A Guide to Guides: An Overview of SpCas9 sgRNA Scaffold Variants and Modifications
Abstract
1. Introduction
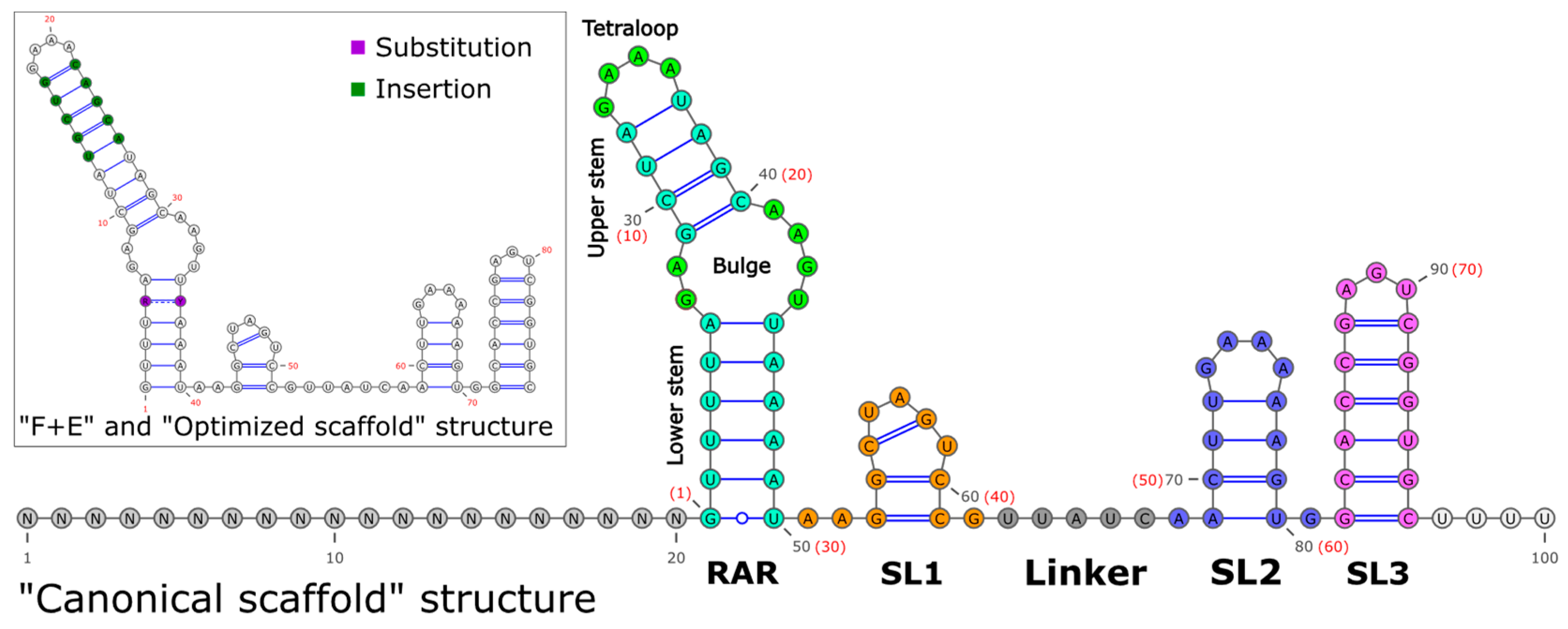
2. Structure of an sgRNA
3. Alternative Scaffolds
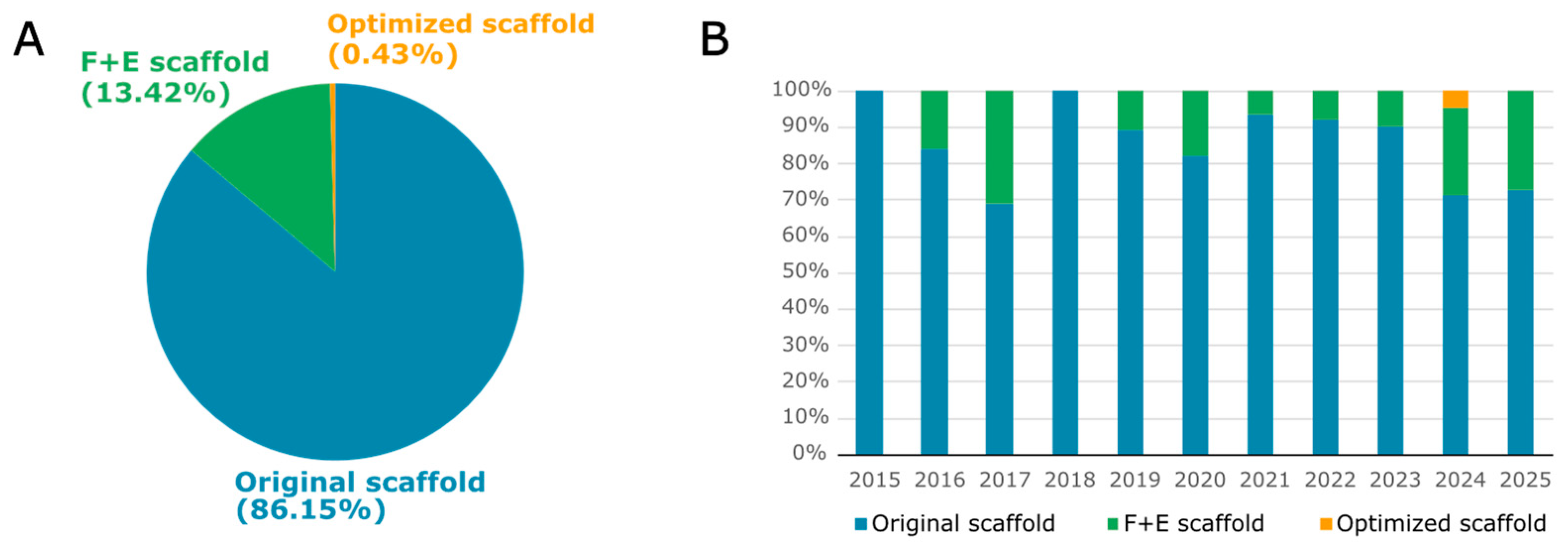
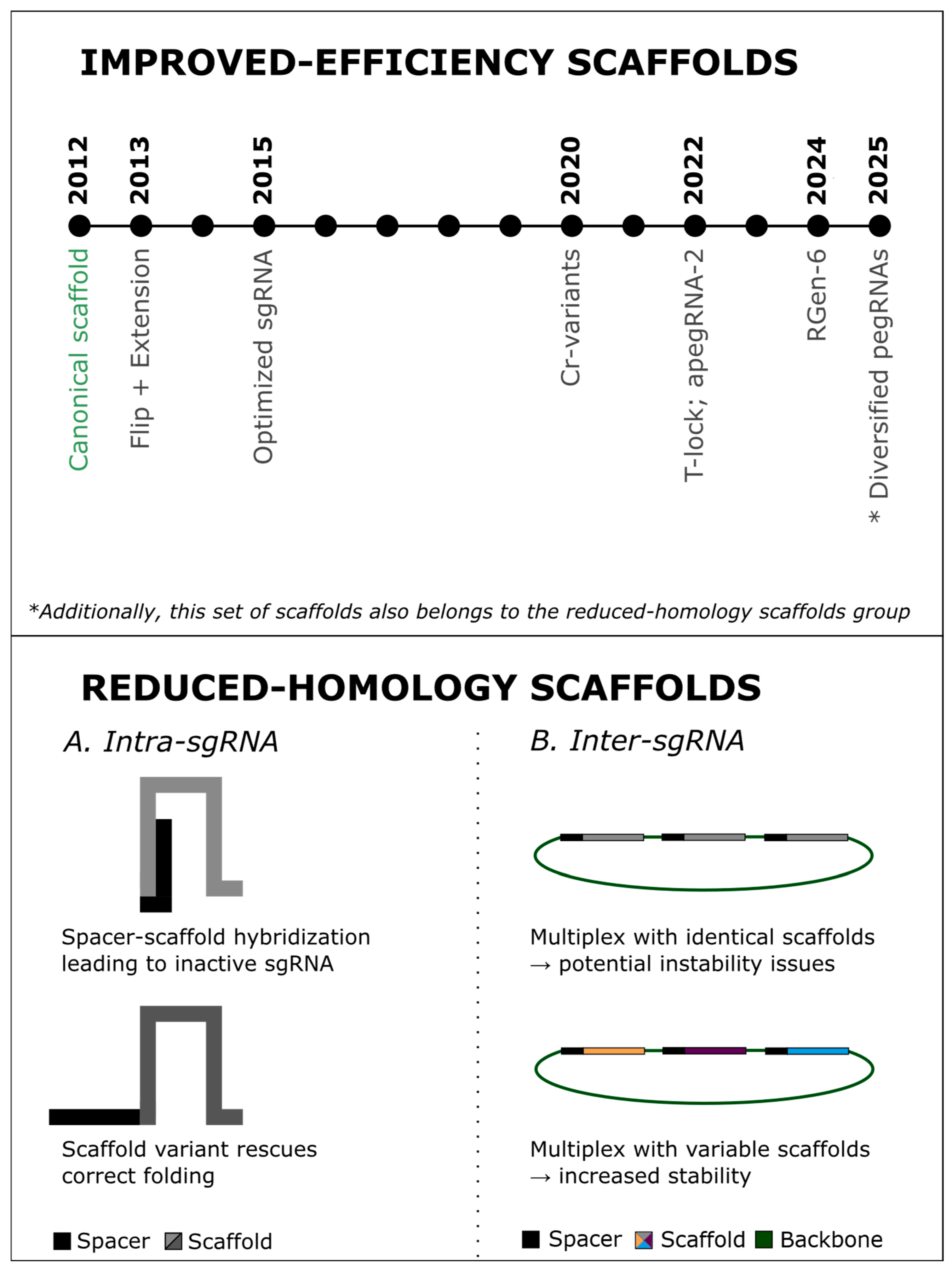
3.1. Scaffolds for Improved Genome Editing Efficiency
3.2. Scaffolds with Reduced Sequence Homology
3.2.1. Spacer–Scaffold Complementarity (Intra-sgRNA Stability)
3.2.2. Recombination in Multiplex sgRNA Arrays (Inter-sgRNA Stability)
3.3. Alternative Scaffolds with Similar Efficiency
4. Other Modifications and Extensions
4.1. Systems for Visualization and Recruitment
4.2. Systems for sgRNA Multiplexing
4.3. Systems to Enhance sgRNA Stability
4.4. DNA Donor Fusions
4.5. Conditional Control Systems
4.6. Fusion with shRNAs, miRNA Elements, and Long Noncoding RNAs
4.7. Guide Barcoding
4.8. Mobile RNA Motifs
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABE | Adenine base editor |
| agRNA | Switchable aptamer–sgRNA |
| AI | Artificial intelligence |
| BE | Base editing |
| Bp | Base pair |
| CBE | Cytosine base editor |
| cgRNA | Circular sgRNA |
| cgRNA | Conditional guide RNA |
| cP | 2′,3′-cyclic phosphate |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CRISPRa | CRISPR activation |
| CRISPRi | CRISPR interference |
| crRNA | CRISPR RNA |
| dCas9 | Dead Cas9 |
| DFHBI | 3,5-Difluoro-4-hydroxybenzylidene imidazolinone |
| ELSA | Extra-long sgRNA array |
| F + E sgRNA | Flip + Extension (F + E) sgRNA |
| fgRNA | Fusion gRNA |
| FT | FLOWERING LOCUS T |
| GOLD-gRNA | Genome-editing Optimized Locked Design-gRNA |
| gRNA | Guide-RNA |
| HBC | Hydroxybenzylidene Cyanine |
| HDR | Homology directed repair |
| HDV | Hepatitis delta virus |
| HEAT scaffold | Hybridization-Extended A–T inversion |
| HH | Hammerhead |
| iSBH-sgRNA | Inducible spacer-blocking hairpin sgRNAs |
| ligRNA | Ligand-responsive sgRNA |
| lncRNA | Long noncoding RNA |
| MCP | MS2 coat protein |
| mFT | Mutant FT |
| miRNA | MicroRNA |
| MMLV | Moloney murine leukemia virus |
| MOI | Multiplicity of infection |
| MVE | Murray Valley encephalitis |
| NHEJ | Non-homologous end-joining |
| NR-RAMBE | Non-repetitive RiboJ-Aided Multiplexed Base Editing |
| nt | Nucleotide |
| PBS | PUF-binding site |
| PBS | Primer-binding site |
| PCP | PP7 coat protein |
| PTG | Polycistronic tRNA–gRNA |
| PE | Prime editing |
| Pri-miRNA | Primary microRNA |
| PUF | Pumilio/FBF RNA-binding protein |
| QCP | Qβ coat protein |
| RAR | Repeat–anti-repeat |
| RGR | Ribozyme–gRNA–Ribozyme |
| RNAi | RNA interference |
| RTT | Reverse transcriptase template |
| sgRNA | Single-guide RNA |
| shRNA | Short hairpin RNA |
| SI-sgRNA | Self-inhibitory sgRNA |
| SL | Stem-loop |
| SMART sgRNA | Small-molecule–Activated Allosteric Aptamer-Regulated sgRNA |
| stgRNA | Self-targeting RNA |
| sTRSV | Satellite tobacco ringspot virus |
| tDeg | Tat Degron |
| thgRNA | Toehold-gated gRNA |
| TLS | tRNA-like structures |
| tracrRNA | Trans-activating CRISPR RNA |
| Tru-gRNA | Truncated gRNA |
| TYMV | Turnip Yellow Mosaic Virus |
| T-stretch | Thymidine stretch |
| U-tail | Uridine-tail |
| x-gRNA | Extended gRNA |
| xrRNA | Exoribonuclease-resistant RNA |
References
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-Programmed Genome Editing in Human Cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.-S. Targeted Genome Engineering in Human Cells with the Cas9 RNA-Guided Endonuclease. Nat. Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.-R.J.; Joung, J.K. Efficient Genome Editing in Zebrafish Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Gostimskaya, I. CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. Biochem. 2022, 87, 777–788. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering. Mol. Biotechnol. 2023, 65, 311–325. [Google Scholar] [CrossRef]
- Villiger, L.; Joung, J.; Koblan, L.; Weissman, J.; Abudayyeh, O.O.; Gootenberg, J.S. CRISPR Technologies for Genome, Epigenome and Transcriptome Editing. Nat. Rev. Mol. Cell Biol. 2024, 25, 464–487. [Google Scholar] [CrossRef]
- Thuma, J.; Chung, Y.-C.; Tu, L.-C. Advances and Challenges in CRISPR-Based Real-Time Imaging of Dynamic Genome Organization. Front. Mol. Biosci. 2023, 10, 1173545. [Google Scholar] [CrossRef]
- Jang, H.; Yim, S.S. Toward DNA-Based Recording of Biological Processes. Int. J. Mol. Sci. 2024, 25, 9223. [Google Scholar] [CrossRef]
- Pujar, A.; Pathania, A.; Hopper, C.; Pandi, A.; Calderón, C.R.; Függer, M.; Nowak, T.; Kushwaha, M. Phage-Mediated Intercellular CRISPRi for Biocomputation in Bacterial Consortia. Nucleic Acids Res. 2025, 53, gkae1256. [Google Scholar] [CrossRef]
