumatol. 2022, 40, 304–308. Available online: https://www.clinexprheumatol.org/abstract.asp?a=17825 (accessed on 17 December 2023). [CrossRef]
- Li, X.; Liu, Y.; Cheng, L.; Huang, Y.; Yan, S.; Li, H.; Zhan, H.; Li, Y. Roles of biomarkers in anti-MDA5-positive dermatomyositis, associated interstitial lung disease, and rapidly progressive interstitial lung disease. J. Clin. Lab. Anal. 2022, 36, e24726. [Google Scholar] [CrossRef]
- Matsushita, T.; Mizumaki, K.; Kano, M.; Yagi, N.; Tennichi, M.; Takeuchi, A.; Okamoto, Y.; Hamaguchi, Y.; Murakami, A.; Hasegawa, M.; et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br. J. Dermatol. 2017, 176, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gono, T.; Masui, K.; Nishina, N.; Kawaguchi, Y.; Kawakami, A.; Ikeda, K.; Kirino, Y.; Sugiyama, Y.; Tanino, Y.; Nunokawa, T.; et al. Risk Prediction Modeling Based on a Combination of Initial Serum Biomarker Levels in Polymyositis/Dermatomyositis–Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021, 73, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.M.; Hodgkinson, L.M.; Wu, T.T.; Li, S.; Huard, C.; Zhao, S.; Bennett, D.; Johnson, J.; Tierney, C.; He, W.; et al. The Type I Interferon Signature Reflects Multiple Phenotypic and Activity Measures in Dermatomyositis. Arthritis Rheumatol. 2023, 75, 1842–1849. [Google Scholar] [CrossRef]
- Ren, F.-P.; Chen, Q.; Yao, S.-S.; Feng, L.; Xue, X.-Y.; Zhao, W.-C.; Wang, D.; Zhao, Z.-L.; Gu, S.-W.; Li, T.; et al. Characteristics and prognostic implications of peripheral blood lymphocyte subsets in patients with anti-MDA5 antibody positive dermatomyositis-interstitial lung disease. BMC Pulm. Med. 2023, 23, 411. [Google Scholar] [CrossRef]
- Mao, M.-M.; Xia, S.; Guo, B.-P.; Qian, W.-P.; Zheng, Z.-X.; Peng, X.-M.; Chen, R.-C.; Luo, Q.; Han, Q. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis /dermatomyositis associated ILD. Respir. Med. 2020, 172, 105983. [Google Scholar] [CrossRef] [PubMed]
- Romero-Bueno, F.; del Campo, P.D.; Trallero-Araguás, E.; Ruiz-Rodríguez, J.; Castellvi, I.; Rodriguez-Nieto, M.; Martínez-Becerra, M.; Sanchez-Pernaute, O.; Pinal-Fernandez, I.; Solanich, X.; et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin. Arthritis Rheum. 2020, 50, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Nakashima, R.; Hosono, Y.; Imura, Y.; Yagita, M.; Yoshifuji, H.; Hirata, S.; Nojima, T.; Sugiyama, E.; Hatta, K.; et al. Multicenter Prospective Study of the Efficacy and Safety of Combined Immunosuppressive Therapy With High-Dose Glucocorticoid, Tacrolimus, and Cyclophosphamide in Interstitial Lung Diseases Accompanied by Anti–Melanoma Differentiation–Associated Gene 5–Positive Dermatomyositis. Arthritis Rheumatol. 2020, 72, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Hosono, Y.; Mimori, T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus 2016, 25, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Go, D.J.; Park, J.K.; Kang, E.H.; Kwon, H.M.; Lee, Y.J.; Song, Y.W.; Lee, E.B. Survival benefit associated with early cyclosporine treatment for dermatomyositis-associated interstitial lung disease. Rheumatol. Int. 2016, 36, 125–131. [Google Scholar] [CrossRef]
- Fujisawa, T. Management of Myositis-Associated Interstitial Lung Disease. Medicina 2021, 57, 347. [Google Scholar] [CrossRef] [PubMed]
- Krämer, B.K.; Montagnino, G.; del Castillo, D.; Margreiter, R.; Sperschneider, H.; Olbricht, C.J.; Krüger, B.; Ortuño, J.; Köhler, H.; Kunzendorf, U.; et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol. Dial. Transplant. 2005, 20, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Ye, S. Tofacitinib in Amyopathic Dermatomyositis—Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 381, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Shirakashi, M.; Nakashima, R.; Tsuji, H.; Tanizawa, K.; Handa, T.; Hosono, Y.; Akizuki, S.; Murakami, K.; Hashimoto, M.; Yoshifuji, H.; et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology 2020, 59, 3284–3292. [Google Scholar] [CrossRef]
- Abe, Y.; Kusaoi, M.; Tada, K.; Yamaji, K.; Tamura, N. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy. Rheumatology 2020, 59, 767–771. [Google Scholar] [CrossRef]
- Femia, A.N.; Eastham, A.B.; Lam, C.; Merola, J.F.; Qureshi, A.A.; Vleugels, R.A. Intravenous immunoglobulin for refractory cutaneous dermatomyositis: A retrospective analysis from an academic medical center. J. Am. Acad. Dermatol. 2013, 69, 654–657. [Google Scholar] [CrossRef]
- Stager, K.; Wise, L. MDA-5 dermatomyositis complicated by interstitial lung disease and cutaneous ulcers: Successful treatment with corticosteroids, mycophenolate mofetil and intravenous immunoglobulin. BMJ Case Rep. 2020, 13, e236431. [Google Scholar] [CrossRef]
