Streamlined Production, Protection, and Purification of Enzyme Biocatalysts Using Virus-like Particles and a Cell-Free Protein Synthesis System
Abstract
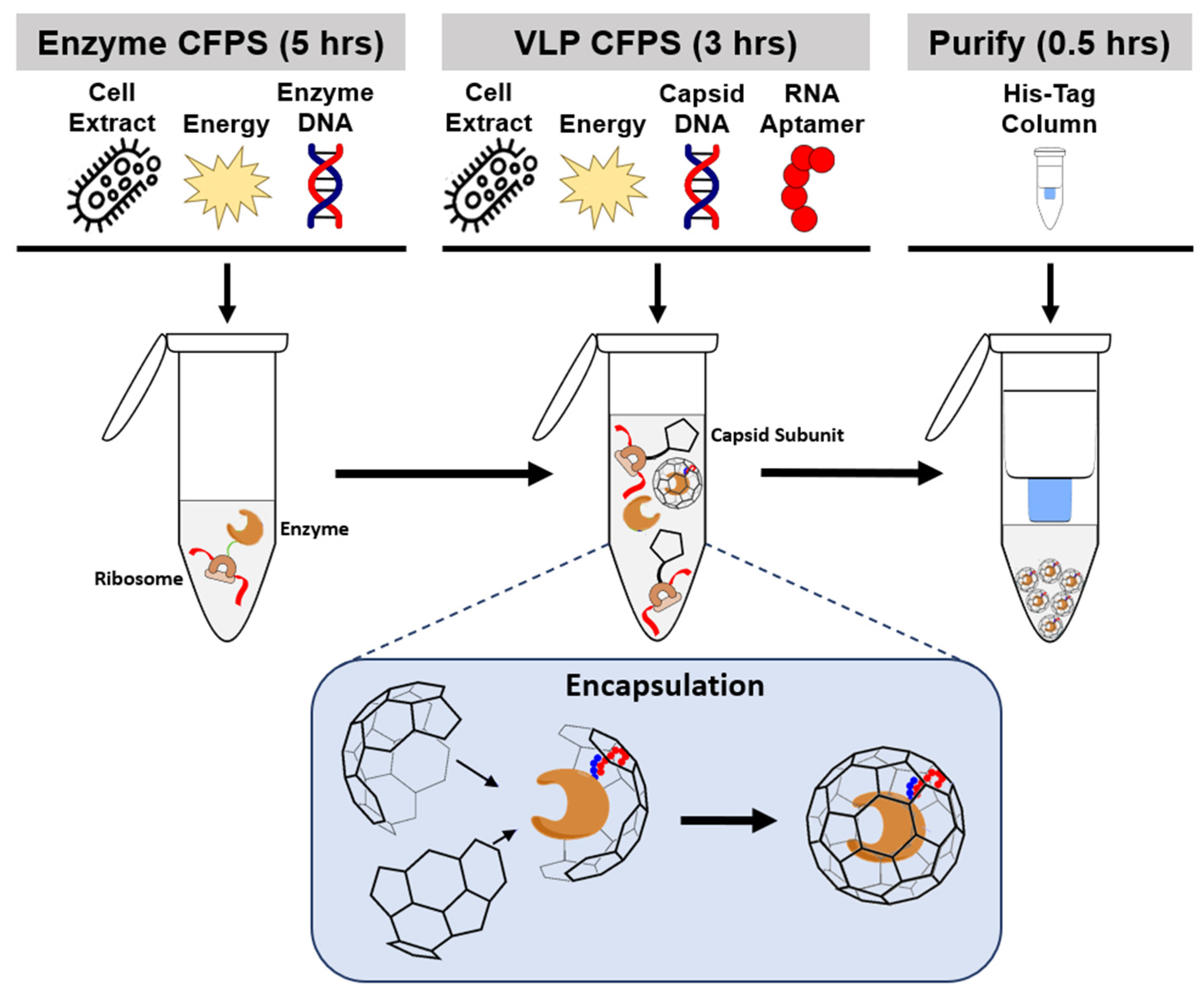
1. Introduction
2. Results
2.1. CFPS Expression of CalB Enzyme
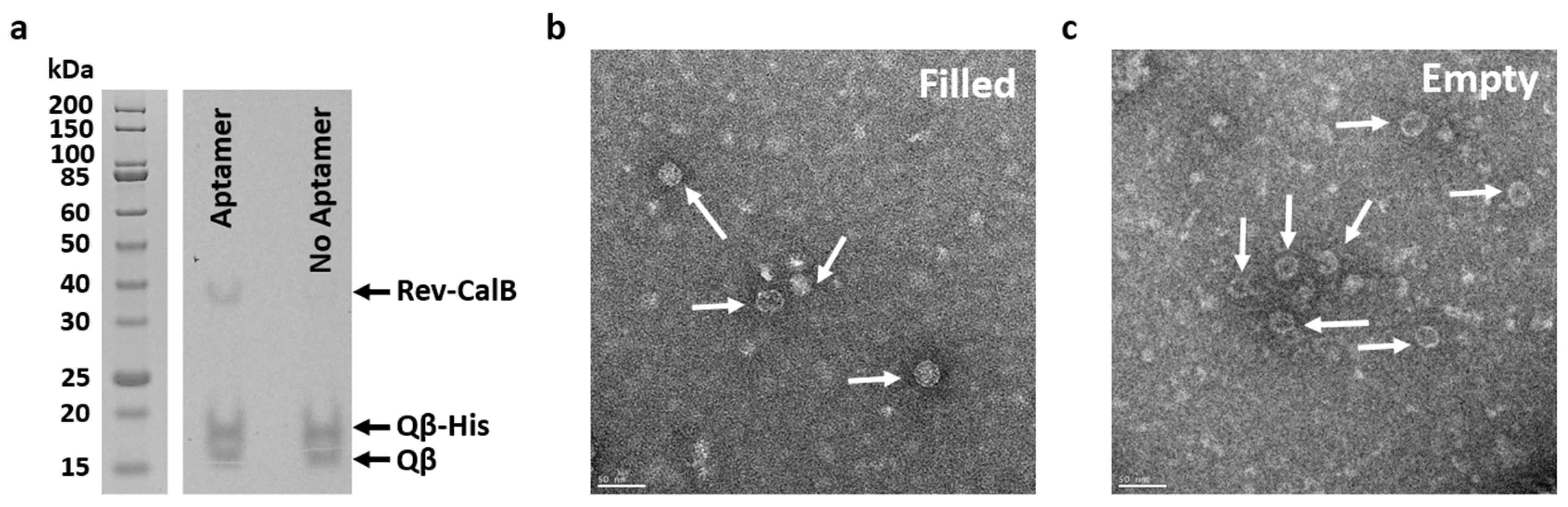
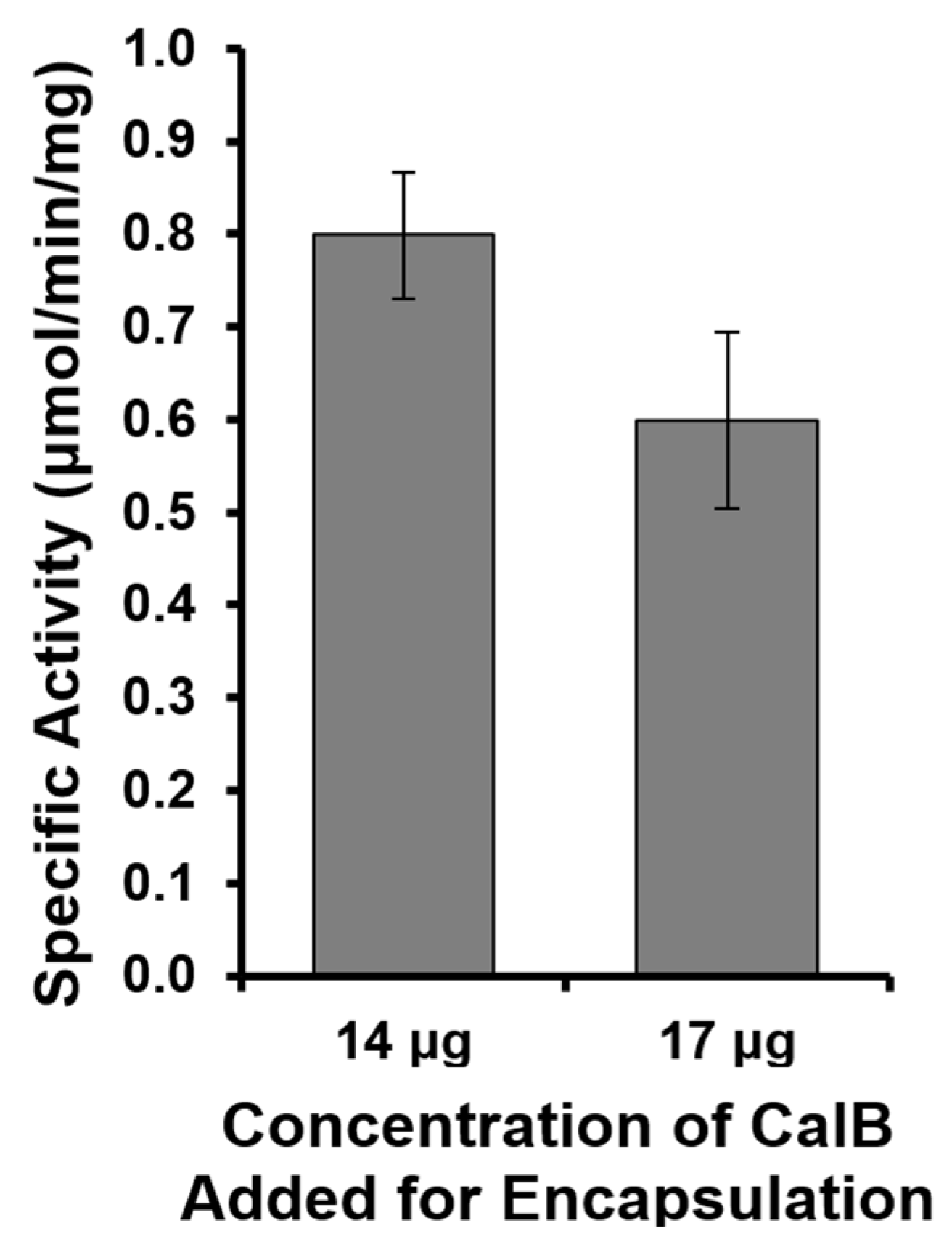
2.2. Targeted Rev-CalB Encapsulation Using Qβ VLP
2.3. Purification of Qβ-Encapsulated CalB via Spin His-Column
2.4. Enzyme Activity
2.5. Enzyme Stability
3. Discussion
3.1. CFPS Expression of CalB Enzyme
3.2. Targeted Rev-CalB Encapsulation Using Qβ VLP
3.3. Purification of Qβ-Encapsulated CalB via Spin His-Column
3.4. Enzyme Activity
3.5. Enzyme Stability
4. Materials and Methods
4.1. Extract Preparation
4.2. Cell-Free Protein Synthesis
4.3. Measuring Protein Concentration
4.4. Bacteriophage Qβ Coat Protein Purification
4.5. RNA Aptamer Transcription
4.6. Lipase Activity
4.7. Thermal Stability
4.8. Protease Stability
4.9. Protein Separation and Autoradiography to Determine the Ratio of VLP and Rev-CalB
4.10. TEM Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, L.; Guo, L.; Wang, K.; Liu, Y.; Xiao, M. β-Galactosidases: A great tool for synthesizing galactose-containing carbohydrates. Biotechnol. Adv. 2020, 39, 107465. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.D.; Gharat, S.A.; Jozwiak, A.; Barbole, R.; Heinicke, S.; Almekias-Siegl, E.; Meir, S.; Rogachev, I.; Connor, S.E.O.; Giri, A.P. A BAHD-type acyltransferase concludes the biosynthetic pathway of non-bitter glycoalkaloids in ripe tomato fruit. Nat. Commun. 2023, 14, 4540. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.P.; Amaral, J.C.; Fernandez-Lafuente, R.; Junior, R.d.S.; Tardioli, P.W. Lipozyme 435-mediated synthesis of xylose oleate in methyl ethyl ketone. Molecules 2021, 26, 3317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, J.; Zhang, F.; Wu, C.; Li, Z.; Wang, D.; Zhuang, J.; Chen, S.; Song, W.; Tang, Y. Self-assembled aldehyde dehydrogenase-activatable nano-prodrug for cancer stem cell-enriched tumor detection and treatment. Nat. Commun. 2024, 15, 9417. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.-J.; Du, S.-Y.; Mu, L.-S.; Fan, J.-J.; Hu, J.-C.; Ye, Y.; Ding, M.; Zhou, W.-Y.; Yu, Q.-H. Artemisinins ameliorate polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction. Science 2024, 384, eadk5382. [Google Scholar] [CrossRef]
