Abstract
Rheumatoid meningitis, a very rare complication, is not well-recognised, and there are few reports describing its treatment. We report the case of a 74-year-old Japanese woman who was diagnosed with rheumatoid meningitis by characteristic brain magnetic resonance imaging (MRI) and was successfully treated with glucocorticoids. We observed fluid-attenuated inversion recovery and diffusion-weighted imaging hyperintensity, which had a meningeal gadolinium-enhancing characteristic of rheumatoid meningitis. We suggest that it is possible to diagnose this disease based on characteristic MRI findings and treat patients early using glucocorticoids.
1. Introduction
Rheumatoid meningitis is a rare disease in which central nervous system complications are unusual. Rheumatoid meningitis mainly involves the pia mater and typically develops in patients with rheumatoid arthritis [1]. Pathological findings include inflammatory cell infiltrates, rheumatoid nodules, and vasculitis that can be found around the pial blood vessels and in the subarachnoid space [2]. If a rheumatoid nodule is observed histologically on a biopsy sample, a definitive diagnosis can be made. However, the frequency of detection of such nodules is low, making it difficult to reach a definitive diagnosis [3]. Previously, rheumatoid meningitis was diagnosed by post-mortem autopsy, but in recent years, its detection has become easier due to advances in magnetic resonance imaging (MRI) [4]. Here, we report a case of rheumatoid meningitis in a 74-year-old Japanese woman who presented with typical MRI findings and had a favourable course of treatment with glucocorticoids.
2. Case Presentation
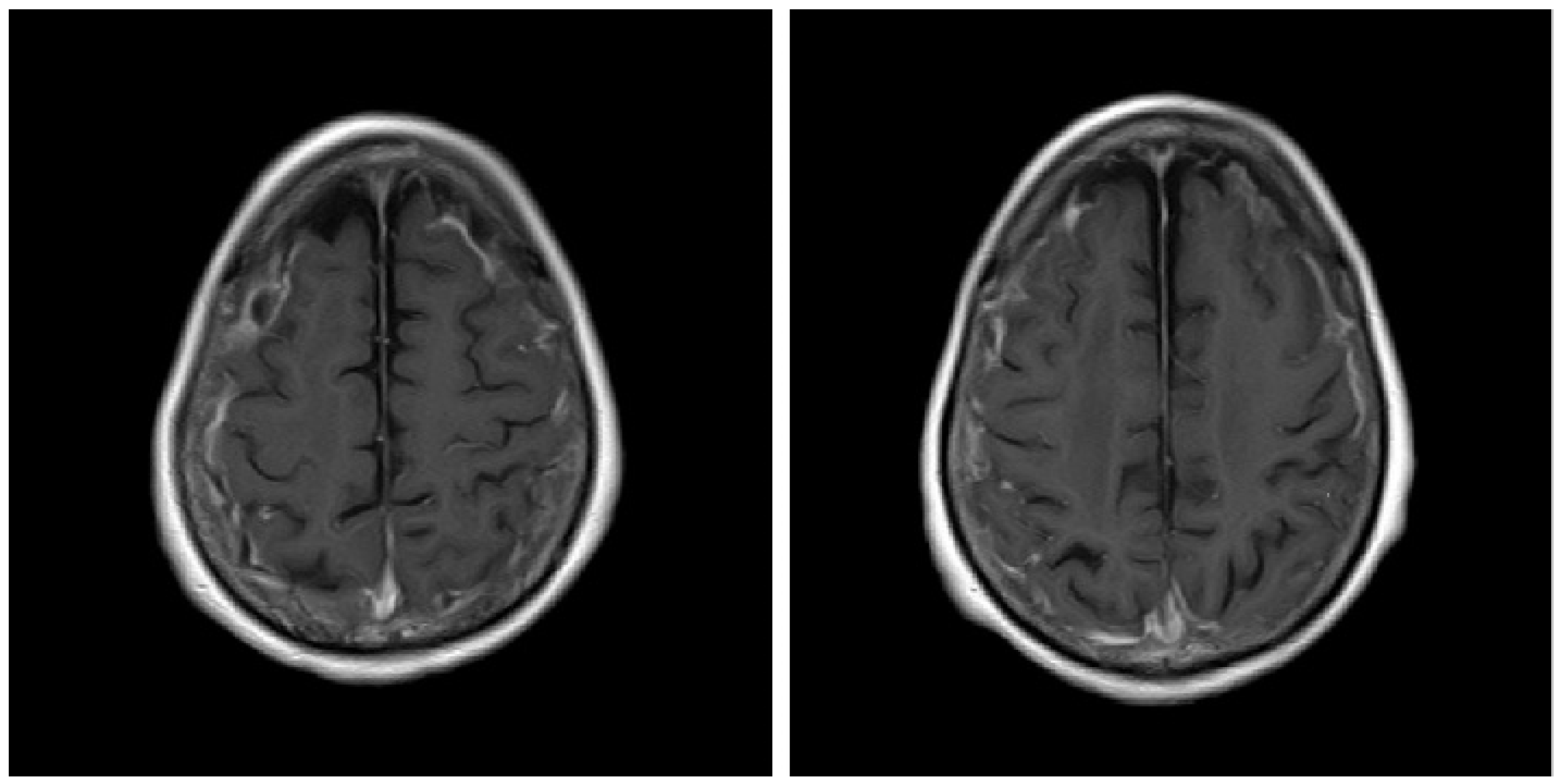
A 74-year-old Japanese woman who was diagnosed with rheumatoid arthritis 12 years earlier was admitted to our hospital. Fourteen months before admission to our hospital, she had discontinued methotrexate treatment due to thrombocytopenia. Nine months before admission, she was admitted to another hospital for status epilepticus, and cerebrospinal fluid (CSF) examination