The Efficacy of Sequential Biologic Agents in Refractory Rheumatoid Arthritis after Failure of Initial DMARD and anti-Tumor Necrosis Factor Therapy
Abstract
:1. Introduction
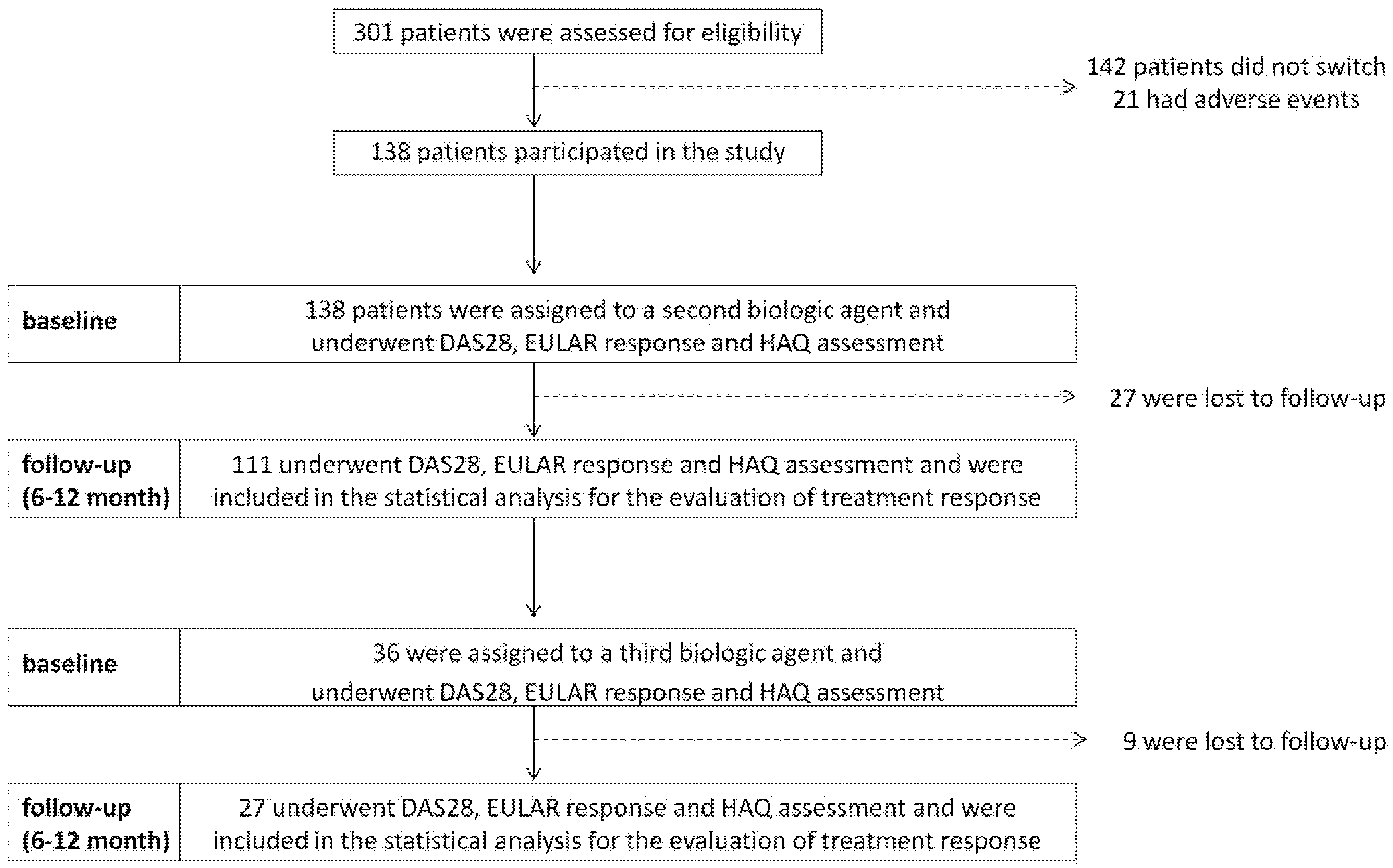
2. Materials and Methods
3. Results
3.1. Drug-Specific Response Rates and Disease Activity-Group I
3.2. Drug-Specific Response Rates and Disease Activity-Group II
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New therapies for treatment of rheumatoid arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Lipsky, P.E.; van der Heijde, D.M.; St. Clair, E.W.; Furst, D.E.; Breedveld, F.C.; Kalden, J.R.; Smolen, J.S.; Weisman, M.; Emery, P.; Feldmann, M.; et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 2000, 343, 1594–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinblatt, M.E.; Kremer, J.M.; Bankhurst, A.D.; Bulpitt, K.J.; Fleischmann, R.M.; Fox, R.I.; Jackson, C.G.; Lange, M.; Burge, D.J. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 1999, 340, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Keystone, E.C.; Furst, D.E.; Moreland, L.; Weisman, M.H.; Birbara, C.A.; Teoh, L.A.; Fischkoff, S.A.; Chartash, E.K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheumatol. 2003, 48, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.; Ritchlin, C.; Mendelsohn, A.; Baker, D.; Kim, L.; Xu, Z.; Han, J.; Taylor, P. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2010, 62, 917–928. [Google Scholar] [CrossRef]
- Genovese, M.C.; McKay, J.D.; Nasonov, E.L.; Mysler, E.F.; da Silva, N.A.; Alecock, E.; Woodworth, T.; Gomez-Reino, J.J. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheumatol. 2008, 58, 2968–2980. [Google Scholar]
- Kremer, J.M.; Dougados, M.; Emery, P.; Durez, P.; Sibilia, J.; Shergy, W.; Steinfeld, S.; Tindall, E.; Becker, J.-C.; Li, T.; et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: Twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 2005, 52, 2263–2271. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.; Mease, P.J.; Brzezicki, J.; Mason, D.; Luijtens, K.; Van Vollenhoven, R.F.; Kavanaugh, A.; Schiff, M.H.; Burmester, G.R.; et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: The RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 2009, 68, 797–804. [Google Scholar] [CrossRef]
- Murray, K.; Turk, M.; Alammari, Y.; Young, F.; Gallagher, P.; Saber, T.; Fearon, U.; Veale, D.J. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res. 2021, 23, 25. [Google Scholar] [CrossRef]
