Abstract
The impact of the microbiota residing in the body on local and systemic immune responses has been increasingly recognized. The major gut microbe metabolites’ short-chain fatty acids (SCFAs) are suggested to regulate the balance between regulatory (Treg) cells and helper T 17 (Th17) cells in physiological and pathological conditions by enhancing regulatory T (Treg) cell function through epigenetic modifications. Patients with Behcet’s disease (BD) exhibited enhanced Th17 cell-mediated immune responses and decreased intestinal relative abundances of SCFA-producing bacteria. Causal correlations between aberrant immune responses and gut microbial composition in patients with BD have been reported in Italy, the Netherlands, Turkey, China, and Japan. We reported that the gut and oral microbiota profiles of patients with BD shared some common features. Immune responses against both commensal and pathogenic microbes may play a crucial role in BD development. This review summarizes the current literature, which was retrieved from public databases, such as PubMed and MEDLINE using search terms, including Behcet’s disease, helper T cells, and microbiota, during 1970–2022, on the potential functional correlation between immune cells and microbiota in patients with BD.
1. Introduction
Behcet’s disease (BD) is a chronic and multisystem inflammatory disorder characterized mainly by recurrent oral and genital ulcerations, repeated attacks of uveitis, and erythema nodosum-like skin lesions [1]. The incidence of BD varies depending on the geographical region. In particular, the prevalence of BD is high in the ancient Silk Road, which includes Italy, Turkey, Israel, Saudi Arabia, Iran, China, Korea, and Japan [2]. HLA-B51 has been detected in approximately 50% of patients with BD [2]. Genome-wide association studies (GWAS) have identified HLA-B51 and ERAP1 as genetic susceptibility factors for BD [3,4,5]. Environmental factors, including several microorganisms, are reported to mediate the pathogenesis of BD [1,2].
In the data of the International Classification of Disease in the United States, the mean hospital length of stay was higher in patients with BD than those in the general population with an admission diagnosis of sepsis, pneumonia, and left upper limb cellulitis [6]. Poor oral health is often correlated with severe BD [7]. For example, Streptococcus sanguinis, which causes dental plaque, is frequently detected in the oral cavity of patients with BD [8]. Conventional sterilization procedures decrease pathergy reactions in patients [9]. This indicates that the skin commensal microbes are associated with the severity of the reaction. Bacterial heat shock proteins (HSPs) exhibit a high degree of sequence homology with mammalian HSPs. Immunoreactivity to HSPs is postulated to be a potential mechanism of BD-related inflammation [10]. Lymphocytes from patients with BD strongly react with the HSP of Mycobacterium bovis compared with those from healthy individuals and patients with other immune diseases such as systemic lupus erythematosus and Sjogren’s syndrome [10].
Recently, the correlation between microbiota and the host immune system was reported to be stronger than was previously assumed. Culturable bacteria and their genes have been identified in human fetal organs, such as the gut, skin, and lungs, from the second trimester of gestation [11]. T cells in the fetal mesenteric lymph nodes react with both several bacterial strains and bacterium-primed dendritic cells during gestation [11].
Tumor-bearing mice gavaged with 11 bacterial strains exhibited enhanced interferon (IFN)-γ production by T cells. Additionally, the effects of immune checkpoint inhibitors were potentiated in these mice, potentially through bacterial antigen-induced T cell expansion [12].
Short-chain fatty acids (SCFAs), which are major gut microbial metabolites, are produced through the fermentation of dietary fibers [13]. Previous studies have reported that SCFAs promote regulatory T (Treg) cell differentiation in mouse models via epigenetic modification [14,15,16,17] and ameliorate the disease activities of colitis [14,15,16], arthritis [17], and encephalomyelitis [18]. Skewed T cell responses and altered gut microbial composition are reported in several human immune disorders [19,20].
This review summarizes the current understanding of the potential functional correlation between immune cells and microbiota in patients with BD. These host and bacterial factors of patients with BD have been suggested to share some common characteristics in different countries.
2. Search Methods
A literature review was performed using the following terms: “Behcet’s disease”, “helper T cells” (retrieved 199 articles with the term “Behcet’s disease”), “gut microbiota” (31 articles), “oral microbiota” (13 articles), “genome wide association study (GWAS)” (96 articles), “single nucleotide polymorphism (SNP) genotyping” (99 articles), “secretory IgA” (7 articles), “short chain fatty acids” (13 articles), “food supplements” (12 articles), “functional food” (6 articles), “herbals” (30 articles), and “medicine” (15 articles were retrieved with the terms Behcet’s disease and intestinal bacteria). Relevant original and review articles were collected through the PubMed, MEDLINE, Cochrane library, and Scopus databases between March and June 2022.
3. Immunopathology of BD
3.1. Lymphoid Cells
The most characteristic feature of the early stage of inflammation in BD is vasculitis with neutrophil infiltration near lesions, such as oral and genital ulcers, cutaneous erythema, and uveitis [1]. HLA-B51 and helper T 17 (Th17) cells are crucial factors for neutrophil activation [2].
3.1.1. Th Cells
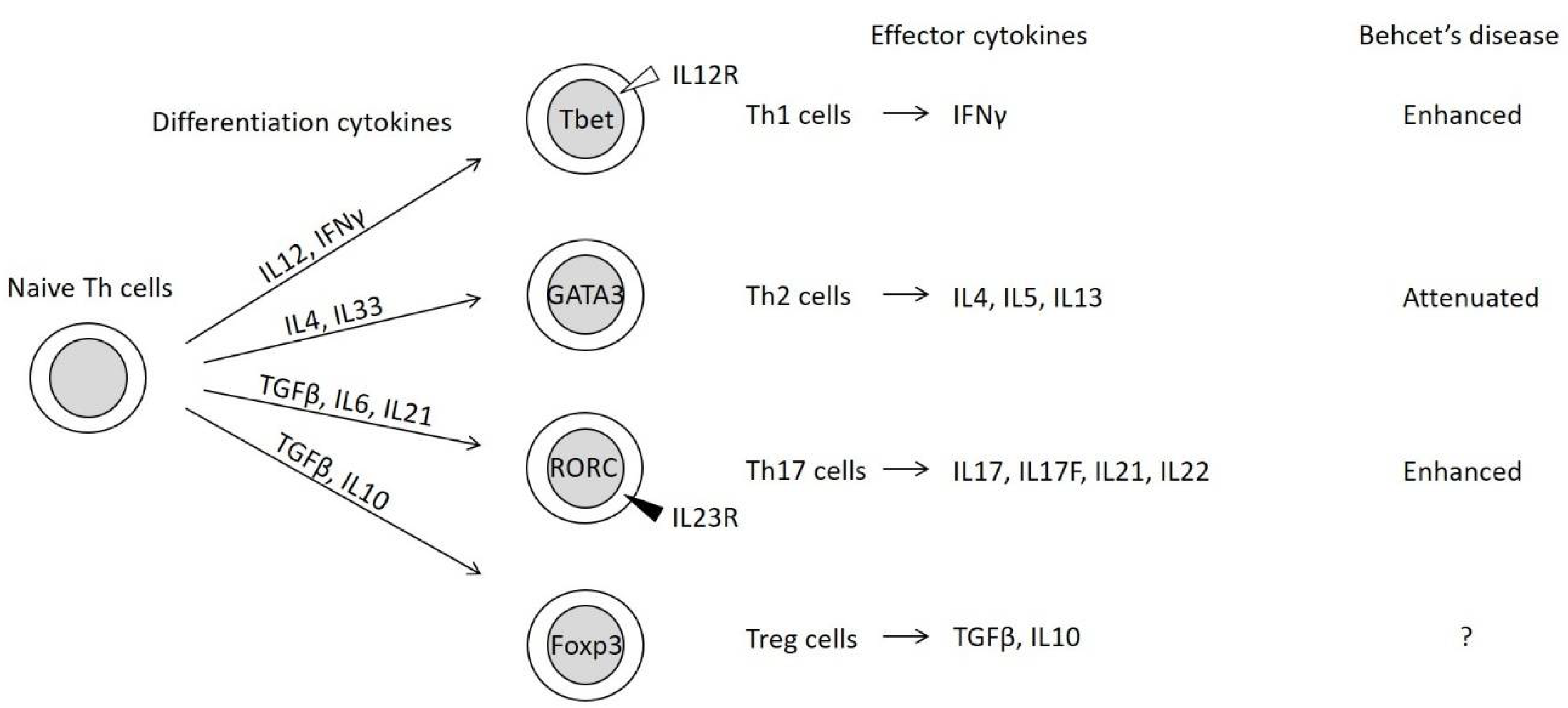
Naive Th cells differentiate into functional effector Th cells depending on the local immune environmental conditions, including cytokine and chemokine levels. Differentiated Th cells are classified into four subsets (Figure 1) [21,22].