- Ghouneimy, A.; Mahas, A.; Marsic, T.; Aman, R.; Mahfouz, M. CRISPR-Based Diagnostics: Challenges and Potential Solutions toward Point-of-Care Applications. ACS Synth. Biol. 2023, 12, 1–16. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 Therapeutics: Progress and Prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Pan, D.; Yu, H.; Zhang, Y.; Chen, W.; Li, F.; Wu, Z.; Ji, Q. Guide RNA Engineering Enables Efficient CRISPR Editing with a Miniature Syntrophomonas palmitatica Cas12f1 Nuclease. Cell Rep. 2022, 40. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lee, J.M.; Moon, S.B.; Chin, H.J.; Park, S.; Lim, Y.; Kim, D.; Koo, T.; Ko, J.-H.; Kim, Y.-S. Efficient CRISPR Editing with a Hypercompact Cas12f1 and Engineered Guide RNAs Delivered by Adeno-Associated Virus. Nat. Biotechnol. 2022, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Omura, S.N.; Nakagawa, R.; Togashi, T.; Takeda, S.N.; Hiramoto, T.; Tasaka, S.; Hirano, H.; Tokuyama, T.; Uosaki, H.; et al. An AsCas12f-Based Compact Genome-Editing Tool Derived by Deep Mutational Scanning and Structural Analysis. Cell 2023, 186, 4920–4935.e23. [Google Scholar] [CrossRef]
- Bai, S.; Cao, X.; Hu, L.; Hu, D.; Li, D.; Sun, Y. Engineering an Optimized Hypercompact CRISPR/Cas12j-8 System for Efficient Genome Editing in Plants. Plant Biotechnol. J. 2025, 23, 1153–1164. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Wu, Z.; Pausch, P.; Al-Shayeb, B.; Amerasekera, J.; Doudna, J.A.; Jacobsen, S.E. Genome Editing in Plants Using the Compact Editor CasΦ. Proc. Natl. Acad. Sci. USA 2023, 120, e2216822120. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Yin, H. Recent Advances in Chemical Modifications of Guide RNA, MRNA and Donor Template for CRISPR-Mediated Genome Editing. Adv. Drug Deliv. Rev. 2021, 168, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Rosenberg, M.; Hendel, A. Using Synthetically Engineered Guide RNAs to Enhance CRISPR Genome Editing Systems in Mammalian Cells. Front. Genome Ed. 2021, 2, 617910. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Helmbrecht, N.; Kanis, P.; Maricic, T.; Pääbo, S. Improved GRNA Secondary Structures Allow Editing of Target Sites Resistant to CRISPR-Cas9 Cleavage. Nat. Commun. 2022, 13, 489. [Google Scholar] [CrossRef]
- Filippova, J.; Matveeva, A.; Zhuravlev, E.; Stepanov, G. Guide RNA Modification as a Way to Improve CRISPR/Cas9-Based Genome-Editing Systems. Biochimie 2019, 167, 49–60. [Google Scholar] [CrossRef]
- Krysler, A.R.; Cromwell, C.R.; Tu, T.; Jovel, J.; Hubbard, B.P. Guide RNAs Containing Universal Bases Enable Cas9/Cas12a Recognition of Polymorphic Sequences. Nat. Commun. 2022, 13, 1617. [Google Scholar] [CrossRef]
- Scott, T.; Urak, R.; Soemardy, C.; Morris, K. V Improved Cas9 Activity by Specific Modifications of the TracrRNA. Sci. Rep. 2019, 9, 16104. [Google Scholar] [CrossRef]
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.-W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic Imaging of Genomic Loci in Living Human Cells by an Optimized CRISPR/Cas System. Cell 2013, 155, 1479–1491. [Google Scholar] [CrossRef]
- Dang, Y.; Jia, G.; Choi, J.; Ma, H.; Anaya, E.; Ye, C.; Shankar, P.; Wu, H. Optimizing SgRNA Structure to Improve CRISPR-Cas9 Knockout Efficiency. Genome Biol. 2015, 16, 280. [Google Scholar] [CrossRef]
- Darty, K.; Denise, A.; Ponty, Y. VARNA: Interactive Drawing and Editing of the RNA Secondary Structure. Bioinformatics 2009, 25, 1974–1975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-X.; Fu, Y.-W.; Zhao, J.-J.; Zhang, F.; Li, S.-A.; Zhao, M.; Wen, W.; Zhang, L.; Cheng, T.; Zhang, J.-P.; et al. Superior Fidelity and Distinct Editing Outcomes of SaCas9 Compared with SpCas9 in Genome Editing. Genom. Proteom. Bioinform. 2023, 21, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas Nuclease Specificity Using Truncated Guide RNAs. Nat. Biotechnol. 2014, 32, 279–284. [Google Scholar] [CrossRef]
- Coelho, M.A.; De Braekeleer, E.; Firth, M.; Bista, M.; Lukasiak, S.; Cuomo, M.E.; Taylor, B.J.M. CRISPR GUARD Protects Off-Target Sites from Cas9 Nuclease Activity Using Short Guide RNAs. Nat. Commun. 2020, 11, 4132. [Google Scholar] [CrossRef] [PubMed]
- Perli, S.D.; Cui, C.H.; Lu, T.K. Continuous Genetic Recording with Self-Targeting CRISPR-Cas in Human Cells. Science 2016, 353, aag0511. [Google Scholar] [CrossRef] [PubMed]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR Guide RNA Design by Deep Learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.T.; Browne, T.S.; Banglorewala, P.N.; Wilson, T.L.; Michael, R.K.; Gloor, G.B.; Edgell, D.R. A Generalizable Cas9/SgRNA Prediction Model Using Machine Transfer Learning with Small High-Quality Datasets. Nat. Commun. 2023, 14, 5514. [Google Scholar] [CrossRef]
- Moreno-Mateos, M.A.; Vejnar, C.E.; Beaudoin, J.-D.; Fernandez, J.P.; Mis, E.K.; Khokha, M.K.; Giraldez, A.J. CRISPRscan: Designing Highly Efficient SgRNAs for CRISPR-Cas9 Targeting In Vivo. Nat. Methods 2015, 12, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, M.; Suzuki, H.I.; Kimura, R.; Suzuki, A. Optimization of Cas9 Activity through the Addition of Cytosine Extensions to Single-Guide RNAs. Nat. Biomed. Eng. 2023, 7, 672–691. [Google Scholar] [CrossRef]
- Herring-Nicholas, A.; Dimig, H.; Roesing, M.R.; Josephs, E.A. Selection of Extended CRISPR RNAs with Enhanced Targeting and Specificity. Commun. Biol. 2024, 7, 86. [Google Scholar] [CrossRef]