- Zink, A.; Listing, J.; Kary, S.; Ramlau, P.; Stoyanova-Scholz, M.; Babinsky, K.; Von Hinueber, U.; Gromnica-Ihle, E.; Wassenberg, S.; Antoni, C.; et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann. Rheum. Dis. 2005, 64, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Monchablon, C.; Gondé, H.; Pouplin, S.; Varin, R.; Vittecoq, O.; Lequerré, T. Assessment of adherence to disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Clin. Rheumatol. 2020, 39, 207–216. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, D.J.; Lee, J.W.; Lee, K.E.; Wen, L.; Kim, T.J.; Park, Y.W.; Lee, S.S. Drug survival rates of tumor necrosis factor inhibitors in patients with rheumatoid arthritis and ankylosing spondylitis. J. Korean Med. Sci. 2014, 29, 1205–1211. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, S.; Cifaldi, M.; Mittendorf, T.; Ganguli, A.; Wolff, M.; Zeidler, J. Biologic TNF inhibiting agents for treatment of rheumatoid arthritis: Persistence and dosing patterns in Germany. Health Econ. Rev. 2014, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vollenhoven, R.; Harju, A.; Brannemark, S.; Klareskog, L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: Data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann. Rheum. Dis. 2003, 62, 1195–1198. [Google Scholar] [CrossRef] [Green Version]
- Coy, N.C.N.; Brown, S.; Bosworth, A.; Davies, C.T.; Emery, P.; Everett, C.C.; Fernandez, C.; Gray, J.C.; Hartley, S.; Hulme, C.; et al. The ‘Switch’ study protocol: A randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet. Disord. 2014, 15, 452. [Google Scholar]
- Furst, D.E.; Gaylis, N.; Bray, V.; Olech, E.; Yocum, D.; Ritter, J.; Weisman, M.; Wallace, D.J.; Crues, J.; Khanna, D.; et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: The opposite study. Ann. Rheum. Dis. 2007, 66, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Migliore, A.; Pompilio, G.; Integlia, D.; Zhuo, J.; Alemao, E. Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: A Bayesian network meta-analysis. Adv. Musculoskelet. Dis. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Virkki, L.M.; Valleala, H.; Takakubo, Y.; Vuotila, J.; Relas, H.; Komulainen, R.; Koivuniemi, R.; Yli-Kerttula, U.; Mali, M.; Sihvonen, S.; et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—A study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin. Rheumatol. 2011, 30, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Hyrich, K.L.; Lunt, M.; Watson, K.D.; Symmons, D.P.; Silman, A.J. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: Results from a large UK national cohort study. Arthritis Rheumatol. 2007, 56, 13–20. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hasegawa, M.; Nishioka, Y.; Minami, Y.; Nishioka, K.; Sudo, A. Clinical outcome in patients with rheumatoid arthritis switched to tocilizumab after etanercept or infliximab failure. Clin. Rheumatol. 2013, 32, 253–259. [Google Scholar] [CrossRef]
- Chatzidionysiou, K.; Askling, J.; Eriksson, J.; Kristensen, L.E.; Van Vollenhoven, R. Effectiveness of TNF inhibitor switch in RA: Results from the national Swedish register. Ann. Rheum. Dis. 2015, 74, 890–896. [Google Scholar] [CrossRef]