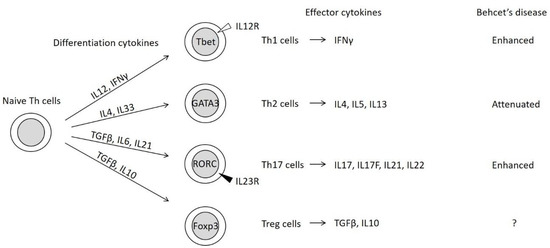
Figure 1.
Four subsets of differentiated helper T (Th) cells [21,22]. Naive Th cells differentiate into functional effector Th cells through the activities of differentiation-related cytokines and the expression of master transcription factor genes (represented within the circles). The master transcription factors repress the expression of alternate Th cell subset-related transcription factors [21]. IL12R, IL12 receptor; IL23R, IL23 receptor.
The concepts of Th1 and Th2 were initially proposed based on the characterization of effector cytokines and their functions [23]. Th1 cells induce the monocyte/macrophage-mediated immunity of monocytes/macrophages through the secretion of IFNγ. Meanwhile, Th2 cells promote humoral immunity and antibody production through the secretion of interleukin (IL)-4 and IL5.
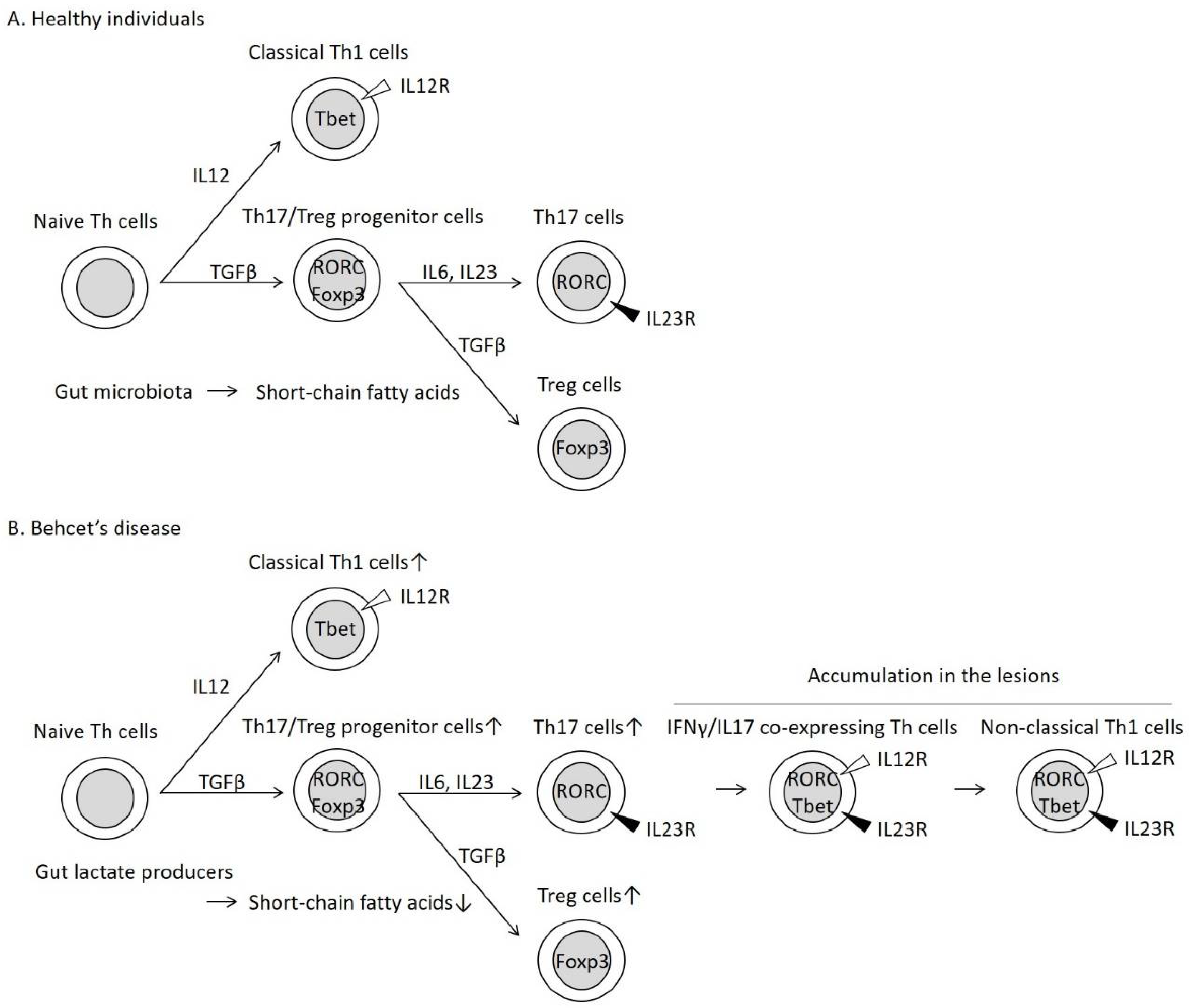
Th17 cells, which are characterized by the presence of the inducing cytokine, IL23, activate epithelial cells and neutrophils through IL17 secretion. Treg cells suppress Th cell function by secreting transforming growth factor (TGF)-β and IL10. The differentiation of Th17 and Treg cells involves common processes, especially the generation of Th17/Treg progenitor cells (Figure 2) [24]. Th17 and Treg cells are often activated in the intestine [22].
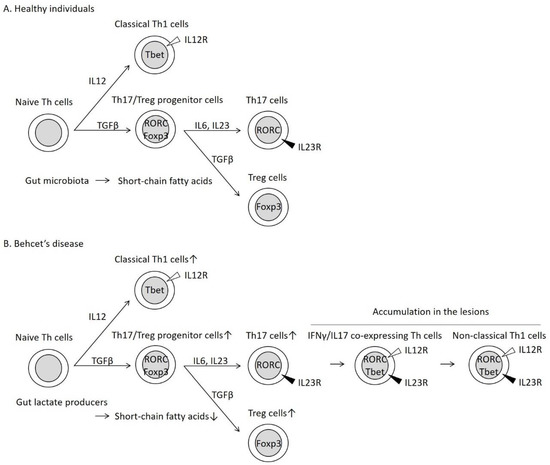
Figure 2.
