Solid-Binding Peptide for Enhancing Biocompatibility of Metallic Biomaterials
Abstract
1. Introduction
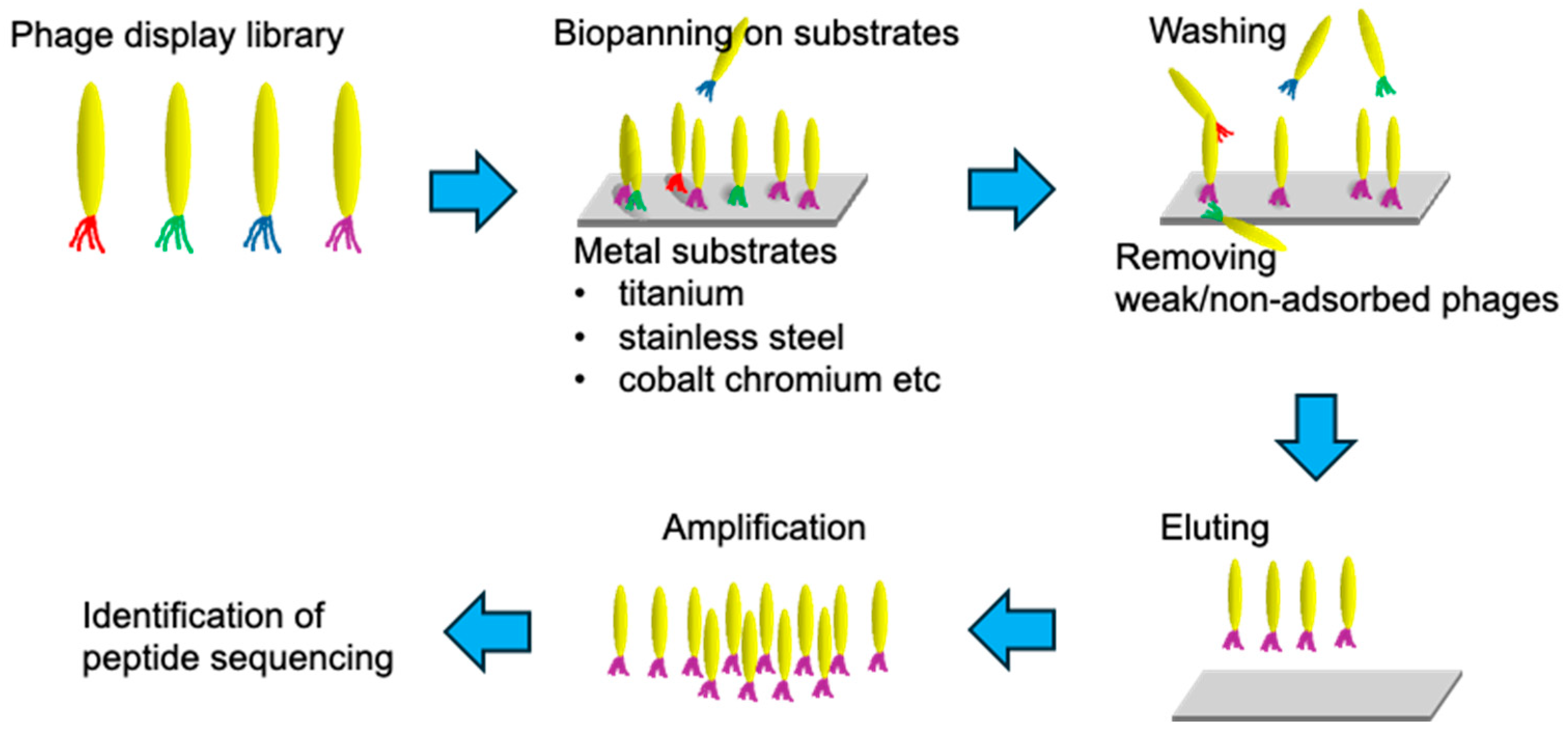
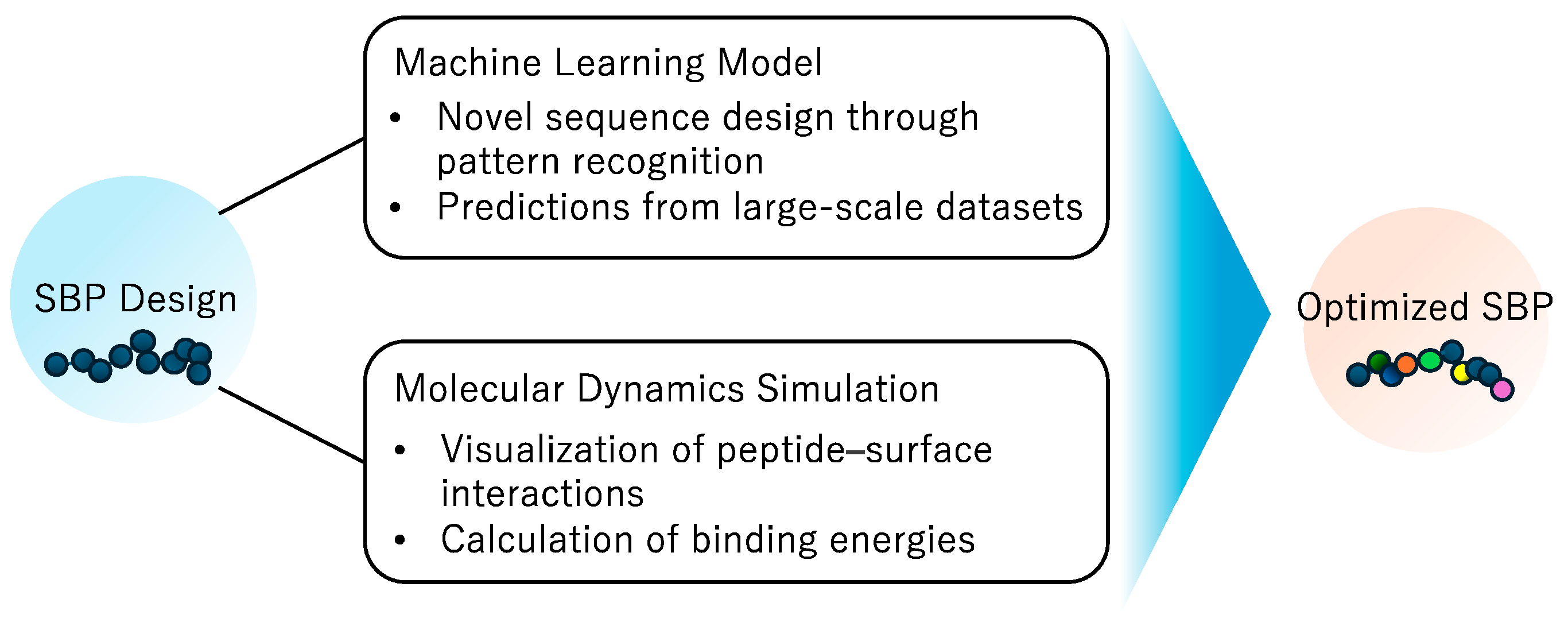
2. Selection and Development of SBPs
2.1. Phage Display Technology
2.2. Computational Approaches for SBP Design and Optimization
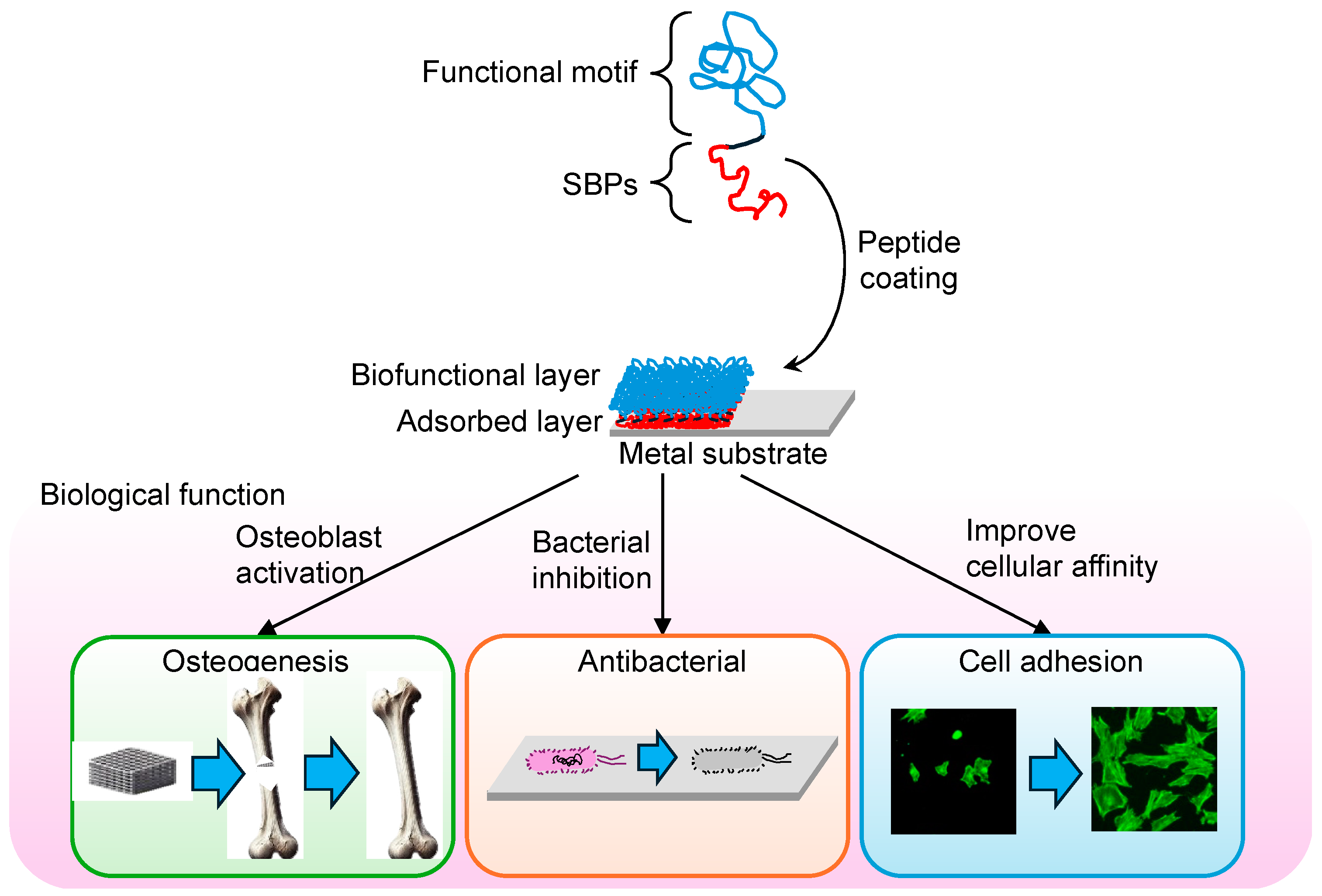
3. Surface Modification of Metal Biomaterials Using SBPs
3.1. Titanium and Titanium Alloys
3.2. Stainless Steel
3.3. Cobalt–Chromium Alloys
3.4. Other Metallic Biomaterials
3.5. Advantages and Challenges of Surface Modification Using SBPs
4. Prospects
4.1. Identification of Peptide Sequences Binding to New Metallic Biomaterials
4.2. Strategies for Enhanced Stability of Peptide Binding
4.3. Multifunctional Surface through Integration with Other Surface Modification Technologies
5. Conclusions
Funding
Conflicts of Interest
References
- Sarikaya, M.; Tamerler, C.; Schwartz, D.T.; Baneyx, F. Materials Assembly and Formation Using Engineered Polypeptides. Annu. Rev. Mater. Res. 2004, 34, 373–408. [Google Scholar] [CrossRef]
- Ruan, Y.; Sohail, M.; Zhao, J.; Hu, F.; Li, Y.; Wang, P.; Zhang, L. Applications of Material-Binding Peptides: A Review. ACS Biomater. Sci. Eng. 2022, 8, 4738–4750. [Google Scholar] [CrossRef]
- Yucesoy, D.T.; Khatayevich, D.; Tamerler, C.; Sarikaya, M. Rationally Designed Chimeric Solid-Binding Peptides for Tailoring Solid Interfaces. Med. Devices Sens. 2020, 3, e10065. [Google Scholar] [CrossRef]
- Gustafsson, E.; Thomée, S.; Grimby-Ekman, A.; Hagberg, M. Texting on Mobile Phones and Musculoskeletal Disorders in Young Adults: A Five-Year Cohort Study. Appl. Ergon. 2017, 58, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Mahdavi, S.; Riahi, R.; Vahdatpour, B.; Kelishadi, R. Association between Sedentary Behavior and Low Back Pain; A Systematic Review and Meta-Analysis. Health Promot. Perspect. 2021, 11, 393–410. [Google Scholar] [CrossRef]
- Alsiwed, K.T.; Alsarwani, R.M.; Alshaikh, S.A.; Howaidi, R.A.; Aljahdali, A.J.; Bassi, M.M. The Prevalence of Text Neck Syndrome and Its Association with Smartphone Use among Medical Students in Jeddah, Saudi Arabia. J. Musculoskelet. Surg. Res. 2021, 5, 266–272. [Google Scholar] [CrossRef]
- Briggs, A.M.; Woolf, A.D.; Dreinhöfer, K.; Homb, N.; Hoy, D.G.; Kopansky-Giles, D.; Åkesson, K.; March, L. Reducing the Global Burden of Musculoskeletal Conditions. Bull. World Health Organ. 2018, 96, 366–368. [Google Scholar] [CrossRef]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56, S243–S255. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving Biocompatibility for next Generation of Metallic Implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef]
- Hanawa, T. Metals and Medicine. Mater. Trans. 2021, 62, 139–148. [Google Scholar] [CrossRef]
