Ultrasound Features across Subtypes of Juvenile Idiopathic Arthritis
Abstract
:1. Introduction
2. Patients and Method
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Methods
2.3.1. Disease Assessment
Assessment of Disease Activity
Assessment of Disease Damage
Assessment of Function
2.3.2. Laboratory Investigations Included
2.3.3. MSUS
- Synovial thickening
- Synovial effusion
- Tenosynovitis
- Bone erosions
- Cartilage thickness in mm
- PD signals
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.L. New advances in juvenile idiopathic arthritis. Chang. Gung Med. J. 2012, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Lovell, D.J.; Ruperto, N.; Goodman, S.; Reiff, A.; Jung, L.; Jarosova, K.; Němcova, D.; Mouy, R.; Sandborg, C.; Bohnsack, J.; et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N. Engl. J. Med. 2008, 359, 810–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheel, A.K.; Hermann, K.G.; Ohrndorf, S.; Werner, C.; Schirmer, C.; Detert, J.; Bollow, M.; Hamm, B.; Müller, G.A.; Burmester, G.R.; et al. Prospective 7-year follow-up imaging study comparing radiography, ultrasonography and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann. Rheum. Dis. 2006, 65, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Jousse-Joulin, S.; Finel, E.; Marhadour, T.; Colin, D.; de Parscau, L.; Devauchelle-Pensec, V. Imaging approaches for evaluating peripheral joint abnormalities in juvenile idiopathic arthritis. Semin. Arthritis Rheum. 2012, 41, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Lanni, S.; Wood, M.; Ravelli, A.; Magni Manzoni, S.; Emery, P.; Wakefield, R.J. Towards a role of ultrasound in children with juvenile idiopathic arthritis. Rheumatology 2013, 52, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Consolaro, A.; Bracciolini, G.; Ruperto, N. Remission, minimal disease activity and acceptable symptom state in juvenile idiopathic arthritis. Arthritis Rheumatol. 2012, 64, 2366–2374. [Google Scholar] [CrossRef]