Helper T 17 (Th17) cell differentiation into IFNγ/IL17 co-expressing Th cells and non-classical Th1 cells in patients with Behcet’s disease (BD). In healthy individuals (A), naive Th cells differentiate into classical Th1 cells through the activities of IL12 [21]. In the presence of TGFβ, naive Th cells are transformed to Th17/Treg progenitor cells, which subsequently differentiate into Th17 cells through the activities of inflammatory cytokines, such as IL6 and IL23 [24]. The same progenitor cells can differentiate into Treg cells without inflammatory cytokines [24]. In patients with BD (B), Th17 cells are unstable in the inflammatory condition and are transformed into IFNγ/IL17-expressing Th cells and non-classical Th1 cells [25]. Non-classical Th1 cells are distinguished from the classical Th1 cells based on the expression of the cytokine receptors [25]. These inflammatory Th cells were observed simultaneously in the skin lesions of patients with BD [26]. Short-chain fatty acids (SCFAs) derived from gut microbiota stabilize the expression of the Treg cell master transcription factor Foxp3 [14,15,16,17] and promote Treg cell differentiation [14,15,16,17,18]. Decreased intestinal SCFAs may enhance skewed Treg cell differentiation in patients with BD.
Inflammatory cytokines, such as IL6 and IL23, promote Th17 cell differentiation from progenitor cells (Figure 2) [24]. In contrast, the same progenitor cells differentiate into Treg cells without inflammatory cytokines [24]. In the periphery, the fate of Th17 cells is unstable, and Th17 cells are transformed into IFNγ/IL17-expressing Th cells (Figure 2) and subsequently into IFNγ-expressing cells (non-classical Th1 cells) (Figure 2) [25]. These Th cells are reported to play an important role in both physiological and pathological immune processes [25]. Thus, Th17 and Treg cells maintain the balance between the pro-inflammatory and anti-inflammatory conditions in the intestinal tract [22].
Previous studies have reported that Th1 and Th17 cell functions are upregulated, whereas Th2 cell function is suppressed in patients with BD [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The numbers of Th17 cells and IFNγ/IL17-expressing Th cells are upregulated in the peripheral blood of patients with BD [30,31,32,33,35,36,37,38,39,40,41,42,44,45]. In addition, to effector Th cells, such as Th1, Th17, and IFNγ/IL17-expressing Th cells [26,27,33,34] and Treg cells [34], have been detected in the erythema nodosum-like skin lesions of BD. The number of Treg cells and the expression levels of Treg-cell-related genes in patients with BD were upregulated when compared with those in healthy donors [34,46]. In contrast, several studies have demonstrated that these Treg-associated components in patients with BD were downregulated in BD patients when compared with those in healthy individuals [38,44,47]. The expression levels of the IL17 and IL23 [43]/IL23 receptor [41] were positively correlated in the Th cells of patients with BD. In the presence of IL1β, IL6, IL23, TGFβ, and tumor necrosis factor (TNF)-α, the production of IL17 in the cultured peripheral Th cells derived from patients with BD was markedly upregulated when compared with that in the cultured peripheral Th cells derived from healthy individuals [41]. Thus, these data indicate that various components of the pathways shown in Figure 2 are simultaneously activated in patients with BD.
3.1.2. γδ T Cells and Innate Lymphoid Cells (ILCs)
γδ T cells express toll-like receptors and react with bacterial antigens to promote bacterial clearance [48]. Similar to Th17 cells, IL17-producing γδ T cells tend to migrate into mucosal tissues, such as the lungs and intestine [49], and induce the rapid infiltration of neutrophils into the lesions of Escherichia coli-infected mice [50] in the presence of IL23 [51]. Vγ9δ2+ T cells, which are a major subset of γδ T cells, recognize bacterial antigens [52], whereas Vδ1+ T cells react with the major histocompatibility complex class I-related chain A (MICA) expressed on the damaged intestinal epithelium [53].
The number of Vγ9δ2+ T cells was upregulated in the peripheral blood of patients with BD. Additionally, Vγ9δ2+ T cells produce TNFα and perforin granules [54]. One study demonstrated that the numbers of Vδ2+ T cells and Vδ1+ T cells were upregulated in the peripheral blood and bronchoalveolar lavage/cerebrospinal fluid, respectively, of patients with BD [55]. Furthermore, Vγ9δ1+ T cells react with Streptococcus sanguinis without an apparent HLA restriction [56]. These data suggest that γδ T cells may also be associated with the development of inflammatory responses in patients with BD.
A similar concept may aid in elucidating the role of ILCs in the pathogenesis of BD. ILCs, which are a subgroup of lymphocytes that lack antigen-specific receptors [57], are divided into three subgroups, ILC1, ILC2, and ILC3. The ILC3 subgroup, which may function as the innate counterpart of Th17 cells, produces IL17 in the presence of IL23 [57].
The peripheral blood levels of the total ILCs and ILC3 in patients with active BD were higher than those in patients with inactive BD and healthy individuals [58].
Thus, these lymphoid cells can initiate BD inflammation by secreting IL17.
3.2. HSPs
Bacterial and mammalian HSPs share a high sequence homology [10,59]. Human HSP60 shares approximately 70% and 40% sequence homology with Chinese hamster HSP60 and mycobacterial HSP65, respectively. Bioinformatic analyses HSP65 has a high binding capacity to HLA-B51 [60].
In the presence of an HSP peptide derived from Mycobacterium bovis, the production of IFNγ, IL12, and TNFα in the peripheral blood lymphocytes of patients with BD was higher than that in the peripheral blood lymphocytes of healthy individuals [27]. Lymphocyte proliferative responses against mycobacterial HSP65 peptides and/or homologous human HSP60 peptides were observed in patients with BD residing in the United Kingdom [10], France [45], Turkey [61], and Japan [60,62]. HSP60 is expressed in the peripheral blood lymphocytes and intestinal tissues of patients with BD but not in those of healthy individuals [28].
These data suggest that bacterial HSPs mimic human HSPs and that cross-reactive T cells play a role in the pathogenesis of BD.
3.3. Pathergy Test
A positive pathergy test has been reported in approximately 30–60% of patients with BD [63]. Surgical sterilization of the skin decreases the positivity rates of tests in patients with BD [9]. The proportion of positive test results varied depending on the body areas with the highest proportion observed in the forearm [64]. Skin pricks with self-collected saliva samples increased the sensitivity of the test [65]. Meanwhile, needle punctures cause tissue damage in the eye [66] and blood vessels [67] in patients with BD. These data suggest that the overactivation of immune cells against multiple commensal microbes may contribute to the pathogenesis of BD.
The numbers of lymphocytes, monocytes, and neutrophils were upregulated in the skin lesions but were not markedly different between patients with BD and healthy individuals at 8 h post-needle prick [68]. At 48 h post-needle prick, the immune responses of the healthy donors were limited or slightly decreased, whereas the migration of the lymphocytes and monocytes was further enhanced in patients with BD. Gene expression analyses of skin tissues revealed that, in addition to Th1 responses (IFNγ and IL12), Treg responses (CD25 and Foxp3) were significantly upregulated in BD lesions [68], which was consistent with the results of previous studies that demonstrated the enhancement of both effector Th and Treg cells in patients with BD [34,46].
4. Potential Correlation between Microbiota and Immunopathology in BD
4.1. Gut Microbiota in Human Disease
Gut microbiota may be associated with the pathogenesis of human diseases, such as obesity, type 2 diabetes, non-alcoholic fatty liver disease, coeliac disease, inflammatory bowel disease, and colorectal cancer, mainly through the production of metabolites [69]. Microbial metabolites primarily comprise SCFAs, bile acids, and trimethylamine N-oxide (TMAO) in humans [69].