- Pisarek, M.; Roguska, A.; Andrzejczuk, M.; Marcon, L.; Szunerits, S.; Lewandowska, M.; Janik-Czachor, M. Effect of Two-Step Functionalization of Ti by Chemical Processes on Protein Adsorption. Appl. Surf. Sci. 2011, 257, 8196–8204. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell Response of Anodized Nanotubes on Titanium and Titanium Alloys. J. Biomed. Mater. Res. 2013, 101A, 2726–2739. [Google Scholar] [CrossRef]
- Berger, M.B.; Cohen, D.J.; Bosh, K.B.; Kapitanov, M.; Slosar, P.J.; Levit, M.M.; Gallagher, M.; Rawlinson, J.J.; Schwartz, Z.; Boyan, B.D. Bone Marrow Stromal Cells Generate an Osteoinductive Microenvironment When Cultured on Titanium–Aluminum–Vanadium Substrates with Biomimetic Multiscale Surface Roughness. Biomed. Mater. 2023, 18, 035001. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.; Kim, K. Surface Modification of Titanium and Titanium Alloys by Ion Implantation. J. Biomed. Mater. Res. 2010, 93B, 581–591. [Google Scholar] [CrossRef]
- Won, S.; Huh, Y.-H.; Cho, L.-R.; Lee, H.-S.; Byon, E.-S.; Park, C.-J. Cellular Response of Human Bone Marrow Derived Mesenchymal Stem Cells to Titanium Surfaces Implanted with Calcium and Magnesium Ions. Tissue Eng. Regen. Med. 2017, 14, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Migita, S.; Araki, K. Effect of Nanometer Scale Surface Roughness of Titanium for Osteoblast Function. AIMS Bioeng. 2017, 4, 162–170. [Google Scholar] [CrossRef]
- Migita, S.; Wakabayashi, K. Analysis of Disordered Abrasive Scratches on Titanium Surfaces and Their Impact on Nuclear Translocation of Yes-Associated Protein. Sci. Rep. 2022, 12, 21705. [Google Scholar] [CrossRef]
- Migita, S.; Okuyama, S.; Araki, K. Sub-Micrometer Scale Surface Roughness of Titanium Reduces Fibroblasts Function. J. Appl. Biomater. Funct. Mater. 2016, 14, 65–69. [Google Scholar] [CrossRef]
- Migita, S. Surface Roughness and Its Role in Mediating Cell Adhesion on Cobalt-Chromium-Molybdenum Alloys. Biosurf. Biotribol. 2023, 9, 161–168. [Google Scholar] [CrossRef]
- Morra, M. Biochemical Modification of Titanium Surfaces: Peptides and ECM Proteins. Eur. Cell Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Chen, J.; Li, T.; Hu, G.; Fang, Z.; Zhou, H.; Guo, K.; Wang, L.; Wang, Y. One-Step Preparation of the Engineered Titanium Implant by Rationally Designed Linear Fusion Peptides with Spacer-Dependent Antimicrobial, Anti-Inflammatory and Osteogenic Activities. Chem. Eng. J. 2021, 424, 130380. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous Fusion Phage: Novel Expression Vectors That Display Cloned Antigens on the Virion Surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage Display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Davidson, T.A.; McGoldrick, S.J.; Kohn, D.H. Phage Display to Augment Biomaterial Function. Int. J. Mol. Sci. 2020, 21, 5994. [Google Scholar] [CrossRef]
- Zambrano-Mila, M.S.; Blacio, K.E.S.; Vispo, N.S. Peptide Phage Display: Molecular Principles and Biomedical Applications. Ther. Innov. Regul. Sci. 2020, 54, 308–317. [Google Scholar] [CrossRef]
