Abstract
The increasing prevalence of multi-drug-resistant (MDR) bacterial pathogens presents a critical global health threat, highlighting the urgent need for innovative approaches to understanding bacterial pathogenesis and developing effective therapies. This review underscores the potential of synthetic biology in elucidating host–pathogen interactions and facilitating the creation of advanced diagnostic tools and targeted therapies to combat MDR infections. We first explore CRISPR-based strategies that modulate essential gene expression, providing insights into the molecular mechanisms underlying host–pathogen interactions. Next, we discuss engineered microbial synthetic circuits for rapid pathogen detection by identifying molecular signatures involved in interspecies communication and facilitating swift pathogen elimination. Additionally, we explore phage therapy (PT), which leverages bacteriophages to selectively target and eliminate specific bacterial pathogens, presenting a targeted and promising approach to combat MDR infections. Finally, we review the application of organ-on-a-chip (OOAC) technology, which overcomes the limitations of animal models in predicting human immune responses by using microfluidic devices that simulate organ-level physiology and pathophysiology, thereby enabling more accurate disease modeling, drug testing, and the development of personalized medicine. Collectively, these synthetic biology tools provide transformative insights into the molecular mechanisms of host–pathogen interactions, advancing the development of precise diagnostic and therapeutic strategies against MDR infections.
1. Introduction
Bacterial infections are a worldwide threat that significantly burdens healthcare systems. It is estimated that bacterial infections caused 7.7 million deaths globally in 2019 [1]. The problem escalated in recent years due to the emergence of multi-drug-resistant (MDR) bacterial strains [2]. According to the Center for Disease Control and Prevention (CDC), 2.8 million antimicrobial resistance infections occur every year, killing about 35,000 people in the United States alone [3]. Mycobacterium tuberculosis, the etiological agent of tuberculosis kills about 2 million people annually, with approximately one-third of the global population serving as carriers of the disease [4]. Similarly, urinary tract infections (UTIs) are becoming increasingly prevalent, with nearly every woman worldwide expected to experience at least one UTI in her lifetime [5]. Alarmingly, reports of multi-drug-resistant strains of M. tuberculosis [6] and uropathogenic Escherichia coli, the primary cause of UTIs [7,8] have been on the rise. Recently, Acinetobacter baumannii, a nosocomially acquired pathogen has emerged as a global threat as it exhibits resistance to most commonly used antimicrobial agents [9]. This highlights an urgent need to detect and combat these pathogens [10]. Traditional methods for deciphering the molecular mechanisms of virulence can be labor-intensive, time-consuming, and often rely on murine infection models; the results from these models may not accurately translate to human drug development due to species-specific differences [11]. For example, traditional approaches using Lambda-Red recombination machinery that has been the gold standard for knocking out non-essential genes in model organisms to study their role in virulence [12,13,14] can be time-consuming as it involves individually replacing the gene of interest with an antibiotic cassette. Other drawbacks of this widely used method include the inability of the technique to knock out essential genes, and this method was developed only for a few model organisms. Transposon insertion sequencing (Tn-seq) was developed to investigate the role of essential genes in determining phenotypes [15,16]. It involves the random insertion of a transposable genetic element across the genome. By growing the Tn-seq library under different conditions, essential genes for a particular growth condition can be identified, as these genes will show little or no transposon insertion. Tn-seq has been widely used to determine interaction between essential and non-essential genes [17] as well as genes involved in antibiotic resistance [18,19]. Although Tn-seq has been a powerful tool to identify essential genes, several reports suggest non-random insertions of the transposon across the genome, especially for organisms with high GC content [20,21,22]. Additionally, the construction of the transposon involves a strong promoter that can lead to overexpression of the genes located downstream of the transposon insertion, which can have impact on the phenotype [20]. Furthermore, the role of smaller genes is often missed due to the low insertion frequency, and the method fails to identify the role of overlapping genes (genes that share at least in part the same genomic location) [23]. Finally, when a bacterial pathogen invades a host, it often encounters other bacterial species of the host microbiota. To establish a successful infection, the invading bacteria must engage in molecular communication with these resident species. However, traditional methodologies have limited utility in elucidating the intricate signaling pathways of the resident microbiota that regulate the complex process within the host, demanding the need for the development of new tools and technologies.
Synthetic biology has emerged as a new tool that integrates engineering and computational principles with biological systems to address critical challenges, including the intricate dynamics of host–pathogen interactions [24,25,26]. These interactions involve a continuous interplay between host immune defenses and bacterial virulence strategies, such as immune evasion, signaling manipulation, and resource exploitation. This complexity is further heightened by co-evolution, environmental factors, and genetic variability within host populations, making traditional methods inadequate for deciphering these processes. By leveraging synthetic biology tools, such as engineered systems that mimic host or pathogen behaviors, synthetic circuits that model gene regulation or immune responses, and genome editing technologies like CRISPR, researchers can unravel these interactions with precision. These approaches not only deepen our understanding of host–pathogen dynamics but also pave the way for transformative therapeutic innovations. For example, to counteract multi-drug-resistant pathogens, CRISPR-based approaches were developed that can knock out or knock down multiple genes of interest (including genes that confer antimicrobial resistance) simultaneously without the involvement of multiple strain construction or antibiotic cassettes [27]. The widely used Type II CRISPR system consists of a chimeric RNA called guide RNA and a single nuclease, Cas9, that can introduce double-stranded breaks in the DNA, a process that is lethal for most bacterial species [28] due to the lack of non-homologous end joining (NHEJ) system needed to repair these breaks. This principle forms the basis of CRISPR-based technologies (described in detail in the CRISPR section). CRISPR-based systems can be inducible, titratable, and can be multiplexed to knock out multiple genes in a high-throughput format [29,30,31]. A variant of CRISPR was created to knock down genes of interest by using a catalytically dead nuclease Cas9 known as CRISPRi [32]. By combining the power of Tn-seq, with CRISPRi, Peters et al. created mobile-CRISPRi [33], which enabled researchers to systematically study the role of essential genes and virulence factors across multiple bacterial pathogens including Pseudomonas aeruginosa [34], Acinetobacter baumannii [35], and Vibrio sp. [36]. While synthetic biology has advanced our understanding of host–pathogen interactions and facilitated precise genetic manipulation through tools like CRISPR as discussed above, it has also enabled innovative diagnostic approaches, addressing critical gaps in early pathogen detection and monitoring within the host.
One of the key aspects of treating a bacterial infection is early diagnosis of the pathogen within the host. For example, the uropathogenic strain of E. coli CFT073 initially infects the urethra. If left untreated, the pathogen migrates upward and infects the urinary bladder causing cystitis [37], followed by infection of the kidney resulting in polynephritis [38]. Currently utilized diagnostic tools exhibit significant limitations, including suboptimal sensitivity, prolonged time requirements for definitive results, and prohibitively high costs, which collectively hinder their clinical efficacy and accessibility [39]. To address this challenge, synthetic biology-based approaches have been adopted to engineer genetic circuits in the bacteria that can act as sensors for biomarkers for multiple pathogens which are collectively called Bacterial Whole Cell Biosensors (BWCBs). BWCBs can easily colonize the human gut, replicate, survive harsh conditions, and establish themselves as residents of the microbiota. The human microbiota is a dynamic milieu, a complex mixture of microorganisms that interact through a rich array of biological processes, significantly influencing host–pathogen interactions. Leveraging this intricate microbial ecosystem, BWCBs are designed to sense bacterial metabolites, cell surface proteins, or lipids and generate detectable output signals—such as fluorescent proteins, biomarkers, or antibodies—that can be utilized for downstream diagnosis [40]. To achieve this, bacteria are engineered with inducible synthetic circuits utilizing transcription factors whose DNA-binding activity is regulated by a specific ligand. These synthetic circuits have allowed researchers to decipher the molecular signatures involved in intra- and inter-species communication [41]. Furthermore, leveraging the natural environmental sensing capabilities of bacteria, researchers have constructed genetic circuits capable of integrating multiple inputs using logic gates, such as AND gates that require all inputs to be present for activation [42], NOR gates [42], or a combination of a diverse array of genetic logic circuits involving OR, NAND, XOR, and XNOR gates, broadening the functional applications of BWCBs [42,43]. While synthetic biology-based tools like BWCBs have significantly advanced diagnostic capabilities and enhanced our understanding of microbial interactions, addressing the challenges posed by bacterial pathogens within the complex microbiota requires additional innovative strategies, such as the development of phage therapy.
One of the key challenges in treating a specific bacterial pathogen stem from its existence within a complex conglomerate of microbiota members. Conventional antimicrobial therapies often employ broad-spectrum antibiotics, which indiscriminately target a wide range of bacteria, including beneficial gut residents, triggering disruptions to the diversity of the gut microbiome. To overcome this obstacle, synthetic biology-based alternative approaches involving bacteriophages have been developed that are collectively referred to as phage therapy [44,45,46,47]. Phage therapy not only offers a targeted approach to bacterial elimination but also provides a unique platform to decipher host–pathogen interactions by exploiting the ability of phages to modulate bacterial populations and interact with the immune system within the microbiota milieu. The main advantage of phage therapy (PT) lies in the inherently narrow host range of phages, which helps preserve commensal microflora and results in fewer adverse effects compared to broad-spectrum antibiotic treatments [46]. In addition, the inherent property of phages to multiply within the host makes them effective at low-input doses [46]. Moreover, phages can coevolve with their bacterial hosts during therapeutic applications, allowing for the effective management of dominant bacterial populations [48]. Although bacterial pathogens may acquire resistance to phages, phage efficacy can be restored or enhanced through mutagenesis [49]. Moreover, bacterial resistance to phages has been shown to come with a trade-off, resulting in a decrease in virulence [50]. The innate ability of phages to infect bacterial cells was exploited by researchers to engineer phages that contain the CRISPR array to target antimicrobial resistance genes of bacterial pathogens [51]. In addition, researchers in synthetic biology have also exploited the property of phages to break open their bacterial host using endolysins. By creating chimeric and conjugated endolysins, researchers targeted a wide variety of bacterial pathogens [52,53,54,55]. Building on the innovative potential of phage therapy in preserving microbiota balance and targeting pathogens, further advancements in microbiota research and infection modeling, such as the development of organ-on-a-chip systems, provide deeper insights into the intricate dynamics between bacterial pathogens, the gut microbiota, and the human host.
Recent studies have highlighted the critical role of the gut microbiota in human health [56,57,58]. The gut microbiota consists of approximately 100 trillion bacteria that engage in complex interactions to regulate various gut processes. Disruptions to the gut microbiota, whether through the introduction of pathogenic bacteria or shifts in the abundance of existing species, can have detrimental effects on the host. Traditional methods for studying the immune response to bacterial pathogens have used the murine infection model. Despite the genetic similarities, mice have significant differences in gene expression profiles and mechanisms of regulation as compared to humans, leading to inconsistent disease modeling, making them less ideal for studying bacterial infection [11]. Furthermore, drugs that work effectively in mice often fail in human clinical trials due to differences in their pharmacodynamic and pharmacokinetic properties [59,60,61]. To solve this problem, organ-on-a-chip was developed utilizing human cell lines in a microfluidic device to mimic the host environment more accurately. Using this method, numerous studies have been conducted to unravel the complex interactions between bacterial pathogens, the host immune system, and antimicrobial agents [62,63].
This review examines the impact of synthetic biology on the study of host–pathogen interactions through rapid, efficient, and systematic approaches enabled by CRISPR-based technology. It also explores the application of synthetic biology in the early diagnosis of pathogens, utilizing bacterial genetics to develop synthetic circuits capable of sensing pathogens within the host. Additionally, this review discusses alternative treatments for multi-drug-resistant bacteria, focusing on phage therapy, and evaluates the role of organ-on-a-chip technology in advancing the understanding of host–pathogen interactions, thereby facilitating the rapid development of novel therapeutics to combat multi-drug-resistant pathogens.
2. CRISPR—A Versatile Tool for Studying Antimicrobial Resistance and Gene Regulation
Bacteria have intrinsic restriction systems that can detect and degrade any foreign DNA as part of their adaptive immune defense system [64]. Notably, in 2007, studies on Streptococcus thermophilus during infections with lytic bacteriophages provided the first experimental evidence supporting CRISPR/Cas-mediated adaptive immunity [65]. This discovery inspired the idea of utilizing the natural CRISPR/Cas systems in bacteria commonly used in the dairy industry for phage immunization, marking the first biotechnological application of CRISPR/Cas [66]. Following this, mature CRISPR RNAs (cr-RNAs) were identified as guide molecules forming a complex with Cas proteins to inhibit viral replication in E. coli [67], and the DNA-targeting activity of the CRISPR/Cas system was demonstrated in the pathogen Staphylococcus epidermidis [68]. The study demonstrates that CRISPR interference effectively blocks conjugation and plasmid transformation by targeting the DNA (nickase gene) of conjugative plasmids. This mechanism helps limit the spread of antibiotic resistance genes among Staphylococcal populations. It is estimated that ~40% of bacterial species use the CRISPR/Cas system that can be divided into two main classes, Class 1 and Class 2, with six types and subtypes distinguished by their structural and functional differences (Table 1). The widely known CRISPR/Cas9 system, classified as Type II, utilizes the Cas9 protein for inducing double-strand breaks in DNA, making it a powerful tool for gene editing. The CRISPR/Cas12 (Type V) system also targets DNA but has a unique ability to cleave single-stranded DNA, offering an alternative approach for genome modification. CRISPR/Cas13 (Type VI), on the other hand, targets RNA rather than DNA, enabling RNA interference and regulation. Type III-A CRISPR/Cas3, found in Class 1, employs helicase-nuclease activity to degrade DNA, while Type IV CRISPR/Cas4 is primarily involved in the adaptation phase of CRISPR immunity. A more recently discovered Type VII system, CRISPR/Cas14, is versatile in targeting both RNA and DNA, and its unique properties make it a promising tool for genetic manipulation. These systems, each with distinct characteristics, contribute to the growing toolbox of CRISPR-based technologies for diverse genetic applications. Unlike other CRISPR types that need multiple proteins to form a complex, the Type II system uses a single protein, Cas9, to cleave DNA. This makes the system easier to use and more efficient in terms of delivery and function. Additionally, Type II CRISPR systems have a well-defined and straightforward mechanism for RNA-guided DNA recognition and cleavage. (Table 1). It utilizes a single nuclease, Cas9, and a guide RNA (gRNA) containing a spacer sequence within its first 20 nucleotides that can form a stable RNA–DNA hybrid with the non-template strand of the target DNA. This RNA–DNA hybrid is subsequently recognized by the Cas9 nuclease through a short protospacer adjacent motif (PAM). Cas9 cleaves the target DNA, causing double-stranded breaks (endonucleolytic cleavage). One of the unique features of the CRISPR system is its inherent flexibility in DNA recognition. By modifying the spacer region of the gRNA, researchers can achieve precise genome editing (Figure 1A). The spacer region is a customizable 20-base-pair sequence complementary to the target DNA, enabling highly specific recognition and cleavage at the desired genomic site. This adaptability allows scientists to target multiple genes or regions for editing, such as deletions, insertions, or replacements. Careful design of the spacer sequence minimizes off-target effects, ensuring accurate and efficient modifications in the genome. Due to its versatility, CRISPR has been widely used for genome editing in bacteria [69], plants [70], and mammals [71,72]. For example, CRISPR/Cas technology has enabled researchers to modify plant genomes to enhance desirable traits such as yield, nutritional content, and resistance to diseases and pests. For instance, CRISPR/Cas has been used to develop crops that can withstand abiotic stresses like drought, salinity, and extreme temperatures, which are becoming increasingly important due to climate change [73]. Additionally, it has facilitated the creation of plants with improved herbicide tolerance and reduced reliance on chemical treatments [74]. Finally, CRISPR/Cas technology is paving the way for sustainable agricultural practices and the development of crops that can meet the growing global food demand [75]. CRISPR/Cas technology has revolutionized mammalian genetic research by enabling precise genome editing. Widely used in creating animal models, particularly mice, it facilitates the study of gene functions in disease development and progression [76]. Additionally, CRISPR/Cas is employed in reproductive biology to edit genes in oocytes and early embryos, facilitating studies on developmental processes and genetic disorders [77]. This technology also holds promise for therapeutic applications, such as correcting genetic mutations in somatic cells to treat inherited diseases [78].