- Weiss, P.F.; Beukelman, T.; Schanberg, L.E.; Kimura, Y.; Colbert, R.A. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: The Childhood Arthritis and Rheumatology Research Alliance Registry. J. Rheumatol. 2012, 39, 2341–2351. [Google Scholar] [CrossRef] [Green Version]
- Viola, S.; Felici, E.; Magni-Manzoni, S.; Pistorio, A.; Buoncompagni, A.; Ruperto, N.; Rossi, F.; Bartoli, M.; Martini, A.; Ravelli, A.; et al. Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheumatol. 2005, 52, 2092–2102. [Google Scholar] [CrossRef]
- Tennant, A.; Kearns, S.; Turner, F.; Wyatt, S.; Haigh, R.; Chamberlain, M.A. Measuring the function of children with juvenile arthritis. Rheumatology 2001, 40, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Collado, P.; Vojinovic, J.; Nieto, J.C.; Windschall, D.; Magni-Manzoni, S.; Bruyn, G.A.; Iagnocco, A.; D’Agostino, M.A.; Naredo, E.; on behalf of the Omeract Ultral Pediatric Group. Toward standardized musculoskeletal ultrasound in pediatric rheumatology: Normal age-related ultrasound findings. Arthritis Care Res. 2016, 68, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, J.; Ravagnani, V.; Backhaus, M.; Balint, P.; Bruns, A.; Bruyn, G.A.; Collado, P.; De La Cruz, L.; Guillaume-Czitrom, S.; Herlin, T.; et al. Preliminary definitions for the Sonographic features of Synovitis in children. Arthritis Care Res. 2017, 69, 1217–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojinovic, J.; Magni-Manzoni, S.; Collado, P.; Windschall, D.; Ravagnani, V.; Hernandez-Diaz, C.; Gonzales, J.C.N.; Malattia, C.; Tzaribachev, N.; Susic, G.; et al. SAT0636 Ultrasonography definitions for synovitis grading in children: The omeract pediatric ultrasound task force. Ann. Rheum. Dis. 2017, 76, 1015. [Google Scholar]