- Sidhu, S.S. Phage Display in Pharmaceutical Biotechnology. Curr. Opin. Biotechnol. 2000, 11, 610–616. [Google Scholar] [CrossRef]
- Kehoe, J.W.; Kay, B.K. Filamentous Phage Display in the New Millennium. Chem. Rev. 2005, 105, 4056–4072. [Google Scholar] [CrossRef]
- Krumpe, L.R.H.; Atkinson, A.J.; Smythers, G.W.; Kandel, A.; Schumacher, K.M.; McMahon, J.B.; Makowski, L.; Mori, T. T7 Lytic Phage-Displayed Peptide Libraries Exhibit Less Sequence Bias than M13 Filamentous Phage-Displayed Peptide Libraries. Proteomics 2006, 6, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Khatayevich, D.; Gungormus, M.; Yazici, H.; So, C.; Cetinel, S.; Ma, H.; Jen, A.; Tamerler, C.; Sarikaya, M. Biofunctionalization of Materials for Implants Using Engineered Peptides. Acta Biomater. 2010, 6, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pfaendtner, J. High-Throughput Computational Screening of Solid-Binding Peptides. J. Chem. Theory Comput. 2024, 20, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.T.; Xiao, X.; Hall, C.K. In Silico Design and Analysis of Plastic-Binding Peptides. J. Phys. Chem. B 2023, 127, 8370–8381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saravanan, K.M.; Wei, Y.; Jiao, Y.; Yang, Y.; Pan, Y.; Wu, X.; Zhang, J.Z.H. Deep Learning-Based Bioactive Therapeutic Peptide Generation and Screening. J. Chem. Inf. Model. 2023, 63, 835–845. [Google Scholar] [CrossRef]
- Janairo, J.I.B. A Machine Learning Classification Model for Gold-Binding Peptides. ACS Omega 2022, 7, 14069–14073. [Google Scholar] [CrossRef]
- Stevens, C.A.; Bachtiger, F.; Kong, X.-D.; Abriata, L.A.; Sosso, G.C.; Gibson, M.I.; Klok, H.-A. A Minimalistic Cyclic Ice-Binding Peptide from Phage Display. Nat. Commun. 2021, 12, 2675. [Google Scholar] [CrossRef]
- Chen, S.; Lovell, S.; Lee, S.; Fellner, M.; Mace, P.D.; Bogyo, M. Identification of Highly Selective Covalent Inhibitors by Phage Display. Nat. Biotechnol. 2021, 39, 490–498. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Huang, J.; He, B. CD47Binder: Identify CD47 Binding Peptides by Combining Next-Generation Phage Display Data and Multiple Peptide Descriptors. Interdiscip. Sci. Comput. Life Sci. 2023, 15, 578–589. [Google Scholar] [CrossRef]
- Alvisi, N.; De Vries, R. Biomedical Applications of Solid-Binding Peptides and Proteins. Mater. Today Bio 2023, 19, 100580. [Google Scholar] [CrossRef]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-Binding Peptides: Smart Tools for Nanobiotechnology. Trend Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef]
- Sano, K.-I.; Shiba, K. A Hexapeptide Motif That Electrostatically Binds to the Surface of Titanium. J. Am. Chem. Soc. 2003, 125, 14234–14235. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Chopra, A.; Schmidt, F. Combined Endothelialization Promoting and Surface Binding Chimeric Conjugate with Low Thrombogenicity. Bioconjug. Chem. 2021, 32, 1602–1605. [Google Scholar] [CrossRef]