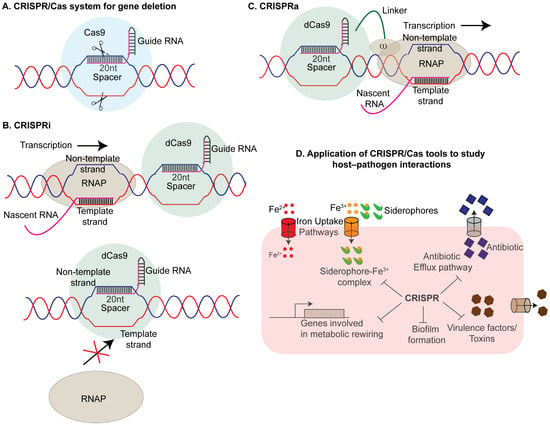
Figure 1.
CRISPR/Cas system and its applications in host–pathogen interaction studies: (A) CRISPR/Cas9-mediated gene knock out: The Type II CRISPR/Cas9 system consists of a single guide RNA (sgRNA) and the Cas9 nuclease (blue circle). The sgRNA guides Cas9 to its target sequence in the pathogen’s genome, where Cas9 induces double-strand breaks. This disruption results in the elimination or functional inactivation of the target gene. (B) CRISPR interference (CRISPRi): CRISPRi uses a catalytically inactive (“dead”) Cas9 (dCas9) (green circle) that retains DNA-binding ability but lacks nuclease activity to knock down the expression of the gene of interest (GOI). When guided by an sgRNA to a target sequence, dCas9 inhibits transcription through steric interference. It achieves this either by binding downstream of the transcriptional elongation complex (TEC), causing TEC stalling (upper panel), or by binding to the promoter region of the gene of interest (GOI), preventing RNA polymerase (RNAP) from binding and initiating transcription (lower panel). (C) CRISPR-associated gene activation (CRISPRa): CRISPRa involves a modified Cas9 from the CRISPRi system fused to the omega subunit of RNA polymerase. The sgRNA-Cas9 complex binds upstream of the target promoter, facilitating the recruitment of RNA polymerase to enhance gene expression. This approach effectively activates transcription of the target gene. (D) Applications in host–pathogen interaction studies: The CRISPR/Cas system is widely used in unraveling the mechanisms of pathogenicity in various bacterial pathogens. It is employed to study the roles of virulence factors, including genes involved in iron acquisition, biofilm formation, toxin production, and antibiotic resistance. Additionally, CRISPR/Cas tools facilitate the investigation of essential metabolic genes critical for pathogen survival in hostile host environments.
2.1. CRISPR Interference (CRISPRi)
While the CRISPR/Cas system has been employed to investigate gene regulation across various bacterial species, studying essential genes presents a significant challenge, as knocking out these genes often results in lethality. Additionally, the binary nature of the system—either editing or not editing—restricts its capacity to precisely modulate gene expression. To overcome these limitations, CRISPR interference (CRISPRi) was developed as a tool to explore gene regulation in bacteria [27,79,80]. By engineering a catalytically inactive variant of the Cas9 protein (dCas9), which retains its DNA-binding capability but is unable to induce double-stranded breaks in the DNA along with a single chimeric RNA, formed by the fusion of CRISPR RNA (cr-RNA) and trans-activating CRISPR RNA (tracr-RNA), researchers achieved the precise fine-tuning of gene expression. The gRNA-dCas9 complex can inhibit transcription either by occluding the RNA polymerase from binding to its cognate promoter DNA or by sterically hindering the elongation complex through interaction with the non-template DNA strand [27] (Figure 1B). Using this approach, researchers successfully studied the functions of virulence genes across multiple pathogenic bacterial species [80,81,82,83,84]. For example, Wang et al. developed an optimized CRISPRi system for Yersinia pestis that successfully inhibited the expression of genes involved in biofilm formation, hmsH, cold shock response, cspB, and virulence, yscB and ail. These knock down mutants exhibited decreased virulence in both HeLa cells and mouse models, consistent with previously observed phenotypes for yscB and ail knock outs [82]. Similarly, Choudhury et al. applied CRISPRi to target essential genes in the pathogenic Mycobacterium tuberculosis and its non-pathogenic counterpart, Mycobacterium smegmatis [81]. In a separate study, functional genomic libraries were constructed to investigate essential genes for bacterial viability in Mycobacterium tuberculosis (Mtb). Using these libraries, the authors identified genes influencing resistance or susceptibility to the antitubercular drug Bedaquiline (BDQ) [85]. Bedaquiline (BDQ) is an antitubercular drug that inhibits ATP synthase in Mycobacterium tuberculosis (Mtb) thereby lowering intracellular ATP level below a critical threshold. To identify genetic factors influencing BDQ efficacy, CRISPR-KO and CRISPRi screenings were conducted on Mtb grown to late-log phase, mimicking in vivo growth conditions. Libraries were treated with BDQ at 250 ng/mL and 1000 ng/mL, resulting in significant bacterial killing. Genomic analysis revealed the dose-dependent recovery of hit genes, with mmpS5-mmpL5 and rv0678 as key genes associated with BDQ resistance. Interestingly, CRISPRi identified essential genes involved in ATP synthesis, such as atpB, atpC, and atpH, which were not detected by CRISPR-KO. Additionally, pks13, which encodes the target of TAM16, a benzofuran compound that inhibits the growth of Mtb, was identified as a potential synergistic drug target. These findings suggest that CRISPR screenings are effective for profiling chemical–genetic interactions, providing insights into potential drug targets and mechanisms of resistance that could enhance TB therapy. The real merit of CRISPRi includes multiplexing and tiltability. By creating a library of gRNAs where each gRNA is specific for a particular gene, the researchers were able to modulate the expression of multiple genes simultaneously. Recently, de Bakker et al. conducted a genome-wide CRISPRi screen in Streptococcus pneumoniae in a murine pneumonia model of infection to identify 269 essential genes, including genes involved in cell wall biosynthesis, pbp2x, DNA replication, dnaA, cell division, ftsZ, and central carbon metabolism, pfkA [83]. In a separate study, Elis et al. developed a novel platform to facilitate the simultaneous knock out of multiple genes in Legionella pneumophila, an intracellular pathogen responsible for legionellosis [84]. This bacterium secretes ~300 effector proteins during infection via the Dot/lcm type IV secretion system. Traditional methods of knocking out individual genes may fail to uncover specific gene contributions to virulence due to functional redundancy. To address this, the authors engineered a CRISPR-based system by designing a plasmid containing dCas9 and MC-10—an array of 10 distinct spacer sequences (lpg2300, lpg1129, lpg1689, lpg0621, lpg1663, lpg218, lpg0921, lpg22344, lpg2793 and lpg0902). Each spacer was tailored to target a specific gene and was placed under the control of a strong inducible Ptet promoter and a box element. To study the effect of CRISPR-array, Lp02(dcas9) strains, either with the empty vector or harboring the multiplexed array, were used to infect U937 macrophages. Intracellular growth was assessed by measuring the average CFU at 2- and 48 h post-infection (hpi). At 48 hpi, bacteria were extracted from the macrophages, and RNA was harvested. The mRNA levels of the ten target genes were quantified using qPCR. This CRISPR array enabled the simultaneous silencing of 10 genes, allowing the study of their collective impact on cellular behavior in anoxic conditions and during macrophage infection. Notably, the silencing efficiency of each spacer was influenced by its position within the array, with the proximal spacers exhibiting the highest potency, which diminished as the spacers were positioned further distally in the array [84]. The potential of multiplexed CRISPR arrays was leveraged to address two critical strategies that pathogens use to develop multi-drug resistance: the utilization of efflux pumps to expel antimicrobial agents and the formation of biofilms, which limit the effectiveness of antimicrobial agents by reducing their access to the pathogens. To combat these bacterial defense mechanisms, CRISPRi-mediated strategies have been implemented in E. coli targeting the AcrAB-TolC efflux pump. By designing recombinant plasmids harboring clustered sgRNAs that can simultaneously target acrA, acrB, and tolC, the authors were able to reduce the transcription of acrA, acrB, and tolC by 78.3%, 90.0%, and 65.4%, respectively, while increasing susceptibility to rifampicin, erythromycin, and tetracycline by 2-, 8-, and 16-fold, respectively. Additionally, it reduced biofilm formation by 11.2% to 58.2%. This study offers valuable insights for the future regulation of efflux pump activity to treat MDR pathogens using CRISPRi. Interestingly, Noirot-Gros et al. used CRISPRi to interrogate genes involved in biofilm formation across various strains of Pseudomonas fluorescens (SBW25, WH6, and Pf0-1). The authors demonstrated the roles of specific genes and operons in biofilm structure, EPS production, and surface attachment, revealing contrasting biofilm phenotypes from silencing c-di-GMP regulators. It also identified the PFLU1114 operon as a potent inhibitor of biofilm formation, providing valuable insights into biofilm dynamics and potential control targets in Pseudomonas species [86]. Similar work was conducted to understand biofilm formation in the pathogenic fungus Candida albicans [87]. Furthermore, a nisin-inducible CRISPRi system was employed in Enterococcus faecalis to investigate the temporal roles of pili in biofilm development. The disruption of pili formation after 24 h of normal growth using this system led to a significant reduction in biofilm development, suggesting that pili play a critical role not only in the initial attachment but also in the maintenance of biofilm structure over time [88]. An alternative strategy was adapted to knock down genes of interest by introducing mismatches within the spacer sequence of the gRNA to reduce its binding affinity to the target DNA. By fine-tuning the degree of mismatches within the gRNA spacer sequence that results in the weakening of the interaction of gRNA with its target, researchers precisely modulated the target gene expression in bacterial systems [79,89] and in human cell lines [90]. For example, using fluorescence as a readout of repression, Bikard et al. demonstrated that increasing the number of mismatches in the cr-RNA leads to the decreased repression of its target gene in E. coli. It is particularly useful when working with essential genes, where complete knock outs can be lethal. By utilizing mismatched crRNA or guide RNA, researchers can fine-tune the expression of essential genes above the critical threshold, enabling the viability of engineered strains for functional studies of these genes. The degree of knock down also depends upon the mode of dCas9 action, i.e., whether dCas9 occludes the RNAP from binding to its promoter element (inhibition of transcriptional initiation) or it stalls the elongation complex by binding to the downstream region on the DNA (inhibition of transcriptional elongation) (Figure 1B).
In addition to the aforementioned strategy, researchers also use inducible dCas9 systems to modulate the degree of target gene repression by adjusting the concentration of the inducer driving dCas9 expression. This was demonstrated by Vigouroux et al., where the author tuned the strength of repression of target genes either by modulating dCas9 expression levels using different aTc concentrations (inducer) or by adjusting spacer complementarity to the target gene with varying numbers of mismatches [89]. The authors demonstrated that the level of complementarity between the guide RNA and the target dictates the rate at which RNA polymerase “kicks out” dCas9 from the target and completes transcription. These strategies demonstrated that output signal (GFP expression) can be precisely controlled by adjusting inducer (aTc) concentration or spacer complementarity, with minimal noise when spacer complementarity is varied, revealing that dCas9-bound targets are transcribed in a complementarity-dependent manner and gene repression remains robust and independent of dCas9 concentration when the target is saturated. Finally, CRISPRi has been used to decipher novel antimicrobial agent targets in various pathogenic bacteria including Acinetobacter bauumanii [91]. Using machine learning algorithms, the authors have identified a compound that targets lipoprotein trafficking, resulting in growth arrest in the murine model of infection. A recent study developed antimicrobial agents targeting the lumazine synthase RibH of Mycobacterium tuberculosis using a CRISPRi-based screen [92]. Interestingly, Peters et al. used an essential gene knock out library against antibiotics to dissect the interaction between genes in response to therapeutics in Bacillus subtilis. The authors found that the enzyme undecaprenyl pyrophosphate synthase, UppS, responsible for producing undecaprenyl pyrophosphate (UPP), a crucial lipid carrier molecule essential for the synthesis of the peptidoglycan of the bacterial cell wall, is the primary target of the antimicrobial agent MAC-0170636 [32]. The overexpression of uppS increased resistance to MAC-0170636 compared to wild-type (WT) cells. Moreover, purified Bacillus subtilis UppS activity was inhibited by MAC-0170636 with an IC50 of 0.79 μM, indicating UppS as the direct target. CRISPR-mediated libraries have been utilized to identify genetic hypersensitivity to the novel antibiotic agent SCH-79797 in both Gram-positive and Gram-negative bacteria [93].