- Das, S.; Zhao, L.; Elofson, K.; Finn, M.G. Enzyme Stabilization by Virus-Like Particles. Biochemistry 2020, 59, 2870–2881. [Google Scholar] [CrossRef]
- Kambiré, M.S.; Gnanwa, J.M.; Boa, D.; Kouadio, E.J.P.; Kouamé, L.P. Modeling of enzymatic activity of free β-glucosidase from palm weevil, Rhynchophorus palmarum Linn. (Coleoptera: Curculionidae) larvae: Effects of pH and temperature. Biophys. Chem. 2021, 276, 106611. [Google Scholar] [CrossRef]
- Caglayan, C.; Taslimi, P.; Türk, C.; Gulcin, İ.; Kandemir, F.M.; Demir, Y.; Beydemir, Ş. Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ. Sci. Pollut. Res. 2020, 27, 10607–10616. [Google Scholar] [CrossRef]
- Minten, I.J.; Claessen, V.I.; Blank, K.; Rowan, A.E.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Catalytic capsids: The art of confinement. Chem. Sci. 2011, 2, 358–362. [Google Scholar] [CrossRef]
- Patterson, D.P.; Prevelige, P.E.; Douglas, T. Nanoreactors by programmed enzyme encapsulation inside the capsid of the bacteriophage P22. ACS Nano 2012, 6, 5000–5009. [Google Scholar] [CrossRef] [PubMed]
- Worsdorfer, B.; Woycechowsky, K.J.; Hilvert, D. Directed evolution of a protein container. Science 2011, 331, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Kermasha, S.; Gill, J.K. Chapter Six—Immobilization of enzymes and their use in biotechnological applications. In Enzymes; Kermasha, S., Eskin, M.N.A., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 133–170. [Google Scholar]
- Yamaguchi, A.; Nakayama, H.; Morita, Y.; Sakamoto, H.; Kitamura, T.; Hashimoto, M.; Suye, S.-i. Enhanced and Prolonged Activity of Enzymes Adsorbed on TEMPO-Oxidized Cellulose Nanofibers. ACS Omega 2020, 5, 18826–18830. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zheng, X.; Li, X.; Zhao, J. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnol. Biofuels 2016, 9, 118. [Google Scholar] [CrossRef]