- Rémy, A.; Avouac, J.; Gossec, L.; Combe, B. Clinical relevance of switching to a second tumour necrosis factor-alpha inhibitor after discontinuation of a first tumour necrosis factor-alpha inhibitor in rheumatoid arthritis: A systematic literature review and meta-analysis. Clin. Exp. Rheumatol. 2011, 29, 96–103. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.; Breedveld, F.C.; Buch, M.; Burmester, G.; Dougados, M.; Emery, P.; Gaujoux-Viala, C.; Gossec, L.; Nam, J.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 2014, 73, 492–509. [Google Scholar] [CrossRef]
- Rayner, F.; Anderson, A.E.; Baker, K.F.; Buckley, C.D.; Dyke, B.; Fenton, S.; Filer, A.; Goodyear, C.S.; Hilkens, C.M.U.; Hiu, S.; et al. BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (the BIO-FLARE study): Protocol for a non-randomised longitudinal cohort study. BMC Rheumatol. 2021, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Baganz, L.; Richter, A.; Kekow, J.; Bussmann, A.; Krause, A.; Stille, C.; Listing, J.; Zink, A.; Strangfeld, A. Long-term effectiveness of tocilizumab in patients with rheumatoid arthritis, stratified by number of previous treatment failures with biologic agents: Results from the German RABBIT cohort. Rheumatol. Int. 2018, 38, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Rendas-Baum, R.; Wallenstein, G.V.; Koncz, T.; Kosinski, M.; Yang, M.; Bradley, J.; Zwillich, S.H. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res. 2011, 13, R25. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.A.; Kristensen, L.E.; Kapetanovic, M.C.; Gulfe, A.; Saxne, T.; Geborek, P. Treatment response to a second or third TNF-inhibitor in RA: Results from the South Swedish Arthritis Treatment Group Register. Rheumatology 2008, 47, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Schiff, M.; Pritchard, C.; Huffstutter, J.E.; Rodriguez-Valverde, V.; Durez, P.; Zhou, X.; Li, T.; Bahrt, K.; Kelly, S.; Le Bars, M.; et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: The ARRIVE trial. Ann. Rheum. Dis. 2009, 68, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malottki, K.; Barton, P.; Tsourapas, A.; Uthman, A.; Liu, Z.; Routh, K.; Connock, M.; Jobanputra, P.; Moore, D.; Fry-Smith, A.; et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: A systematic review and economic evaluation. Health Technol. Assess. 2011, 15, 1–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, P.; Keystone, E.; Tony, H.P.; Cantagrel, A.; Van Vollenhoven, R.; Sanchez, A.; Alecock, E.; Lee, J.; Kremer, J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008, 67, 1516–1523. [Google Scholar] [CrossRef] [PubMed]

| First Switch (Group I) | Second Switch (Group II) | |
|---|---|---|
| Age (y), (mean ± SD) | 54 ± 8.47 | 53 ± 7.93 |
| BMI (Kg/m2), (mean ± SD) | 25.02 ± 4.04 | 25.14 ± 4.99 |
| Female (%) | 89 (80) | 22 (82) |
| Duration of disease (y), (mean ± SD) * | 13.37 ± 6.12 | 27 ± 7.56 |
| DAS28, (mean ± SD) | 5.63 ± 1.30 | 5.33 ± 2.19 |
| HAQ, (mean ± SD) | 2.07 ± 0.50 | 1.94 ± 0.67 |
| Concurrent corticosteroid use | ||
| None, n (%) | 22 (20) | 5 (19) |