2.2. CRISPR Activation
CRISPR activation (CRISPRa) is a robust tool that contrasts with CRISPRi by upregulating the expression of specific target genes. In E. coli, the CRISPRa system comprises a fusion of dCas9 with the ω-subunit of RNA polymerase [79] (Figure 1C). This complex binds to the upstream promoter element, facilitating the recruitment of RNA polymerase. However, a limitation of this approach is this CRISPRa system operates only in a rpoZ-deletion strain. rpoZ encodes the omega subunit of RNA polymerase and is involved in the stringent stress response [94]. Deleting rpoZ can alter metabolic pathways, potentially affecting the outcomes of CRISPRa-mediated gene expression. Additionally, studies have reported the accumulation of suppressor mutants in such systems, which may outcompete knock out strains within the same culture [95]. To address this limitation, Dong et al. developed a CRISPRa method in which the guide RNA is extended to form scaffold RNA (scRNA) that can bind the RNA binding protein, MS2 coat protein, MCP. MCP in turn can recruit transcriptional activators to this complex. To screen for effector proteins that can activate transcription by binding to this CRISPR/MCP complex, a GFP-based reporter assay was conducted in E. coli. The authors identified SoxS, the oxidative stress-responsive global regulator in E. coli, to be the best candidate to act as an activator. Interestingly, the authors observed a clear relationship between the distance of the CRISPRa target site to the transcription start site (TSS), and effective gene activation with the most efficient activation was achieved when the gRNA target sites were positioned approximately 60–90 bases upstream of the TSS of a reporter gene [96]. One challenge in studying host–pathogen interactions in bacterial systems is the absence of suitable PAM sites in target genes, complicating the use of CRISPRa for gene regulation. To address this, Kiattisewee et al. systematically evaluated PAM-flexible dCas9 variants for their gene activation potential [97]. The authors found that dxCas9-NG exhibited a high dynamic range at NGN PAM sites, while dSpRY enabled modest activity across nearly all PAMs. However, increased PAM flexibility reduced CRISPR interference (CRISPRi) efficacy, which could be partially rescued by multiplexing sgRNAs. This study offers a valuable framework for optimizing dCas9 variants to expand CRISPRa/i applications in bacterial gene regulation.
2.3. Mobile CRISPRi
Mobile CRISPRi is a flexible and modular system for gene knock down or knock out, leveraging CRISPR interference (CRISPRi) technology in conjunction with specific transport mechanisms such as T7 transposases for Gram-negative bacteria and the ICEBs1 conjugative element for Gram-positive bacteria [31,89]. In Gram-negative bacteria, the CRISPR array is delivered to recipient strains through tri-parental conjugation. This process involves a diaminopimelic acid (DAP)-dependent donor strain of Escherichia coli, which transfers the CRISPRi machinery via the RP4 conjugation system, while a second donor provides a helper plasmid carrying Tn7 transposition genes required for integration into the bacterial genome. Once inside the recipient cell, the transposases excise the CRISPRi machinery and facilitate its integration downstream of the glmS genes through recombination. For Gram-positive organisms, the CRISPRi machinery is delivered via biparental conjugation between donor and recipient strains. Upon entry, the CRISPRi machinery is integrated downstream of the trnS-leu2 site with the assistance of an integrase. Importantly, integration of the CRISPRi system downstream of either glnS or trnS-leu2 does not disrupt the function of these genes. Mobile-CRISPRi screens have been used to decipher gene functions in a wide variety of bacterial pathogens [34,51]. In one study, mobile-CRISPRi was used to knock down essential and non-essential genes in five different species of Vibrio [36]. Interestingly, the authors observed a decline in knock down efficiency as the binding site of the guide RNA shifted from the 5′ end to the 3′ end of the target gene. In a recent study, using mobile CRISPRi, the authors were able to identify the essential genes of Pseudomonas aeruginosa in murine model of infection. The authors demonstrated that the knock down of exsA, a gene that activates the Type III secretion system (T3SS), results in attenuated virulence in mice [34]. Recently, mobile CRISPRi was used to decipher essential genes in Acinetobacter baumannii [35]. Taken together, CRISPR-based approaches have proven effective in uncovering the roles of key factors involved in host–pathogen interactions (Figure 1D).
Although CRISPR-based approaches have revolutionized bacterial research by enabling precise gene editing and functional studies, several limitations hinder their broader application. One major challenge is the variability in CRISPR system efficiency across different bacterial species, particularly in non-model organisms with complex genetic architectures or limited genomic data. Additionally, off-target effects pose a significant risk, potentially leading to unintended genetic alterations that compromise the accuracy of experimental outcomes [98]. Another limitation is the potential development of resistance to CRISPR/Cas systems in bacterial populations through mutations in protospacer adjacent motifs (PAMs) or other target sites [22]. Moreover, ethical and biosafety concerns arise when deploying CRISPRs for therapeutic purposes, such as concerns about unintended ecological impacts when used for microbial population control. Addressing these limitations requires further advancements in CRISPR delivery methods, target specificity, and the development of tools optimized for diverse bacterial species.

Table 1.
Overview of CRISPR systems, their targets, and associated Cas protein types.
Table 1.
Overview of CRISPR systems, their targets, and associated Cas protein types.
| Type | Subtype | Key Features | Target | Cas Protein(s) | Reference |
|---|---|---|---|---|---|
| I | I-A to I-F | Multiprotein cascade complex for crRNA processing and target recognition; Cas3 nuclease for target degradation; uses helicase-nuclease activity to degrade target DNA | DNA | Cas3, Cas5, Cas6, Cas7, Cas8 | Makarova et al. (2011) [99] |
| II | II-A to II-C | Single-protein Cas9 for crRNA processing and target cleavage; requires tracrRNA | DNA | Cas9 | Jinek et al. (2012) [100] |
| III | III-A to III-D | Multiprotein Csm or Cmr complex for crRNA processing and target cleavage; some subtypes target RNA | DNA and RNA | Cas10, Csm2-5, or Cmr2-6 | Makarova et al. (2015) [101] |
| IV | Cas1 and Cas2 present; involved in adaptation processes; contains a nuclease activity | Unknown | Cas1, Cas2; may associate with Cas4, IHF, Csn2, and Cas9 | Makarova et al. (2011) [99] | |
| V | V-A to V-U | Diverse Cas proteins with different domain architectures; can cleave single-stranded DNA | DNA or RNA | Cas12, Cas13, Cas14 | Shmakov et al. (2015) [102] |
| VI | VI-A to VI-D | Single-protein Cas13 with HEPN domains that target RNA | RNA | Cas13 | Abudayyeh et al. (2016) [103] |
3. Engineered Microbes as Synthetic Biosensors for Detection and Elimination of Pathogens
Detecting pathogens in humans presents several challenges, including the demand for rapid and precise identification, the sensitivity required to detect minimal pathogen levels, and the ability to distinguish between closely related species. Traditional detection methods are often slow and may lack the necessary sensitivity, causing delays in diagnosis and treatment. Furthermore, the emergence of new pathogens and the ongoing evolution of existing ones add complexity to detection efforts. To address these challenges, synthetic biology approaches have also been developed to detect and respond to specific pathogens or pathogen-associated molecular patterns (PAMPs) involved in host–pathogen interactions [26,40,104,105]. By engineering synthetic circuits in bacteria, researchers created Bacterial Whole Cell Biosensors (BWCBs) that are capable of sensing infection biomarkers and producing easily detectable signals (Figure 2) [106,107,108]. BWCBs offer several advantages over traditional pathogen detection methods. Conventional approaches, such as isolating bacteria from stool samples, are often time-consuming [109]. Moreover, the molecular signatures associated with a pathogen’s virulence can be transient, localized to specific gut regions, and absent from stool samples. BWCBs address these limitations by providing a sensitive, rapid, and noninvasive method to identify key molecular markers of pathogenicity at physiologically relevant concentrations [110]. These engineered microbes typically incorporate synthetic circuits composed of three main modules. The first is the “sensor” module, designed to detect specific signals or stimuli, such as pathogen-associated metabolites or secreted toxins. Upon signal detection, the sensor module transmits the information to the second component, the “synthetic circuit,” which contains a response regulator. This regulator receives the signal from the sensor and triggers downstream processes to activate the appropriate elements of the final module, the “Output” module. The output module then generates detectable signals, which can be bioluminescence, fluorescence, or colorimetric in nature (Figure 2A). For example, E. coli was engineered to create a genetic memory system capable of sensing anhydrotetracycline (ATC) [111]. This engineered E. coli system consists of three key components that work together: the “bistable switch”, “the trigger element”, and the “memory element”. The bistable switch is the lambda cI/Cro genetic circuit, which can exist in two stable states: the cI state or the Cro state, akin to a toggle switch. Initially, the circuit is in the cI state. The trigger element, a tetracycline-inducible promoter, acts like the mechanism that flips the switch. When ATC is present, the promoter activates, driving the transcription of the Cro gene and flipping the switch to the Cro state. The memory element, derived from the cI/Cro region, ensures that the bacteria remain in the Cro state even after ATC is no longer present, effectively “remembering” the switch’s position. This integration allows E. coli to detect ATC, maintain the state change, and provide a stable response. When this engineered Escherichia coli was administered to mice that was treated with anhydrotetracycline, the recovered bacteria consistently switched to the Cro state, whereas those given to untreated mice retained the cI state. Notably, the engineered E. coli established itself robustly in the murine gut environment, suggesting the possibility of using similar genetic circuits to monitor complex, undefined environments and advancing the potential for developing living diagnostics and therapeutics [111]. Interestingly, Mimee et al. integrated engineered biological systems with advanced electronics to create a bio-electronic device known as the Ingestible Micro-Bio-Electronic Device (IMBED) for use in diagnostic applications [112]. IMBED combines a probiotic bacterial sensor with ultra-low-power microelectronics to detect biomarkers of the gastrointestinal tract. As a “proof-of-concept”, IMBED was designed to identify gastrointestinal bleeding in a porcine model by sensing heme released from lysed blood cells. The engineered E. coli Nissle 1917 strain responded to increasing blood levels by emitting bioluminescence as a readout, which was detected by phototransistors and subsequently converted to photocurrent signals. Using this device, the authors were able to detect gut infection with 100% specificity at 120 min. The IMBED platform has also been adapted to detect other disease-relevant molecules, such as thiosulfate, a biomarker for gut inflammation, and N-acyl homoserine lactone (AHL), an indicator of bacterial infections [112]. This technology underscores the potential of ingestible bio-electronic devices for non-invasive and real-time medical diagnostics.
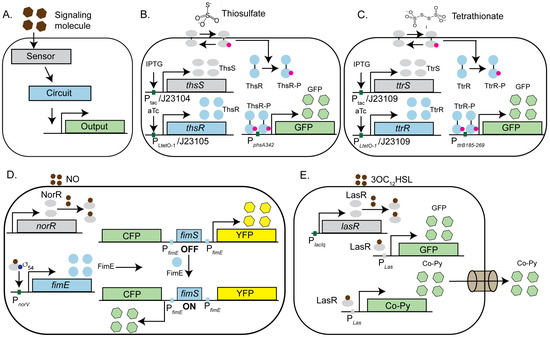
Figure 2.
Engineered microbes as tools to study host–pathogen interactions: (A) Schematic representation of an engineered microbial system designed to detect specific pathogens or their metabolites using synthetic circuits. The system comprises of a sensor (represented by gray) that responds to a target signal, an inducible, titratable, gated or bistable synthetic circuit, and a downstream reporter gene. Upon sensing the signal, the sensor activates a response regulator (represented by blue), which triggers a gene cascade to produce a detectable output signal (colorimetric, fluorescent, or bioluminescent, (represented by green or yellow). (B,C) The E. coli Nissle strain is engineered to act as a biosensor for gut inflammation by detecting thiosulfate (B) and tetrathionate (C), biomarkers of Salmonella-induced inflammation. Detection involves specific sensors (ThsS for thiosulfate and TtrS for tetrathionate) that phosphorylate their respective response regulators (ThsR or TtrR) in the presence of the signal. The phosphorylated response regulators activate reductase operons that can utilize these metabolites. Sensors and response regulators are driven by either inducible promoters (induced by Isopropyl β-D-1-thiogalactopyranoside, IPTG, or ATC) in vitro or constitutive promoters (J23104, J23105, and J23109) in murine infection models. (D) The E. coli strain EA3020 is engineered to sense nitric oxide (NO), a biomarker of inflammatory bowel disease (IBD), with cyan fluorescent protein (CFP) as the output. In the presence of NO, the NorR sensor activates the recombinase FimE, which switches the FimS invertible promoter element from an OFF to an ON state, driving CFP expression. (E) E. coli was designed to detect Pseudomonas aeruginosa by sensing its quorum-sensing molecule, 3OC12HSL. The LasR sensor activates the detection module, resulting in GFP fluorescence, confirming pathogen presence. Additionally, LasR activates the expression of chimeric bacteriocin Co-Py, which is secreted through the FlgM-mediated Type IV secretion system to eliminate the target pathogen.
3.1. Engineered Microbes as Biosensors for Gut Inflammation
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions of the gastrointestinal tract. These diseases are linked to an imbalance in the gut microbiome, known as dysbiosis, and the resulting immune response, which involves genetic susceptibility, microbial factors, and environmental triggers. Gastrointestinal pathogens are a common cause of dysbiosis and have been implicated in both the onset and exacerbation of IBD. One of the key aspects of treating IBD is early detection of its biomarkers. To address this, the E. coli Nissle strain was also engineered to develop biosensors capable of detecting markers of gut inflammation in response to Salmonella infection [113]. Previous studies have shown that Salmonella species can exploit the macrophage-mediated inflammatory response to facilitate the establishment of an infection. Reactive oxygen species generated during inflammation react with luminal sulfur compounds, such as H2S, to produce thiosulphate (S2O32−), which can be further oxidized to tetrathionate (S4O62⁻). The ability to utilize tetrathionate as a terminal electron acceptor provides S. Typhimurium with a unique growth advantage over competing species in the inflamed gut lumen. Using synthetic biology tools, Daeffler et al. have modified the widely used probiotic E. coli Nissle strain to sense both the markers of inflammation (thiosulfate and tetrathionate) in a dextran sodium sulfate (DSS) murine model of colitis [113]. To accomplish this, the authors employed a thiosulfate and tetrathionate sensor, along with their respective response regulators, from the marine bacteria Shewanella species as there was no previous report of genetically encoded thiosulfate sensor available, and the only known tetrathionate sensor is the TtrSR two-component system (TCS) from Salmonella typhimurium. In the presence of a signaling molecule (thiosulfate or tetrathionate), the sensor protein (ThsS or TtrS) phosphorylates its cognate response regulator (ThsR or TtrR), which in turn activates the downstream reductase operon that can utilize thiosulfate or tetrathionate, respectively (Figure 2B, C). Although thiosulfate reductase is regulated by the catabolite repressor protein (CRP), the tetrathionate reductase operon is naturally regulated by oxygen and nitrate levels through the global regulator FNR, making it prone to cross-repression in the gut environment. Since oxygen and nitrate levels are elevated during inflammation, these regulatory complexities reduce the reliability of the TtrSR system as a sensor for tetrathionate in this context. To overcome this limitation, the authors engineered the reductase operon under the control of catabolite repressor protein (CRP) instead of FNR, thus eliminating the influence of fluctuating O2 levels in the gut. GFP fluorescence was used as a readout to detect the presence of thiosulfate or tetrathionate in a murine infection model. Using flow cytometry, the authors demonstrated the activation of the thiosulfate sensor in response to gut inflammation during the murine infection, confirming the validity of the assay [113]. In summary, these engineered microbes have the potential for early disease detection to facilitate therapeutic intervention promptly.
