Angiotensin-Converting Enzyme Activity May Predict Disease Severity in Psoriasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Populations
2.2. DNA and Enzyme Activity Determination
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langley, R.G.B. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii18–ii23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Ryder, K.W.; Epinette, W.W.; Jay, S.J.; Ransburg, R.C.; Glick, M.R. Serum angiotensin converting enzyme activity in patients with psoriasis. Clin. Chim. Acta 1985, 153, 143–146. [Google Scholar] [CrossRef]
- Huskić, J.; Mulabegović, N.; Alendar, F.; Ostojić, L.; Ostojić, Z.; Simić, D.; Milicević, R.; Naletilić, M. Serum and tissue angiotensin converting enzyme in patients with psoriasis. Coll. Antropol. 2008, 32, 1215–1219. [Google Scholar] [PubMed]
- Coto-Segura, P.; Alvarez, V.; Soto-Sã¡nchez, J.; Morales, B.; Coto, E.; Santos-Juanes, J. Lack of association between angiotensin I-converting enzyme insertion/deletion polymorphism and psoriasis or psoriatic arthritis in Spain. Int. J. Dermatol. 2009, 48, 1320–1323. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Wu, W.-M.; Chen, C.-H.; Lee, S.-H.; Hong, H.-S.; Hsu, L.-A. Association between the insertion/deletion polymorphism of the angiotensin I-converting enzyme gene and risk for psoriasis in a Chinese population in Taiwan. Br. J. Dermatol. 2007, 156, 642–645. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, F.; Li, F.; Su, J.; Wen, S.; Liu, Y.; Feng, D. Angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to psoriasis in a Chinese population. J. Renin-Angiotensin-Aldosterone Syst. 2013, 15, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Özkur, M.; Erbagci, Z.; Nacak, M.; Tuncel, A.; Alasehirli, B.; Aynacioglu, A. Association of insertion/deletion polymorphism of the angiotensin-converting enzyme gene with psoriasis. Br. J. Dermatol. 2004, 151, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Weger, W.; Hofer, A.; Wolf, P.; El-Shabrawi, Y.; Renner, W.; Kerl, H.; Salmhofer, W. The angiotensin-converting enzyme insertion/deletion and the endothelin -134 3A/4A gene polymorphisms in patients with chronic plaque psoriasis. Exp. Dermatol. 2007, 16, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Veletza, S.; Karpouzis, A.; Giassakis, G.; Caridha, R.; Papaioakim, M. Assessment of insertion/deletion polymorphism of the angiotensin converting enzyme gene in psoriasis. J. Dermatol. Sci. 2008, 49, 85–87. [Google Scholar] [CrossRef]
- Rigat, B.; Hubert, C.; Corvol, P.; Soubrier, R. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res. 1992, 20, 1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Investig. 1990, 86, 1343–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haznedaroglu, I.C.; Öztürk, M. Towards the understanding of the local hematopoietic bone marrow renin-angiotensin system. Int. J. Biochem. Cell Biol. 2003, 35, 867–880. [Google Scholar] [CrossRef]