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149. [Google Scholar] [CrossRef] [Green Version]
- Lotfy, H.; Seif El Dienb, H.; El Minawia, N.; Abdel Aziz, H. The role of Doppler Ultrasonography in evaluating disease activity in a group of juvenile idiopathic arthritis patients. Egypt. J. Radiol. Nucl. Med. 2018, 49, 1036–1042. [Google Scholar] [CrossRef]
- Collado, P.; Gamir, M.L.; López-Robledillo, J.C.; Merino, R.; Modesto, C.; Monteagudo, I. Detection of synovitis by ultrasonography in clinically inactive juvenile idiopathic arthritis on and off medication. Clin. Exp. 2014, 32, 597–603. [Google Scholar]
- Ventura-Ríos, L.; Faugier, E.; Barzola, L.; De La Cruz-Becerra, L.B.; Sánchez-Bringas, G.; García, A.R.; Maldonado, R.; Roth, J.; Hernández-Día, C.Z. Reliability of ultrasonography to detect inflammatory lesions and structural damage in juvenile idiopathic arthritis. Pediatr. Rheumatol. 2018, 16, 58. [Google Scholar] [CrossRef]
- Darwish, A.; Ismaela, F.; Ell-Labana, A.; Hamed, A.; Kader, M.A.; Osman, A. Implementation of musculoskeletal ultrasonography in detection of early juvenile idiopathic arthritis. Eur. J. Radiol. 2016, 3, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Laurell, L.; Court-Payen, M.; Nielsen, S.; Zak, M.; Thomsen, C.; Miguel-Pérez, M.; Fasth, A. Ultrasonography and color Doppler of proximal gluteal enthesitis in juvenile idiopathic arthritis: A descriptive study. Pediatr. Rheumatol. Online J. 2011, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Pradsgaard, D.Ø.; Spannow, A.H.; Heuck, C.; Herlin, T. Decreased cartilage thickness in juvenile idiopathic arthritis assessed by ultrasonography. J. Rheumatol. 2013, 40, 1596–1603. [Google Scholar] [CrossRef]