- Watanabe, M.; Bhawal, U.K.; Takemoto, S.; Nishiyama, N.; Nakahara, Y.; Tatematsu, K.; Sezutsu, H.; Kuwabara, N.; Minamisawa, T.; Shiba, K.; et al. Bio-Functionalized Titanium Surfaces with Modified Silk Fibroin Carrying Titanium Binding Motif to Enhance the Ossific Differentiation of MC3T3-E1. Biotechnol. Bioeng. 2021, 118, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Xue, Q.; Wang, Y.-J.; Zhang, M.; Chen, Y.-J.; Zhang, Q. Engineered Chimeric Peptides with IGF-1 and Titanium-Binding Functions to Enhance Osteogenic Differentiation In Vitro under T2DM Condition. Materials 2022, 15, 3134. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Kokubu, E.; Kokubun, K.; Matsuzaka, K.; Shiba, K.; Kashiwagi, K.; Inoue, T. An Artificial Fusion Protein between Bone Morphogenetic Protein 2 and Titanium-Binding Peptide Is Functional In Vivo. J. Biomed. Mater. Res. 2014, 102, 1180–1186. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, S.; Wu, C.; Li, X.; Ma, X.; Hu, H.; Wu, J.; Wang, Y.; Liu, Z. Chimeric Peptides Quickly Modify the Surface of Personalized 3D Printing Titanium Implants to Promote Osseointegration. ACS Appl. Mater. Interfaces 2021, 13, 33981–33994. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Xu, S.; Ma, X.; Zhao, Z.; Hu, H.; Deng, J.; Peng, C.; Wang, Y.; Ma, S. A Drug Delivery System Constructed by a Fusion Peptide Capturing Exosomes Targets to Titanium Implants Accurately Resulting the Enhancement of Osseointegration Peri-Implant. Biomater. Res. 2022, 26, 89. [Google Scholar] [CrossRef]
- Geng, H.; Sun, X.; Zhang, X.; Yuan, Y. Efficient Titanium Surface Modified Using Bifunctional Chimeric Peptides to Prevent Biofilm Formation by Multiple Microorganisms. Colloids Surf. B Biointerfaces 2023, 230, 113534. [Google Scholar] [CrossRef]
- Drexelius, M.; Arnold, R.; Meinberger, D.; Wilhelm, M.; Mathur, S.; Neundorf, I. Rational Design of Bifunctional Chimeric Peptides That Combine Antimicrobial and Titanium Binding Activity. J. Pept. Sci. 2023, 29, e3481. [Google Scholar] [CrossRef]
- Yucesoy, D.T.; Hnilova, M.; Boone, K.; Arnold, P.M.; Snead, M.L.; Tamerler, C. Chimeric Peptides as Implant Functionalization Agents for Titanium Alloy Implants with Antimicrobial Properties. JOM 2015, 67, 754–766. [Google Scholar] [CrossRef]
- Wisdom, C.; Chen, C.; Yuca, E.; Zhou, Y.; Tamerler, C.; Snead, M.L. Repeatedly Applied Peptide Film Kills Bacteria on Dental Implants. JOM 2019, 71, 1271–1280. [Google Scholar] [CrossRef]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl. Mater. Interfaces 2016, 8, 5070–5081. [Google Scholar] [CrossRef]
- Wisdom, E.C.; Zhou, Y.; Chen, C.; Tamerler, C.; Snead, M.L. Mitigation of Peri-Implantitis by Rational Design of Bifunctional Peptides with Antimicrobial Properties. ACS Biomater. Sci. Eng. 2020, 6, 2682–2695. [Google Scholar] [CrossRef]