- Fleming, I. Signaling by the Angiotensin-Converting Enzyme. Circ. Res. 2006, 98, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Schoemaker, R.G.; van Gilst, W.; Roks, A.J.M. The role of the renin–angiotensin–aldosterone system in cardiovascular progenitor cell function. Clin. Sci. 2009, 116, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, P.B.; Alvarenga, C.; Siqueira, P.D.; Paredes-Gamero, E.J.; Sabatini, R.A.; Morais, R.L.; Reis, R.I.; Santos, E.L.; Teixeira, L.G.; Casarini, D.E.; et al. Angiotensin II Binding to Angiotensin I–Converting Enzyme Triggers Calcium Signaling. Hypertension 2011, 57, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Yeung, H.; Takeshita, J.; Mehta, N.N.; Kimmel, S.E.; Ogdie, A.; Margolis, D.J.; Shin, D.B.; Attor, R.; Troxel, A.; Gelfand, J.M. Psoriasis severity and the prevalence of major medical comorbidity. JAMA Dermatol. 2013, 149, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, K.M.; Armstrong, A.W. Updates on cardiovascular comorbidities associated with psoriatic diseases: Epidemiology and mechanisms. Rheumatol. Int. 2016, 37, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Dann, F. Comorbidities in patients with psoriasis. Am. J. Med. 2009, 122, 1150.e1–1150.e9. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2015, 38, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. A tale of two plaques: Convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis. Exp. Dermatol. 2011, 20, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Akhyani, M.; Ehsani, A.; Robati, R.; Robati, A. The lipid profile in psoriasis: A controlled study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Tekin, N.S.; Tekin, I.O.; Barut, F.; Sipahi, E.Y. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediat. Inflamm. 2006, 2007, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mallbris, L.; Granath, F.; Hamsten, A.; Ståhle, M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J. Am. Acad. Dermatol. 2006, 54, 614–621. [Google Scholar] [CrossRef]
- Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.; Gelfand, J. Prevalence of cardiovascular risk factors in patients with psoriasis. J. Am. Acad. Dermatol. 2006, 55, 829–835. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Kim, D.-H.; Song, D.-K. Serum tumor necrosis factor-α levels and components of the metabolic syndrome in obese adolescents. Metabolism 2004, 53, 863–867. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, N.N.; Li, R.; Krishnamoorthy, P.; Yu, Y.; Farver, W.; Rodrigues, A.; Raper, A.; Wilcox, M.; Baer, A.; DerOhannesian, S.; et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012, 224, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Sheth, N.; Krishnamoorthy, P.; Saboury, B.; Raper, A.; Baer, A.; Ochotony, R.; Doveikis, J.; Derohannessian, S.; Van Voorhees, A.S.; et al. Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: A pilot study. Am. J. Cardiovasc. Dis. 2012, 2, 285–292. [Google Scholar]