- Kiris, A.; Kaya, A.; Ozgocmen, S.; Kocakoc, E. Assessment of enthesitis in ankylosing spondylitis by power Doppler ultrasonography. Skelet. Radiol. 2006, 35, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Magni-Manzoni, S.; Epis, O.; Ravelli, A. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheumatol. 2009, 61, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, H.; Nada, D.; Hussein, M.; Hablas, S.; Darwish, N.; Abu-Zaid, M.; Gadou, S.E. Role of musculoskeletal ultrasonography in the detection of subclinical synovitis in oligo and polyarticular juvenile idiopathic arthritis children. Egypt. Rheumatol. 2019, 41, 151–155. [Google Scholar] [CrossRef]
- Borzani, I.; Di Landro, G.; Ravelli, A.; Corona, F.; Filocamo, G. SAT0506 Towards the Development of an Ultrasound Composite Disease Activity Score for Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2015, 74, 843. [Google Scholar] [CrossRef]
- Nielsen, H.E.; Strandberg, C.; Andersen, S.; Kinnander, C.; Erichsen, G. Ultrasonographic examination in juvenile idiopathic arthritis is better than clinical examination for identification of intraarticular disease. Dan. Med. J. 2013, 60, A4669. [Google Scholar] [PubMed]
- Beck, M.C.; Glimm, A.M.; Ohrndorf, S.; Minden, K.; Trauzeddel, R.; Werner, S.; Horneff, G.; Backhaus, M.; Burmester, G.R.; Kallinich, T.; et al. Fluorescence optical imaging in pediatric patients with inflammatory and non-inflammatory joint diseases: A comparative study with ultrasonography. Arthritis Res. Ther. 2017, 19, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojinovic, J. Toward a Multibiomarker Panel to Optimize Outcome and Predict Respons. J. Rheumatol. 2018, 45, 451–453. [Google Scholar] [CrossRef] [Green Version]

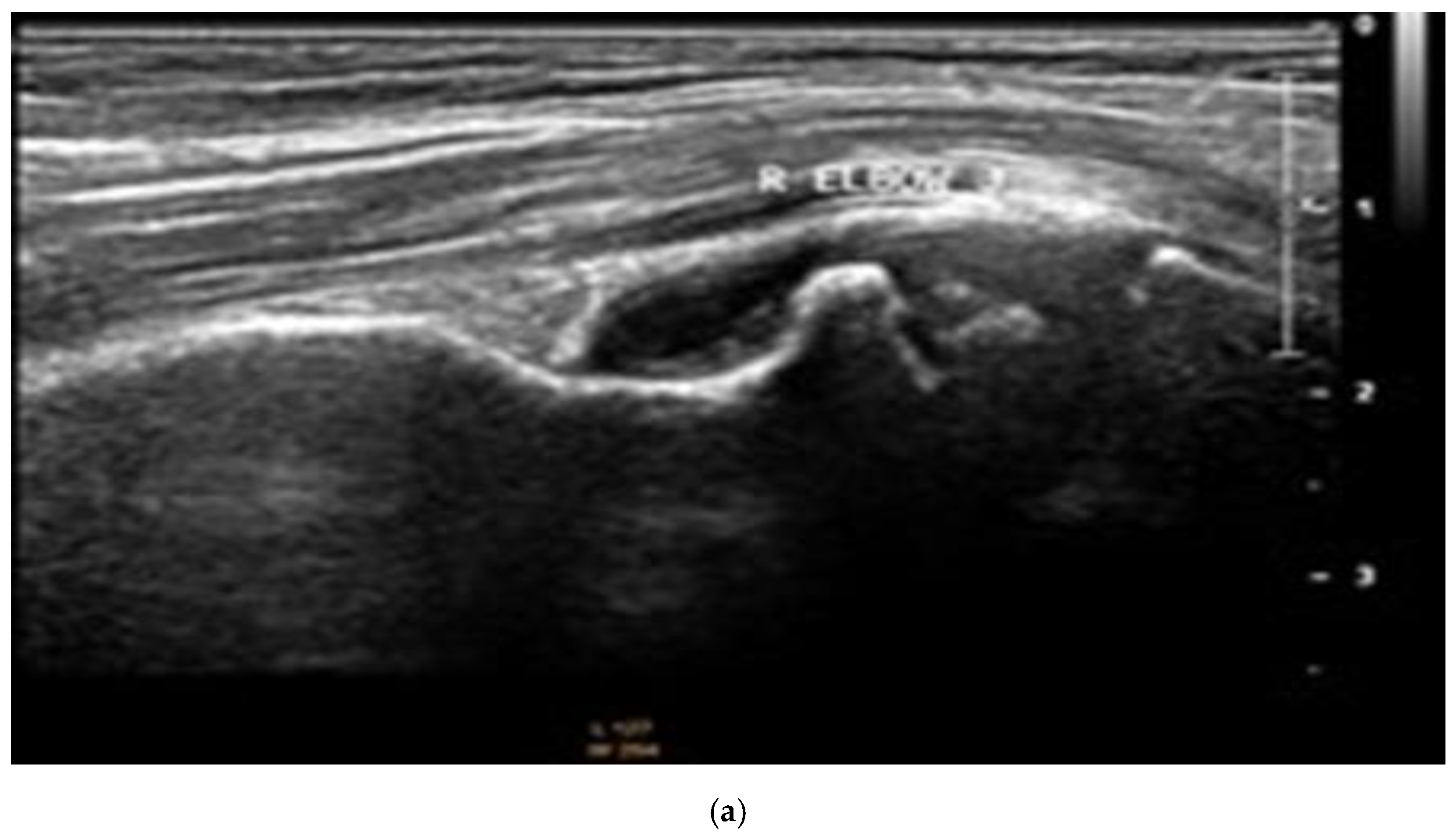
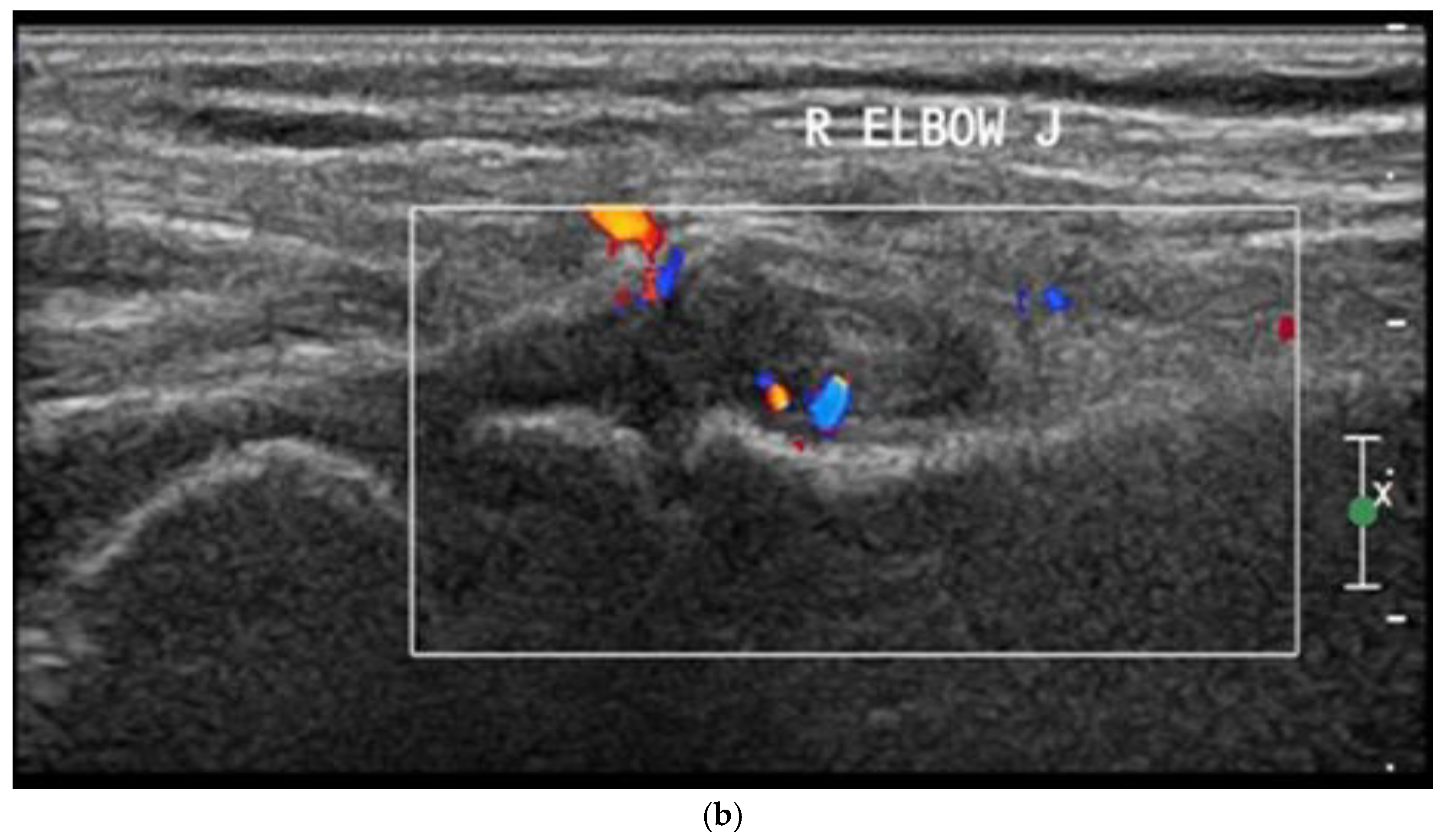
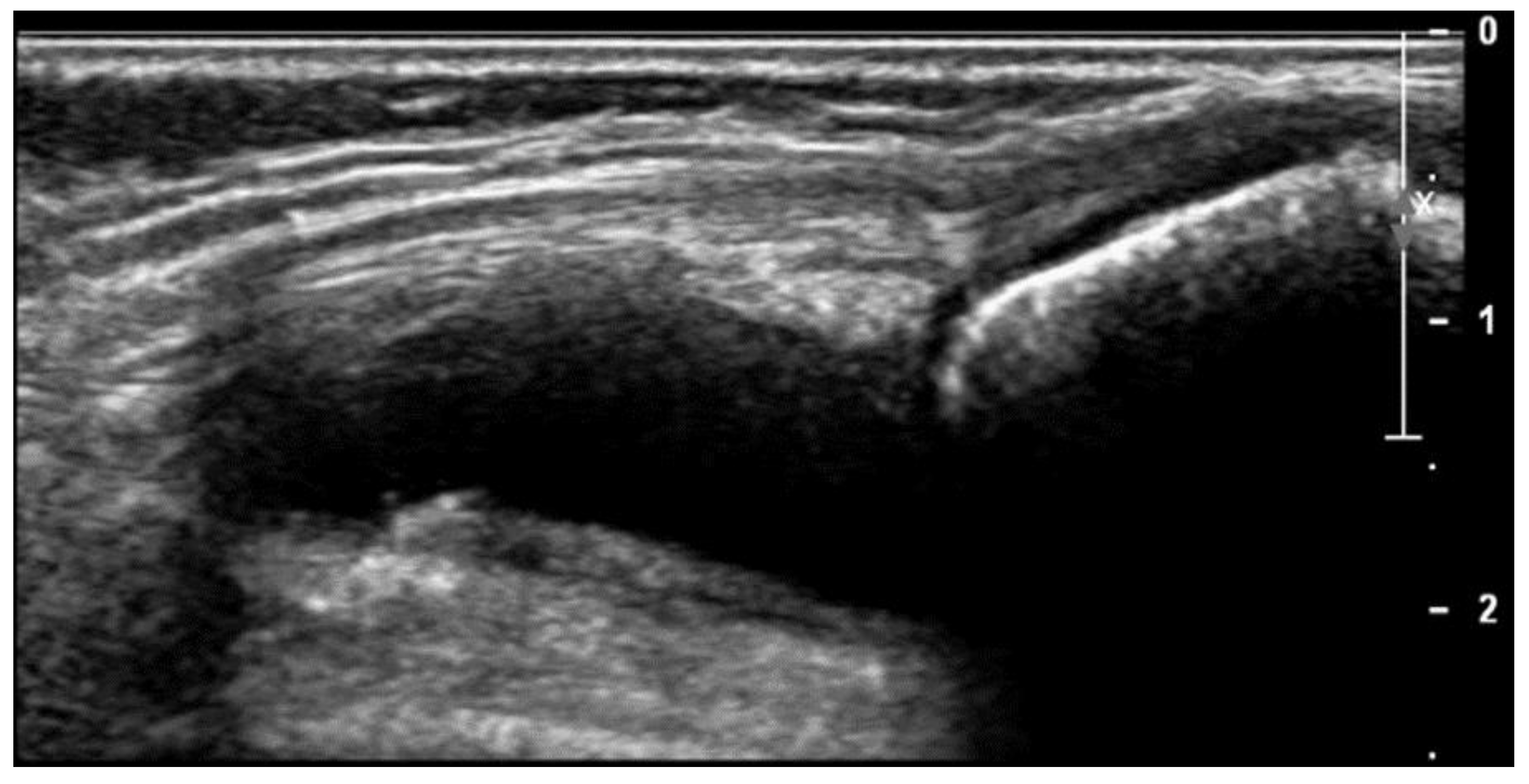



| Clinical Data | Subtypes of JIA | ||||||
|---|---|---|---|---|---|---|---|