- Yazici, H.; Fong, H.; Wilson, B.; Oren, E.E.; Amos, F.A.; Zhang, H.; Evans, J.S.; Snead, M.L.; Sarikaya, M.; Tamerler, C. Biological Response on a Titanium Implant-Grade Surface Functionalized with Modular Peptides. Acta Biomater. 2013, 9, 5341–5352. [Google Scholar] [CrossRef]
- Vidal, G.; Blanchi, T.; Mieszawska, A.J.; Calabrese, R.; Rossi, C.; Vigneron, P.; Duval, J.-L.; Kaplan, D.L.; Egles, C. Enhanced Cellular Adhesion on Titanium by Silk Functionalized with Titanium Binding and RGD Peptides. Acta Biomater. 2013, 9, 4935–4943. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Jones, S.E.; Cai, Y.; Ahmad, G.; Naik, R.R.; Kröger, N.; Sandhage, K.H. Identification and Design of Peptides for the Rapid, High-Yield Formation of Nanoparticulate TiO2 from Aqueous Solutions at Room Temperature. Chem. Mater. 2008, 20, 1578–1584. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, J.; Zhou, B.; Wei, W.; Gong, S. Peptide Aptamers against Titanium-Based Implants Identified through Phage Display. J. Mater. Sci Mater. Med. 2010, 21, 1103–1107. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Végh, A.G.; Martin, M.; Vladimirov, B.; Larroque, C.; Gergely, C.; Cuisinier, F.J.G.; Estephan, E. Improving Dental Epithelial Junction on Dental Implants with Bioengineered Peptides. Front. Bioeng. Biotechnol. 2023, 11, 1165853. [Google Scholar] [CrossRef]
- Khoo, X.; Hamilton, P.; O’Toole, G.A.; Snyder, B.D.; Kenan, D.J.; Grinstaff, M.W. Directed Assembly of PEGylated-Peptide Coatings for Infection-Resistant Titanium Metal. J. Am. Chem. Soc. 2009, 131, 10992–10997. [Google Scholar] [CrossRef]
- Kodama, T.; Yoshihara, A.; Goel, I.; Sekino, M.; Kuwahata, A.; Yoshimori, A.; Murayama, Y.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B.; et al. Identification of Metal-Binding Peptides and Their Conjugation onto Nanoparticles of Superparamagnetic Iron Oxides and Liposomes. ACS Appl. Mater. Interfaces 2020, 12, 24623–24634. [Google Scholar] [CrossRef] [PubMed]
- Vreuls, C.; Zocchi, G.; Genin, A.; Archambeau, C.; Martial, J.; De Weerdt, C.V. Inorganic-Binding Peptides as Tools for Surface Quality Control. J. Inorg. Biochem. 2010, 104, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi-Mikami, A.; Fujimoto, K.; Taguchi, T.; Isao, K.; Yamazaki, T. A Novel Biofunctionalizing Peptide for Metallic Alloy. Biotechnol. Lett. 2020, 42, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Migita, S.; Sakashita, K.; Saito, Y.; Suyalatu; Yamazaki, T. Co–Cr–Mo Alloy Binding Peptide as Molecular Glue for Constructing Biomedical Surfaces. J. Appl. Biomater. Funct. Mater. 2020, 18, 228080002092473. [Google Scholar] [CrossRef]
- Migita, S.; Wakabayashi, K.; Yamazaki, T. Binding Stability of Peptides to Co–Cr–Mo Alloy Affects Proliferation and Differentiation of Osteoblast. Biotechnol. Bioeng. 2022, 119, 1157–1163. [Google Scholar] [CrossRef]
- Hnilova, M.; Oren, E.E.; Seker, U.O.S.; Wilson, B.R.; Collino, S.; Evans, J.S.; Tamerler, C.; Sarikaya, M. Effect of Molecular Conformations on the Adsorption Behavior of Gold-Binding Peptides. Langmuir 2008, 24, 12440–12445. [Google Scholar] [CrossRef]