- Salahuddin, T.; Natarajan, B.; Playford, M.P.; Joshi, A.A.; Teague, H.; Masmoudi, Y.; Selwaness, M.; Chen, M.Y.; Bluemke, D.A.; Mehta, N.N. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur. Heart J. 2015, 36, 2662–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, K.; Hernandez, W.; Ansari, R.A.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015, 6, 209–217. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Cosenso-Martin, L.N.; Vaz-De-Melo, R.O.; Pereira, L.R.; Cesarino, C.B.; Yugar-Toledo, J.C.; Cipullo, J.P.; Pinhel, M.A.D.S.; Souza, D.R.S.; Vilela-Martin, J.F. Angiotensin-converting enzyme insertion/deletion polymorphism, 24-h blood pressure profile and left ventricular hypertrophy in hypertensive individuals: A cross-sectional study. Eur. J. Med. Res. 2015, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dhar, S.; Ray, S.; Dutta, A.; Sengupta, B.; Chakrabarti, S. Polymorphism of ACE gene as the genetic predisposition of coronary artery disease in Eastern India. Indian Heart J. 2012, 64, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2010, 303, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Danser, A.J.; Schalekamp, M.A.; Bax, W.A.; Brink, A.M.V.D.; Saxena, P.R.; Riegger, G.A.; Schunkert, H. Angiotensin-converting enzyme in the human heart. Circulation 1995, 92, 1387–1388. [Google Scholar] [CrossRef]
- Boutin, A.T.; Weidemann, A.; Fu, Z.; Mesropian, L.; Gradin, K.; Jamora, C.; Wiesener, M.; Eckardt, K.-U.; Koch, C.J.; Ellies, L.G.; et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 2008, 133, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, W.O.; Souza dos Santos, R.A.; Faria-Silva, R.; da Mata Machado, L.T.; Schiffrin, E.L.; Touyz, R.M. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via akt-dependent pathways. Hypertension 2007, 49, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed]
- Kinosian, B.; Glick, H.; Garland, G. Cholesterol and coronary heart disease: Predicting risks by levels and ratios. Ann. Intern. Med. 1994, 121, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Ascaso, J.F.; Santos, P.G.; Mijares, A.H.; Rojas, A.M.; Masana, L.; Millan, J.; Pallardo, L.F.; Pedrobotet, J.; Jimenez, F.P.; Pinto, X.; et al. Management of dyslipidemia in the metabolic syndrome. Am. J. Cardiovasc. Drugs 2007, 7, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Rimm, E.B.; Hankinson, S.E.; Curhan, G.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Ma, J. multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women. Circulation 2004, 110, 2824–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, S.H.; Pan, D.; Berookim, P.; Lee, M.L. A Population-based, cross-sectional comparison of lipid-related indexes for symptoms of atherosclerotic disease. Am. J. Cardiol. 2006, 98, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Zapater, P.; Novalbos, J.; Gallego-Sandín, S.; Hernández, F.T.; Abad-Santos, F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J. Cardiovasc. Pharmacol. 2004, 43, 737–744. [Google Scholar] [CrossRef]
| PCR Conditions | PCR Components | Gel Conditions | Genotypes | |
|---|---|---|---|---|
| ACE I/D Polymorphism | 35 cycles Denaturation: 45 s at 94 °C Annealing: 45 s at 58 °C Extension: 45 s at 72 °C Final extension: 32 s at 72 °C | DNA: 10 uL (200 mg) Primer F: 1 uL, 10 pmol Primer R: 1 uL, 10 pmol Master Mix: 12.5 uL Dimethyl sulfoxide: 1.25 uL | 2% 110 volts 60 min | II–477 pb ID–477 + 190 pb DD–190 pb |