- Li, T.; Guo, L.; Chen, Z.; Gu, L.; Sun, F.; Tan, X.; Chen, S.; Wang, X.; Ye, S. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci. Rep. 2016, 6, 33226. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, H.; Ichiyasu, H.; Hirooka, S.; Akaike, K.; Kojima, K.; Jodai, T.; Sakamoto, Y.; Ideguchi, H.; Hamada, S.; Yoshida, C.; et al. Clinical effects of direct hemoperfusion using a polymyxin B-immobilized fiber column in clinically amyopathic dermatomyositis-associated rapidly progressive interstitial pneumonias. BMC Pulm. Med. 2017, 17, 134. [Google Scholar] [CrossRef]
- Leclair, V.; Labirua-Iturburu, A.; Lundberg, I.E. Successful Lung Transplantation in a Case of Rapidly Progressive Interstitial Lung Disease Associated with Antimelanoma Differentiation-associated Gene 5 Antibodies. J. Rheumatol. 2018, 45, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Levy, R.D.; Avina-Zubieta, J.A. Successful lung transplant in rapid progressive interstitial lung disease associated with anti-melanoma differentiation associated gene 5. Rheumatology 2020, 59, 2161–2163. [Google Scholar] [CrossRef] [PubMed]
- Rapidly progressive interstitial lung disease due to anti-melanoma differentiation associated protein-5 requiring a bilateral lung transplant, and complicated by kennel cough. Respir. Med. Case Rep. 2019, 28, 100886. [CrossRef]
- Lian, Q.-Y.; Chen, A.; Zhang, J.-H.; Xu, X.; Huang, D.-X.; Luo, Q.; He, J.-X.; Ju, C.-R. Lung transplantation for anti-MDA5-positive dermatomyositis-associated rapid progressive interstitial lung disease: Report of two cases and review of the literature. Clin. Rheumatol. 2023, 42, 941–947. [Google Scholar] [CrossRef]





| Medications | Dose * | Side Effects |
|---|---|---|
| Calcineurin inhibitors | Cyclosporine: 3–5 mg/kg/day (target trough 150–200 ng/mL) Tacrolimus: 0.075 mg/kg/day (target trough 5–10 ng/mL) [44,47] | Cyclosporine: Hyperglycemia, gingival hyperplasia, thrombotic microangiopathy, hepatotoxicity, hyperkalemia, hypertension, hirsutism, hyperlipidemia, nephrotoxicity Tacrolimus: Similar to cyclosporine, reduced risk of gingival hyperplasia and hirsutism |
| Glucocorticoids | IV methylprednisolone pulse (1000 mg for 3 days) or 0.75–1 mg/kg Prednisone for 4 weeks followed by gradual taper every 2–4 weeks [2,3,38,45] | May lead to increased risk of infections, osteoporosis, hyperglycemia, hypertension, mood changes, weight gain |
| Cyclophosphamide | 300–1000 mg/m2 IV every 2–4 weeks, recommended total of one to six doses [45,47] | Bone marrow suppression, infection risk, infertility, hemorrhagic cystitis |
| Rituximab | 375 mg/m2 at 0 and 14 days or 100 mg weekly for 4 weeks [27,42] | Infusion-related reactions, hypogammaglobulinemia and increased infection risk, reactivation of hepatitis B, progressive multifocal leukoencephalopathy |
| JAK inhibitors | Tofacitinib: 5 mg twice daily [49] | Bone marrow suppression, increased cardiovascular risk, gastrointestinal perforation, increased risk of infections, liver enzyme abnormalities |
| Plasma exchange | 1–3 times/week for 3–15 weeks (1–1.3 volumes of plasma removed per session and replaced with fresh frozen plasma) [50] | Vascular access related-bleeding, infection, thrombosis, replacement-related complications: dyspnea, pruritus, urticaria, fever, tachycardia |
| Intravenous immunoglobulin | 2 g/kg every 4 weeks, given as 1 g/kg/day for 2 consecutive days [52] | Generally well tolerated but potential for headache, nausea, allergic reactions, elevated liver enzymes, chest pain, tachycardia, hypertension |
| Antifibrotic Therapy | Pirfenidone: target dose of 1800 mg/day (started at 200 mg tid and was increased to the target dose of 600 mg tid over a 2-week period [54] | Nausea, fatigue, rash, diarrhea, elevated liver enzymes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.; Zickuhr, L.; Baral, M.R.; Bhalla, S.; Jones, H.; Bucelli, R.; Sen, D. A Review of MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. Rheumato 2024, 4, 33-48. https://doi.org/10.3390/rheumato4010004
Bhandari S, Zickuhr L, Baral MR, Bhalla S, Jones H, Bucelli R, Sen D. A Review of MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. Rheumato. 2024; 4(1):33-48. https://doi.org/10.3390/rheumato4010004
Chicago/Turabian StyleBhandari, Sambhawana, Lisa Zickuhr, Maun Ranjan Baral, Sanjeev Bhalla, Heather Jones, Robert Bucelli, and Deepali Sen. 2024. "A Review of MDA-5 Dermatomyositis and Associated Interstitial Lung Disease" Rheumato 4, no. 1: 33-48. https://doi.org/10.3390/rheumato4010004
APA StyleBhandari, S., Zickuhr, L., Baral, M. R., Bhalla, S., Jones, H., Bucelli, R., & Sen, D. (2024). A Review of MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. Rheumato, 4(1), 33-48. https://doi.org/10.3390/rheumato4010004