SCFAs, such as acetate, propionate, and butyrate, are produced through the bacterial fermentation of indigestible carbohydrates (dietary fiber) and are absorbed into epithelial cells as an energy source [13]. Based on physiological metabolism, SCFAs increase the expression of tight junction proteins [70]. A low-fiber diet reduces the colonic mucus barrier function owing to the prevalence of mucin-degrading bacteria [71]. High intestinal permeability in genetically engineered mucus-deficient mice increases susceptibility to colitis with transmissible gut microbiota alterations in cohabitants [72]. The leakage of lipopolysaccharides from the intestine [72] may be related to type 2 diabetes, atherosclerosis, and several types of cancer [73,74].
In the gastrointestinal tract, enteroendocrine cells express receptors for SCFAs and secrete glucagon-like peptides (GLPs) and peptide YY in response to stimulation, suggesting a close relationship between intestinal SCFAs and host glucose metabolism [13]. Therefore, SCFAs in the intestine improve insulin secretion and the sensitivity of the host [73,74].
Similar protective effects on host glucose homeostasis have been reported in bile acid metabolism via the secretion of GLP1 and insulin [75]. A high-fat diet promotes the production of other major bacterial metabolites and secondary bile acids, which are reported to be associated with tumorigenesis, especially in the intestine [73,74,76].
Choline and L-carnitine in the intestine induce the production of trimethylamine (TMA), a newly discovered bacterial metabolite. Subsequently, TMA is bioactivated to TMAO in the liver under high-fat diet conditions [77]. The plasma level of TMAO is correlated with the risk of death, myocardial infarction, and stroke [78].
Thus, these data suggest highly complex interactions between bacterial metabolites and host organs in human diseases.
4.2. Physiological Correlation between Gut Microbiota and Th Cell Function through SCFAs and the Pathology of Rheumatic Diseases
SCFAs promote Treg cell differentiation via epigenetic modification of Foxp3 (Figure 2) [14,15,16,17,22].
Th17 and Treg cells may regulate the balance between pro-inflammatory and anti-inflammatory immune responses [22]. Patients with hyper-immunoglobulin E syndrome exhibit Th17 cell deficiencies and are highly susceptible to Candida albicans and Staphylococcus aureus infections, resulting in skin and lung inflammation [79]. Mutations in the Treg cell master transcription factor-encoding FoxP3 (Figure 1 and Figure 2) result in severe immune dysregulation and autoimmunity in humans (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, and increased numbers of Th17 cells) [80,81].
SCFAs may be among the most effective bacterial metabolites involved in the maintenance of the Th cell balance for the host immune responses [22]. In several human immune disorders, such as rheumatoid arthritis and psoriatic arthritis, enhanced Th17 differentiation in the peripheral blood and a decreased abundance of SCFA-producing bacteria in the intestine have been reported. The causal relationship between these factors has been speculated [19,20].
These data will enable the elucidation of the correlation between gut microbiota and immune function in patients with BD.
4.3. Gut Microbiota Profile in Japanese Patients with BD
Gut microbiota analyses were performed with Japanese patients with BD [82,83]. The bacterial species composition was markedly different between patients with BD and healthy donors.
The abundances of the genera Eggerthella, Bifidobacterium, and Lactobacillus were significantly upregulated in patients with BD. Meanwhile, the abundances of the genera Megamonas, Butyrivibrio, and Phascolarctobacterium were significantly upregulated in healthy individuals.
Butyrivibrio species, which were enriched in patients with BD, produce butyrate from wheat bran (dietary fiber) in the in vitro fermentation system of human microbiota [84]. Phascolarctobacterium species produce propionate [85] by utilizing lactate [86]. Bifidobacterium and Lactobacillus species are the major lactate-producing bacteria [86].
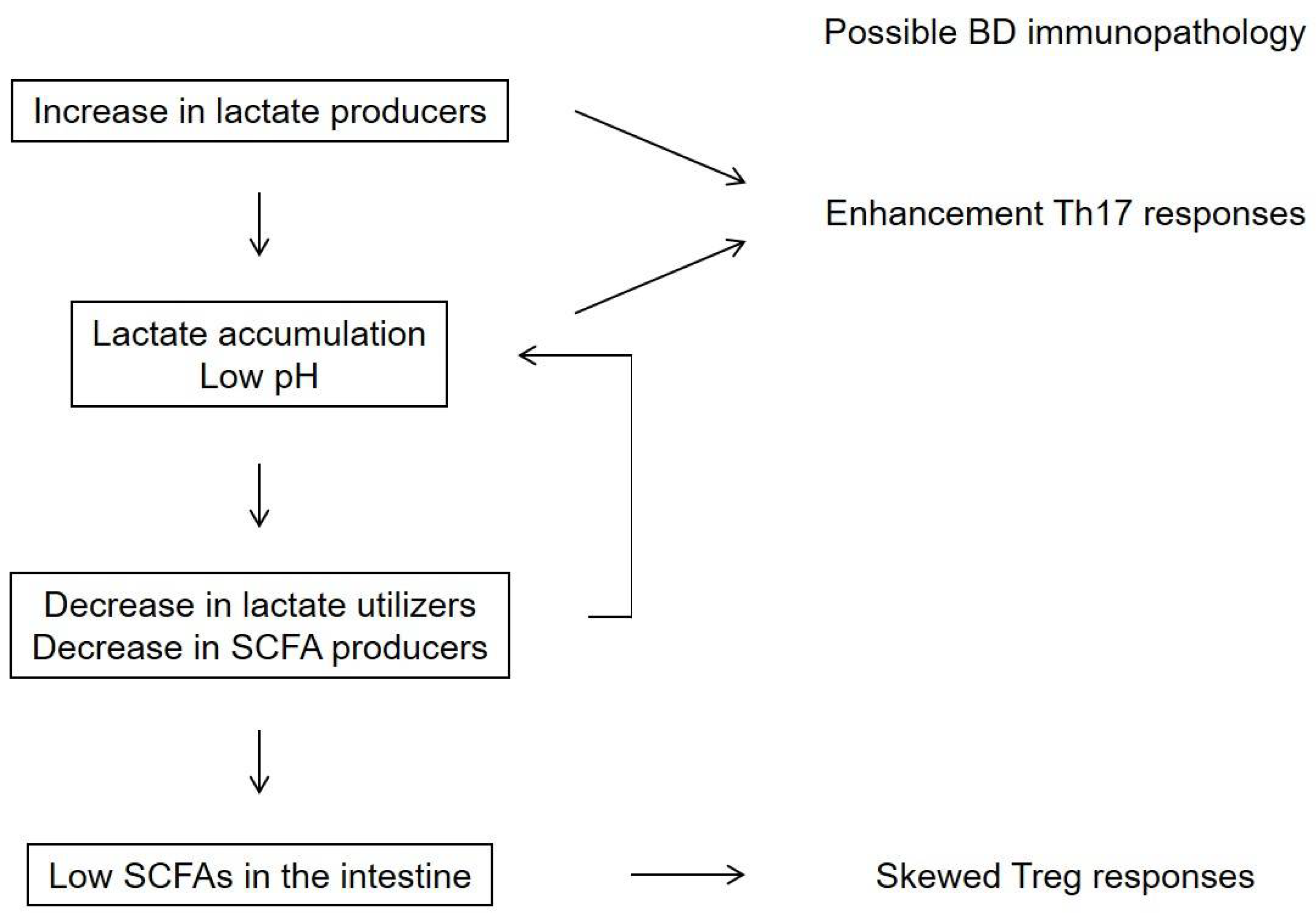
Recently, lactate-producing, lactate-utilizing, and SCFA-producing bacteria have been suggested to play a role in maintaining the immune equilibrium of the colon through the production of various metabolites [86]. Several bacterial species in the intestine produce lactate, whereas limited numbers of bacteria produce SCFA with some utilizing lactate for SCFA production [85,86,87]. Low pH conditions in culture experiments on human gut microbiota resulted in decreased lactate consumption and SCFA concentrations with lactate accumulation (Figure 3) [86,87,88].