Additionally, recombinase-based circuits have been utilized to engineer the E. coli strain EA3020 to sense nitric oxide, a biomarker of inflammatory bowel disease (IBD), with cyan fluorescent protein (CFP) serving as the output signal [114]. To achieve this, a bidirectional memory switch was engineered using the canonical E. coli fimbriae (Fim) phase variation system. In the OFF state, the fimS promoter is oriented towards the Inverted Repeat Right (IRR), leading to the constitutive expression of yellow fluorescent protein (YFP). Upon inversion of the switch to the ON state, fimS reorient toward the Inverted Repeat Left (IRL), resulting in the constitutive expression of cyan fluorescent protein (CFP). This inversion is mediated by the DNA recombinase FimE, which specifically binds to the inverted repeat sequences in the promoter region of CFP. By replacing the native promoter of FimE with a nitric acid sensor, NorR-regulated PnorV, the authors were able to design a synthetic circuit that allowed them to detect the presence of nitric oxide through a NorR-regulated synthetic circuit in the murine model of infection (Figure 2D). In a separate study, the Vibrio harveyi strain BB170 was engineered to detect Autoinducer-2 (AI-2), a quorum-sensing molecule previously linked to Irritable Bowel Syndrome (IBS), using ex vivo saliva and stool samples from patients. The engineered bacteria sense AI-2 through the periplasmic sensor protein LuxP. LuxP subsequently activates the response regulator LuxQ via phosphorylation, which triggers the downstream luxCDABE cascade to produce bioluminescence as the output signal [115]. In addition to acting as a sensor for biomarkers, engineered microbes have emerged as pivotal tools in therapeutics. These innovative devices integrate biological sensing elements with the targeted delivery of effector molecules, enabling the selective elimination of the desired pathogen with high sensitivity and specificity as discussed in the following section.
3.2. Engineered Microbes as Pathogen-Killing Machines
Synthetic biology has facilitated the development of engineered microbes with potential prophylactic and therapeutic applications. For instance, engineered E. coli has been designed to detect Pseudomonas aeruginosa by sensing its quorum-sensing molecule 3OC12HSL [116]. Upon detecting the molecule, the sensor LasR of the “detection module” activates the expression of GFP fluorescence, confirming the pathogen’s presence. The second component, a LasR controlled “destruction module,” synthesizes a chimeric bacteriocin called CoPy, which was engineered by replacing the receptor and translocase domains of Colicin E3 with those of Pyocin S3 to target P. aeruginosa specifically. The final component, a “secretion module,” utilizes the Type IV secretion system (FlgM) to release CoPy into the environment (Figure 2E). The Type IV secretion system (T4SS) in bacteria is crucial for transferring DNA and proteins, playing a key role in bacterial virulence and horizontal gene transfer. Targeting T4SS can disrupt the pathogenicity of bacteria like Legionella pneumophila and Helicobacter pylori, offering a potential therapeutic strategy to combat infections and reduce antibiotic resistance [117]. Using this engineered E. coli strain, the authors demonstrated the growth inhibition of P. aeruginosa strain PA01 in co-culture, paving the way for developing novel therapeutic bacterial strains. In a recent study, Hwang et al. engineered the E. coli Nissle strain to target and kill P. aeruginosa by delivering an antimicrobial peptide and a biofilm-dispersing enzyme [118]. Additionally, a CRISPRi-based synthetic circuit was developed in Bacteroides thetaiotaomicron, a key member of the human gut microbiome, to modulate gene expression and antimicrobial resistance, highlighting the broader potential of synthetic biology in combating pathogens [119]. In conclusion, biosensors represent a transformative advancement in therapeutic applications, offering precise, real-time monitoring and the targeted delivery of treatments. Their integration into healthcare systems has the potential to significantly enhance patient outcomes by enabling personalized medicine and improving the efficacy of therapeutic interventions. As research continues to evolve, the development of more sophisticated biosensors will likely address current challenges, such as sensitivity and specificity, further solidifying their role as indispensable tools in modern medicine.
4. Phage Therapy-Based Approaches to Decipher Host–Pathogen Interactions
Bacteriophages have long been employed as valuable tools for generating gene knock outs across various bacterial species. With advances in synthetic biology, phages have been further repurposed for the detection and elimination of multi-drug-resistant bacterial strains [44,120,121]. This has been achieved through CRISPR interference (CRISPRi)-based approaches [122,123] or by inducing bacterial cell lysis using endolysins [45,52,53,54] as discussed below.
4.1. Application of CRISPRi-Based Methods on Phage Therapy
CRISPRi technology has also emerged as a powerful tool for enhancing phage therapy, a treatment strategy that uses bacteriophages to combat bacterial infections. By integrating CRISPR/Cas systems into engineered phages, researchers can precisely target and silence the expression of targets, increasing the specificity and efficacy of phage therapy. CRISPR-enhanced phages can eliminate antibiotic-resistant bacteria by targeting resistance genes, thereby restoring the effectiveness of existing antibiotics. Additionally, CRISPR systems can be programmed to silence virulence factors, rendering pathogenic bacteria less harmful. CRISPR interference (CRISPRi) has revolutionized phage therapy by enabling the precise and multiplex targeting of bacterial genes. This capability allows for the simultaneous disruption of multiple essential genes, significantly reducing the likelihood of resistance development, and offering a powerful tool to combat pathogenic bacteria within complex microbiomes. This method offers a significant advantage over antibiotics, the latter often causing the loss of beneficial bacteria in the microbiome due to their broad-spectrum effects [124]. Additionally, CRISPRi can be used to engineer bacteriophages that deliver RNA-guided nucleases (RGNs) to disrupt essential bacterial functions, leading to targeted bacterial cell death. For example, Citorik et al. developed a CRISPRi-based-PT system in which RGNs were delivered into E. coli strain EMG2 via the phagemid targeting blaNDM-1 and blaSHV-18 antibiotic resistance genes, conferring β-lactam resistance, or a mutated DNA gyrase gene (gyrAD87G) associated with quinolone resistance [123]. Once inside the pathogen, the cr-RNA directed nucleases to the target DNA, inducing double-stranded breaks that led to plasmid loss and bacterial death (Figure 3A). Importantly, this method demonstrated specificity, as bacteria lacking the targeted sequences remained unaffected. The authors further tested their CRISPRi-based method in vivo using Galleria mellonella larvae infected with Enterohemorrhagic E. coli O157:H7 (EHEC). By targeting the eae gene, which encodes a virulence factor critical for the pathogen’s attachment to the host epithelial cells, the authors demonstrated the enhanced survival of Galleria mellonella larvae treated with the engineered phage compared to the control. This in vivo study highlighted the potential of CRISPRi-based phage therapy in improving host survival by specifically targeting bacterial virulence factors. Similarly, Bikard et al. successfully treated Staphylococcus aureus infections in a murine skin model by targeting key virulence factors, including the aminoglycoside phosphotransferase gene (aph), the methicillin resistance gene (mecA), and the enterotoxin gene (sek), using the CRISPR/Cas based approach that was delivered by a bacteriophage (Figure 3A) [124]. These studies showcase the potential of CRISPR-based phage therapy as a precise antimicrobial strategy to treat multi-drug-resistant bacterial pathogens, particularly carbapenem-resistant Enterobacteriaceae (CRE), that pose a significant threat to public health. Highlighted by the US Center for Disease Control and Prevention as one of the most urgent antibiotic-resistant threats, CRE infections are becoming increasingly difficult to treat due to the spread of extended-spectrum β-lactamases and enzymes like New-Delhi metallo-β-lactamase 1 (NDM-1). These enzymes confer broad resistance to β-lactam antibiotics and are often found with other resistance genes on mobile plasmids, facilitating rapid dissemination. There is an urgent need for new treatments that can target these resistant pathogens specifically without affecting beneficial bacteria, as current broad-spectrum antibiotics are becoming less effective. One promising approach to addressing this challenge involves the use of phage-based antimicrobials, particularly endolysins, as discussed in the following section.
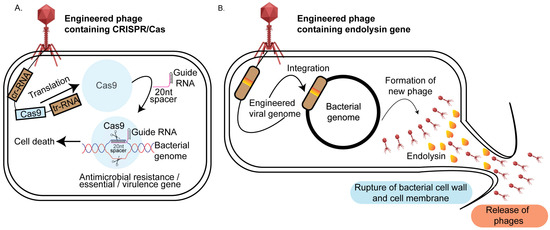
Figure 3.
Phage therapy-based approaches for targeted pathogen elimination: (A) CRISPR/Cas phage engineering: The bacteriophage genome is modified to include CRISPR/Cas genes. Upon infection, the phage transfers its genome into the bacterial host where the Cas9 gene is translated by the host translational machinery and guided by a specific gRNA to target sequences that can be an antimicrobial resistance gene, a virulence factor, or an essential gene. Cas9-induced double-stranded breaks in the target DNA result in bacterial cell death. (B) Endolysin phage engineering: The bacteriophage genome is engineered to carry endolysin genes. During the lytic phase, these genes are expressed by the bacterial host, producing endolysins that degrade the peptidoglycan layer of the cell wall. This leads to bacterial lysis, pathogen death, and the release of new phage progeny.
4.2. Phage-Based Antimicrobial Endolysins
One promising approach to combating pathogenic bacteria involves modifying the native endolysins of bacteriophages [125]. Endolysins are enzymes produced by bacteriophages during the lytic phase. These enzymes degrade the peptidoglycan layer of the bacterial host cell wall from within, leading to cell lysis and the subsequent release of progeny virions (Figure 3B). Endolysins exhibit structural variability depending on its target. For example, endolysins targeting Gram-positive bacteria typically exhibit a modular structure, which enhances their effectiveness in degrading bacterial cell walls. This modular architecture consists of two main components: the catalytic domain (CD) and the cell wall-binding domain (CBD). The catalytic domain is responsible for the enzymatic activity that breaks down the peptidoglycan layer of the bacterial cell wall, leading to cell lysis. This domain encompasses various enzymes, including N-acetylmuramoyl-L-alanine amidases, N-acetylglucosaminidases, or endopeptidases, each targeting specific bonds within the peptidoglycan matrix. The cell wall-binding domain, on the other hand, ensures that the endolysin binds effectively and specifically to the bacterial cell wall. This domain recognizes and attaches to unique components of the Gram-positive bacterial cell wall, such as teichoic acids or peptidoglycan, thereby localizing the catalytic activity to the site of action. In contrast, endolysins targeting Gram-negative bacteria are generally smaller, single-domain proteins without a distinct CBD. The outer membrane of Gram-negative bacteria restricts access to the peptidoglycan layer, necessitating different structural adaptations in the endolysins [125]. Previous studies have demonstrated that recombinantly purified endolysins are effective in killing both Gram-positive and Gram-negative bacteria [126,127]. Building on this, researchers engineered a chimeric endolysin, Cpl-7-11, by combining the catalytic, linker, and cell wall-binding domains of two separate endolysins, Cpl1 and Cpl7S. This chimeric endolysin was shown to be 50% more effective against Streptococcus pneumoniae in a murine infection model compared to the parental Cpl1 alone [128]. Similar studies were conducted with chimeric endolysins to treat Streptococcus sp. [129,130], Staphylococcus sp. [131], and Listeria sp. [132]. While chimeric endolysins have shown success in treating Gram-positive bacteria, their application to Gram-negative bacteria has been limited by the presence of the outer membrane. To overcome this challenge, researchers have used permeabilizing agents such as EDTA, citric acid, polymyxin B, and poly-L-lysine. A combination of EDTA, citric acid, and malic acid was found to be particularly effective against Pseudomonas aeruginosa strain PA01 and Pseudomonas fluorescens when treated with the Salmonella phage endolysin Lys68 [133]. Another innovative approach involved fusing endolysins with lipopolysaccharide (LPS)-destabilizing peptides, leading to the creation of “Artilysins”. These engineered enzymes combine the antimicrobial activity of endolysins with the ability to disrupt the outer membrane of Gram-negative bacteria, enhancing their effectiveness in targeting a broader range of pathogens. In one study, polycationic nonapeptides (PCNPs) were fused with the endolysin OBPgp279, isolated from the P. fluorescens phage OBP, to create an Artilysin that was found to be effective against Pseudomonas aeruginosa [134]. Similarly, Lood et al. discovered the endolysin PlyF307, which was effective against Acinetobacter baumannii in a murine infection model [135]. This strategy of combining endolysins with LPS-destabilizing peptides shows promise in enhancing the efficacy of phage-derived therapies, especially against difficult-to-treat Gram-negative pathogens. Building on these advancements, recent developments in phage-based pathogen detection have addressed some limitations of traditional methods, which often fail to identify pathogens present in low numbers due to the enrichment step as outlined below.
4.3. Application of Engineered Phages for Bacterial Detection and Diagnostics
Recent developments in phage-based pathogen detection have addressed some limitations of traditional methods, which often fail to identify pathogens present in low numbers due to the enrichment step [136]. To overcome this, researchers have created luminescence-based assays by fusing a luciferase gene with a phage protein through homologous recombination. Upon infection, the engineered phage produces light, which can be detected using luminescence assays. For example, Sarkis et al. engineered a phage by cloning the luciferase gene into the tRNA region of the L5 mycobacteriophage, which successfully detected live Mycobacterium smegmatis [137]. Similarly, a luminescent assay for detecting Listeria monocytogenes was created by fusing the major capsid protein gene of Listeria phage A511 with the luxA and luxB genes from Vibrio harveyi, enabling detection at concentrations below 1 CFU/g in contaminated food [138]. This innovative approach allows for the quick identification of Listeria contamination in food and environmental samples, which is crucial for preventing foodborne illnesses, as its high sensitivity and specificity ensure that even low levels of Listeria can be detected, reducing the risk of outbreaks. Additionally, the use of bioluminescence as a detection mechanism simplifies the testing process, making it more accessible and cost-effective for routine screening.