| Oligoarticular n = 7 | Extended-Oligo n = 4 | Polyarticular n = 22 | SJIA n = 21 | ERA n = 6 | Kruskal–Wallis Test, p Value | ||
| Total Number of Examined Joints | 280 | 160 | 880 | 840 | 240 | ||
| Active joint count | Range | 0–4 | 0–6 | 0–14 | 0–40 | 2–10 | 0.135 |
| Median | 2 | 3.5 | 1 | 4 | 3 | ||
| (IQR) | (1) | (5.8) | (4) | (10) | (3.5) | ||
| n, percent | 13 (4.6%) | 13 (8.1%) | 58 (6.6%) | 166 (19.8%) | 24 (10%) | ||
| Tender joint count | Range | 0–4 | 1–8 | 0–14 | 0–40 | 2–10 | 0.135 |
| Median | 2 | 6 | 2 | 4 | 4 | ||
| (IQR) | (3) | (5.3) | (6) | (12.5) | (5) | ||
| n, percent | 15 (5.4%) | 21 (13.1%) | 83 (9.4%) | 181 (21.5%) | 28 (11%) | ||
| Swollen joint count | Range | 0–4 | 0–6 | 0–26 | 0–40 | 2–10 | 0.164 |
| Median | 2 | 3.5 | 2 | 4 | 3 | ||
| (IQR) | (1) | (5.8) | (6) | (9.5) | (3.5) | ||
| n, percent | 13 (4.6%) | 13 (8.1%) | 94 (10.7%) | 168 (20%) | 24 (10%) | ||
| Limited joint count | Range | 0–2 | 0–6 | 0–20 | 0–40 | 0–10 | 0.320 |
| Median | 0 | 2 | 1 | 2 | 4 | ||
| (IQR) | (0) | (5.5) | (4) | (8) | (5.5) | ||
| n, percent | 2 (0.7%) | 10 (6.3%) | 68 (7.7%) | 164 (17.4%) | 22 (9.2%) | ||
| JADAS-10 | Range | 0–11 | 2.2–22 | 0–38 | 0.3–40 | 6.5–40 | 0.050 * |
| Median | 4.5 | 12.9 | 7.9 | 21.3 | 13 | ||
| (IQR) | (4.3) | (19.1]) | (7.6) | (33.9) | (21.3) | ||
| C-HAQ | Range | 0–1 | 0–2 | 0–2.5 | 0–3 | 0–3 | 0.035 * |
| Median | 0 | 1 | 1 | 2 | 1.5 | ||
| (IQR) | (0) | (1.75) | (2) | (2.5) | (1.5) | ||
| JAD-articular | Range | 0–2 | 0–4 | 0–30 | 0–40 | 0–6 | 0.722 |
| Median | 0 | 2 | 0 | 0 | 3 | ||
| (IQR) | (0) | (4) | (4) | (5.5) | (4.5) | ||
| JAD-extra articular | Range | 0–2 | 0–2 | 0–5 | 0–5 | 0–1 | 0.618 |
| Median | 0 | 0 | 1 | 1 | 1 | ||
| (IQR) | (2) | (1.5) | (1) | (3) | (0.25) | ||
| Laboratory Data | Subtypes of JIA | ||||||
|---|---|---|---|---|---|---|---|