- Jost, M.; Santos, D.A.; Saunders, R.A.; Horlbeck, M.A.; Hawkins, J.S.; Scaria, S.M.; Norman, T.M.; Hussmann, J.A.; Liem, C.R.; Gross, C.A.; et al. Titrating Gene Expression Using Libraries of Systematically Attenuated CRISPR Guide RNAs. Nat. Biotechnol. 2020, 38, 355–364. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Briner, A.E.; Donohoue, P.D.; Gomaa, A.A.; Selle, K.; Slorach, E.M.; Nye, C.H.; Haurwitz, R.E.; Beisel, C.L.; May, A.P.; Barrangou, R. Guide RNA Functional Modules Direct Cas9 Activity and Orthogonality. Mol. Cell 2014, 56, 333–339. [Google Scholar] [CrossRef]
- Gao, Z.; Herrera-Carrillo, E.; Berkhout, B. Delineation of the Exact Transcription Termination Signal for Type 3 Polymerase III. Mol. Ther. Nucleic Acids 2018, 10, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Landrieux, E.; Alic, N.; Ducrot, C.; Acker, J.; Riva, M.; Carles, C. A Subcomplex of RNA Polymerase III Subunits Involved in Transcription Termination and Reinitiation. EMBO J. 2006, 25, 118–128. [Google Scholar] [CrossRef]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering Complex Synthetic Transcriptional Programs with CRISPR RNA Scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef]
- Li, C.; Zong, Y.; Jin, S.; Zhu, H.; Lin, D.; Li, S.; Qiu, J.-L.; Wang, Y.; Gao, C. SWISS: Multiplexed Orthogonal Genome Editing in Plants with a Cas9 Nickase and Engineered CRISPR RNA Scaffolds. Genome Biol. 2020, 21, 141. [Google Scholar] [CrossRef]
- Mikami, M.; Toki, S.; Endo, M. In Planta Processing of the SpCas9–GRNA Complex. Plant Cell Physiol. 2017, 58, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Shechner, D.M.; Hacisuleyman, E.; Younger, S.T.; Rinn, J.L. Multiplexable, Locus-Specific Targeting of Long RNAs with CRISPR-Display. Nat. Methods 2015, 12, 664–670. [Google Scholar] [CrossRef]
- Ma, H.; Tu, L.-C.; Naseri, A.; Chung, Y.-C.; Grunwald, D.; Zhang, S.; Pederson, T. CRISPR-Sirius: RNA Scaffolds for Signal Amplification in Genome Imaging. Nat. Methods 2018, 15, 928–931. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Gao, B.-Q.; Li, G.; Wang, X.; Wang, Y.; Wei, J.; Han, W.; Wang, Z.; Li, J.; et al. Highly Efficient Prime Editing by Introducing Same-Sense Mutations in PegRNA or Stabilizing Its Structure. Nat. Commun. 2022, 13, 1669. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, R.; Chao, L.; Xu, G.; Zhang, Y.; Zhou, G.; Yin, D.; Guo, Y.; Fu, Y.; Yang, Y.; et al. RNAGenesis: A Generalist Foundation Model for Functional RNA Therapeutics. bioRxiv 2025. [Google Scholar] [CrossRef]
- McDiarmid, T.A.; Taylor, M.L.; Chen, W.; Chardon, F.M.; Choi, J.; Liao, H.; Li, X.; Kim, H.; Lalanne, J.-B.; Li, T.; et al. A Parts List of Promoters and GRNA Scaffolds for Mammalian Genome Engineering and Molecular Recording. Nat. Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Li, J.; Liu, X.; Xu, R.; Yang, J.; Wei, P. SpCas9-NG Self-Targets the SgRNA Sequence in Plant Genome Editing. Nat. Plants 2020, 6, 197–201. [Google Scholar] [CrossRef]
- Chey, Y.C.J.; Gierus, L.; Lushington, C.; Arudkumar, J.C.; Geiger, A.B.; Staker, L.G.; Robertson, L.J.; Pfitzner, C.; Kennedy, J.G.; Lee, R.H.B.; et al. Optimal SpCas9- and SaCas9-Mediated Gene Editing by Enhancing GRNA Transcript Levels through Scaffold Poly-T Tract Reduction. BMC Genom. 2025, 26, 138. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Li, X.; Chu, V.T.; Rajewsky, K. SgRNA Sequence Motifs Blocking Efficient CRISPR/Cas9-Mediated Gene Editing. Cell Rep. 2019, 26, 1098–1103.e3. [Google Scholar] [CrossRef]
- Cross, B.C.S.; Lawo, S.; Archer, C.R.; Hunt, J.R.; Yarker, J.L.; Riccombeni, A.; Little, A.S.; McCarthy, N.J.; Moore, J.D. Increasing the Performance of Pooled CRISPR–Cas9 Drop-out Screening. Sci. Rep. 2016, 6, 31782. [Google Scholar] [CrossRef]
- Čermák, T.; Curtin, S.J.; Gil-Humanes, J.; Čegan, R.; Kono, T.J.Y.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Liu, Q.; Li, J.; Wang, K. Increasing the Efficiency of CRISPR-Cas9-VQR Precise Genome Editing in Rice. Plant Biotechnol. J. 2018, 16, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Sanson, K.R.; Hanna, R.E.; Hegde, M.; Donovan, K.F.; Strand, C.; Sullender, M.E.; Vaimberg, E.W.; Goodale, A.; Root, D.E.; Piccioni, F.; et al. Optimized Libraries for CRISPR-Cas9 Genetic Screens with Multiple Modalities. Nat. Commun. 2018, 9, 5416. [Google Scholar] [CrossRef]
- Vos, P.D.; Gandadireja, A.P.; Rossetti, G.; Siira, S.J.; Mantegna, J.L.; Filipovska, A.; Rackham, O. Mutational Rescue of the Activity of High-Fidelity Cas9 Enzymes. Cell Rep. Methods 2024, 4, 100756. [Google Scholar] [CrossRef]
- Liu, M.-S.; Gong, S.; Yu, H.-H.; Jung, K.; Johnson, K.A.; Taylor, D.W. Engineered CRISPR/Cas9 Enzymes Improve Discrimination by Slowing DNA Cleavage to Allow Release of off-Target DNA. Nat. Commun. 2020, 11, 3576. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.W.; Randolph, P.B.; Shen, S.P.; Everette, K.A.; Chen, P.J.; Anzalone, A.V.; An, M.; Newby, G.A.; Chen, J.C.; Hsu, A.; et al. Engineered PegRNAs Improve Prime Editing Efficiency. Nat. Biotechnol. 2021, 40, 402–410. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Thyme, S.B.; Akhmetova, L.; Montague, T.G.; Valen, E.; Schier, A.F. Internal Guide RNA Interactions Interfere with Cas9-Mediated Cleavage. Nat. Commun. 2016, 7, 11750. [Google Scholar] [CrossRef]