- Smith, M.T.; Wu, J.C.; Varner, C.T.; Bundy, B.C. Enhanced protein stability through minimally invasive, direct, covalent, and site-specific immobilization. Biotechnol. Prog. 2013, 29, 247–254. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Wu, J.C.; Hutchings, C.H.; Lindsay, M.J.; Werner, C.J.; Bundy, B.C. Enhanced enzyme stability through site-directed covalent immobilization. J. Biotechnol. 2015, 193, 83–90. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, V.; Majumdar, D.K. Entrapment of alpha-Amylase in Agar Beads for Biocatalysis of Macromolecular Substrate. Int. Sch. Res. Not. 2014, 2014, 936129. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, M.; Fang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Xu, J. Recent advancements in encapsulation of chitosan-based enzymes and their applications in food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 11044–11062. [Google Scholar] [CrossRef]
- Bhushan, B.; Pal, A.; Jain, V. Improved Enzyme Catalytic Characteristics upon Glutaraldehyde Cross-Linking of Alginate Entrapped Xylanase Isolated from Aspergillus flavus MTCC 9390. Enzym. Res. 2015, 2015, 210784. [Google Scholar] [CrossRef] [PubMed]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Field, R.A.; Prousoontorn, M.; Pichyangkura, R. Cross-linked enzyme aggregates (combi-CLEAs) derived from levansucrase and variant inulosucrase are highly efficient catalysts for the synthesis of levan-type fructooligosaccharides. Mol. Catal. 2023, 535, 112827. [Google Scholar] [CrossRef]
- González-Davis, O.; Villagrana-Escareño, M.V.; Trujillo, M.A.; Gama, P.; Chauhan, K.; Vazquez-Duhalt, R. Virus-like nanoparticles as enzyme carriers for Enzyme Replacement Therapy (ERT). Virology 2023, 580, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Olivares-Medina, C.N.; Villagrana-Escareño, M.V.; Juárez-Moreno, K.; Cadena-Nava, R.D.; Rodríguez-Hernández, A.G.; Vazquez-Duhalt, R. Targeted Enzymatic VLP-Nanoreactors with β-Glucocerebrosidase Activity as Potential Enzyme Replacement Therapy for Gaucher’s Disease. ChemMedChem 2022, 17, e202200384. [Google Scholar] [CrossRef]