Additionally, GFP-based phages were engineered to detect the enterohemorrhagic strain of E. coli O157:H7. In this engineered phage, the gene encoding green fluorescent protein (GFP) was recombined into the genome of a T4 phage mutant that lacks lytic activity. Upon encountering the target pathogen, GFP fluorescence is expressed on the small outer capsid (soc), with the fluorescence intensity directly proportional to the infection time [139]. In a different approach, Piuri et al. engineered the mycobacteriophage TM4 to emit GFP or ZsYellow in the presence of Mycobacterium tuberculosis, enabling detection using flow cytometry. This assay was further used to detect multi-drug-resistant strains of M. tuberculosis. The addition of Rifampicin decreased fluorescence intensity in susceptible bacterial populations, while any remaining fluorescence after Rifampicin treatment indicated the presence of drug-resistant bacteria, providing a rapid detection method for drug-resistant M. tuberculosis [140]. Finally, a T7 coliphage was engineered to display a biotinylated peptide on its major capsid protein. These biotinylated phages were conjugated to streptavidin-coated quantum dots (QDs), which emit fluorescence only after infection. In the absence of the host, the biotinylated phages were not produced, offering a novel method for pathogen detection [141]. These advancements not only enhance the efficiency of monitoring and controlling the above listed pathogens in various settings but also set a precedent for developing similar detection systems for other pathogenic bacteria, thereby improving overall food safety and public health surveillance.
Despite its versatility, phage therapy has several limitations that hinder their widespread use as therapeutic agents. Their narrow cleavage spectrum restricts them to target only specific bacteria, which makes them less effective against infections caused by multiple bacterial species. Additionally, lysogenic bacteriophages can transfer toxins and antibiotic resistance genes, complicating their therapeutic use. There is also a lack of standardized policies and regulations for bacteriophage therapy (PT), as well as insufficient guidance on phage isolation, purification, and clinical application. Importantly, bacteria can evolve resistance to bacteriophages through various mechanisms, such as the CRISPR/Cas system, which complicates their long-term effectiveness. Furthermore, the lack of pharmacokinetic data, the difficulty of maintaining effective phage concentrations in the body, and potential interactions with the immune system or the release of bacterial toxins pose significant challenges for PT’s clinical application [142].
5. Organ-on-a-Chip—An Emerging Platform for Investigating Host–Pathogen Interactions
Traditional approaches to studying host–pathogen interactions are limited by the challenges in accessing human organs, ethical concerns, and the lack of translatability of murine models to human systems [11]. Additionally, traditional methods such as fecal microbiota analysis fail to capture real-time microbial dynamics under physiological or disease conditions [143]. To address these challenges, organ-on-a-chip technology was developed to replicate human organ-level functions for accurate drug testing and disease modeling while reducing dependence on animal models [62,144]. By integrating shear stress conditions that replicate the in vivo environment, this microfluidic-based approach offers a unique platform for investigating pathogen interactions with various human organ tissues, including the gut, lungs, liver, intestine, and kidney [62,63,145,146,147,148,149] (Figure 4). This methodology enables the generation of physiologically relevant insights as discussed below.
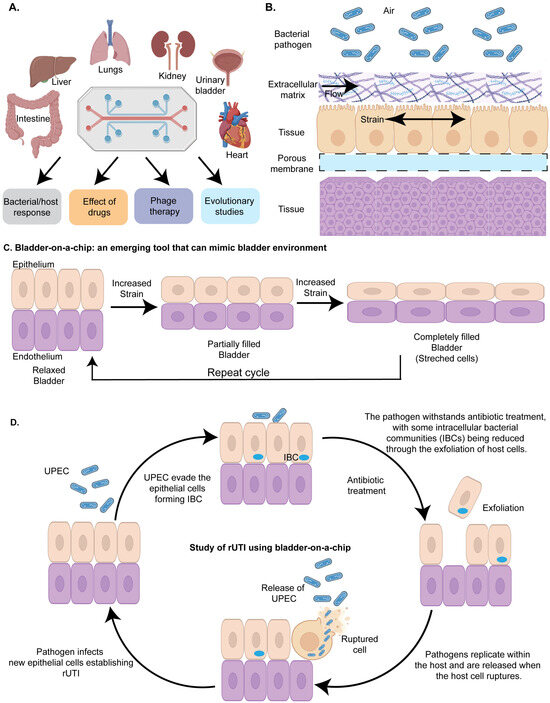
Figure 4.
Organ-on-a-chip to study host–pathogen interactions: (A) Overview of various organs modeled using organ-on-a-chip technology to investigate the molecular mechanisms of infectious diseases and the potential applications of this approach. Organ-on-a-chip is used to decipher bacterial and host response, to study the effect of drugs, antimicrobials and engineered bacteriophages (phage therapy) on bacterial pathogens, and to study genetic and epigenetic changes in response to the environment. (B) A simplified diagram of a model organ-on-chip illustrating the layered structure of the chip. The top layer contains the pathogenic bacteria of interest, followed by the extracellular matrix. Nutrients, growth factors, and drugs flow through this layer to replicate the host environment (e.g., gut). Beneath this, the first host cell layer is represented, such as umbrella cells in a urinary bladder model. The chip design includes a mechanical strain to simulate physiological conditions, such as peristaltic motion in a gut model or the stress that bladder epithelial cells undergo during filling and voiding in a urinary bladder model. A porous membrane is located below the host cell layer, enabling the efficient exchange of chemicals with the underlying tissue layer. (C) Bladder-on-a-chip: a tool mimicking the urinary bladder environment: The bladder-on-a-chip is an innovative device designed to replicate the environment of the urinary bladder. It consists of an upper chamber lined with bladder epithelial cells and a lower chamber containing endothelial cells. By incorporating mechanical strain that simulates the natural expansion of bladder cells during urine storage and their subsequent relaxation during micturition, this device closely mimics the in vivo conditions of the urinary bladder. This realistic model provides a valuable platform for studying host–pathogen interactions. (D) Bladder-on-a-chip in recurrent UTI studies: The bladder-on-a-chip is used to investigate the mechanisms underlying recurrent urinary tract infections (rUTIs). Uropathogenic Escherichia coli (UPEC) are introduced into the upper chamber, where they adhere to the epithelial cells. Some UPEC evade the host immune response by invading the epithelial cells and forming intracellular bacterial communities (IBCs). Upon antibiotic treatment, extracellular UPEC that failed to invade the cells are eliminated, but those within IBCs are shielded from the antibiotics. These protected bacteria multiply within the host cells and are released into the external environment upon host cell rupture. The released UPEC then infect new epithelial cells, perpetuating the cycle of recurrent UTIs.
5.1. Organ-on-a-Chip as a Tool to Study the Interaction of Pathogens with the Host
Organ-on-a-chip technology has been widely applied to explore the adaptative strategies employed by pathogens to counteract the host immune defense (Figure 4A) [62,63,150]. It typically consists of two main compartments: an upper compartment and a lower compartment, separated by a porous membrane. The upper compartment is often lined with human epithelial cells, simulating the tissue interface, while the lower compartment contains a different type of cell, such as endothelial cells, or immune or stromal cells, to replicate the underlying tissue environment. To investigate the interactions between pathogens (UPEC, Mycobacterium tuberculosis, Staphylococcus aureus and Pseudomonas sp.) and the host, the bacterial pathogens are introduced into the upper compartment, where they first engage with the epithelial cells. The porous membrane between these compartments allows for the exchange of nutrients, gases, and signaling molecules, facilitating realistic cell–cell interactions and mimicking the dynamic environment of human organs (Figure 4B). This design enables researchers to study complex biological processes, such as host–pathogen interactions, in a controlled and physiologically relevant setting, providing valuable insights into disease mechanisms and potential therapeutic interventions.
Previous work has deciphered that Uropathogenic Escherichia coli (UPEC) evade host immune defenses and antibiotic treatment by forming intracellular bacterial communities (IBCs) within the umbrella cells of the urinary bladder [151]. Commonly used antibiotics fail to eliminate IBCs, allowing the persistent population to reactivate within the host and cause recurrent UTIs. Understanding the molecular mechanisms of IBC formation could pave the way for developing novel therapeutics to effectively treat recurrent UTIs. One of the significant challenges for studying the IBC formation in real time within the host environment is the inaccessibility of the urinary bladder. To address this problem, Sharma et al. developed a chip to investigate bacterial persistence by uropathogenic E. coli [152]. The microfluidic chip harbors a layer of bladder cells at the base of a channel filled with diluted human urine. The bladder cells were infected with uropathogenic Escherichia coli (UPEC) and imaged over time to observe bacterial movement, interactions with the bladder cells, and aggregation. Immune cells derived from human blood were introduced into a vascular channel beneath the bladder tissue, lined with endothelial cells mimicking blood vessel architecture. The immune cells swiftly crossed the endothelial barrier into the bladder tissue, targeting infection sites. To mimic the bladder filling due to the accumulation of urine and quick voiding due to micturition, the authors designed a duty cycle (periodic application of mechanical or biochemical stimuli to mimic the natural rhythms and cycles experienced by tissues in the body) of 6 hr. The cycle included a linear increase in strain to simulate bladder filling (0–2 h), sustained stretching to represent a filled bladder (2–4 h), rapid strain relaxation over 2 min to mimic voiding (4:02 h), and maintenance without strain to reflect a voided bladder (4:02–6 h). To facilitate the attachment of the pathogen to the epithelial cells, the flow rate of the urine harboring the pathogen was selected to be 1.2 mL/h, which corresponded to a shear stress of 0.02 dyne cm−2 for a period of 1.5–2 h (Figure 4C). Using time-lapse microscopy, the authors observed a steady increase in bacterial attachment to the epithelial cells over this period. However, by the end of this period, the typical infectious dose remained low, with fewer than one bacterium per epithelial cell probably due to the failure of the uropathogen to infect the host. Furthermore, the authors observed the formation of intracellular bacterial communities (IBCs) in uroepithelial cells that exhibited heterogeneous dynamics that influence their persistence and response to antibiotics. Successive rounds of ampicillin treatment under flow conditions eliminated planktonic bacteria but allowed intracellular UPEC within IBCs to survive, grow, and seed new infections (Figure 4D). Time-lapse imaging revealed diverse outcomes for IBCs, including bacterial shedding, cell exfoliation, and persistent growth, with morphologies ranging from coccoid to filamentous. Moreover, antibiotic effectiveness varied based on the bacterial niche and drug type, with Fosfomycin showing higher clearance rates than ampicillin. The bladder filling and voiding cycle further increased bacterial burden, highlighting its role in UPEC uptake and proliferation. This study underscores the protective nature of IBCs in persistent UTIs and the potential of bladder-chip models to investigate host–pathogen interactions and antibiotic dynamics in physiologically relevant settings (Figure 4D).
Organ-on-a-chip models have also been proven valuable in investigating the host immune response to Mycobacterium tuberculosis (Mtb). Pulmonary surfactants, which comprise dipalmitoylphosphatidylcholine (a unique phospholipid) and four surfactant-associated proteins—SP-A, SP-B, SP-C, and SP-D—play a crucial role in host defense. It lines the alveolar wall and facilitates the binding and clearance of various pathogens, including Mycobacterium tuberculosis [153]. Previous studies indicated that mice deficient in pulmonary surfactant production exhibit increased susceptibility to Mycobacterium tuberculosis [154], highlighting the importance of pulmonary surfactant in mitigating tuberculosis. To explore the molecular mechanisms behind these interactions, Thacker et al. recently developed a lung-on-a-chip model incorporating alveolar cells and macrophages to investigate the early dynamics of Mycobacterium tuberculosis (Mtb) infection at an air–liquid interface [155]. This lung-on-a-chip technology offers several advantages over ex vivo lung tissue and 3D lung organoid models. One of the primary benefits is its ability to accurately replicate the in vivo lung environment, including the mechanical and biochemical cues that are essential for proper lung function. This micro-engineered device incorporates microfluidic channels that simulate airways and blood vessels, allowing for realistic air and blood flow dynamics. Additionally, akin to the bladder-on-a-chip described above, lung-on-a-chip models can integrate mechanical stretching to mimic the expansion and contraction of lung tissues during breathing, which is difficult to achieve with ex vivo tissues or 3D organoids. This feature enhances the physiological relevance of the model. Furthermore, lung-on-a-chip systems enable the real-time monitoring of cellular responses through integrated sensors, providing continuous data on lung cell function and overall device performance, making it a preferred choice for host–pathogen interaction studies. Utilizing time-lapse microscopy, the study revealed a crucial role of pulmonary surfactant in controlling tuberculosis by mitigating bacterial growth within the macrophages. The study highlights that Mtb growth rates measured in a lung-on-chip model align closely with mouse infection data, with a net growth rate of 0.37, corresponding to a doubling time of 45 h. Surfactant levels significantly affect infection outcomes: reduced surfactant in Deficient Surfactant (DS) conditions increases intracellular Mtb replication, whereas adding exogenous surfactant (Curosurf) suppresses growth. Additionally, Curosurf partially removes virulence-associated lipids like sulfoglycolipids and trehalose dimycolate from Mtb surfaces, offering a potential mechanism for attenuating growth in macrophages. The lung-on-chip model under normal surfactant conditions effectively mirrors in vivo Mtb infection dynamics, demonstrating its utility in studying early infection phases and host–pathogen interactions. Interestingly, lung-on-a-chip technology was also employed to study pathogen dynamics under diseased host conditions. For example, in one study, Pseudomonas aeruginosa demonstrated enhanced growth in lung airway chips engineered to replicate cystic fibrosis (CF) airways, effectively mirroring critical features of the disease. These chips incorporate the primary epithelium isolated from CF patients and effectively replicate the CF airway’s characteristics, such as increased cilia density, mucus accumulation, ciliary beating frequency, and IL-8 secretion. As a result, there is greater adhesion of circulating immune cells to the endothelium and their transmigration into the airway compartment, compared to healthy airway chips [156]. Organ-on-a-chip technology has applications in studying secondary bacterial infections, such as Staphylococcus aureus infections following influenza. Pneumonia, often exacerbated by bacterial co-infections, significantly increases morbidity and mortality during influenza epidemics. A microfluidic chip system was developed to model these interactions by coculturing alveolar epithelial cells (NCI-H441) and monocyte-derived macrophages in the upper chamber under air-phase conditions for 14 days, while human umbilical vein endothelial cells (HUVECs) were cultured in the lower chamber with the medium perfused via a peristaltic pump. The authors observed significant damage to the endothelial layer following Staphylococcus aureus infection. This platform allowed the spatiotemporal monitoring of S. aureus spread and host immune responses, offering an advanced tool for studying pneumonia pathophysiology and exploring potential therapeutic targets [157].