- Huszár, K.; Welker, Z.; Györgypál, Z.; Tóth, E.; Ligeti, Z.; Kulcsár, P.I.; Dancsó, J.; Tálas, A.; Krausz, S.L.; Varga, É.; et al. Position-Dependent Sequence Motif Preferences of SpCas9 Are Largely Determined by Scaffold-Complementary Spacer Motifs. Nucleic Acids Res. 2023, 51, 5847–5863. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Oliveira, P.H.; Prazeres, D.M.F.; Monteiro, G.A. High Frequency Plasmid Recombination Mediated by 28 Bp Direct Repeats. Mol. Biotechnol. 2008, 40, 252–260. [Google Scholar] [CrossRef]
- Bi, X.; Liu, L.F. RecA-Independent and RecA-Dependent Intramolecular Plasmid Recombination: Differential Homology Requirement and Distance Effect. J. Mol. Biol. 1994, 235, 414–423. [Google Scholar] [CrossRef]
- Lovett, S.T. Encoded Errors: Mutations and Rearrangements Mediated by Misalignment at Repetitive DNA Sequences. Mol. Microbiol. 2004, 52, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kyaw, H.; Tong, Z.; Yang, Y.; Wang, Z.; Zhang, L.; Deng, L.; Zhang, Z.; Xiao, B.; Quick, W.P.; et al. A Simple and Efficient CRISPR/Cas9 System Permits Ultra-Multiplex Genome Editing in Plants. Crop J. 2024, 12, 569–582. [Google Scholar] [CrossRef]
- Yuan, Q.; Gao, X. Multiplex Base- and Prime-Editing with Drive-and-Process CRISPR Arrays. Nat. Commun. 2022, 13, 2771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Liu, M.; Zhou, Y.; Li, F. Guide RNA Scaffold Variants Enabled Easy Cloning of Large GRNA Cluster for Multiplexed Gene Editing. Plant Biotechnol. J. 2024, 22, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.; Halper, S.M.; Vezeau, G.E.; Cetnar, D.P.; Hossain, A.; Clauer, P.R.; Salis, H.M. Simultaneous Repression of Multiple Bacterial Genes Using Nonrepetitive Extra-Long SgRNA Arrays. Nat. Biotechnol. 2019, 37, 1294–1301. [Google Scholar] [CrossRef]
- Karvelis, T.; Gasiunas, G.; Miksys, A.; Barrangou, R.; Horvath, P.; Siksnys, V. CrRNA and TracrRNA Guide Cas9-Mediated DNA Interference in Streptococcus Thermophilus. RNA Biol. 2013, 10, 841–851. [Google Scholar] [CrossRef]
- Woo, S.-G.; Kim, S.K.; Kim, T.H.; Kim, S.; Kim, Y.; Lee, S.-G.; Lee, D.-H. RiboJ-Assisted Non-Repeated SgRNA Arrays for Enhanced CRISPR Multiplex Genome Engineering in Escherichia Coli. Chem. Eng. J. 2025, 512, 162336. [Google Scholar] [CrossRef]
- Xu, Z.; Kuang, Y.; Ren, B.; Yan, D.; Yan, F.; Spetz, C.; Sun, W.; Wang, G.; Zhou, X.; Zhou, H. SpRY Greatly Expands the Genome Editing Scope in Rice with Highly Flexible PAM Recognition. Genome Biol. 2021, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Corsi, G.I.; Yan, A.C.; Haynes, K.; Layzer, J.M.; Zhou, J.H.; Llanga, T.; Gorodkin, J.; Sullenger, B.A. Utilizing Directed Evolution to Interrogate and Optimize CRISPR/Cas Guide RNA Scaffolds. Cell Chem. Biol. 2023, 30, 879–892.e5. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Fontana, J.; Patel, A.; Carothers, J.M.; Zalatan, J.G. Synthetic CRISPR-Cas Gene Activators for Transcriptional Reprogramming in Bacteria. Nat. Commun. 2018, 9, 2489. [Google Scholar] [CrossRef] [PubMed]
- Konermann, S.; Brigham, M.D.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.S.; Nishimasu, H.; et al. Genome-Scale Transcriptional Activation by an Engineered CRISPR-Cas9 Complex. Nature 2015, 517, 583–588. [Google Scholar] [CrossRef]
- Dong, F.; Xie, K.; Chen, Y.; Yang, Y.; Mao, Y. Polycistronic TRNA and CRISPR Guide-RNA Enables Highly Efficient Multiplexed Genome Engineering in Human Cells. Biochem. Biophys. Res. Commun. 2017, 482, 889–895. [Google Scholar] [CrossRef]
- Kurata, M.; Wolf, N.K.; Lahr, W.S.; Weg, M.T.; Kluesner, M.G.; Lee, S.; Hui, K.; Shiraiwa, M.; Webber, B.R.; Moriarity, B.S. Highly Multiplexed Genome Engineering Using CRISPR/Cas9 GRNA Arrays. PLoS ONE 2018, 13, e0198714. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, Z.; Zhang, Y.; Shi, S.; Nielsen, J.; Liu, Z. A GRNA-TRNA Array for CRISPR-Cas9 Based Rapid Multiplexed Genome Editing in Saccharomyces Cerevisiae. Nat. Commun. 2019, 10, 1053. [Google Scholar] [CrossRef]
- Nahar, S.; Sehgal, P.; Azhar, M.; Rai, M.; Singh, A.; Sivasubbu, S.; Chakraborty, D.; Maiti, S. A G-Quadruplex Motif at the 3′ End of SgRNAs Improves CRISPR–Cas9 Based Genome Editing Efficiency. Chem. Commun. 2018, 54, 2377–2380. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Huang, S.; Qu, S.; Cheng, D.; Yao, Y.; Ji, Q.; Wang, X.; Huang, X.; Liu, J. Enhancement of Prime Editing via XrRNA Motif-Joined PegRNA. Nat. Commun. 2022, 13, 1856. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Liang, J.; Xu, R.; Jiang, Y.; Li, Y.; Ding, J.; Li, M.; Qin, R.; Wei, P. Development of a Highly Efficient Prime Editor 2 System in Plants. Genome Biol. 2022, 23, 161. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Li, J.; Zhao, D.; Li, S.; Jiang, G.; Wang, J.; Chen, X.; Bi, C.; Zhang, X. Circular Guide RNA for Improved Stability and CRISPR-Cas9 Editing Efficiency In Vitro and in Bacteria. ACS Synth. Biol. 2023, 12, 350–359. [Google Scholar] [CrossRef]
- Bao, Z.; Xiao, H.; Liang, J.; Zhang, L.; Xiong, X.; Sun, N.; Si, T.; Zhao, H. Homology-Integrated CRISPR–Cas (HI-CRISPR) System for One-Step Multigene Disruption in Saccharomyces Cerevisiae. ACS Synth. Biol. 2015, 4, 585–594. [Google Scholar] [CrossRef]