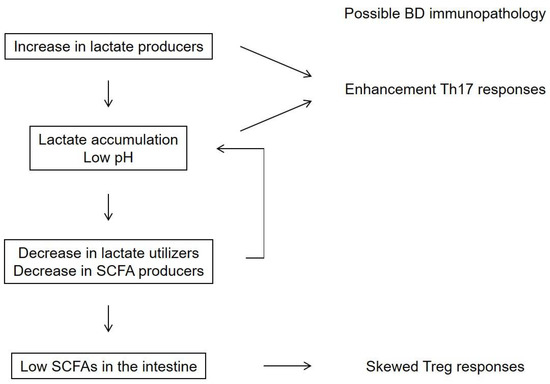
Figure 3.
A hypothesis for the mechanism underlying the downregulation of short-chain fatty acids (SCFAs) in the intestine of patients with Behcet’s disease (BD). A high prevalence of lactate-producing bacteria may lead to a low pH in the intestine and a decreased abundance of lactate-utilizing bacteria [86]. Lactate-producing bacteria and extracellular lactate were reported to enhance Th17 responses [89,90]. These metabolite processes may be associated with low SCFA concentrations in the intestine and subsequently skewed Treg responses in patients with BD [20].
Lactate is reported to accumulate in the intestine of patients with severe colitis [91,92]. Previous studies have reported that lactate-producing bacteria and extracellular lactate enhance Th17 responses [89,90]. These metabolite processes may be associated with low SCFA concentrations in the intestine and subsequently skewed Treg responses in patients with BD [20].
4.4. Comparison among Countries
The microbiota of patients with BD has been analyzed in other countries [93,94,95,96,97,98]. The composition of gut microbiota is diverse in human populations [99]. Thus, the microbial profile data of patients with BD are considered valuable. Table 1 summarizes the gut and oral microbial profiles in patients with BD from various countries.

Table 1.
Summary of the gut and oral microbial profiles in patients with BD in different countries.
Several lactate-producing gut and oral bacteria have been detected in the microbiota of patients with BD. Meanwhile, several SCFA-producing gut and oral bacteria have been detected in the microbiota of healthy individuals. These data support the hypothesis that intestinal lactate concentrations, SCFA concentrations, gut microbial composition, and skewed T cell responses are correlated in patients with BD. The fecal levels of butyrate in patients with BD are lower than those in healthy individuals [93].
In mouse experiments, orally administered bacteria hardly colonized the intestine, which can be attribute to the competition with commensals [100]. Ingested Klebsiella species colonized the intestine and expanded both Th1 cells and IFNγ/IL17-expressing Th cells in the colonic lamina propria in IL10-deficient mice (spontaneous colitis model) [101]. These mechanisms may contribute to the species similarity in the gut and oral microbiota of patients with BD.
4.5. Immunoglobulin-A (IgA) Sequencing (IgA-seq)
Secretory IgA in the intestine is critical for distinguishing commensals from pathogens under the regulation of Th cells [102]. The fecal level of secretory IgA in patients with BD are higher than those in healthy individuals [82]. These data suggest that secretory IgA concentrations in patients with BD are associated with the persistent dysregulation of the intestinal mucosal immune system.
The binding of IgA to gut microbes is assessed using cell sorting, while pathogens are detected using IgA-seq [103]. These analyses have been performed on patients with BD in Italy and the Netherlands [94]. One of the most predominant IgA-coated bacteria in patients with BD belonged to the genus Bifidobacterium, a major lactate producer [86] and a prevalent bacterium in Japanese patients with BD [82,83] (Table 1). Replication studies and an understanding of the exact role of secretory IgA in the intestine of patients with BD are required.
4.6. Potential Immunomodulatory Effects of Dietary Supplementation and Herbal Medicine on BD
Various novel therapeutic strategies involving dietary supplementation and herbal medicines are being developed for patients with BD.
The dysregulation of zinc, an essential micronutrient for cellular processes, is reported to be associated with skin manifestations [104]. The serum zinc levels were downregulated and inversely correlated with pathergy test positivity grades in patients with BD when compared with those in healthy individuals [105]. Zinc supplementation improved several activity indices and downregulated the expression levels of toll-like receptor (TLR)-2 [106] and Nod-like receptor-3 (NLRP3) [107] in the white blood cells of patients.
Meta-analyses demonstrated that, compared with those in the control group, the total effective rates of Chinese herbal medicines were higher and the disease recurrence rates were lower in patients with BD [108,109]. The modulatory effects of the herbal medicine decoctions on T cell balance in other human diseases are described in the discussion sections.
A case report on BD revealed that the administration of capsules containing seven strains of live bacteria and concentrated fructo-oligosaccharide, which is one of the most well-studied fermentable dietary fibers [110], gradually ameliorated anterior uveitis [111]. The authors suggested preferable properties of the remedies for the regulation of T cell balance in patients [112].
Recently, a clinical trial was initiated comparing the effects of a vegetarian diet, Mediterranean diet, and Mediterranean diet supplemented with butyrate in patients with BD [113]. Further investigations of food-based interventions are needed to develop new prophylactic or therapeutic strategies for chronic and recurrent inflammatory diseases such as BD.
5. Conclusions
BD is highly prevalent in some areas, which may be attributed to the HLA type. Th cell responses and gut microbiota composition were similar in patients with BD from different countries as described in this review (Table 1), suggesting that these biological factors are also affected by HLA.
Similar correlations between human immune disorders and HLA molecules have been reported in ankylosing spondylitis and psoriasis, which are classified as MHC class I-opathies. Patients with ankylosing spondylitis and psoriasis have characteristic alterations in the gut microbiota.
Further studies in this field will contribute to the development of novel therapeutic strategies for not only BD but also ankylosing spondylitis and psoriasis based on the modification of intestinal lymphocytes and microbes.