Furthermore, the researchers designed a device that mimics the human intestine, incorporating the flowing liquid stress (shear stress) and the squeezing and relaxing motions of intestinal muscles (peristaltic movement, causing tensile stress). Using this setup, they demonstrated that Shigella flexneri invades gut cells from the top side, leading to a breakdown of the protective intestinal barrier [158]. This effect was further aggravated by physical deformities caused by peristaltic movement-induced stress on the cells. In a separate study, the importance of tensile stress was highlighted using enterotoxigenic E. coli, which increased the secretion of cGMP by human jejunal enteroids in response to enterotoxins released by the pathogen. The study provided new insights into the mechanisms underlying enterotoxin-induced diarrhea [159].
5.2. Organ Chips as a Tool to Decipher Host–Microbiota Interactions
There is growing evidence about the significant role of gut microbiota in human health [56,57], with disruptions linked to conditions such as inflammatory bowel disease (IBD) [160], celiac disease [161], and gastrointestinal malignancies [162]. Understanding the crosstalk between individual microbes, gut epithelial cells, and immune cells under standard and disease conditions could open new avenues for drug development. Recent studies have demonstrated the potential of organ-on-a-chip models to uncover these interactions. For example, Kim et al. conducted a “proof-of-concept” study where the authors incubated eight probiotic bacterial strains with intestinal epithelial cells in an organ-on-a-chip model [163]. The authors found that peristaltic motion is crucial in regulating microbiota overgrowth in the gut. In addition, the authors also found that the exogenous addition of lipopolysaccharide (LPS) endotoxin-induced the production of proinflammatory cytokines (IL-8, IL-6, IL-1B, and TNF-α), leading to villus restructuring and impaired barrier function. Interestingly, this damage could be reversed by adding probiotic bacteria.
Recently, significant progress has been made in replicating the human gut microbiome in vitro. Using an advanced two-channel intestine-on-a-chip model, the authors successfully co-cultured a microbiome containing over 200 bacterial species, closely mimicking the diversity and complexity of a human stool microbiome, in direct contact with the human intestinal epithelium and its naturally secreted mucus layer for five days [164]. These models were lined with either Caco-2 cells or primary human organoid-derived epithelium, interfaced with the primary intestinal microvascular endothelium. This was accomplished by creating a hypoxia gradient across the epithelial–endothelial interface, replicating the in vivo conditions, which was confirmed using integrated on-chip oxygen sensors. The presence of living bacteria in the system either maintained or improved intestinal barrier function, underscoring the microbiome’s role in supporting intestinal health within this model. This setup provides a sophisticated platform for investigating host–microbiome interactions in a controlled, physiologically relevant environment. In a separate study, Shin et al. deciphered DSS-induced oxidative stress that caused changes in the villus microarchitecture and barrier function in a gut-inflammation-on-a-chip model, further underscoring the importance of gut homeostasis in disease [165]. The addition of non-pathogenic E. coli or lipopolysaccharide (LPS) results in the production of proinflammatory cytokines and the recruitment of immune cells. Additionally, the inclusion of probiotic bacteria was also shown to reduce oxidative stress, further highlighting the importance of gut microbiota dynamics on human health. Finally, Tovaglieri et al. investigated the relationship between host pathophysiology and the gut microbiome using a human colonic epithelium gut-chip model to simulate enterohemorrhagic E. coli (EHEC) infection [166]. The authors found that human microbiome-derived metabolites caused greater epithelial cell damage compared to those from mice and identified four metabolites that triggered the expression of flagellin, a bacterial protein essential for motility. These findings suggest that specific metabolites in the human gut microbiome may influence the host’s immune response, offering insights into host–microbiome interactions that could enhance the translational relevance of microbial research for human health.
The organ-on-chip platform, like any emerging technology, faces several challenges that need to be addressed for broader adoption and effective application. Notably, organ chips can exhibit significant variability across different production batches, laboratories, and users [167]. Interestingly, Polydimethylsiloxane (PDMS), the material used in the fabrication of these chips despite being favored for its high biocompatibility, transparency, and oxygen permeability, has been shown in multiple studies to adsorb proteins onto its surface and can interfere with the interaction of drugs or stimulatory substances with the cells within the chip [168]. Additionally, organ-on-a-chip devices often require specialized equipment adding complexity and cost to their setup.
6. Conclusions and Future Perspective
In the past decade, the emergence of multi-drug-resistant (MDR) bacterial strains has become a significant global health challenge, driven by factors such as de novo mutations, horizontal gene transfer, and the inappropriate use of antibiotics in both human medicine and livestock farming [169,170]. These factors foster conditions that promote bacterial resistance, enabling them to survive in sublethal concentrations of antibiotics. Furthermore, to counteract host immune responses, bacteria have evolved a range of virulence factors, sensors, and response regulators that activate downstream stress response pathways to counteract the host-mediated nutritional immunity [171,172]. For instance, the Ferric Uptake Regulator (Fur) plays a crucial role in equipping uropathogenic E. coli with the necessary adaptations to survive the nutrient-limited urinary tract [172]. Similarly, the global Fe-S cluster regulator, IscR, is pivotal in the survival and virulence of Yersinia pestis [173]. Additionally, Mycobacterium tuberculosis has developed several adaptive strategies to evade antimicrobial treatments, including its ability to enter a dormant state and reactivate under immunocompromised conditions, establishing chronic infections. Central to this adaptation is the DevR/DosR response regulator, which is crucial for the virulence, dormancy adaptation, and antibiotic tolerance of M. tuberculosis. By modulating the dormancy regulon, DevR/DosR facilitates the persistence of M. tuberculosis in the host, enabling it to endure environmental stresses that promote dormancy [174]. Moreover, Poly Phosphate Kinase 1 (PPK1) plays a key role during phosphate starvation, likely through a bistable signaling pathway [175], while the transcription factor MtrA regulates dormancy and reactivation, promoting the long-term survival of M. tuberculosis [176]. We previously developed recombinant tools to study the role of transcription factors in M. tuberculosis under in vitro [177] and in vivo conditions [178]. Using these methods, we identified the role of the novel transcription factor Rv1222 in M. tuberculosis [179]. Additionally, these tools have proven valuable in discovering a novel anti-tuberculosis molecule, D-AAP1, to treat drug-resistant M. tuberculosis [180]. With the rise in resistant strains, new tools are urgently needed to understand the governing roles of these factors in host–pathogen interactions.
Synthetic biology-based strategies have greatly enhanced our ability to dissect the roles of virulence factors and essential genes in bacterial pathogens [24,38,91,92]. Many pathogenic bacteria contain horizontally acquired genes located within their genomic pathogenicity islands. These islands frequently encode genes critical for bacterial survival and virulence, enabling pathogens to counteract the host immune response. For instance, the uropathogenic Escherichia coli strain CFT073 harbors 13 pathogenicity islands, which include genes involved in iron acquisition, toxin production and transport, biofilm formation, and antimicrobial resistance [172,181]. The CRISPR/Cas systems have emerged as powerful tools in bacterial research due to their versatility in targeting bacterial defense mechanisms. These methods can be utilized to elucidate the roles of pathogenicity island genes with unknown functions, thereby revealing novel adaptive strategies underlying host–pathogen interactions. Mobile CRISPRi has emerged as a new player to study host–pathogen interactions. Currently, this method can be only utilized in a limited number of Gram-positive pathogens. Further development of mobile CRISPRi-based platforms will unlock the untapped potential to study a greater range of pathogens. Furthermore, CRISPR-based functional genomics techniques, such as CRISPR interference (CRISPRi), combined with sequencing technologies like CRISPRi-seq, have proven effective in identifying key gene subsets and disrupting essential processes involved in host–pathogen interactions [83]. Although the application of CRISPRi-seq is still limited, its integration with other techniques, such as Tn-seq, has the potential to reveal niche-specific metabolic processes and identify synergistic antibiotic targets that may be concealed by genetic redundancy. Finally, CRISPR-mediated transposition (CAST) [182] and CRISPR activation (CRISPRa) systems provide innovative methods for large-scale bacterial genome analysis, offering promising solutions to the limitations of current methods. In addition to these genetic tools, the development of engineered microbes has further expanded our capabilities in studying bacterial pathogens.
These engineered microbes, designed to detect microbial pathogens and pathogen-associated metabolites in vivo, have opened new avenues for understanding the molecular signatures of disease progression, providing valuable insights for drug development. For instance, BWCBs have proven to be an effective method for identifying both intra- and inter-species communication signals (quorum-sensing molecules) within the gut microbiota. These reporter gene-based synthetic circuits allow for bacterial imaging, but, over time, the signal intensity may diminish, often due to the depletion of essential exogenous substrates required for bioluminescent bacteria [183]. Furthermore, fluorescence imaging is hindered by background autofluorescence, which can compromise sensitivity [184]. To address these limitations, strategies to correct autofluorescence can be employed, or alternative optical imaging techniques, such as bioluminescence or photoacoustic imaging, can be explored to improve accuracy and reliability. While engineered microbes and BWCBs have advanced our ability to detect and study microbial interactions within the host, complementary approaches like phage therapy offer innovative solutions for targeting antibiotic-resistant pathogens, bridging diagnostic capabilities with therapeutic advancements in combating complex infections.
Phage therapy (PT) has emerged as a promising alternative to traditional antibiotics, utilizing bacteriophages to target and eliminate antibiotic-resistant pathogens [44,120,121]. While it holds significant promise, its widespread adoption is hindered by challenges such as the need for extensive clinical research, regulatory approvals, and overcoming misconceptions regarding the potential for phages to cause infections. PT is especially promising in personalized medicine, where phage biobanks are used to match specific phages to bacterial strains [142]. Despite these hurdles, PT could serve as a valuable adjunct to conventional antibiotics, particularly in treating infections resistant to standard therapies. Additionally, advancements in phage therapy, driven by clinical trials and biotechnological innovations, provide a foundation for developing alternative anti-infectives. However, challenges such as the longevity of phages, their interactions with antibiotics, pharmacokinetics, and safety concerns regarding endotoxins and protein toxins remain. Efforts to develop large phage banks and improve isolation and purification methods are crucial to ensuring the safety and efficacy of PT, while genetic engineering and sequencing technologies offer hope for the future, provided that logistical and regulatory hurdles are addressed. Building on the innovative approaches of phage therapy, the integration of advanced technologies like organ-on-a-chip models provides a powerful platform for exploring host–pathogen interactions, evaluating therapeutic efficacy, and addressing complex challenges in antimicrobial resistance and personalized medicine.