| Oligoarticular | Extended-Oligo | Polyarticular | SJIA | ERA | Kruskal–Wallis Test, p Value | ||
| n = 7 | n = 4 | n = 22 | n = 21 | n = 6 | |||
| ESR 1st hour (mm) | median | 30.0 | 25.5 | 26 | 60.0 | 20 | 0.096 |
| (IQR) | (7.0) | (6.2) | (31.25) | (71.0) | (39) | ||
| CRP (mg/dL) | median | 0.5 | 0.5 | 0.65 | 24.0 | 17.4 | 0.034 * |
| (IQR) | (5.5) | (5.5) | (23.5) | (40.0) | (15.2) | ||
| n | % | |
|---|---|---|
| Elbow | 92 | 12.6 |
| Wrist | 100 | 13.7 |
| MCP | 98 | 13.4 |
| PIP | 76 | 10.4 |
| Knee | 110 | 15 |
| Ankle | 93 | 12.7 |
| Subtalar | 78 | 10.7 |
| MTP | 83 | 11.4 |
| US Features | Subtypes of JIA | ||||||
|---|---|---|---|---|---|---|---|
| Oligoarticular n = 7 | Extended-Oligo n = 4 | Polyarticular n = 22 | SJIA n = 21 | ERA n = 6 | Kruskal–Wallis Test, p Value | ||
| Total Number of Examined Joints | 280 | 160 | 880 | 840 | 240 | ||
| Number joints with effusion | Range | 0–12 | 4–37 | 0–26 | 0–40 | 2–16 | 0.017 * |
| Median | 4 | 30 | 7.5 | 14 | 5.5 | ||
| (IQR) | (3) | (24.75) | (12.5) | (20.5) | (9.5) | ||
| n, percent | 30 (10.7%) | 101 (63.1%) | 191 (21.7%) | 334 (40 %) | 41 (17%) | ||
| Number joints with synovial thickening | Range | 1–12 | 4–30 | 0–21 | 2–40 | 2–16 | 0.025 * |
| Median | 4 | 22.5 | 7 | 16 | 4 | ||
| (IQR) | (3) | (23.25) | (92.5) | (20.5) | (8) | ||
| n, percent | 32 (11.4%) | 79 (49.4%) | 181 (20.6%) | 345 (41%) | 36 (15%) | ||
| Number joints with tenosynovitis | Range | 0 | 0–3 | 0–2 | 0–2 | 0–3 | 0.070 |
| Median | 0 | 2 | 0 | 0 | 0.5 | ||
| (IQR) | (0) | (2.25) | (1.25) | (2) | (2.25) | ||
| n, percent | 0 | 7 (4.4%) | 13 (1.5%) | 12 (1.4%) | 6 (2.5%) | ||
| Number joints with erosion | Range | 0 | 0 | 0–3 | 0–5 | 0–2 | 0.382 |
| Median | 0 | 0 | 0 | 0 | 0 | ||
| (IQR) | (0) | (0) | (0.25) | (1) | (1.25) | ||
| n, percent | 0 | 0 | 7 (0.8%) | 14 (1.7%) | 3 (0.8%) | ||