- McNeale, D.; Dashti, N.; Cheah, L.C.; Sainsbury, F. Protein cargo encapsulation by virus-like particles: Strategies and applications. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1869. [Google Scholar] [CrossRef]
- Jordan, P.C.; Patterson, D.P.; Saboda, K.N.; Edwards, E.J.; Miettinen, H.M.; Basu, G.; Thielges, M.C.; Douglas, T. Self-assembling biomolecular catalysts for hydrogen production. Nat. Chem. 2016, 8, 179–185. [Google Scholar] [CrossRef]
- Rampoldi, A.; Crooke, S.N.; Preininger, M.K.; Jha, R.; Maxwell, J.; Ding, L.; Spearman, P.; Finn, M.G.; Xu, C. Targeted Elimination of Tumorigenic Human Pluripotent Stem Cells Using Suicide-Inducing Virus-like Particles. ACS Chem. Biol. 2018, 13, 2329–2338. [Google Scholar] [CrossRef]
- Su, Y.; Liu, B.; Huang, Z.; Teng, Z.; Yang, L.; Zhu, J.; Huo, S.; Liu, A. Virus-like particles nanoreactors: From catalysis towards bio-applications. J. Mater. Chem. B 2023, 11, 9084–9098. [Google Scholar] [CrossRef]
- Sharma, J.; Uchida, M.; Miettinen, H.M.; Douglas, T. Modular interior loading and exterior decoration of a virus-like particle. Nanoscale 2017, 9, 10420–10430. [Google Scholar] [CrossRef]
- Fiedler, J.D.; Fishman, M.R.; Brown, S.D.; Lau, J.; Finn, M. Multifunctional enzyme packaging and catalysis in the Qβ protein nanoparticle. Biomacromolecules 2018, 19, 3945–3957. [Google Scholar] [CrossRef]
- Garenne, D.; Bowden, S.; Noireaux, V. Cell-free expression and synthesis of viruses and bacteriophages: Applications to medicine and nanotechnology. Curr. Opin. Syst. Biol. 2021, 28, 100373. [Google Scholar] [CrossRef]
- Schwarz, B.; Uchida, M.; Douglas, T. Biomedical and catalytic opportunities of virus-like particles in nanotechnology. Adv. Virus Res. 2017, 97, 1–60. [Google Scholar] [PubMed]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like particles: Fundamentals and biomedical applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.D.; Higginson, C.; Hovlid, M.L.; Kislukhin, A.A.; Castillejos, A.; Manzenrieder, F.; Campbell, M.G.; Voss, N.R.; Potter, C.S.; Carragher, B.; et al. Engineered mutations change the structure and stability of a virus-like particle. Biomacromolecules 2012, 13, 2339–2348. [Google Scholar] [CrossRef]