Organ-on-a-chip models offer significant advantages for studying conditions that are challenging to replicate in humans or animals, including genetic disorders and the effects of severe radiation exposure. These models provide a human-relevant alternative to genetically modified mice, facilitating more accurate therapeutic development. Organ chips also present a safer alternative for investigating pregnancy-related drug effects on the placental barrier [144]. Recent advancements have led to the development of multi-organ-on-a-chip systems designed to explore how various organs respond under normal and diseased conditions [185]. These systems allow for the more accurate modeling of inter-organ interactions and disease progression. By simulating complex drug metabolism and pharmacokinetics, they will provide a more relevant approach to preclinical drug testing. The potential of multi-organ chips to transform personalized medicine is immense, providing insights into individual responses to therapeutics. Looking forward, the development of bacterial platforms for antimicrobial therapy and the combination of innovative strategies such as phage therapy, CRISPR-based genomics, and synthetic microbial communities offer promising directions to address antibiotic resistance and enhance therapeutic approaches. These emerging technologies have the potential to reshape treatments for infectious diseases, heralding a pivotal shift in the fight against antimicrobial resistance.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Rahn, D.D. Urinary tract infections: Contemporary management. Urol. Nurs. 2008, 28, 333–341; quiz 342. [Google Scholar] [PubMed]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold. Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef]
- Khan, M.I.; Xu, S.; Ali, M.M.; Ali, R.; Kazmi, A.; Akhtar, N.; Bilal, M.; Hu, Y.; Li, F. Assessment of multidrug resistance in bacterial isolates from urinary tract-infected patients. J. Radiat. Res. Appl. Sci. 2020, 13, 267–275. [Google Scholar] [CrossRef]
- Brumbaugh, A.R.; Mobley, H.L. Preventing urinary tract infection: Progress toward an effective Escherichia coli vaccine. Expert. Rev. Vaccines 2012, 11, 663–676. [Google Scholar] [CrossRef]
- Clark, N.M.; Zhanel, G.G.; Lynch, J.P., 3rd. Emergence of antimicrobial resistance among Acinetobacter species: A global threat. Curr. Opin. Crit. Care 2016, 22, 491–499. [Google Scholar] [CrossRef]
- Chua, H.C.; Tse, A.; Smith, N.M.; Mergenhagen, K.A.; Cha, R.; Tsuji, B.T. Combatting the Rising Tide of Antimicrobial Resistance: Pharmacokinetic/Pharmacodynamic Dosing Strategies for Maximal Precision. Int. J. Antimicrob. Agents 2021, 57, 106269. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Winzeler, E.A.; Shoemaker, D.D.; Astromoff, A.; Liang, H.; Anderson, K.; Andre, B.; Bangham, R.; Benito, R.; Boeke, J.D.; Bussey, H.; et al. Functional Characterization of the S. cerevisiae Genome by Gene Deletion and Parallel Analysis. Science 1999, 285, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-M.; Kritikos, G.; Farelli, J.D.; Todor, H.; Tong, K.; Kimsey, H.; Wapinski, I.; Galardini, M.; Cabal, A.; Peters, J.M.; et al. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst. 2017, 4, 291–305.e297. [Google Scholar] [CrossRef] [PubMed]
- van Opijnen, T.; Bodi, K.L.; Camilli, A. Tn-seq: High-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 2009, 6, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.K.; Barquist, L.; Goodman, A.L.; Paulsen, I.T.; Parkhill, J.; van Opijnen, T. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet. 2020, 21, 526–540. [Google Scholar] [CrossRef]
- Jana, B.; Liu, X.; Dénéréaz, J.; Park, H.; Leshchiner, D.; Liu, B.; Gallay, C.; Zhu, J.; Veening, J.-W.; van Opijnen, T. CRISPRi–TnSeq maps genome-wide interactions between essential and non-essential genes in bacteria. Nat. Microbiol. 2024, 9, 2395–2409. [Google Scholar] [CrossRef]
- Leshchiner, D.; Rosconi, F.; Sundaresh, B.; Rudmann, E.; Ramirez, L.M.N.; Nishimoto, A.T.; Wood, S.J.; Jana, B.; Buján, N.; Li, K.; et al. A genome-wide atlas of antibiotic susceptibility targets and pathways to tolerance. Nat. Commun. 2022, 13, 3165. [Google Scholar] [CrossRef]
- Gallagher, L.A.; Shendure, J.; Manoil, C. Genome-Scale Identification of Resistance Functions in Pseudomonas aeruginosa Using Tn-seq. mBio 2011, 2, e00315-10. [Google Scholar] [CrossRef]
- DeJesus Michael, A.; Gerrick Elias, R.; Xu, W.; Park Sae, W.; Long Jarukit, E.; Boutte Cara, C.; Rubin Eric, J.; Schnappinger, D.; Ehrt, S.; Fortune Sarah, M.; et al. Comprehensive Essentiality Analysis of the Mycobacterium tuberculosis Genome via Saturating Transposon Mutagenesis. mBio 2017, 8, e02133-16. [Google Scholar] [CrossRef]
- Green, B.; Bouchier, C.; Fairhead, C.; Craig, N.L.; Cormack, B.P. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mob. DNA 2012, 3, 3. [Google Scholar] [CrossRef]
- Javaid, N.; Choi, S. CRISPR/Cas System and Factors Affecting Its Precision and Efficiency. Front. Cell Dev. Biol. 2021, 9, 761709. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Harris, D.J.; Posada, D. Origin and Length Distribution of Unidirectional Prokaryotic Overlapping Genes. G3 2014, 4, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Rivera, P.M.; Lin, A.; Del Vecchio, D.; Weiss, R. A load driver device for engineering modularity in biological networks. Nat. Biotechnol. 2014, 32, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Sismour, A.M. Synthetic biology. Nat. Rev. Genet. 2005, 6, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ruder, W.C.; Lu, T.; Collins, J.J. Synthetic Biology Moving into the Clinic. Science 2011, 333, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Sharda, M.; Badrinarayanan, A.; Seshasayee, A.S.N. Evolutionary and Comparative Analysis of Bacterial Nonhomologous End Joining Repair. Genome Biol. Evol. 2020, 12, 2450–2466. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- McNeil, M.B.; Keighley, L.M.; Cook, J.R.; Cheung, C.Y.; Cook, G.M. CRISPR interference identifies vulnerable cellular pathways with bactericidal phenotypes in Mycobacterium tuberculosis. Mol. Microbiol. 2021, 116, 1033–1043. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef]
- Peters, J.M.; Colavin, A.; Shi, H.; Czarny, T.L.; Larson, M.H.; Wong, S.; Hawkins, J.S.; Lu, C.H.S.; Koo, B.-M.; Marta, E.; et al. A Comprehensive, CRISPR-based Functional Analysis of Essential Genes in Bacteria. Cell 2016, 165, 1493–1506. [Google Scholar] [CrossRef]
- Peters, J.M.; Koo, B.-M.; Patino, R.; Heussler, G.E.; Hearne, C.C.; Qu, J.; Inclan, Y.F.; Hawkins, J.S.; Lu, C.H.S.; Silvis, M.R.; et al. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nat. Microbiol. 2019, 4, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Prasad, N.K.; Yu, M.A.; Chen, S.; Lyden, A.; Herrera, N.; Silvis, M.R.; Crawford, E.; Looney, M.R.; Peters, J.M.; et al. Modulating Pathogenesis with Mobile-CRISPRi. J. Bacteriol. 2019, 201, e00304-19. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Tran, J.S.; Banta, A.B.; Bacon, E.E.; Rose, W.E.; Peters, J.M. Essential gene knockdowns reveal genetic vulnerabilities and antibiotic sensitivities in Acinetobacter baumannii. mBio 2024, 15, e02051-23. [Google Scholar] [CrossRef] [PubMed]
- Geyman, L.J.; Tanner, M.P.; Rosario-Meléndez, N.; Peters, J.M.; Mandel, M.J.; van Kessel, J.C. Mobile-CRISPRi as a powerful tool for modulating Vibrio gene expression. Appl. Environ. Microbiol. 2024, 90, e0006524. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
- Fu, E.; Yager, P.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. Perspective on diagnostics for global health. IEEE Pulse 2011, 2, 40–50. [Google Scholar] [CrossRef]
- Rogers, J.K.; Taylor, N.D.; Church, G.M. Biosensor-based engineering of biosynthetic pathways. Curr. Opin. Biotechnol. 2016, 42, 84–91. [Google Scholar] [CrossRef]
- Kumari, A.; Pasini, P.; Deo, S.K.; Flomenhoft, D.; Shashidhar, H.; Daunert, S. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 2006, 78, 7603–7609. [Google Scholar] [CrossRef]
- Bonnet, J.; Yin, P.; Ortiz, M.E.; Subsoontorn, P.; Endy, D. Amplifying genetic logic gates. Science 2013, 340, 599–603. [Google Scholar] [CrossRef]
- Wang, B.; Kitney, R.I.; Joly, N.; Buck, M. Engineering modular and orthogonal genetic logic gates for robust digital-like synthetic biology. Nat. Commun. 2011, 2, 508. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lammens, E.-M.; Nikel, P.I.; Lavigne, R. Exploring the synthetic biology potential of bacteriophages for engineering non-model bacteria. Nat. Commun. 2020, 11, 5294. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Samson, J.E.; Magadán, A.H.; Sabri, M.; Moineau, S. Revenge of the phages: Defeating bacterial defences. Nat. Rev. Microbiol. 2013, 11, 675–687. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Weigle, J.J. Induction of Mutations in a Bacterial Virus*. Proc. Natl. Acad. Sci. USA 1953, 39, 628–636. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Enright, A.L.; Heelan, W.J.; Ward, R.D.; Peters, J.M. CRISPRi functional genomics in bacteria and its application to medical and industrial research. Microbiol. Mol. Biol. Rev. 2024, 88, e0017022. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef]
- Belete, M.A.; Tadesse, S.; Tilahun, M.; Alemayehu, E.; Saravanan, M. Phage endolysins as new therapeutic options for multidrug resistant Staphylococcus aureus: An emerging antibiotic-free way to combat drug resistant infections. Front. Cell. Infect. Microbiol. 2024, 14, 1397935. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, J.A.; García, J.L.; García, P. Taking aim on bacterial pathogens: From phage therapy to enzybiotics. Curr. Opin. Microbiol. 2007, 10, 461–472. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Andes, D.; Craig, W.A. Animal model pharmacokinetics and pharmacodynamics: A critical review. Int. J. Antimicrob. Agents 2002, 19, 261–268. [Google Scholar] [CrossRef]
- Moore, J.N.; Poon, L.; Pahwa, S.; Bensman, T.; Wei, X.; Danielsen, Z.Y.; Jang, S. Animal pharmacokinetics/pharmacodynamics (PK/PD) infection models for clinical development of antibacterial drugs: Lessons from selected cases. J. Antimicrob. Chemother. 2023, 78, 1337–1343. [Google Scholar] [CrossRef]
- Baddal, B.; Marrazzo, P. Refining Host-Pathogen Interactions: Organ-on-Chip Side of the Coin. Pathogens 2021, 10, 203. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Tock, M.R.; Dryden, D.T.F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005, 8, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Barrangou, R.; Horvath, P. CRISPR: New Horizons in Phage Resistance and Strain Identification. Annu. Rev. Food Sci. Technol. 2012, 3, 143–162. [Google Scholar] [CrossRef]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; van der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef]
- Mojica, F.J.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 2000, 36, 244–246. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant. Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Xu, X.; Chemparathy, A.; Zeng, L.; Kempton, H.R.; Shang, S.; Nakamura, M.; Qi, L.S. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol. Cell 2021, 81, 4333–4345.e4. [Google Scholar] [CrossRef]
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef]
- Kumar, M.; Prusty, M.R.; Pandey, M.K.; Singh, P.K.; Bohra, A.; Guo, B.; Varshney, R.K. Application of CRISPR/Cas9-mediated gene editing for abiotic stress management in crop plants. Front. Plant. Sci. 2023, 14, 1157678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaur, A.; Pandey, A.; Mamrutha, H.M.; Singh, G.P. CRISPR-based genome editing in wheat: A comprehensive review and future prospects. Mol. Biol. Rep. 2019, 46, 3557–3569. [Google Scholar] [CrossRef] [PubMed]
- Hwarari, D.; Radani, Y.; Ke, Y.; Chen, J.; Yang, L. CRISPR/Cas genome editing in plants: Mechanisms, applications, and overcoming bottlenecks. Funct. Integr. Genom. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, F.J.; Jacks, T. Applications of the CRISPR-Cas9 system in cancer biology. Nat. Rev. Cancer 2015, 15, 387–395. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal. Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401–2415. [Google Scholar] [CrossRef]
- Bikard, D.; Jiang, W.; Samai, P.; Hochschild, A.; Zhang, F.; Marraffini, L.A. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013, 41, 7429–7437. [Google Scholar] [CrossRef]
- Liu, X.; Gallay, C.; Kjos, M.; Domenech, A.; Slager, J.; van Kessel, S.P.; Knoops, K.; Sorg, R.A.; Zhang, J.R.; Veening, J.W. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol. Syst. Biol. 2017, 13, 931. [Google Scholar] [CrossRef]
- Choudhary, E.; Thakur, P.; Pareek, M.; Agarwal, N. Gene silencing by CRISPR interference in mycobacteria. Nat. Commun. 2015, 6, 6267. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Zhang, Q.; Cao, S.; Li, X.; Qi, Z.; Tan, Y.; You, Y.; Bi, Y.; Song, Y.; et al. Reversible Gene Expression Control in Yersinia pestis by Using an Optimized CRISPR Interference System. Appl. Environ. Microbiol. 2019, 85, e00097-19. [Google Scholar] [CrossRef]
- de Bakker, V.; Liu, X.; Bravo, A.M.; Veening, J.-W. CRISPRi-seq for genome-wide fitness quantification in bacteria. Nat. Protoc. 2022, 17, 252–281. [Google Scholar] [CrossRef] [PubMed]
- Ellis, N.A.; Kim, B.; Tung, J.; Machner, M.P. A multiplex CRISPR interference tool for virulence gene interrogation in Legionella pneumophila. Commun. Biol. 2021, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-Y.; Zheng, D.; Li, S.-S.; Ding, X.-Y.; Wang, C.-L.; Guo, X.-P.; Zhan, L.; Jin, Q.; Yang, J.; Sun, Y.-C. Application of combined CRISPR screening for genetic and chemical-genetic interaction profiling in Mycobacterium tuberculosis. Sci. Adv. 2022, 8, eadd5907. [Google Scholar] [CrossRef] [PubMed]
- Noirot-Gros, M.-F.; Forrester, S.; Malato, G.; Larsen, P.E.; Noirot, P. CRISPR interference to interrogate genes that control biofilm formation in Pseudomonas fluorescens. Sci. Rep. 2019, 9, 15954. [Google Scholar] [CrossRef]
- Gervais, N.C.; La Bella, A.A.; Wensing, L.F.; Sharma, J.; Acquaviva, V.; Best, M.; Cadena López, R.O.; Fogal, M.; Uthayakumar, D.; Chavez, A.; et al. Development and applications of a CRISPR activation system for facile genetic overexpression in Candida albicans. G3 2023, 13, jkac301. [Google Scholar] [CrossRef]
- Afonina, I.; Ong, J.; Chua, J.; Lu, T.; Kline, K.A. Multiplex CRISPRi System Enables the Study of Stage-Specific Biofilm Genetic Requirements in Enterococcus faecalis. mBio 2020, 11, e01101-20. [Google Scholar] [CrossRef]
- Vigouroux, A.; Oldewurtel, E.; Cui, L.; Bikard, D.; van Teeffelen, S. Tuning dCas9’s ability to block transcription enables robust, noiseless knockdown of bacterial genes. Mol. Syst. Biol. 2018, 14, e7899. [Google Scholar] [CrossRef]