showed normal numbers of cells and protein levels and was negative for fungi, bacteria, tuberculosis, and herpes simplex virus. Three weeks before admission, a brain MRI was performed; fluid-attenuated inversion recovery (FLAIR) MR images showed hyperintensity along the sulci of both fornices, and T1-weighted (T1W1) images showed high signal after gadolinium (Gd) contrast agent administration (Figure 1). Therefore, she was diagnosed with meningitis and admitted to our hospital. On admission, she was taking prednisolone (8 mg/day) for arthritis.
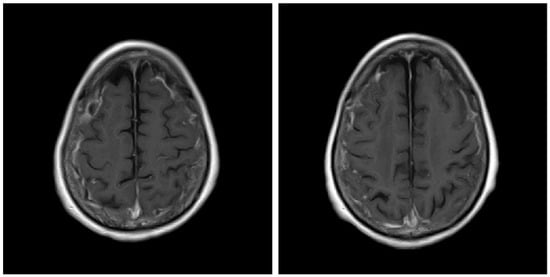
Figure 1.
Gadolinium-enhanced T1-weighted (T1W1) brain magnetic resonance imaging scan before treatment.
On admission, her height was 155 cm, body weight was 38.4 kg, the Glasgow Coma Scale (GCS) was E2V1M5, body temperature was elevated at 38.0 °C, pulse rate was 78 bpm, and blood pressure was 110/60 mmHg. There were no active lesions, such as swelling in the joints, but bilateral finger tendon rupture was observed. CSF examination showed an absence of red blood cells and was negative for xanthochromia, with levels of total protein of 32 mg/dL (normal range: 10–40 mg/dL), glucose of 53 mg/dL (normal range: 50–75 mg/dL), adenosine deaminase of <2.0 U/L (normal range: 5.0–20.0 U/L), IgG of 6.13 mg/dL (normal range: 1–3 mg/dL), IgG index of 0.42 (normal range: <0.73), albumin quotient evaluation of 6.3 (normal range: <8.0), and IL-6 of 4.6 pg/mL (normal range: 0–4 pg/mL). Blood tests showed levels of total proteins of 7.4 g/dL (normal range: 6.6–8.1 g/dL), albumin of 2.5 g/dL (normal range: 4.1–5.1 g/dL), C-reactive protein of 3.90 mg/dL (normal range: 0–0.14 mg/dL), rheumatoid factor of 877.9 U/mL (normal range: 0–14.9 U/mL), and IgG of 2348 mg/dL (normal range: 680–1620 mg/dL). The patient showed negativity for antinuclear antibody, anti-double-stranded deoxyribonucleic acid (ds-DNA) antibody, anti-Smith (Sm) antibody, anti-SS-A antibody, anti-ribonucleoprotein (RNP) antibody, proteinase 3-antineutrophil cytoplasmic antibodies (ANCA), and myeloperoxidase-ANCA.