- Smith, M.T.; Varner, C.T.; Bush, D.B.; Bundy, B.C. The incorporation of the A2 protein to produce novel Qbeta virus-like particles using cell-free protein synthesis. Biotechnol. Prog. 2012, 28, 549–555. [Google Scholar] [CrossRef]
- Weber, H. The binding site for coat protein on bacteriophage Qbeta RNA. Biochim. et Biophys. Acta 1976, 418, 175–183. [Google Scholar] [CrossRef]
- Witherell, G.W.; Uhlenbeck, O.C. Specific RNA binding by Q beta coat protein. Biochemistry 1989, 28, 71–76. [Google Scholar] [CrossRef]
- Fiedler, J.D.; Brown, S.D.; Lau, J.L.; Finn, M.G. RNA-directed packaging of enzymes within virus-like particles. Angew. Chem. (Int. Ed. Engl.) 2010, 49, 9648–9651. [Google Scholar] [CrossRef]
- Golmohammadi, R.; Fridborg, K.; Bundule, M.; Valegard, K.; Liljas, L. The crystal structure of bacteriophage Q beta at 3.5 A resolution. Structure 1996, 4, 543–554. [Google Scholar] [CrossRef]
- Boles, K.S.; Kannan, K.; Gill, J.; Felderman, M.; Gouvis, H.; Hubby, B.; Kamrud, K.I.; Venter, J.C.; Gibson, D.G. Digital-to-biological converter for on-demand production of biologics. Nat. Biotechnol. 2017, 35, 672–675. [Google Scholar] [CrossRef]
- Smith, M.T.; Wilding, K.M.; Hunt, J.M.; Bennett, A.M.; Bundy, B.C. The emerging age of cell-free synthetic biology. FEBS Lett. 2014, 588, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.S.; Smith, M.T.; Bennett, A.M.; Williams, J.B.; Pitt, W.G.; Bundy, B.C. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol. J. 2015, 11, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Smith, M.T.; Bundy, B.C. Cell-free unnatural amino acid incorporation with alternative energy systems and linear expression templates. New Biotechnol. 2014, 31, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.S.; Earl, C.C.; Muhlestein, C.; Bundy, B.C. Escherichia coli-based cell-free extract development for protein-based cancer therapeutic production. Int. J. Dev. Biol. 2016, 60, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Blank, K.; Morfill, J.; Gumpp, H.; Gaub, H.E. Functional expression of Candida antarctica lipase B in Eschericha coli. J. Biotechnol. 2006, 125, 474–483. [Google Scholar] [CrossRef]