- Lin, B.; An, Y.; Meng, L.; Zhang, H.; Song, J.; Zhu, Z.; Liu, W.; Song, Y.; Yang, C. Control of CRISPR-Cas9 with Small Molecule-Activated Allosteric Aptamer Regulating SgRNAs. Chem. Commun. 2019, 55, 12223–12226. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, R.S.; Ozdilek, B.A.; Garst, A.D.; Choudhury, A.; Batey, R.T. Small Molecule Regulated SgRNAs Enable Control of Genome Editing in E. Coli by Cas9. Nat. Commun. 2020, 11, 1394. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, K.; Xing, J.; Zhang, T.; Wang, X.; Wei, Z.; Ren, C.; Liu, Z.; Shao, S.; Zhang, Z. Multiplex CRISPR/Cas9-Based Genome Engineering Enhanced by Drosha-Mediated SgRNA-ShRNA Structure. Sci. Rep. 2016, 6, 38970. [Google Scholar] [CrossRef]
- Wang, X.-W.; Hu, L.-F.; Hao, J.; Liao, L.-Q.; Chiu, Y.-T.; Shi, M.; Wang, Y. A MicroRNA-Inducible CRISPR–Cas9 Platform Serves as a MicroRNA Sensor and Cell-Type-Specific Genome Regulation Tool. Nat. Cell Biol. 2019, 21, 522–530. [Google Scholar] [CrossRef]
- Replogle, J.M.; Norman, T.M.; Xu, A.; Hussmann, J.A.; Chen, J.; Cogan, J.Z.; Meer, E.J.; Terry, J.M.; Riordan, D.P.; Srinivas, N.; et al. Combinatorial Single-Cell CRISPR Screens by Direct Guide RNA Capture and Targeted Sequencing. Nat. Biotechnol. 2020, 38, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cao, Z.; Liu, Z.; He, Y.; Wang, Y.; Yuan, P.; Li, W.; Tian, F.; Bao, Y.; Wei, W. Guide RNAs with Embedded Barcodes Boost CRISPR-Pooled Screens. Genome Biol. 2019, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Ellison, E.E.; Nagalakshmi, U.; Gamo, M.E.; Huang, P.; Dinesh-Kumar, S.; Voytas, D.F. Multiplexed Heritable Gene Editing Using RNA Viruses and Mobile Single Guide RNAs. Nat. Plants 2020, 6, 620–624. [Google Scholar] [CrossRef]
- Yang, L.; Machin, F.; Wang, S.; Saplaoura, E.; Kragler, F. Heritable Transgene-Free Genome Editing in Plants by Grafting of Wild-Type Shoots to Transgenic Donor Rootstocks. Nat. Biotechnol. 2023, 41, 958–967. [Google Scholar] [CrossRef]
- Zhang, Z.; Rong, X.; Xie, T.; Li, Z.; Song, H.; Zhen, S.; Wang, H.; Wu, J.; Jaffrey, S.R.; Li, X. Fluorogenic CRISPR for Genomic DNA Imaging. Nat. Commun. 2024, 15, 934. [Google Scholar] [CrossRef]
- Ma, H.; Tu, L.-C.; Naseri, A.; Huisman, M.; Zhang, S.; Grunwald, D.; Pederson, T. CRISPR-Cas9 Nuclear Dynamics and Target Recognition in Living Cells. J. Cell Biol. 2016, 214, 529–537. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Su, N.; Bao, B.; Xie, X.; Zuo, F.; Yang, L.; Wang, H.; Jiang, L.; Lin, Q.; et al. Visualizing RNA Dynamics in Live Cells with Bright and Stable Fluorescent RNAs. Nat. Biotechnol. 2019, 37, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, X.; Shi, Y.; Wang, W.; Huang, Z.; Tong, Y.; Zou, X.; Xu, Y.; Dai, Z. CRISPR/Pepper-TDeg: A Live Imaging System Enables Non-Repetitive Genomic Locus Analysis with One Single-Guide RNA. Adv. Sci. 2024, 11, 2402534. [Google Scholar] [CrossRef] [PubMed]
- Su-Tobon, Q.; Fan, J.; Goldstein, M.; Feeney, K.; Ren, H.; Autissier, P.; Wang, P.; Huang, Y.; Mohanty, U.; Niu, J. CRISPR-Hybrid: A CRISPR-Mediated Intracellular Directed Evolution Platform for RNA Aptamers. Nat. Commun. 2025, 16, 595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Rocha, P.P.; Luo, V.M.; Raviram, R.; Deng, Y.; Mazzoni, E.O.; Skok, J.A. CRISPR-DCas9 and SgRNA Scaffolds Enable Dual-Colour Live Imaging of Satellite Sequences and Repeat-Enriched Individual Loci. Nat. Commun. 2016, 7, 11707. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tu, L.-C.; Naseri, A.; Huisman, M.; Zhang, S.; Grunwald, D.; Pederson, T. Multiplexed Labeling of Genomic Loci with DCas9 and Engineered SgRNAs Using CRISPRainbow. Nat. Biotechnol. 2016, 34, 528–530. [Google Scholar] [CrossRef]
- Hirao, I.; Spingola, M.; Peabody, D.; Ellington, A.D. The Limits of Specificity: An Experimental Analysis with RNA Aptamers to MS2 Coat Protein Variants. Mol. Divers. 1998, 4, 75–89. [Google Scholar] [CrossRef]
- Wang, S.; Su, J.-H.; Zhang, F.; Zhuang, X. An RNA-Aptamer-Based Two-Color CRISPR Labeling System. Sci. Rep. 2016, 6, 26857. [Google Scholar] [CrossRef]
- Truong, V.A.; Hsu, M.-N.; Kieu Nguyen, N.T.; Lin, M.-W.; Shen, C.-C.; Lin, C.-Y.; Hu, Y.-C. CRISPRai for Simultaneous Gene Activation and Inhibition to Promote Stem Cell Chondrogenesis and Calvarial Bone Regeneration. Nucleic Acids Res. 2019, 47, e74. [Google Scholar] [CrossRef]
- Cheng, A.W.; Jillette, N.; Lee, P.; Plaskon, D.; Fujiwara, Y.; Wang, W.; Taghbalout, A.; Wang, H. Casilio: A Versatile CRISPR-Cas9-Pumilio Hybrid for Gene Regulation and Genomic Labeling. Cell Res. 2016, 26, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Varani, G. Engineering RNA-Binding Proteins for Biology. FEBS J. 2013, 280, 3734–3754. [Google Scholar] [CrossRef] [PubMed]
- Clow, P.A.; Du, M.; Jillette, N.; Taghbalout, A.; Zhu, J.J.; Cheng, A.W. CRISPR-Mediated Multiplexed Live Cell Imaging of Nonrepetitive Genomic Loci with One Guide RNA per Locus. Nat. Commun. 2022, 13, 1871. [Google Scholar] [CrossRef]
- Yao, R.; Liu, D.; Jia, X.; Zheng, Y.; Liu, W.; Xiao, Y. CRISPR-Cas9/Cas12a Biotechnology and Application in Bacteria. Synth. Syst. Biotechnol. 2018, 3, 135–149. [Google Scholar] [CrossRef]