| Number joints with active synovitis | Range | 0–4 | 0–30 | 0–19 | 0–40 | 0–8 | 0.115 |
| Median | 1 | 17 | 2 | 6 | 5 | ||
| (IQR) | (3) | (29) | (7.25) | (21.5) | (5) | ||
| n, percent | 10 (3.6%) | 64 (40%) | 85 (9.7%) | 261 (31%) | 26 (10.8%) | ||
| Number joints with US synovitis | Range | 1–12 | 4–37 | 0–26 | 2–40 | 2–16 | 0.015 * |
| Median | 4 | 30 | 7.5 | 17 | 6 | ||
| (IQR) | (3) | (24.25) | (11.5) | (20.5) | (9.5) | ||
| n, percent | 32 (11.4%) | 101 (63.1%) | 205 (23.3%) | 350 (41.7%) | 43 (17.9%) | ||
| Number joints with subclinical synovitis | Range | 0–8 | 3–20 | 0–13 | 0–15 | 0–9 | 0.017 * |
| Median | 0 | 10 | 3.2 | 6 | 3 | ||
| (IQR) | (3) | (12.75) | (4.55) | (6.3) | (8) | ||
| n, percent | 9 (3.2%) | 58 (36.25%) | 102 (11.6%) | 126 (15%) | 17 (7.08%) | ||
| Number of joints with low cartilage thickness | Range | 0–8 | 0–12 | 0–12 | 0–8 | 0–12 | 0.393 |
| median | 2 | 0 | 2 | 0 | 0 | ||
| (IQR) | (8) | (9) | (4.5) | (2) | (4.5) | ||
| n, percent | 26 (9.3%) | 12 (7.5%) | 66 (7.5%) | 30 (3.6%) | 14 (5.8%) | ||
| Number of enthesitis | Range | 0–2 | 0 | 0 | 0 | 0–6 | <0.001 * |
| Median | 0 | 0 | 0 | 0 | 5 | ||
| (IQR) | (1.25) | (0) | (0) | (0) | (3) | ||
| Synovial effusion score | Range | 0–15 | 4–64 | 0–45 | 2–120 | 2–16 | 0.018 * |
| Median | 4 | 55 | 12 | 18 | 7.5 | ||
| (IQR) | (4) | (47.5) | (16.25) | (26.5) | (9.5) | ||
| Synovial hypertrophy score | Range | 1–13 | 7–40 | 0–30 | 2–80 | 2–16 | 0.013 * |
| Median | 4 | 24.5 | 8.5 | 20 | 6.5 | ||
| (IQR) | (4) | (27.5) | (11.5) | (27) | (9.5) | ||
| Active synovitis score | Range | 0–7 | 0–30 | 0–38 | 0–80 | 0–16 | 0.157 |
| Median | 1 | 19 | 2.5 | 8 | 5 | ||
| (IQR) | (4) | (28) | (8) | (35.5) | (8.5) | ||
| Total US severity score | Range | 2–35 | 11–120 | 0–96 | 4–280 | 4–38 | 0.026 * |
| Median | 11 | 105.5 | 23.5 | 53 | 20 | ||