- Park, C.G.; Kim, T.W.; Oh, I.S.; Song, J.K.; Kim, D.M. Expression of functional Candida antarctica lipase B in a cell-free protein synthesis system derived from Escherichia coli. Biotechnol. Prog. 2009, 25, 589–593. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst-many applications: The use of Candida antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Van Tassel, L.; Moilanen, A.; Ruddock, L.W. Efficient production of wild-type lipase B from Candida antarctica in the cytoplasm of Escherichia coli. Protein Expr. Purif. 2020, 165, 105498. [Google Scholar] [CrossRef]
- Lu, C.; Peng, X.; Lu, D.; Liu, Z. Global and Kinetic Profiles of Substrate Diffusion in Candida antarctica Lipase B: Molecular Dynamics with the Markov-State Model. ACS Omega 2020, 5, 9806–9812. [Google Scholar] [CrossRef]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Wilding, K.M.; Hunt, J.P.; Wilkerson, J.W.; Funk, P.J.; Swensen, R.L.; Carver, W.C.; Christian, M.L.; Bundy, B.C. Endotoxin-free E. coli-based cell-free protein synthesis: Pre-expression endotoxin removal approaches for on-demand cancer therapeutic production. Biotechnol. J. 2018, 14, 1800271. [Google Scholar] [CrossRef] [PubMed]
- Burdette, R.A.; Quinn, D.M. Interfacial reaction dynamics and acyl-enzyme mechanism for lipoprotein lipase-catalyzed hydrolysis of lipid p-nitrophenyl esters. J. Biol. Chem. 1986, 261, 12016–12021. [Google Scholar] [CrossRef] [PubMed]
- Ulker, C.; Gokalp, N.; Guvenilir, Y. Immobilization of Candida antarctica lipase B (CALB) on surface-modified rice busk ashes (RHA) via physical adsorption and cross-linking methods. Biocatal. Biotransform. 2016, 34, 172–180. [Google Scholar] [CrossRef]
- Zhang, N.; Suen, W.C.; Windsor, W.; Xiao, L.; Madison, V.; Zaks, A. Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution. Protein Eng. 2003, 16, 599–605. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, Y.C.; Lee, H.H.; Jeon, S.T.; Min, W.K.; Seo, J.H. Simple amino acid tags improve both expression and secretion of Candida antarctica lipase B in recombinant Escherichia coli. Biotechnol. Bioeng. 2015, 112, 346–355. [Google Scholar] [CrossRef]