- Seker, U.O.S.; Wilson, B.; Dincer, S.; Kim, I.W.; Oren, E.E.; Evans, J.S.; Tamerler, C.; Sarikaya, M. Adsorption Behavior of Linear and Cyclic Genetically Engineered Platinum Binding Peptides. Langmuir 2007, 23, 7895–7900. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Mohamed, A.; Rajeev, A.S. Clinical Outcomes and Complications of Titanium versus Stainless Steel Elastic Nail in Management of Paediatric Femoral Fractures—A Systematic Review. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 157–167. [Google Scholar] [CrossRef]
- Goyal, N.; Aggarwal, A.N.; Mishra, P.; Jain, A. Randomized Controlled Trial Comparing Stabilization of Fresh Close Femoral Shaft Fractures in Children with Titanium Elastic Nail System versus Stainless Steel Elastic Nail System. Acta Orthop. Belg. 2014, 80, 69–75. [Google Scholar] [PubMed]
- Wall, E.J.; Jain, V.; Vora, V.; Mehlman, C.T.; Crawford, A.H. Complications of Titanium and Stainless Steel Elastic Nail Fixation of Pediatric Femoral Fractures. J. Bone Jt. Surg. 2008, 90, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Mojarad Shafiee, B.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220. [Google Scholar] [CrossRef]
- Stranz, M.; Kastang, E.S. A Review of pH and Osmolarity. Int. J. Pharm. Compd. 2002, 6, 216–220. [Google Scholar]
- Varmaziar, S.; Atapour, M.; Hedberg, Y.S. Corrosion and Metal Release Characterization of Stainless Steel 316L Weld Zones in Whey Protein Solution. NPJ Mater. Degrad. 2022, 6, 19. [Google Scholar] [CrossRef]
- Aherwar, A.; Singh, A.K.; Patnaik, A. Cobalt Based Alloy: A Better Choice Biomaterial for Hip Implants. Trends Biomater. Artif. Organs 2016, 30, 50–55. [Google Scholar]
- Fu, W.; Liu, S.; Jiao, J.; Xie, Z.; Huang, X.; Lu, Y.; Liu, H.; Hu, S.; Zuo, E.; Kou, N.; et al. Wear Resistance and Biocompatibility of Co-Cr Dental Alloys Fabricated with CAST and SLM Techniques. Materials 2022, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, U.; Keck, J.; Krüger, S.; Arabmotlagh, M.; Rauschmann, M.; Schilling, C. Biomechanical Comparison of Different Rod-to-Rod Connectors to a Conventional Titanium- and Cobalt Chromium Posterior Spinal Fixation System. Brain Spine 2023, 3, 101708. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, Y.; Li, Z.; Cao, C.; Liu, G.; Liu, Q.; Zhang, X.; Dai, D.; Zheng, Z.; Zhao, C.; et al. Study on the Micro Removal Process of Inner Surface of Cobalt Chromium Alloy Cardiovascular Stent Tubes. Micromachines 2022, 13, 1374. [Google Scholar] [CrossRef]
- Poh, C.K.; Shi, Z.; Tan, X.W.; Liang, Z.C.; Foo, X.M.; Tan, H.C.; Neoh, K.G.; Wang, W. Cobalt Chromium Alloy with Immobilized BMP Peptide for Enhanced Bone Growth. J. Orthop. Res. 2011, 29, 1424–1430. [Google Scholar] [CrossRef]