| (IQR) | (9) | (89) | (34.5) | (86.5) | (31) | ||
| Synovial Effusion Score | Synovial Hypertrophy Score | Active Synovitis Score | Total US Severity Score | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Hemoglobin(gm/dL) | −0.176 | 0.179 | −0.286 | 0.057 | −0.251 | 0.056 | −0.243 | 0.061 |
| WBCs | 0.418 | <0.001 * | 0.366 | <0.001 * | 0.422 | <0.001 * | 0.408 | <0.001 * |
| Platelets | 0.484 | <0.001 * | 0.582 | <0.001 * | 0.636 | <0.001 * | 0.574 | <0.001 * |
| ESR 1st hour | 0.492 | <0.001 * | 0.614 | <0.001 * | 0.680 | <0.001 * | 0.614 | <0.001 * |
| CRP | 0.463 | <0.001 * | 0.501 | <0.001 * | 0.565 | <0.001 * | 0.525 | <0.001 * |
| US Activity Score | AJC | ||
|---|---|---|---|
| Optimal Cut-off point | Value | 3 | 4 |
| AUC | Value | 85.9 | 89.7 |
| 95% CI | 73.2–95.6 | 81.2–98.1 | |
| Sensitivity % | Value | 85.7 | 78.57 |
| 95% CI | 67.33–95.97 | 59.05–91.70 | |
| Specificity % | Value | 78.75 | 90.62 |
| 95% CI | 49.99–86.88 | 74.98–98.02 | |
| PPV % | Value | 70.59 | 88.00 |
| 95% CI | 58.41–80.40 | 71.05–95.63 | |
| NPV % | Value | 84.62 | 82.86 |
| 95% CI | 68.31–93.35 | 70.22–90.83 | |
| Accuracy % | Value | 83.67 | 85.00 |
| 95% CI | 63.96–89.62 | 73.43–92.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosa, D.M.; Abdelrahman, A.M.; El-Bahnasawy, A.S. Ultrasound Features across Subtypes of Juvenile Idiopathic Arthritis. Rheumato 2022, 2, 2-14. https://doi.org/10.3390/rheumato2010002
Mosa DM, Abdelrahman AM, El-Bahnasawy AS. Ultrasound Features across Subtypes of Juvenile Idiopathic Arthritis. Rheumato. 2022; 2(1):2-14. https://doi.org/10.3390/rheumato2010002
Chicago/Turabian StyleMosa, Doaa Mosad, Ashraf M. Abdelrahman, and Amany S. El-Bahnasawy. 2022. "Ultrasound Features across Subtypes of Juvenile Idiopathic Arthritis" Rheumato 2, no. 1: 2-14. https://doi.org/10.3390/rheumato2010002