- Xu, C.; Suo, H.; Xue, Y.; Qin, J.; Chen, H.; Hu, Y. Experimental and theoretical evidence of enhanced catalytic performance of lipase B from Candida antarctica acquired by the chemical modification with amino acid ionic liquids. Mol. Catal. 2021, 501, 111355. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, X.-G.; Lu, Z.-P.; Xu, C.; Xu, H.-J.; Hu, Y. Enhancing the Catalytic Performance of Candida antarctica Lipase B by Chemical Modification with Alkylated Betaine Ionic Liquids. Front. Bioeng. Biotechnol. 2022, 10, 850890. [Google Scholar] [CrossRef]
- Xiao, D.; Li, X.; Zhang, Y.; Wang, F. Efficient Expression of Candida antarctica Lipase B in Pichia pastoris and Its Application in Biodiesel Production. Appl. Biochem. Biotechnol. 2023, 195, 5933–5949. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Hendil-Forssell, P.; Volkov, A.; Krog, A.; Malmo, J.; Aune, T.E.V.; Knecht, W.; Miskelly, I.R.; Moody, T.S.; Svedendahl Humble, M. Streamlined Preparation of Immobilized Candida antarctica Lipase B. ACS Omega 2017, 2, 8674–8677. [Google Scholar] [CrossRef]
- Ujiie, A.; Nakano, H.; Iwasaki, Y. Extracellular production of Pseudozyma (Candida) antarctica lipase B with genuine primary sequence in recombinant Escherichia coli. J. Biosci. Bioeng. 2016, 121, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; An, J.; Yang, G.; Wu, G.; Zhang, Y.; Cui, L.; Feng, Y. Enhanced enzyme kinetic stability by increasing rigidity within the active site. J. Biol. Chem. 2014, 289, 7994–8006. [Google Scholar] [CrossRef] [PubMed]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.; Rowan, A.E.; Blank, K.G. Interfacial activation of Candida antarctica lipase B: Combined evidence from experiment and simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Gorzelnik, K.V.; Cui, Z.; Reed, C.A.; Jakana, J.; Young, R.; Zhang, J. Asymmetric cryo-EM structure of the canonical Allolevivirus Qβ reveals a single maturation protein and the genomic ssRNA in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 11519–11524. [Google Scholar] [CrossRef]