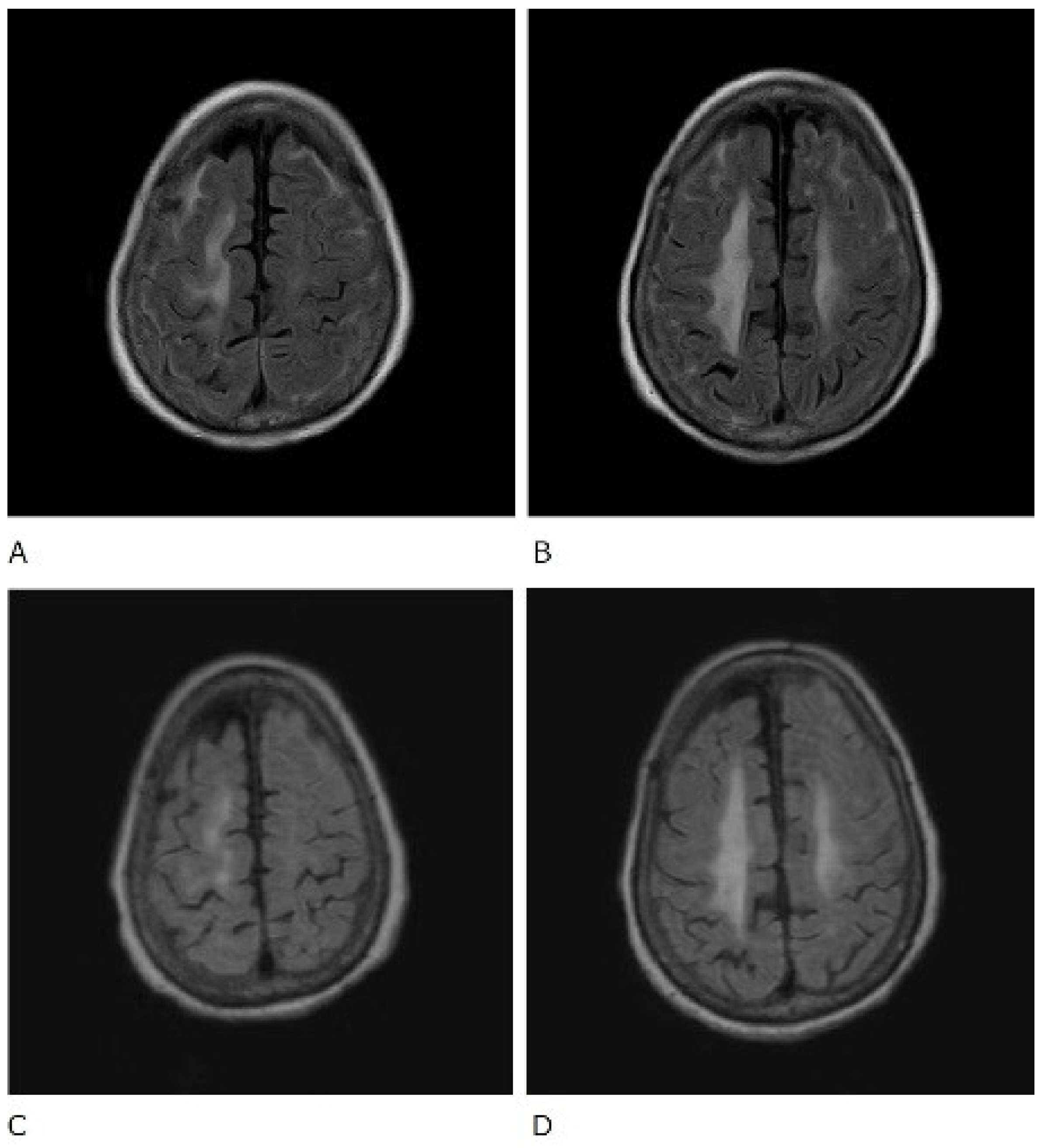
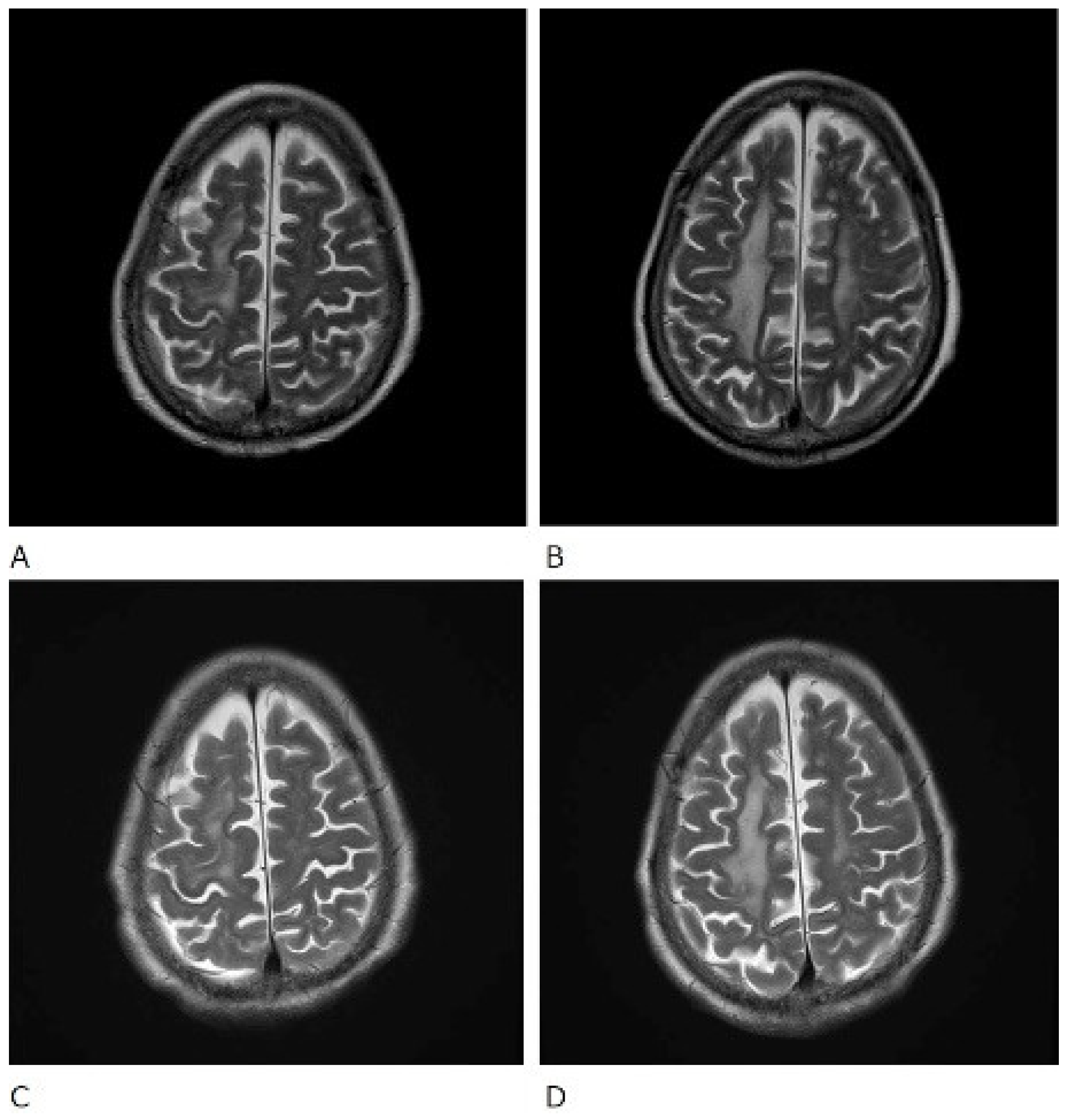
The patient was also tested for infectious diseases but was negative for herpes simplex virus 1, varicella-zoster virus, cytomegalovirus, Epstein–Barr virus, hepatitis B virus, hepatitis C virus, and cryptococcus. Differential diagnosis of non-infectious granulomatous meningitis includes sarcoidosis and tuberculosis. However, there were no other clinical findings, such as bilateral hilar lymphadenopathy, that were indicative of sarcoidosis. Moreover, she had no history of tuberculosis, and a current tuberculosis infection was excluded because no mycobacteria were detected in the CSF; her T-SPOT-TB was negative. Therefore, the patient was diagnosed with rheumatoid meningitis. Prednisolone sodium succinate (40 mg/day; 1 mg/kg/day) was administered by infusion on day 1 to day 9 after admission, and her consciousness level gradually improved. No antibiotics were used. MRI was performed on the 10th day of hospitalisation and showed that the high signal that had been observed in the previous FLAIR sequence had almost disappeared, except in the left frontal region. In contrast, no change was observed in diffuse hyperintensity (T2-WI, FLAIR) of cerebral white matter showing leukoaraiosis (Figure 2 and Figure 3). Since the level of consciousness had improved, her medication was changed to prednisolone 30 mg/day on Day 10 of hospitalisation. On Day 16, her consciousness level recovered to E4V4M6 on the GCS, and she was transferred to another hospital.