- Kor, S.D.; Chowdhury, N.; Keot, A.K.; Yogendra, K.; Chikkaputtaiah, C.; Sudhakar Reddy, P. RNA Pol III Promoters—Key Players in Precisely Targeted Plant Genome Editing. Front. Genet. 2023, 13, 989199. [Google Scholar] [CrossRef]
- Deguchi, M.; Sinclair, K.M.; Patel, A.; Coile, M.; Ortega, M.A.; Bewg, W.P.; Tsai, C.-J. A Compendium of Nonredundant Short Polymerase III Promoters for CRISPR Applications. Plant Physiol. 2025, 198, kiaf294. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Debray, K.; Herwegh, D.; Develtere, W.; Impens, L.; Schaumont, D.; Vandeputte, W.; Aesaert, S.; Coussens, G.; De Boe, Y.; et al. BREEDIT: A Multiplex Genome Editing Strategy to Improve Complex Quantitative Traits in Maize. Plant Cell 2023, 35, 218–238. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y. Self-Processing of Ribozyme-Flanked RNAs into Guide RNAs In Vitro and In Vivo for CRISPR-Mediated Genome Editing. J. Integr. Plant Biol. 2014, 56, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Y.-L.; Wang, D.-F.; Wang, Y.-L.; Zhang, T.-P.; Li, H.; Liang, F.; Zhao, Y.; Zhang, G.-Y. SgRNA Expression of CRIPSR-Cas9 System Based on MiRNA Polycistrons as a Versatile Tool to Manipulate Multiple and Tissue-Specific Genome Editing. Sci. Rep. 2017, 7, 5795. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Kumar, J.; Alok, A.; Tuli, R. RNA-Guided Genome Editing for Target Gene Mutations in Wheat. G3 Genes|Genomes|Genet. 2013, 3, 2233–2238. [Google Scholar] [CrossRef]
- Cody, W.B.; Scholthof, H.B.; Mirkov, T.E. Multiplexed Gene Editing and Protein Overexpression Using a Tobacco Mosaic Virus Viral Vector. Plant Physiol. 2017, 175, 23–35. [Google Scholar] [CrossRef]
- Uranga, M.; Aragonés, V.; Selma, S.; Vázquez-Vilar, M.; Orzáez, D.; Daròs, J.-A. Efficient Cas9 Multiplex Editing Using Unspaced SgRNA Arrays Engineering in a Potato Virus X Vector. Plant J. 2021, 106, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Cody, W.B.; Scholthof, H.B. Native Processing of Single Guide RNA Transcripts to Create Catalytic Cas9/Single Guide RNA Complexes in Planta. Plant Physiol. 2020, 184, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Fujii, W.; Ogawa, T.; Sugiura, K.; Naito, K. Development of a Mono-Promoter-Driven CRISPR/Cas9 System in Mammalian Cells. Sci. Rep. 2015, 5, 18341. [Google Scholar] [CrossRef] [PubMed]
- Nissim, L.; Perli, S.D.; Fridkin, A.; Perez-Pinera, P.; Lu, T.K. Multiplexed and Programmable Regulation of Gene Networks with an Integrated RNA and CRISPR/Cas Toolkit in Human Cells. Mol. Cell 2014, 54, 698–710. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, X.; Qi, Y.; Zhang, D.; Cheng, Y.; Tang, A.; Voytas, D.F.; Zhang, Y. A Single Transcript CRISPR-Cas9 System for Efficient Genome Editing in Plants. Mol. Plant 2016, 9, 1088–1091. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Huang, S.; Li, X.; Wang, X.; Li, G.; Chi, T.; Chen, Y.; Huang, X.; Wang, X. Enhancing Prime Editing by Csy4-Mediated Processing of PegRNA. Cell Res. 2021, 31, 1134–1136. [Google Scholar] [CrossRef]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 Multiplex Editing Capability with the Endogenous TRNA-Processing System. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, H.-L.; Wang, Z.-P.; Zhang, H.-Y.; Yang, F.; Wang, X.-C.; Chen, Q.-J. Potential High-Frequency off-Target Mutagenesis Induced by CRISPR/Cas9 in Arabidopsis and Its Prevention. Plant Mol. Biol. 2018, 96, 445–456. [Google Scholar] [CrossRef]
- Port, F.; Bullock, S.L. Augmenting CRISPR Applications in Drosophila with TRNA-Flanked SgRNAs. Nat. Methods 2016, 13, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, T.; Kawakami, K. A TRNA-Based Multiplex SgRNA Expression System in Zebrafish and Its Application to Generation of Transgenic Albino Fish. Sci. Rep. 2018, 8, 13366. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, D.; Cieśla, M.; Boguta, M. Regulation of TRNA Synthesis by the General Transcription Factors of RNA Polymerase III-TFIIIB and TFIIIC, and by the MAF1 Protein. Biochim. Biophys. Acta—Gene Regul. Mech. 2018, 1861, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.J.H.F.; Michaels, Y.S.; Jamilly, M.; Ferry, Q.R.V.; Barbosa, H.; Milne, T.A.; Fulga, T.A. Decoupling TRNA Promoter and Processing Activities Enables Specific Pol-II Cas9 Guide RNA Expression. Nat. Commun. 2019, 10, 1490. [Google Scholar] [CrossRef] [PubMed]
- Lowder, L.; Malzahn, A.; Qi, Y. Rapid Evolution of Manifold CRISPR Systems for Plant Genome Editing. Front. Plant Sci. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Jiang, C.; Geng, L.; Wang, J.; Liang, Y.; Guo, X.; Liu, C.; Zhao, Y.; Jin, J.; Liu, Z.; Mu, Y. Multiplexed Gene Engineering Based on DCas9 and GRNA-TRNA Array Encoded on Single Transcript. Int. J. Mol. Sci. 2023, 24, 8535. [Google Scholar] [CrossRef]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR Screens in Plants: Approaches, Guidelines, and Future Prospects. Plant Cell 2021, 33, 794–813. [Google Scholar] [CrossRef]
- Kweon, J.; Jang, A.-H.; Kim, D.; Yang, J.W.; Yoon, M.; Rim Shin, H.; Kim, J.-S.; Kim, Y. Fusion Guide RNAs for Orthogonal Gene Manipulation with Cas9 and Cpf1. Nat. Commun. 2017, 8, 1723. [Google Scholar] [CrossRef]