Author Contributions
J.S. conceived and prepared the manuscript; M.A.M., Y.M. and N.S. prepared and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakane, T.; Takeno, M.; Suzuki, N.; Inaba, G. Behçet’s disease. N. Engl. J. Med. 1999, 341, 1284–1291. [Google Scholar] [CrossRef]
- Yazici, Y.; Hatemi, G.; Bodaghi, B.; Cheon, J.H.; Suzuki, N.; Ambrose, N.; Yazici, H. Behcet syndrome. Nat. Rev. Dis. Primers 2021, 7, 67. [Google Scholar] [CrossRef]
- Remmers, E.F.; Cosan, F.; Kirino, Y.; Ombrello, M.J.; Abaci, N.; Satorius, C.; Le, J.M.; Yang, B.; Korman, B.D.; Cakiris, A.; et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet’s disease. Nat. Genet. 2010, 42, 698–702. [Google Scholar] [CrossRef]
- Mizuki, N.; Meguro, A.; Ota, M.; Ohno, S.; Shiota, T.; Kawagoe, T.; Ito, N.; Kera, J.; Okada, E.; Yatsu, K.; et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet’s disease susceptibility loci. Nat. Genet. 2010, 42, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Bertsias, G.; Ishigatsubo, Y.; Mizuki, N.; Tugal-Tutkun, I.; Seyahi, E.; Ozyazgan, Y.; Sacli, F.S.; Erer, B.; Inoko, H.; et al. Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat. Genet. 2013, 45, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Naramala, S.; Konala, V.M.; Adapa, S.; Gayam, V.; Sidhu, J.; Biswas, S.; Balla, M.; Merugu, G.P.; Pattanaik, D. Trends in hospitalization and inpatient outcomes of Behçet’s disease: A nationwide inpatient sample study. Cureus 2020, 12, e7470. [Google Scholar] [CrossRef] [PubMed]
- Mumcu, G.; Ergun, T.; Inanc, N.; Fresko, I.; Atalay, T.; Hayran, O.; Direskeneli, H. Oral health is impaired in Behcet’s disease and is associated with disease severity. Rheumatol. Oxf. 2004, 43, 1028–1033. [Google Scholar] [CrossRef]
- Yokota, K.; Hayashi, S.; Araki, Y.; Isogai, E.; Kotake, S.; Yoshikawa, K.; Fujii, N.; Hirai, Y.; Oguma, K. Characterization of Streptococcus sanguis isolated from patients with Behcet’s disease. Microbiol. Immunol. 1995, 39, 729–732. [Google Scholar] [CrossRef]
- Fresko, I.; Yazici, H.; Bayramiçli, M.; Yurdakul, S.; Mat, C. Effect of surgical cleaning of the skin on the pathergy phenomenon in Behçet’s syndrome. Ann. Rheum. Dis. 1993, 52, 619–620. [Google Scholar] [CrossRef]
- Pervin, K.; Childerstone, A.; Shinnick, T.; Mizushima, Y.; van der Zee, R.; Hasan, A.; Vaughan, R.; Lehner, T. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behçet’s disease. J. Immunol. 1993, 151, 2273–2282. [Google Scholar]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184, 3394–3409.e20. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2016, 44, 951–953. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in rheumatic diseases: An overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Tuganbaev, T.; Skelly, A.N.; Honda, K. T cell responses to the microbiota. Annu. Rev. Immunol. 2022, 40, 559–587. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Zhou, L.; Lopes, J.E.; Chong, M.M.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Maggi, L.; Liotta, F.; Cosmi, L.; Annunziato, F. Biological and clinical significance of T helper 17 cell plasticity. Immunology 2019, 158, 287–295. [Google Scholar] [CrossRef]
- Nagafuchi, H.; Takeno, M.; Yoshikawa, H.; Kurokawa, M.S.; Nara, K.; Takada, E.; Masuda, C.; Mizoguchi, M.; Suzuki, N. Excessive expression of Txk, a member of the Tec family of tyrosine kinases, contributes to excessive Th1 cytokine production by T lymphocytes in patients with Behcet’s disease. Clin. Exp. Immunol. 2005, 139, 363–370. [Google Scholar] [CrossRef]
- Imamura, Y.; Kurokawa, M.S.; Yoshikawa, H.; Nara, K.; Takada, E.; Masuda, C.; Tsukikawa, S.; Ozaki, S.; Matsuda, T.; Suzuki, N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin. Exp. Immunol. 2005, 139, 371–378. [Google Scholar] [CrossRef]
- Chi, W.; Zhu, X.; Yang, P.; Liu, X.; Lin, X.; Zhou, H.; Huang, X.; Kijlstra, A. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3058–3064. [Google Scholar] [CrossRef]
- Chi, W.; Yang, P.; Zhu, X.; Wang, Y.; Chen, L.; Huang, X.; Liu, X. Production of interleukin-17 in Behcet’s disease is inhibited by cyclosporin A. Mol. Vis. 2010, 16, 880–886. [Google Scholar]
- Geri, G.; Terrier, B.; Rosenzwajg, M.; Wechsler, B.; Touzot, M.; Seilhean, D.; Tran, T.A.; Bodaghi, B.; Musset, L.; Soumelis, V.; et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J. Allergy Clin. Immunol. 2011, 128, 655–664. [Google Scholar] [CrossRef]
- Hamzaoui, K.; Bouali, E.; Ghorbel, I.; Khanfir, M.; Houman, H.; Hamzaoui, A. Expression of Th-17 and RORγt mRNA in Behcet’s disease. Med. Sci. Monit. 2011, 17, CR227–CR234. [Google Scholar] [CrossRef]
- Shimizu, J.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Yoshikawa, H.; Kaneko, F.; Suzuki, T.; Suzuki, N. Excessive CD4+ T cells co-expressing interleukin-17 and interferon-γ in patients with Behcet’s disease. Clin. Exp. Immunol. 2012, 168, 68–74. [Google Scholar] [CrossRef]
- Shimizu, J.; Izumi, T.; Arimitsu, N.; Fujiwara, N.; Ueda, Y.; Wakisaka, S.; Yoshikawa, H.; Kaneko, F.; Suzuki, T.; Takai, K.; et al. Skewed TGFβ/Smad signalling pathway in T cells in patients with Behcet’s disease. Clin. Exp. Rheumatol. 2012, 30 (Suppl. 72), 35–39. [Google Scholar]
- Na, S.Y.; Park, M.J.; Park, S.; Lee, E.S. Up-regulation of Th17 and related cytokines in Behçet’s disease corresponding to disease activity. Clin. Exp. Rheumatol. 2013, 31 (Suppl. 77), 32–40. [Google Scholar]
- Shimizu, J.; Kaneko, F.; Suzuki, N. Skewed helper T-cell responses to IL-12 family cytokines produced by antigen-presenting cells and the genetic background in Behcet’s disease. Genet. Res. Int. 2013, 2013, 363859. [Google Scholar] [CrossRef]
- Wang, C.; Tian, Y.; Ye, Z.; Kijlstra, A.; Zhou, Y.; Yang, P. Decreased interleukin 27 expression is associated with active uveitis in Behçet’s disease. Arthritis Res. Ther. 2014, 16, R117. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Z.; Kijlstra, A.; Zhou, Y.; Yang, P. Decreased expression of the aryl hydrocarbon receptor in ocular Behcet’s disease. Mediat. Inflamm. 2014, 2014, 195094. [Google Scholar] [CrossRef]
- Aktas Cetin, E.; Cosan, F.; Cefle, A.; Deniz, G. IL-22-secreting Th22 and IFN-γ-secreting Th17 cells in Behçet’s disease. Mod. Rheumatol. 2014, 24, 802–807. [Google Scholar] [CrossRef]
- Emmi, G.; Silvestri, E.; Bella, C.D.; Grassi, A.; Benagiano, M.; Cianchi, F.; Squatrito, D.; Cantarini, L.; Emmi, L.; Selmi, C.; et al. Cytotoxic Th1 and Th17 cells infiltrate the intestinal mucosa of Behcet patients and exhibit high levels of TNF-α in early phases of the disease. Med. Baltim. 2016, 95, e5516. [Google Scholar] [CrossRef]
- Ye, Z.; Deng, B.; Wang, C.; Zhang, D.; Kijlstra, A.; Yang, P. Decreased B and T lymphocyte attenuator in Behcet’s disease may trigger abnormal Th17 and Th1 immune responses. Sci. Rep. 2016, 6, 20401. [Google Scholar] [CrossRef]