- Tan, H.C.; Poh, C.K.; Cai, Y.; Soe, M.T.; Wang, W. Covalently Grafted BMP-7 Peptide to Reduce Macrophage/Monocyte Activity: An in Vitro Study on Cobalt Chromium Alloy. Biotechnol. Bioeng. 2013, 110, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.C.; Poh, C.K.; Cai, Y.; Wang, W. Anti-Fibrosis Effect of BMP-7 Peptide Functionalization on Cobalt Chromium Alloy. J. Orthop. Res. 2013, 31, 983–990. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, X.; Yang, L.; Qin, G.; Zhang, E. A Novel Ti-Au Alloy with Strong Antibacterial Properties and Excellent Biocompatibility for Biomedical Application. Biomater. Adv. 2022, 133, 112653. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Lazić, V.; Grgur, B. Development of a New AuCuZnGe Alloy and Determination of Its Corrosion Properties. Metals 2022, 12, 1284. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Yang, L.; Cui, Y. Materials and Biomedical Applications of Implantable Electronic Devices. Adv. Mater. Technol. 2023, 8, 2200853. [Google Scholar] [CrossRef]
- Pramounmat, N.; Yan, K.; Wolf, J.; Renner, J. Platinum-Binding Peptides: Understanding of Selective Binding and Multifunctionality. Multifunct. Mater. 2022, 5, 012002. [Google Scholar] [CrossRef]
- Hörsted-Bindslev, P. Amalgam Toxicity—Environmental and Occupational Hazards. J. Dent. 2004, 32, 359–365. [Google Scholar] [CrossRef]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.-Y.; Schulten, K.; Baneyx, F. Molecular Biomimetics: Nanotechnology through Biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Care, A.; Lord, M.S.; Walsh, T.R.; Sunna, A. Experimental and Theoretical Tools to Elucidate the Binding Mechanisms of Solid-Binding Peptides. New Biotechnol. 2019, 52, 9–18. [Google Scholar] [CrossRef]
- Takaichi, A.; Suyalatu; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; et al. Microstructures and Mechanical Properties of Co–29Cr–6Mo Alloy Fabricated by Selective Laser Melting Process for Dental Applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef]
- Migita, S.; Sakashita, K.; Suyalatu. Osteoblast Compatibility of 3D Printed Co–Cr–Mo Alloys with Different Building Direction. Biosurf. Biotribol. 2019, 5, 67–70. [Google Scholar] [CrossRef]
- Patel, M.S.; McCormick, J.R.; Ghasem, A.; Huntley, S.R.; Gjolaj, J.P. Tantalum: The next Biomaterial in Spine Surgery? J. Spine Surg. 2020, 6, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Porter, D.; Grove, K.; Collins, S.; Ornberg, A.; Shulfer, R. A Comprehensive Review of Biological and Materials Properties of Tantalum and Its Alloys. J. Biomed. Mater. Res. 2022, 110, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.; Zhang, J.; Cai, X.; Li, L.; Liu, G.; Liu, J.; Cui, L.; Huang, J. The Progress on Physicochemical Properties and Biocompatibility of Tantalum-Based Metal Bone Implants. SN Appl. Sci. 2020, 2, 671. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Tsutsumi, Y.; Nomura, N.; Hanawa, T. Bioinspired Low-Magnetic Zr Alloy with High Strength and Ductility. Scr. Mater. 2021, 199, 113856. [Google Scholar] [CrossRef]