- Perroud, P.-F.; Guyon-Debast, A.; Casacuberta, J.M.; Paul, W.; Pichon, J.-P.; Comeau, D.; Nogué, F. Improved Prime Editing Allows for Routine Predictable Gene Editing in Physcomitrium Patens. J. Exp. Bot. 2023, 74, 6176–6187. [Google Scholar] [CrossRef]
- Litke, J.L.; Jaffrey, S.R. Highly Efficient Expression of Circular RNA Aptamers in Cells Using Autocatalytic Transcripts. Nat. Biotechnol. 2019, 37, 667–675. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Meng, J.; Huang, X. Synthetic Circular GRNA Mediated Biological Function of CRISPR-(d)Cas9 System. Front. Cell Dev. Biol. 2022, 10, 86431. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, Y.; Xue, X.; Liu, L.; Wei, X.; Wang, H. 5′ Capped and 3′ PolyA-Tailed SgRNAs Enhance the Efficiency of CRISPR-Cas9 System. Protein Cell 2019, 10, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Chiu, S.; Voje, W.E.; Carothers, J.M.; Moon, T.S. Conditional Guide RNA Deactivation by MRNA and Small Molecule Triggers in Saccharomyces Cerevisiae. N. Biotechnol. 2025, 89, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, E.K.; Vandoros, L.A.; Huang, M.; Lackey, P.E.; Marzluff, W.F.; Asokan, A. Controlling MRNA Stability and Translation with the CRISPR Endoribonuclease Csy4. RNA 2015, 21, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, S.; Mo, Q.; Liu, P.; Xiao, X.; Ma, H. Enhancing Prime Editing Efficiency and Flexibility with Tethered and Split PegRNAs. Protein Cell 2023, 14, 304–308. [Google Scholar] [CrossRef]
- Butcher, S.E. Structure and Function of the Small Ribozymes. Curr. Opin. Struct. Biol. 2001, 11, 315–320. [Google Scholar] [CrossRef]
- Hoffmann, U.A.; Lichtenberg, E.; Rogh, S.N.; Bilger, R.; Reimann, V.; Heyl, F.; Backofen, R.; Steglich, C.; Hess, W.R.; Wilde, A. The Role of the 5’ Sensing Function of Ribonuclease E in Cyanobacteria. RNA Biol. 2024, 21, 373–390. [Google Scholar] [CrossRef]
- Chapman, E.G.; Moon, S.L.; Wilusz, J.; Kieft, J.S. RNA Structures That Resist Degradation by Xrn1 Produce a Pathogenic Dengue Virus RNA. eLife 2014, 3, e01892. [Google Scholar] [CrossRef]
- Shigematsu, M.; Kawamura, T.; Kirino, Y. Generation of 2′,3′-Cyclic Phosphate-Containing RNAs as a Hidden Layer of the Transcriptome. Front. Genet. 2018, 9, 562. [Google Scholar] [CrossRef]
- Mullally, G.; van Aelst, K.; Naqvi, M.M.; Diffin, F.M.; Karvelis, T.; Gasiunas, G.; Siksnys, V.; Szczelkun, M.D. 5′ Modifications to CRISPR–Cas9 GRNA Can Change the Dynamics and Size of R-Loops and Inhibit DNA Cleavage. Nucleic Acids Res. 2020, 48, 6811–6823. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; HamediRad, M.; Xue, P.; Xiao, H.; Tasan, I.; Chao, R.; Liang, J.; Zhao, H. Genome-Scale Engineering of Saccharomyces Cerevisiae with Single-Nucleotide Precision. Nat. Biotechnol. 2018, 36, 505–508. [Google Scholar] [CrossRef]
- Simone, B.W.; Lee, H.B.; Daby, C.L.; Ata, H.; Restrepo-Castillo, S.; Martínez-Gálvez, G.; Kar, B.; Gendron, W.A.C.; Clark, K.J.; Ekker, S.C. Chimeric RNA: DNA TracrRNA Improves Homology-Directed Repair In Vitro and In Vivo. Cris. J. 2021, 5, 40–52. [Google Scholar] [CrossRef]
- Ghosh, A.; Myacheva, K.; Riester, M.; Schmidt, C.; Diederichs, S. Chimeric Oligonucleotides Combining Guide RNA and Single-Stranded DNA Repair Template Effectively Induce Precision Gene Editing. RNA Biol. 2022, 19, 588–593. [Google Scholar] [CrossRef]
- Lee, K.; Mackley, V.A.; Rao, A.; Chong, A.T.; Dewitt, M.A.; Corn, J.E.; Murthy, N. Synthetically Modified Guide RNA and Donor DNA Are a Versatile Platform for CRISPR-Cas9 Engineering. eLife 2017, 6, e25312. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime Editing for Precise and Highly Versatile Genome Manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, Y.; He, R.; Han, X.; Li, S.; Liu, M.; Xi, X.; Liu, Z.; Wang, H.; Xie, S.; et al. EXPERT Expands Prime Editing Efficiency and Range of Large Fragment Edits. Nat. Commun. 2025, 16, 1592. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Hu, J.H.; Liu, D.R. Aptazyme-Embedded Guide RNAs Enable Ligand-Responsive Genome Editing and Transcriptional Activation. Nat. Commun. 2017, 8, 15939. [Google Scholar] [CrossRef] [PubMed]
- Kundert, K.; Lucas, J.E.; Watters, K.E.; Fellmann, C.; Ng, A.H.; Heineike, B.M.; Fitzsimmons, C.M.; Oakes, B.L.; Qu, J.; Prasad, N.; et al. Controlling CRISPR-Cas9 with Ligand-Activated and Ligand-Deactivated SgRNAs. Nat. Commun. 2019, 10, 2127. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Sheng, Y.; Wang, Y.; Dou, X. Synthetic Molecular Sensors Based on CRISPR-Cas9 Redirect Anticancer Signal Flows to Treat Retinoblastomas. Clin. Transl. Med. 2021, 11, e618. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Qi, Q.; Lei, H.; Zhang, Y.; Shen, W.; Fu, F.; Tian, T.; Zhou, X. G-Quadruplex-Guided RNA Engineering to Modulate CRISPR-Based Genomic Regulation. Nucleic Acids Res. 2022, 50, 11387–11400. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, W.; Qi, Q.; Zhang, Y.; Ji, H.; Cui, S.; An, J.; Sun, X.; Yin, H.; Tian, T.; et al. Rational Guide RNA Engineering for Small-Molecule Control of CRISPR/Cas9 and Gene Editing. Nucleic Acids Res. 2022, 50, 4769–4783. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-H.; Chen, W. Riboregulated Toehold-Gated GRNA for Programmable CRISPR–Cas9 Function. Nat. Chem. Biol. 2019, 15, 217–220. [Google Scholar] [CrossRef]