- Shimizu, J.; Takai, K.; Takada, E.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Possible association of proinflammatory cytokines including IL1β and TNFα with enhanced Th17 cell differentiation in patients with Behcet’s disease. Clin. Rheumatol. 2016, 35, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Hwang, J.H.; Zheng, Z.; Bang, D.; Kim, D.Y. Enhancement of Th1/Th17 inflammation by TRIM21 in Behçet’s disease. Sci. Rep. 2017, 7, 3018. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, C.; Yucel, A.A.; Yesil, T.H.; Kucuk, H.; Sezgin, B.; Mercan, R.; Yucel, A.E.; Demirel, G.Y. Correlation between IL-17A/F, IL-23, IL-35 and IL-12/-23 (p40) levels in peripheral blood lymphocyte cultures and disease activity in Behcet’s patients. Clin. Rheumatol. 2018, 37, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Yousefi, M.; Abbaspour-Aghdam, S.; Dolati, S.; Aghebati-Maleki, L.; Eghbal-Fard, S.; Khabbazi, A.; Rostamzadeh, D.; Alipour, S.; Shabani, M.; et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet’s disease. J. Cell Physiol. 2019, 234, 3985–3994. [Google Scholar] [CrossRef]
- Filleron, A.; Tran, T.A.; Hubert, A.; Letierce, A.; Churlaud, G.; Koné-Paut, I.; Saadoun, D.; Cezar, R.; Corbeau, P.; Rosenzwajg, M. Regulatory T cell/Th17 balance in the pathogenesis of paediatric Behçet disease. Rheumatol. Oxf. 2021, 61, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, K.; Hamzaoui, A.; Houman, H. CD4+CD25+ regulatory T cells in patients with Behcet’s disease. Clin. Exp. Rheumatol. 2006, 24 (Suppl. 42), 71–78. [Google Scholar]
- Liu, X.; Li, W.; Liu, X.; Luo, J.; Gao, C.; Li, X. Low-dose IL-2 effectively restored decreased regulatory T cells in patients with Behçet’s disease. Clin. Exp. Rheumatol. 2021, 39, 746–752. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. γδ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue adaptations of memory and tissue-resident gamma delta T cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Shibata, K.; Yamada, H.; Hara, H.; Kishihara, K.; Yoshikai, Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007, 178, 4466–4472. [Google Scholar] [CrossRef]
- Nakamura, R.; Shibata, K.; Yamada, H.; Shimoda, K.; Nakayama, K.; Yoshikai, Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by γδ T cells. J. Immunol. 2008, 181, 2071–2075. [Google Scholar] [CrossRef]
- Altincicek, B.; Moll, J.; Campos, N.; Foerster, G.; Beck, E.; Hoeffler, J.F.; Grosdemange-Billiard, C.; Rodríguez-Concepción, M.; Rohmer, M. Cutting edge: Human γδ T cells are activated by intermediates of the 2-C-methyl-D-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J. Immunol. 2001, 166, 3655–3658. [Google Scholar] [CrossRef]
- Groh, V.; Steinle, A.; Bauer, S.; Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 1998, 279, 1737–1740. [Google Scholar] [CrossRef]
- Yamashita, N.; Kaneoka, H.; Kaneko, S.; Takeno, M.; Oneda, K.; Koizumi, H.; Kogure, M.; Inaba, G.; Sakane, T. Role of γδ T lymphocytes in the development of Behçet’s disease. Clin. Exp. Immunol. 1997, 107, 241–247. [Google Scholar] [CrossRef]
- Hamzaoui, K.; Hamzaoui, A.; Hentati, F.; Kahan, A.; Ayed, K.; Chabbou, A.; Ben Hamida, M.; Hamza, M. Phenotype and functional profile of T cells expressing γδ receptor from patients with active Behçet’s disease. J. Rheumatol. 1994, 21, 2301–2306. [Google Scholar]
- Mochizuki, M.; Suzuki, N.; Takeno, M.; Nagafuchi, H.; Harada, T.; Kaneoka, H.; Yamashita, N.; Hirayama, K.; Nakajima, T.; Mizushima, Y.; et al. Fine antigen specifi city of human γδ T cell lines (Vγ9+) established by repetitive stimulation with a serotype (KTH-1) of a gram-positive bacterium, Streptococcus sanguis. Eur. J. Immunol. 1994, 24, 1536–1543. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Gelmez, M.Y.; Cinar, S.; Cetin, E.A.; Ozcit-Gürel, G.; Babuna-Kobaner, G.; Erdugan, M.; Gul, A.; Akdag-Kose, A.; Deniz, G. Inflammatory status might direct ILC and NK cells to IL-17 expressing ILC3 and NK subsets in Behcet’s disease. Immunol. Lett. 2021, 235, 1–8. [Google Scholar] [CrossRef]
- Dudani, A.K.; Gupta, R.S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect. Immun. 1989, 57, 2786–2793. [Google Scholar] [CrossRef]
- Kaburaki, T.; Nakahara, H.; Tanaka, R.; Okinaga, K.; Kawashima, H.; Hamasaki, Y.; Rungrotmongkol, T.; Hannongbua, S.; Noguchi, H.; Aihara, M.; et al. Lymphocyte proliferation induced by high-affinity peptides for HLA-B*51:01 in Behçet’s uveitis. PLoS ONE 2019, 14, e0222384. [Google Scholar] [CrossRef]
- Direskeneli, H.; Ekşioglu-Demiralp, E.; Yavuz, S.; Ergun, T.; Shinnick, T.; Lehner, T.; Akoglu, T. T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behçet’s disease. J. Rheumatol. 2000, 27, 708–713. [Google Scholar] [PubMed]
- Kaneko, S.; Suzuki, N.; Yamashita, N.; Nagafuchi, H.; Nakajima, T.; Wakisaka, S.; Yamamoto, S.; Sakane, T. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behcet’s disease (BD) in Japan. Clin. Exp. Immunol. 1997, 108, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Shahram, F.; Chams-Davatchi, C.; Shams, H.; Nadji, A.; Akhlaghi, M.; Faezi, T.; Ghodsi, Z.; Faridar, A.; Ashofteh, F.; et al. Behcet’s disease: From East to West. Clin. Rheumatol. 2010, 29, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Balevi, S.; Deniz, F.; Mevlitoğlu, I. Pathergy reaction in different body areas in Behçet’s disease. Clin. Exp. Dermatol. 2007, 32, 85–87. [Google Scholar] [CrossRef]
- Togashi, A.; Saito, S.; Kaneko, F.; Nakamura, K.; Oyama, N. Skin prick test with self-saliva in patients with oral aphthoses: A diagnostic pathergy for Behcet’s disease and recurrent aphthosis. Inflamm. Allergy Drug Targets 2011, 10, 164–170. [Google Scholar] [CrossRef][Green Version]
- Yalçindağ, F.N.; Batioğlu, F. Pathergy-like reaction following intravitreal triamcinolone acetonide injection in a patient with Behçet disease. Ocul. Immunol. Inflamm. 2008, 16, 181–183. [Google Scholar] [CrossRef]
- Alpagut, U.; Ugurlucan, M.; Dayioglu, E. Major arterial involvement and review of Behcet’s disease. Ann. Vasc. Surg. 2007, 21, 232–239. [Google Scholar] [CrossRef]
- Melikoglu, M.; Uysal, S.; Krueger, J.G.; Kaplan, G.; Gogus, F.; Yazici, H.; Oliver, S. Characterization of the divergent wound-healing responses occurring in the pathergy reaction and normal healthy volunteers. J. Immunol. 2006, 177, 6415–6421. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Chen, E.S.; Xiao, S.; Snapper, S.B.; Bao, B.; An, D.; Blumberg, R.S.; et al. Foxo1 controls gut homeostasis and commensalism by regulating mucus secretion. J. Exp. Med. 2021, 218, e20210324. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef]
- Ahmad, T.R.; Haeusler, R.A. Bile acids in glucose metabolism and insulin signalling—Mechanisms and research needs. Nat. Rev. Endocrinol. 2019, 15, 701–712. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018, 16, 171–181. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Milner, J.D.; Brenchley, J.M.; Laurence, A.; Freeman, A.F.; Hill, B.J.; Elias, K.M.; Kanno, Y.; Spalding, C.; Elloumi, H.Z.; Paulson, M.L.; et al. Impaired Th17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008, 452, 773–776. [Google Scholar] [CrossRef]