- Ashcroft, A.E.; Lago, H.; Macedo, J.M.; Horn, W.T.; Stonehouse, N.J.; Stockley, P.G. Engineering thermal stability in RNA phage capsids via disulphide bonds. J. Nanosci. Nanotechnol. 2005, 5, 2034–2041. [Google Scholar] [CrossRef]
- Hou, Q.; Rooman, M.; Pucci, F. Enzyme stability-activity trade-off: New insights from protein stability weaknesses and evolutionary conservation. J. Chem. Theory Comput. 2023, 19, 3664–3671. [Google Scholar] [CrossRef]
- Bundy, B.C.; Swartz, J.R. Efficient disulfide bond formation in virus-like particles. J. Biotechnol. 2011, 154, 230–239. [Google Scholar] [CrossRef]
- Jewett, M.C.; Swartz, J.R. Rapid expression and purification of 100 nmol quantities of active protein using cell-free protein synthesis. Biotechnol. Prog. 2004, 20, 102–109. [Google Scholar] [CrossRef]
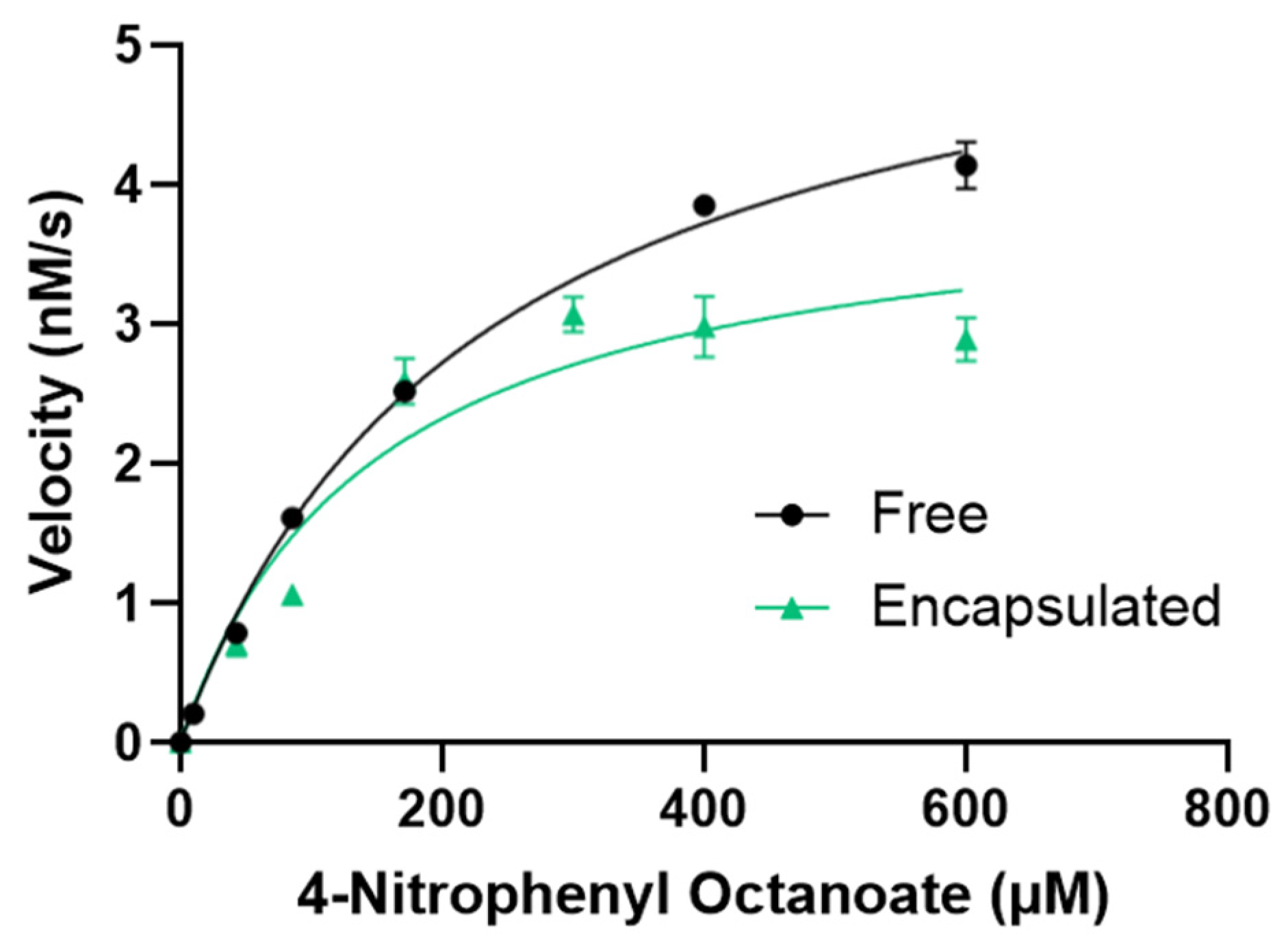

| Vmax (nM/s) | Kcat (s−1) | Km (μM) | Kcat/Km (M−1 s−1) | |
|---|---|---|---|---|
| Free | 5.88 ± 0.16 | 0.71 ± 0.02 | 232 ± 15 | 3080 ± 210 |
| Encapsulated | 4.05 ± 0.34 | 0.49 ± 0.04 | 149 ± 36 | 3300 ± 850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.O.; Talley, J.P.; Nielsen, G.H.; Wilding, K.M.; Bundy, B.C. Streamlined Production, Protection, and Purification of Enzyme Biocatalysts Using Virus-like Particles and a Cell-Free Protein Synthesis System. SynBio 2025, 3, 5. https://doi.org/10.3390/synbio3010005
Yang SO, Talley JP, Nielsen GH, Wilding KM, Bundy BC. Streamlined Production, Protection, and Purification of Enzyme Biocatalysts Using Virus-like Particles and a Cell-Free Protein Synthesis System. SynBio. 2025; 3(1):5. https://doi.org/10.3390/synbio3010005
Chicago/Turabian StyleYang, Seung O., Joseph P. Talley, Gregory H. Nielsen, Kristen M. Wilding, and Bradley C. Bundy. 2025. "Streamlined Production, Protection, and Purification of Enzyme Biocatalysts Using Virus-like Particles and a Cell-Free Protein Synthesis System" SynBio 3, no. 1: 5. https://doi.org/10.3390/synbio3010005
APA StyleYang, S. O., Talley, J. P., Nielsen, G. H., Wilding, K. M., & Bundy, B. C. (2025). Streamlined Production, Protection, and Purification of Enzyme Biocatalysts Using Virus-like Particles and a Cell-Free Protein Synthesis System. SynBio, 3(1), 5. https://doi.org/10.3390/synbio3010005