- Hunt, V.M.; Chen, W. A MicroRNA-Gated ThgRNA Platform for Multiplexed Activation of Gene Expression in Mammalian Cells. Chem. Commun. 2022, 58, 6215–6218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gao, Y.; Zhou, N.; Wang, B. CRISPR-Powered RNA Sensing In Vivo. Trends Biotechnol. 2024, 42, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Pelea, O.; Fulga, T.A.; Sauka-Spengler, T. RNA-Responsive GRNAs for Controlling CRISPR Activity: Current Advances, Future Directions, and Potential Applications. Cris. J. 2022, 5, 642–659. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Zhang, R.; Ding, S.; Zhang, T.; Yuan, Q.; Guan, G.; Chen, X.; Zhang, T.; Zhuang, H.; et al. The GRNA-MiRNA-GRNA Ternary Cassette Combining CRISPR/Cas9 with RNAi Approach Strongly Inhibits Hepatitis B Virus Replication. Theranostics 2017, 7, 3090–3105. [Google Scholar] [CrossRef]
- Paajanen, P.; Tomkins, M.; Hoerbst, F.; Veevers, R.; Heeney, M.; Thomas, H.R.; Apelt, F.; Saplaoura, E.; Gupta, S.; Frank, M.; et al. Re-Analysis of Mobile MRNA Datasets Raises Questions about the Extent of Long-Distance MRNA Communication. Nat. Plants 2025, 11, 977–984. [Google Scholar] [CrossRef]
- Lucas, W.J.; Yoo, B.-C.; Kragler, F. RNA as a Long-Distance Information Macromolecule in Plants. Nat. Rev. Mol. Cell Biol. 2001, 2, 849–857. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long Distance RNA Movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef]
- Kitagawa, M.; Tran, T.M.; Jackson, D. Traveling with Purpose: Cell-to-Cell Transport of Plant MRNAs. Trends Cell Biol. 2024, 34, 48–57. [Google Scholar] [CrossRef]
- Heeney, M.; Frank, M.H. The MRNA Mobileome: Challenges and Opportunities for Deciphering Signals from the Noise. Plant Cell 2023, 35, 1817–1833. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Zeng, X.; Jackson, S.; Zhou, Y.; Hong, Y. A Cis Element within Flowering Locus T MRNA Determines Its Mobility and Facilitates Trafficking of Heterologous Viral RNA. J. Virol. 2009, 83, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Mach, J. Ticket to Ride: TRNA-Related Sequences and Systemic Movement of MRNAs. Plant Cell 2016, 28, 1231–1232. [Google Scholar] [CrossRef]
- Ghoshal, B.; Vong, B.; Picard, C.L.; Feng, S.; Tam, J.M.; Jacobsen, S.E. A Viral Guide RNA Delivery System for CRISPR-Based Transcriptional Activation and Heritable Targeted DNA Demethylation in Arabidopsis Thaliana. PLoS Genet. 2020, 16, e1008983. [Google Scholar] [CrossRef]
- Chatzou, M.; Magis, C.; Chang, J.-M.; Kemena, C.; Bussotti, G.; Erb, I.; Notredame, C. Multiple Sequence Alignment Modeling: Methods and Applications. Brief. Bioinform. 2016, 17, 1009–1023. [Google Scholar] [CrossRef]
- Lohse, S. Scientific Inertia in Animal-Based Research in Biomedicine. Stud. Hist. Philos. Sci. Part A 2021, 89, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rappa, M.; Debackere, K. Youth and Scientific Innovation: The Role of Young Scientists in the Development of a New Field. Minerva 1993, 31, 1–20. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. The Roles of Dimensionality, Canopies and Complexity in Ecosystem Monitoring. PLoS ONE 2011, 6, e27307. [Google Scholar] [CrossRef]
- Kulcsár, P.I.; Tálas, A.; Ligeti, Z.; Tóth, E.; Rakvács, Z.; Bartos, Z.; Krausz, S.L.; Welker, Á.; Végi, V.L.; Huszár, K.; et al. A Cleavage Rule for Selection of Increased-Fidelity SpCas9 Variants with High Efficiency and No Detectable off-Targets. Nat. Commun. 2023, 14, 5746. [Google Scholar] [CrossRef] [PubMed]
| Category | Representative Elements | Integration Site | Role | Typical Application | Validated In |
|---|---|---|---|---|---|
| Visualization and recruitment (Section 4.1) |
|
| Effector or fluorophore recruitment | Different modalities | |
| Multiplexing (Section 4.2) |
|
| Transcript processing | Multiplexing | |
| Stability (Section 4.3) |
|
| Lower degradation | Prime editing (other modalities possible) | |
| DNA donor fusion (Section 4.4) |
|
| Colocalization of donor and SpCas9 | HDR |
|
| Conditional control (Section 4.5) |
|
| Conditional sgRNA control | Classical CRISPR (other modalities possible) | |
| Fusion with other RNA elements (Section 4.6) |
|
| Regulatory control | Different modalities | |
| Barcoding (Section 4.7) |
|
| sgRNA tracking | Pooled screens | |
| Mobile RNA motifs (Section 4.8) |
|
| Systemic mobility | Classical CRISPR (other modalities possible) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Saeger, J. A Guide to Guides: An Overview of SpCas9 sgRNA Scaffold Variants and Modifications. SynBio 2025, 3, 19. https://doi.org/10.3390/synbio3040019
De Saeger J. A Guide to Guides: An Overview of SpCas9 sgRNA Scaffold Variants and Modifications. SynBio. 2025; 3(4):19. https://doi.org/10.3390/synbio3040019
Chicago/Turabian StyleDe Saeger, Jonas. 2025. "A Guide to Guides: An Overview of SpCas9 sgRNA Scaffold Variants and Modifications" SynBio 3, no. 4: 19. https://doi.org/10.3390/synbio3040019
APA StyleDe Saeger, J. (2025). A Guide to Guides: An Overview of SpCas9 sgRNA Scaffold Variants and Modifications. SynBio, 3(4), 19. https://doi.org/10.3390/synbio3040019