| ≤5 mg, n (%) | 67 (60) | 15 (56) |
| >5 mg, n (%) | 15 (18) | 6 (22) |
| Concurrent MTX use, n (%) | 69 (62) | 16 (60) |
| Weekly MTX dose (mg), (mean ± SD) | 8.80 ± 2.40 | 9.2 ± 2.20 |
| Concurrent other DMARDs use, n (%) | 22 (20) | 6 (22) |
| First Switch (Group I) | Second Switch (Group II) | |||
|---|---|---|---|---|
| 6 Months | 12 Months | 6 Months | 12 Months | |
| Tocilizumab (n = 24) | Tocilizumab (n = 6) | |||
| No response, n (%) | 3 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Moderate response, n (%) | 21 (87.5) | 6 (25) | 3 (50) | 3 (50) |
| Good response, n (%) | 0 (0) | 18 (75) | 3 (50) | 3 (50) |
| Abatacept (n = 24) | Abatacept (n = 9) | |||
| No response, n (%) | 6 (25) | 0 (0) | 3 (33) | 0 (0) |
| Moderate response, n (%) | 15 (62.5) | 18 (75) | 3 (33) | 9 (100) |
| Good response, n (%) | 3 (12.5) | 6 (25) | 3 (33) | 0 (0) |
| Adalimumab (n = 12) | Adalimumab (n = 3) | |||
| No response, n (%) | 0 (0) | 0 (0) | 2 (66) | 0 (0) |
| Moderate response, n (%) | 9 (75) | 6 (50) | 1 (33) | 3 (100) |
| Good response, n (%) | 3 (25) | 6 (50) | 0 (0) | 0 (0) |
| Etanercept (n = 33) | Etanercept (n = 9) | |||
| No response, n (%) | 0 (0) | 0 (0) | 9 (100) | 0 (0) |
| Moderate response, n (%) | 30 (91) | 6 (18) | 0 (0) | 6 (66) |
| Good response, n (%) | 3 (9) | 27 (82) | 0 (0) | 3 (33) |
| Infliximab (n = 15) | ||||
| No response, n (%) | 0 (0) | 0 (0) | ||
| Moderate response, n (%) | 15 (100) | 0 (0) | ||
| Good response, n (%) | 0 (0) | 15 (100) | ||
| Group I (n = 111) | Group II (n = 27) | |
|---|---|---|
| HAQ0 | 2.07 (0.51) | 1.94 (0.47) |
| HAQ6 | 0.98 (0.37) * | 1.01 (0.46) * |
| HAQ12 | 0.53 (0.18) * | 0.78 (0.37) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Versace, A.G.; Aragona, C.O.; La Rosa, D.; Chiappalone, M.; Tringali, M.C.; De Gaetano, A.; Moore, C.F., Jr.; Sangari, D.; Roberts, W.N.; Bagnato, G. The Efficacy of Sequential Biologic Agents in Refractory Rheumatoid Arthritis after Failure of Initial DMARD and anti-Tumor Necrosis Factor Therapy. Rheumato 2021, 1, 22-30. https://doi.org/10.3390/rheumato1010005
Versace AG, Aragona CO, La Rosa D, Chiappalone M, Tringali MC, De Gaetano A, Moore CF Jr., Sangari D, Roberts WN, Bagnato G. The Efficacy of Sequential Biologic Agents in Refractory Rheumatoid Arthritis after Failure of Initial DMARD and anti-Tumor Necrosis Factor Therapy. Rheumato. 2021; 1(1):22-30. https://doi.org/10.3390/rheumato1010005
Chicago/Turabian StyleVersace, Antonio Giovanni, Caterina Oriana Aragona, Daniela La Rosa, Marianna Chiappalone, Maria Concetta Tringali, Alberta De Gaetano, Charles Frederick Moore, Jr., Donatella Sangari, William Neal Roberts, and Gianluca Bagnato. 2021. "The Efficacy of Sequential Biologic Agents in Refractory Rheumatoid Arthritis after Failure of Initial DMARD and anti-Tumor Necrosis Factor Therapy" Rheumato 1, no. 1: 22-30. https://doi.org/10.3390/rheumato1010005