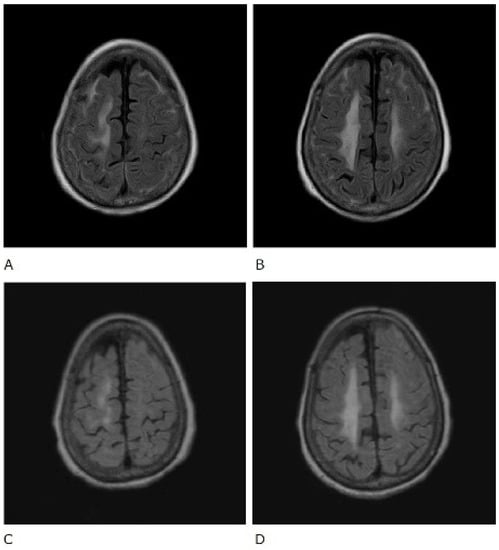
Figure 2.
Fluid-attenuated inversion recovery (FLAIR) sequence of brain magnetic resonance imaging. Before treatment (A,B) and after treatment (C,D).
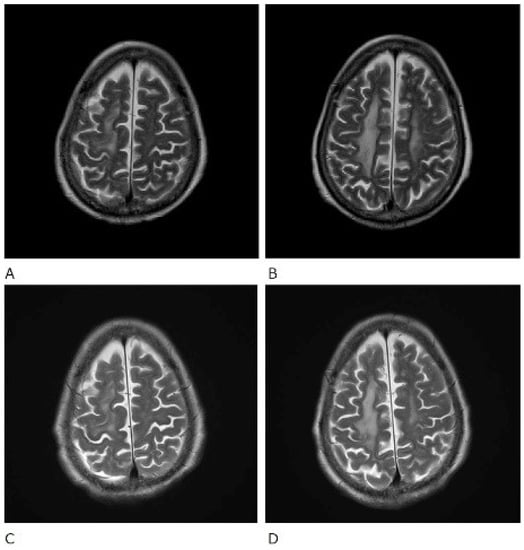
Figure 3.
T2-weighted (T2WI) magnetic resonance imaging scans of the brain. Before treatment (A,B) and after treatment (C,D).
3. Discussion
The typical neurological complications of rheumatoid arthritis are synovitis and subluxation of the joints, resulting in damage to the spinal cord and peripheral nerves. Meningitis with direct central nervous system involvement is extremely rare in rheumatoid arthritis [5]. The precise pathology remains unclear. However, it is associated with the infiltration of mononuclear cells around small vessels in the leptomeninges [6]. Rheumatoid nodules within the meninges are specific for rheumatoid meningitis but are not always present. The nodules consist of focal areas of necrosis surrounded by palmoplantar epithelial giant cells, and granulomatous inflammation and vasculitis may be present [7,8]. However, rheumatoid meningitis has no specific pathology, and there is no clear difference in treatment and outcome between patients who undergo biopsy and those who do not [9].
In contrast, rheumatoid meningitis presents with characteristic findings on MRI. Brain MRI findings of rheumatoid meningitis are mostly unilateral supratentorial lesions characterised by a high signal on FLAIR and diffusion-weighted imaging (DWI) sequences in the subarachnoid space and a Gd-enhancing effect of the meninges, composed mainly of the pia mater [10]. DWI hyperintensity often persists after the symptoms improve and the Gd-enhancing effect disappears and therefore does not necessarily indicate high activity [10]. In our case, FLAIR and DWI hyperintensity, which had a meningeal Gd-enhancing characteristic of rheumatoid meningitis, was also observed (Figure 1). Therefore, the patient was correctly diagnosed and started early on treatment. Central nervous system disorders associated with rheumatoid arthritis may involve both the pia mater and the dura mater. In cases with dura mater involvement, clinical symptoms such as headaches and cranial neuropathy are common [1]. In cases where the pia mater is affected, convulsion, paralysis, psychiatric symptoms, and gait disorders are common [1]. There are no guidelines for the treatment of rheumatoid meningitis, but previous reports have indicated that the administration of glucocorticoids alone led to improvement [11]. Similarly, our patient was treated with glucocorticoids alone and improved. Recurrent rheumatoid meningitis has also been reported to develop during the administration of infliximab, and anti-TNF-α inhibitors may be ineffective in these instances [12]. Therefore, it is still unclear what strategy is required for recurrence suppression and maintenance. However, rituximab has been shown to be effective in the suppression of rheumatoid arthritis and may potentially be effective in rheumatoid meningitis [12]. Other reports have indicated that the use of immunosuppressants such as azathioprine and cyclophosphamide in combination with glucocorticoids can also successfully treat rheumatoid meningitis [13].