- Bacchetta, R.; Barzaghi, F.; Roncarolo, M.G. From IPEX syndrome to FOXP3 mutation: A lesson on immune dysregulation. Ann. N. Y. Acad. Sci. 2018, 1417, 5–22. [Google Scholar] [CrossRef]
- D’Hennezel, E.; Ben-Shoshan, M.; Ochs, H.D.; Torgerson, T.R.; Russell, L.J.; Lejtenyi, C.; Noya, F.J.; Jabado, N.; Mazer, B.; Piccirillo, C.A. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N. Engl. J. Med. 2009, 361, 1710–1713. [Google Scholar] [CrossRef]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Bifidobacteria abundance-featured gut microbiota compositional change in patients with Behcet’s disease. PLoS ONE 2016, 11, e0153746. [Google Scholar] [CrossRef]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Relative abundance of Megamonas hypermegale and Butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with Behcet’s disease. Clin. Rheumatol. 2019, 38, 1437–1445. [Google Scholar] [CrossRef]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Rubio, L.A.; Duncan, S.H.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P.; et al. Pivotal roles for pH, lactate, and lactate-utilizing bacteria in the stability of a human colonic microbial ecosystem. mSystems 2020, 5, e00645–20. [Google Scholar] [CrossRef]
- Sheridan, P.O.; Louis, P.; Tsompanidou, E.; Shaw, S.; Harmsen, H.J.; Duncan, S.H.; Flint, H.J.; Walker, A.W. Distribution, organization and expression of genes concerned with anaerobic lactate utilization in human intestinal bacteria. Microb. Genom. 2022, 8, 000739. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 2007, 73, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Hove, H.; Nordgaard-Andersen, I.; Mortensen, P.B. Faecal DL-lactate concentration in 100 gastrointestinal patients. Scand. J. Gastroenterol. 1994, 29, 255–259. [Google Scholar] [CrossRef]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate Is a Metabolic Mediator That Shapes Immune Cell Fate and Function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Consolandi, C.; Turroni, S.; Emmi, G.; Severgnini, M.; Fiori, J.; Peano, C.; Biagi, E.; Grassi, A.; Rampelli, S.; Silvestri, E.; et al. Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun. Rev. 2015, 14, 269–276. [Google Scholar] [CrossRef]
- Van der Houwen, T.B.; van Laar, J.A.M.; Kappen, J.H.; van Hagen, P.M.; de Zoete, M.R.; van Muijlwijk, G.H.; Berbers, R.M.; Fluit, A.C.; Rogers, M.; Groot, J.; et al. Behçet’s disease under microbiotic surveillance? A combined analysis of two cohorts of Behçet’s disease patients. Front. Immunol. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Yasar Bilge, N.S.; Pérez Brocal, V.; Kasifoglu, T.; Bilge, U.; Kasifoglu, N.; Moya, A.; Dinleyici, E.C. Intestinal microbiota composition of patients with Behçet’s disease: Differences between eye, mucocutaneous and vascular involvement. The Rheuma-BIOTA study. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 127), 60–68. [Google Scholar]
- Ye, Z.; Zhang, N.; Wu, C.; Zhang, X.; Wang, Q.; Huang, X.; Du, L.; Cao, Q.; Tang, J.; Zhou, C.; et al. A metagenomic study of the gut microbiome in Behcet’s disease. Microbiome 2018, 6, 135. [Google Scholar] [CrossRef]
- Seoudi, N.; Bergmeier, L.A.; Drobniewski, F.; Paster, B.; Fortune, F. The oral mucosal and salivary microbial community of Behçet’s syndrome and recurrent aphthous stomatitis. J. Oral. Microbiol. 2015, 7, 27150. [Google Scholar] [CrossRef]
- Coit, P.; Mumcu, G.; Ture-Ozdemir, F.; Unal, A.U.; Alpar, U.; Bostanci, N.; Ergun, T.; Direskeneli, H.; Sawalha, A.H. Sequencing of 16S rRNA reveals a distinct salivary microbiome signature in Behçet’s disease. Clin. Immunol. 2016, 169, 28–35. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Seedorf, H.; Griffin, N.W.; Ridaura, V.K.; Reyes, A.; Cheng, J.; Rey, F.E.; Smith, M.I.; Simon, G.M.; Scheffrahn, R.H.; Woebken, D.; et al. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 2014, 159, 253–266. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Bendelac, A. IgA responses to microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Pearson, C.; Ilott, N.E.; Huus, K.E.; Hegazy, A.N.; Webber, J.; Finlay, B.B.; Macpherson, A.J.; Powrie, F.; Lam, L.H. Accurate identification and quantification of commensal microbiota bound by host immunoglobulins. Microbiome 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and skin: An update. J. Dtsch. Dermatol. Ges. 2019, 17, 589–596. [Google Scholar] [CrossRef]
- Najim, R.A.; Sharquie, K.E.; Abu-Raghif, A.R. Oxidative stress in patients with Behcet’s disease: I correlation with severity and clinical parameters. J. Dermatol. 2007, 34, 308–314. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Khabbazi, A.; Baradaran, B.; Khajebishak, Y.; Baghbani, E.; Noorolyai, S.; Rahmani, S.; Seyyed Shoura, S.M.; Alipour, M.; Alipour, B. Immunomodulatory and clinical responses to zinc gluconate supplementation in patients with Behçet’s disease: A double-blind, randomized placebo-controlled clinical trial. Clin. Nutr. 2022, 41, 1083–1092. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Baradaran, B.; Khabbazi, A.; Abdoli Shadbad, M.; Papi, S.; Faghfuri, E.; Khajebishak, Y.; Rahmani, S.; Tolou Hayat, P.; Alipour, B. Regulation of NLRP3 inflammasome by zinc supplementation in Behçet’s disease patients: A double-blind, randomized placebo-controlled clinical trial. Int. Immunopharmacol. 2022, 109, 108825. [Google Scholar] [CrossRef]
- Yan, J.; Yan, Y.; Young, A.; Yan, Z.; Yan, Z. Effectiveness and safety of Chinese medicine decoctions for Behcet’s disease: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2021, 2021, 8202512. [Google Scholar] [CrossRef]
- Jun, J.H.; Ang, L.; Choi, T.Y.; Lee, H.W.; Lee, M.S. Integrative medicine (herbal medicine combined with drug therapy) for Behcet’s disease: A systematic review and meta-analysis of randomized controlled trials. Pharmaceutics 2021, 13, 476. [Google Scholar] [CrossRef]
- Askari, G.; Moravejolahkami, A.R. Synbiotic supplementation may relieve anterior uveitis, an ocular manifestation in Behcet’s syndrome. Am. J. Case Rep. 2019, 20, 548–550. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.H.; Kim, Y.J.; Jeong, H.J.; Ryu, J.S.; Lee, H.J.; Kim, T.W.; Im, S.H.; Oh, J.Y.; Kim, M.K. Clinical effect of IRT-5 probiotics on immune modulation of autoimmunity or alloimmunity in the eye. Nutrients 2017, 9, 1166. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Fiorillo, C.; Becatti, M.; Turroni, S.; Emmi, G.; Sofi, F. Modulation of gut microbiota through nutritional interventions in Behçet’s syndrome patients (the MAMBA study): Study protocol for a randomized controlled trial. Trials 2020, 21, 511. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).