| Psoriatic Patients | Controls | ||
|---|---|---|---|
| ACE I/D polymorphism p = 0.777 | II | 11.5 (7) | 13.3 (59) |
| ID | 41.0 (25) | 43.8 (194) | |
| DD | 47.5 (29) | 42.9 (190) | |
| ACE allele p = 0.480 | I | 32.0 (39) | 35.2 (312) |
| D | 68.0 (83) | 64.8 (574) |
| Psoriatic Patients | Controls | Univariate (p-Value) | Multivariate * | ||
|---|---|---|---|---|---|
| ACE activity | Total | 19.09 ± 2.86 (20) | 11.85 ± 0.40 (276) | p = 0.015 | B = 6.618, CI 95% 3.176–10.060, p < 0.001 |
| Female | 17.38 ± 3.13 (6) | 11.62 ± 0.44 (220) | p = 0.037 | ||
| Male | 19.83 ± 3.92 (14) | 12.76 ± 0.93 (56) | p = 0.063 |
| ACE Activity | |||
|---|---|---|---|
| ACE I/D polymorphism | II | 7.75 ± 0.67 (24) | p < 0.001 |
| ID | 11.27 ± 0.56 (101) | ||
| DD | 14.57 ± 0.90 (93) | ||
| Post-hoc tests | II–ID | p = 0.003 | |
| II–DD | p < 0.001 | ||
| ID–DD | p = 0.005 | ||
| PASI ≤ 10 | PASI > 10 | Univariate (p-Value) | Multivariate * (p-Value) | |
|---|---|---|---|---|
| Total cholesterol | 196.60 ± 46.20 (20) | 196 ± 38.42 (9) | p = 0.814 | p = 0.447 |
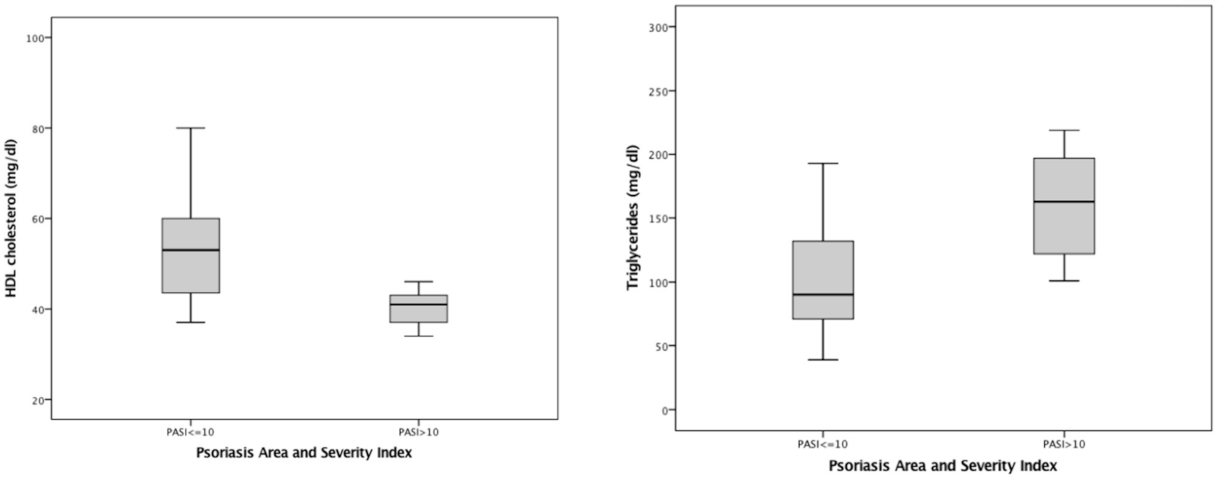
| HDL cholesterol | 54.26 ± 14.72 (19) | 41.67 ± 11.04 (9) | p = 0.028 | B = −12.596, CI 95% −23.987–−1.206, p = 0.032 |
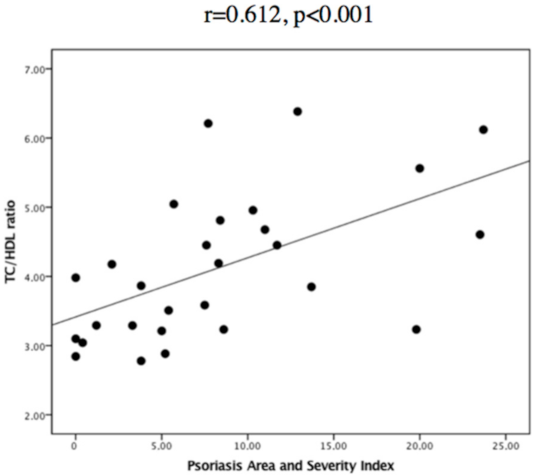
| TC/HDL ratio | 3.76 ± 0.89 (19) | 4.88 ± 1.02 (9) | p = 0.012 | B = 0.792, CI 95% 0.025–1.560, p = 0.044 |
| Non-HDL cholesterol | 143.74 ± 39.51 (19) | 154.78 ± 32.57 (9) | p = 0.279 | p = 0.257 |
| LDL cholesterol | 121.42 ± 37.41 (19) | 121.13 ± 36.60 (8) | p = 0.811 | p = 0.547 |
| LDL/HDL ratio | 2.31 ± 0.69 (19) | 3.01 ± 0.89 (8) | p = 0.035 | p = 0.059 |
| Triglycerides | 113.38 ± 66.66 (21) | 163.56 ± 44.95 (9) | p = 0.021 | B = 50.175, CI 95% 0.187–100.162, p = 0.049 |
| ACE activity | 10.24 ± 8.22 (5) | 17.20 ± 11.03 (4) | p = 0.327 | p = 0.436 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandeira, M.; Gil, Â.; Santos, A.C.; Romão, V.C.; Mascarenhas, M.R.; Filipe, P.; Fonseca, J.E.; Bicho, M. Angiotensin-Converting Enzyme Activity May Predict Disease Severity in Psoriasis. Rheumato 2022, 2, 15-23. https://doi.org/10.3390/rheumato2010003
Bandeira M, Gil Â, Santos AC, Romão VC, Mascarenhas MR, Filipe P, Fonseca JE, Bicho M. Angiotensin-Converting Enzyme Activity May Predict Disease Severity in Psoriasis. Rheumato. 2022; 2(1):15-23. https://doi.org/10.3390/rheumato2010003
Chicago/Turabian StyleBandeira, Matilde, Ângela Gil, Ana Carolina Santos, Vasco C. Romão, Mário Rui Mascarenhas, Paulo Filipe, João Eurico Fonseca, and Manuel Bicho. 2022. "Angiotensin-Converting Enzyme Activity May Predict Disease Severity in Psoriasis" Rheumato 2, no. 1: 15-23. https://doi.org/10.3390/rheumato2010003
APA StyleBandeira, M., Gil, Â., Santos, A. C., Romão, V. C., Mascarenhas, M. R., Filipe, P., Fonseca, J. E., & Bicho, M. (2022). Angiotensin-Converting Enzyme Activity May Predict Disease Severity in Psoriasis. Rheumato, 2(1), 15-23. https://doi.org/10.3390/rheumato2010003