According to previous reports, pathological findings revealed that vasculitis is involved in the mechanism of rheumatoid meningitis [1,2,13]. It has been postulated that immunosuppressants are effective for rheumatoid meningitis, as seen in other extra-articular vasculitis manifestations of rheumatoid arthritis [13]. Glucocorticoids remain the treatment of choice for initial and long-term treatment. Although glucocorticoids have an anti-inflammatory effect, the mechanism of action of glucocorticoids in rheumatoid meningitis is not well-studied.
To conclude, MRI is a useful tool in the diagnosis of rheumatoid meningitis. Unlike biopsy, MRI has the advantage of being non-invasive and relatively easy to perform. It is therefore possible to diagnose this disease from characteristic MRI findings and to treat patients early with glucocorticoids. We hope that there will be more reports on such cases in the future for a better understanding of this condition.
Author Contributions
T.T. was the first author of this article and was the inpatient doctor; H.K. (Hirotoshi Kikuchi) was the inpatient doctor and developed the treatment policy; K.A. was treating patients in the same team; H.K. (Hajime Kono) is the corresponding author and developed the treatment policy. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from a Japan Research Committee of the Ministry of Health, Labour and Welfare for Intractable Vasculitis (Hajime Kono).
Institutional Review Board Statement
All procedures performed in this report were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. An ethical approval statement was not required for this manuscript.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Servioli, M.J.; Chugh, C.; Lee, J.M.; Biller, J. Rheumatoid meningitis. Front. Neurol. 2011, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Bathon, J.M.; Moreland, L.W.; DiBartolomeo, A.G. Inflammatory central nervous system involvement in rheumatoid arthritis. Semin. Arthritis Rheum. 1989, 18, 258–266. [Google Scholar] [CrossRef]
- Abe, T.; Mishima, K.; Uchino, A.; Sasaki, A.; Tanahashi, N.; Takao, M. A case of anti-cyclic citrullinated peptides antibody positive rheumatoid meningitis without arthritis at the onset of neurological symptoms. Rinsho Shinkeigaku (Clin. Neurol.) 2016, 56, 627–632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Padjen, I.; Mayer, M.; Habek, M.; Kolenc, D.; Dotlic, S. Redefining a diagnosis: From meningeal plasma cell granuloma to rheumatoid meningitis. Report of a patient follow-up. Neurol. Sci. 2015, 36, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Belsey, N.A.; McLoud, T.C.; Mullins, M.E. Rheumatoid meningitis: Radiologic and Pathilogic Correlation. Am. J. Roentgenol. 2006, 186, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.; Romand, X.; Gilson, M.; Vaillant, M.; Guerne, P.A.; Hayem, G.; Bertolini, E.; Baillet, A.; Gaudin, P. Rheumatoid Meningitis a Rare Extra-Articular Manifestation of Rheumatoid Arthritis: Report of 6 Cases and Literature Review. J. Clin. Med. 2020, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Lee Ching, C.; Kenyon, L.; Berk, M.; Park, C. Rheumatoid meningitis sine arthritis. J. Neuroimmunol. 2019, 328, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Ho Park, Y.; Kim, J.A.; Han, J.H.; Choe, G.; Kim, S. Pearls & Oy-sters: Asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Case Rep. 2017, 88, e108–e110. [Google Scholar]
- Parsons, A.M.; Aslam, F.; Grill, M.F.; Aksamit, A.J.; Goodman, B.P. Rheumatoid Meningitis: Clinical Characteristics, Diagnostic Evaluation, and Treatment. Neurohospitalist 2020, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Terasaki, Y.; Sakaguchi, M.; Nakatsuji, Y.; Yoshizaki, K.; Mochizuki, H. A case of rheumatoid meningitis presented with generalized seizure in whom MRI images were helpful for the diagnosis. Rinsho Shinkeigaku (Clin. Neurol.) 2015, 55, 926–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, K.; Matsui, T.; Kawakami, M. Diffuse chronic leptomeningitis with seropositive rheumatoid arthritis: Report of case successfully treated as rheumatoid leptomeningitis. Mod. Rheumatol. 2009, 19, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.C.; Henson, J.W.; Tian, D. Successful treatment of rheumatoid meningitis with cyclophosphamide but not infliximab. Ann. Rheum. 2006, 65, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hoshi, K.; Sekijima, Y. Rheumatoid meningitis: An autopsy report and review of the literature. Clin. Rheumatol. 2003, 22, 475–480. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).