- Jost, M.; Santos, D.A.; Saunders, R.A.; Horlbeck, M.A.; Hawkins, J.S.; Scaria, S.M.; Norman, T.M.; Hussmann, J.A.; Liem, C.R.; Gross, C.A.; et al. Titrating gene expression using libraries of systematically attenuated CRISPR guide RNAs. Nat. Biotechnol. 2020, 38, 355–364. [Google Scholar] [CrossRef]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef]
- Singh, M.; Dhanwal, A.; Verma, A.; Augustin, L.; Kumari, N.; Chakraborti, S.; Agarwal, N.; Sriram, D.; Dey, R.J. Discovery of potent antimycobacterial agents targeting lumazine synthase (RibH) of Mycobacterium tuberculosis. Sci. Rep. 2024, 14, 12170. [Google Scholar] [CrossRef]
- Martin, J.K.; Sheehan, J.P.; Bratton, B.P.; Moore, G.M.; Mateus, A.; Li, S.H.-J.; Kim, H.; Rabinowitz, J.D.; Typas, A.; Savitski, M.M.; et al. A Dual-Mechanism Antibiotic Kills Gram-Negative Bacteria and Avoids Drug Resistance. Cell 2020, 181, 1518–1532.e1514. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Chatterji, D. The evolving story of the omega subunit of bacterial RNA polymerase. Trends Microbiol. 2006, 14, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.R.; Gautam, S.; Chatterji, D. Validation of Omega Subunit of RNA Polymerase as a Functional Entity. Biomolecules 2020, 10, 1588. [Google Scholar] [CrossRef]
- Dong, C.; Fontana, J.; Patel, A.; Carothers, J.M.; Zalatan, J.G. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat. Commun. 2018, 9, 2489. [Google Scholar] [CrossRef]
- Kiattisewee, C.; Karanjia, A.V.; Legut, M.; Daniloski, Z.; Koplik, S.E.; Nelson, J.; Kleinstiver, B.P.; Sanjana, N.E.; Carothers, J.M.; Zalatan, J.G. Expanding the Scope of Bacterial CRISPR Activation with PAM-Flexible dCas9 Variants. ACS Synth. Biol. 2022, 11, 4103–4112. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F.; et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011, 9, 467–477. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, S.; van Oudenaarden, A. Synthetic biology: Understanding biological design from synthetic circuits. Nat. Rev. Genet. 2009, 10, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Collins, J.J. Synthetic biology: Applications come of age. Nat. Rev. Genet. 2010, 11, 367–379. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Naydich, A.D.; Nangle, S.N.; Bues, J.J.; Trivedi, D.; Nissar, N.; Inniss, M.C.; Niederhuber, M.J.; Way, J.C.; Silver, P.A.; Riglar, D.T. Synthetic Gene Circuits Enable Systems-Level Biosensor Trigger Discovery at the Host-Microbe Interface. mSystems 2019, 4, e00125-19. [Google Scholar] [CrossRef]
- Hernández-Sancho, J.M.; Boudigou, A.; Alván-Vargas, M.V.G.; Freund, D.; Arnling Bååth, J.; Westh, P.; Jensen, K.; Noda-García, L.; Volke, D.C.; Nikel, P.I. A versatile microbial platform as a tunable whole-cell chemical sensor. Nat. Commun. 2024, 15, 8316. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Park, M.; Tsai, S.L.; Chen, W. Microbial biosensors: Engineered microorganisms as the sensing machinery. Sensor 2013, 13, 5777–5795. [Google Scholar] [CrossRef]
- Kotula, J.W.; Kerns, S.J.; Shaket, L.A.; Siraj, L.; Collins, J.J.; Way, J.C.; Silver, P.A. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc. Natl. Acad. Sci. USA 2014, 111, 4838–4843. [Google Scholar] [CrossRef]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 360, 915–918. [Google Scholar] [CrossRef]
- Daeffler, K.N.M.; Galley, J.D.; Sheth, R.U.; Ortiz-Velez, L.C.; Bibb, C.O.; Shroyer, N.F.; Britton, R.A.; Tabor, J.J. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol. Syst. Biol. 2017, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.J.; Robinson, A.B.; Süel, G.M. Engineered E. coli that detect and respond to gut inflammation through nitric oxide sensing. ACS Synth. Biol. 2012, 1, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.; Pasini, P.; Daunert, S. Deciphering bacterial universal language by detecting the quorum sensing signal, autoinducer-2, with a whole-cell sensing system. Anal. Chem. 2013, 85, 9604–9609. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bram, E.E.; Weiss, R. Genetically programmable pathogen sense and destroy. ACS Synth. Biol. 2013, 2, 715–723. [Google Scholar] [CrossRef]
- Sgro, G.G.; Oka, G.U.; Souza, D.P.; Cenens, W.; Bayer-Santos, E.; Matsuyama, B.Y.; Bueno, N.F.; Dos Santos, T.R.; Alvarez-Martinez, C.E.; Salinas, R.K.; et al. Bacteria-Killing Type IV Secretion Systems. Front. Microbiol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Koh, E.; Wong, A.; March, J.C.; Bentley, W.E.; Lee, Y.S.; Chang, M.W. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat. Commun. 2017, 8, 15028. [Google Scholar] [CrossRef]
- Zheng, L.; Tan, Y.; Hu, Y.; Shen, J.; Qu, Z.; Chen, X.; Ho, C.L.; Leung, E.L.-H.; Zhao, W.; Dai, L. CRISPR/Cas-Based Genome Editing for Human Gut Commensal Bacteroides Species. ACS Synth. Biol. 2022, 11, 464–472. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B. Phage Therapy-Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Dörner, P.J.; Anandakumar, H.; Röwekamp, I.; Fiocca Vernengo, F.; Millet Pascual-Leone, B.; Krzanowski, M.; Sellmaier, J.; Brüning, U.; Fritsche-Guenther, R.; Pfannkuch, L.; et al. Clinically used broad-spectrum antibiotics compromise inflammatory monocyte-dependent antibacterial defense in the lung. Nat. Commun. 2024, 15, 2788. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A.; Marraffini, L.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The Recombinant Phage Lysin LysK Has a Broad Spectrum of Lytic Activity against Clinically Relevant Staphylococci, Including Methicillin-Resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, Y.; Zhang, Q.; Yuan, Y.; Qi, Q.; Lu, X. Characterization of Salmonella endolysin XFII produced by recombinant Escherichia coli and its application combined with chitosan in lysing Gram-negative bacteria. Microb. Cell Factories 2022, 21, 171. [Google Scholar] [CrossRef]
- Díez-Martínez, R.; De Paz, H.D.; García-Fernández, E.; Bustamante, N.; Euler, C.W.; Fischetti, V.A.; Menendez, M.; García, P. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2015, 70, 1763–1773. [Google Scholar] [CrossRef]
- Becker, S.C.; Foster-Frey, J.; Stodola, A.J.; Anacker, D.; Donovan, D.M. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 2009, 443, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, J.; Yang, H.; Wei, C.; Yu, J.; Zhang, Y.; Huang, Y.; Zhang, X.E.; Wei, H. Construction of a chimeric lysin Ply187N-V12C with extended lytic activity against staphylococci and streptococci. Microb. Biotechnol. 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Yu, J.; Huang, Y.; Zhang, X.-E.; Wei, H. Novel Chimeric Lysin with High-Level Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus In Vitro and In Vivo. Antimicrob. Agents Chemother. 2014, 58, 536–542. [Google Scholar] [CrossRef]
- Schmelcher, M.; Tchang, V.S.; Loessner, M.J. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb. Biotechnol. 2011, 4, 651–662. [Google Scholar] [CrossRef]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Puyenbroeck, V.V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Lazcka, O.; Campo, F.J.D.; Muñoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, G.J.; Jacobs, W.R., Jr.; Hatfull, G.F. L5 luciferase reporter mycobacteriophages: A sensitive tool for the detection and assay of live mycobacteria. Mol. Microbiol. 1995, 15, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Rees, C.E.; Stewart, G.S.; Scherer, S. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 1996, 62, 1133–1140. [Google Scholar] [CrossRef]
- Oda, M.; Morita, M.; Unno, H.; Tanji, Y. Rapid detection of Escherichia coli O157:H7 by using green fluorescent protein-labeled PP01 bacteriophage. Appl. Environ. Microbiol. 2004, 70, 527–534. [Google Scholar] [CrossRef]
- Piuri, M.; Jacobs, W.R., Jr.; Hatfull, G.F. Fluoromycobacteriophages for Rapid, Specific, and Sensitive Antibiotic Susceptibility Testing of Mycobacterium tuberculosis. PLoS ONE 2009, 4, e4870. [Google Scholar] [CrossRef]
- Edgar, R.; McKinstry, M.; Hwang, J.; Oppenheim, A.B.; Fekete, R.A.; Giulian, G.; Merril, C.; Nagashima, K.; Adhya, S. High-sensitivity bacterial detection using biotin-tagged phage and quantum-dot nanocomplexes. Proc. Natl. Acad. Sci. USA 2006, 103, 4841–4845. [Google Scholar] [CrossRef]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of Phage Therapy and Corresponding Optimization Strategies: A Review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Lkhagva, E.; Jung, S.; Kim, H.-J.; Chung, H.-J.; Hong, S.-T. Fecal Microbiome Does Not Represent Whole Gut Microbiome. Cell. Microbiol. 2023, 2023, 6868417. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef]
- Feaugas, T.; Sauvonnet, N. Organ-on-chip to investigate host-pathogens interactions. Cell. Microbiol. 2021, 23, e13336. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Xu, N.; Liu, Z.-F.; Pang, D.-W.; Zhang, Z.-L. A virus-induced kidney disease model based on organ-on-a-chip: Pathogenesis exploration of virus-related renal dysfunctions. Biomaterials 2019, 219, 119367. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Nasiri, R.; Barros, N.R.; Tebon, P.; Thakor, J.; Goudie, M.; Shamloo, A.; Martin, M.G.; Khademhosseini, A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 2020, 255, 120196. [Google Scholar] [CrossRef]
- Yokoi, F.; Deguchi, S.; Takayama, K. Organ-on-a-chip models for elucidating the cellular biology of infectious diseases. Biochim. Biophys. Acta BBA Mol. Cell Res. 2023, 1870, 119504. [Google Scholar] [CrossRef]
- Nielubowicz, G.R.; Mobley, H.L.T. Host–pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.W.; McKinney, J.D. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. eLife 2021, 10, e66481. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Chroneos, Z.C.; Midde, K.; Sever-Chroneos, Z.; Jagannath, C. Pulmonary surfactant and tuberculosis. Tuberculosis 2009, 89 (Suppl. 1), S10–S14. [Google Scholar] [CrossRef]
- Thacker, V.V.; Dhar, N.; Sharma, K.; Barrile, R.; Karalis, K.; McKinney, J.D. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLife 2020, 9, e59961. [Google Scholar] [CrossRef]
- Plebani, R.; Potla, R.; Soong, M.; Bai, H.; Izadifar, Z.; Jiang, A.; Travis, R.N.; Belgur, C.; Dinis, A.; Cartwright, M.J.; et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J. Cyst. Fibros. 2022, 21, 606–615. [Google Scholar] [CrossRef]
- Deinhardt-Emmer, S.; Rennert, K.; Schicke, E.; Cseresnyés, Z.; Windolph, M.; Nietzsche, S.; Heller, R.; Siwczak, F.; Haupt, K.F.; Carlstedt, S.; et al. Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication 2020, 12, 025012. [Google Scholar] [CrossRef]
- Grassart, A.; Malardé, V.; Gobaa, S.; Sartori-Rupp, A.; Kerns, J.; Karalis, K.; Marteyn, B.; Sansonetti, P.; Sauvonnet, N. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host. Microbe 2019, 26, 565. [Google Scholar] [CrossRef] [PubMed]
- Sunuwar, L.; Yin, J.; Kasendra, M.; Karalis, K.; Kaper, J.; Fleckenstein, J.; Donowitz, M. Mechanical Stimuli Affect Escherichia coli Heat-Stable Enterotoxin-Cyclic GMP Signaling in a Human Enteroid Intestine-Chip Model. Infect. Immun. 2020, 88, e00866-19. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Kim, H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host–microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA 2018, 115, E10539–E10547. [Google Scholar] [CrossRef]
- Tovaglieri, A.; Sontheimer-Phelps, A.; Geirnaert, A.; Prantil-Baun, R.; Camacho, D.M.; Chou, D.B.; Jalili-Firoozinezhad, S.; de Wouters, T.; Kasendra, M.; Super, M.; et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Developmentally inspired human ‘organs on chips’. Development 2018, 145, dev156125. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Ho, C.M. Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Microfluid. Nanofluidics 2009, 7, 291–306. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Banerjee, R.; Askenasy, I.; Mettert, E.L.; Kiley, P.J. Iron-sulfur Rrf2 transcription factors: An emerging versatile platform for sensing stress. Curr. Opin. Microbiol. 2024, 82, 102543. [Google Scholar] [CrossRef]
- Banerjee, R.; Weisenhorn, E.; Schwartz, K.J.; Myers, K.S.; Glasner, J.D.; Perna, N.T.; Coon, J.J.; Welch, R.A.; Kiley, P.J. Tailoring a Global Iron Regulon to a Uropathogen. mBio 2020, 11, e00351-20. [Google Scholar] [CrossRef]
- Balderas, D.; Mettert, E.; Lam, H.N.; Banerjee, R.; Gverzdys, T.; Alvarez, P.; Saarunya, G.; Tanner, N.; Zoubedi, A.; Wei, Y.; et al. Genome Scale Analysis Reveals IscR Directly and Indirectly Regulates Virulence Factor Genes in Pathogenic Yersinia. mBio 2021, 12, e0063321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, R.; Jain, A.; Kumar, M.; Gauttam, R.; Banerjee, R.; Mukhopadhyay, J.; Tyagi, J.S. Functional insights into Mycobacterium tuberculosis DevR-dependent transcriptional machinery utilizing Escherichia coli. Biochem. J. 2021, 478, 3079–3098. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.; Banerjee, S.K.; Banerjee, R.; Mukhopadhyay, J.; Kundu, M. Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology 2013, 159, 2074–2086. [Google Scholar] [CrossRef]
- Sharma, A.K.; Chatterjee, A.; Gupta, S.; Banerjee, R.; Mandal, S.; Mukhopadhyay, J.; Basu, J.; Kundu, M. MtrA, an essential response regulator of the MtrAB two-component system, regulates the transcription of resuscitation-promoting factor B of Mycobacterium tuberculosis. Microbiology 2015, 161, 1271–1281. [Google Scholar] [CrossRef]
- Banerjee, R.; Rudra, P.; Prajapati, R.K.; Sengupta, S.; Mukhopadhyay, J. Optimization of recombinant Mycobacterium tuberculosis RNA polymerase expression and purification. Tuberculosis 2014, 94, 397–404. [Google Scholar] [CrossRef]
- Banerjee, R.; Rudra, P.; Saha, A.; Mukhopadhyay, J. Recombinant reporter assay using transcriptional machinery of Mycobacterium tuberculosis. J. Bacteriol. 2015, 197, 646–653. [Google Scholar] [CrossRef]
- Rudra, P.; Prajapati, R.K.; Banerjee, R.; Sengupta, S.; Mukhopadhyay, J. Novel mechanism of gene regulation: The protein Rv1222 of Mycobacterium tuberculosis inhibits transcription by anchoring the RNA polymerase onto DNA. Nucleic Acids Res. 2015, 43, 5855–5867. [Google Scholar] [CrossRef][Green Version]
- Lin, W.; Mandal, S.; Degen, D.; Liu, Y.; Ebright, Y.W.; Li, S.; Feng, Y.; Zhang, Y.; Mandal, S.; Jiang, Y.; et al. Structural Basis of Mycobacterium tuberculosis Transcription and Transcription Inhibition. Mol. Cell 2017, 66, 169–179.e8. [Google Scholar] [CrossRef]
- Lloyd, A.L.; Henderson, T.A.; Vigil, P.D.; Mobley, H.L. Genomic islands of uropathogenic Escherichia coli contribute to virulence. J. Bacteriol. 2009, 191, 3469–3481. [Google Scholar] [CrossRef]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 2019, 571, 219–225. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Surre, J.; Saint-Ruf, C.; Collin, V.; Orenga, S.; Ramjeet, M.; Matic, I. Strong increase in the autofluorescence of cells signals struggle for survival. Sci. Rep. 2018, 8, 12088. [Google Scholar] [CrossRef] [PubMed]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach To Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).