- Suzuki, A.K.; Campo, K.N.; Fonseca, E.B.; Araújo, L.C.; Gandra, F.C.G.; Lopes, É.S.N. Appraising the Potential of Zr-Based Biomedical Alloys to Reduce Magnetic Resonance Imaging Artifacts. Sci. Rep. 2020, 10, 2621. [Google Scholar] [CrossRef]
- Xue, R.; Wang, D.; Yang, D.; Zhang, L.; Xu, X.; Liu, L.; Wu, D. Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Materials 2020, 13, 5130. [Google Scholar] [CrossRef]
- Sun, X.; Liu, D.; Chen, M.; Zhou, W.; Nomura, N.; Hanawa, T. Influence of Annealing Treatment on the Microstructure, Mechanical Performance and Magnetic Susceptibility of Low Magnetic Zr–1Mo Parts Manufactured via Laser Additive Manufacturing. Mater. Sci. Eng. A 2021, 804, 140740. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Micro + Nano: Conserving the Gold Standard Microroughness to Nanoengineer Zirconium Dental Implants. ACS Biomater. Sci. Eng. 2021, 7, 3069–3074. [Google Scholar] [CrossRef]
- Rothenstein, D.; Shopova-Gospodinova, D.; Bakradze, G.; Jeurgens, L.P.H.; Bill, J. Generation of Luminescence in Biomineralized Zirconia by Zirconia-Binding Peptides. CrystEngComm 2015, 17, 1783–1790. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yoshinari, M.; Matsuzaka, K.; Shiba, K.; Inoue, T. Identification of Peptide Motif That Binds to the Surface of Zirconia. Dent. Mater. J. 2011, 30, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Sharath Kumar, J.; Kumar, R.; Verma, R. Surface Modification Aspects for Improving Biomedical Properties in Implants: A Review. Acta Metall. Sin. (Engl. Lett.) 2023, 37, 213–241. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-Based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on Magnesium and Magnesium-Based Alloys as Biomaterials for Bone Immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Gutiérrez Púa, L.D.C.; Rincón Montenegro, J.C.; Fonseca Reyes, A.M.; Zambrano Rodríguez, H.; Paredes Méndez, V.N. Biomaterials for Orthopedic Applications and Techniques to Improve Corrosion Resistance and Mechanical Properties for Magnesium Alloy: A Review. J. Mater. Sci. 2023, 58, 3879–3908. [Google Scholar] [CrossRef]
- Sun, Y.; Helmholz, H.; Willumeit-Römer, R. Peri-Implant Gas Accumulation in Response to Magnesium-Based Musculoskeletal Biomaterials: Reframing Current Evidence for Preclinical Research and Clinical Evaluation. J. Magnes. Alloys 2024, 12, 59–71. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kikuta, A. Development of a Model System for Gas Cavity Formation Behavior of Magnesium Alloy Implantation. ACS Biomater. Sci. Eng. 2022, 8, 2437–2444. [Google Scholar] [CrossRef]
- Khoo, X.; O’Toole, G.A.; Nair, S.A.; Snyder, B.D.; Kenan, D.J.; Grinstaff, M.W. Staphylococcus Aureus Resistance on Titanium Coated with Multivalent PEGylated-Peptides. Biomaterials 2010, 31, 9285–9292. [Google Scholar] [CrossRef]
- Huang, J.; Best, S.M.; Bonfield, W.; Buckland, T. Development and Characterization of Titanium-Containing Hydroxyapatite for Medical Applications. Acta Biomater. 2010, 6, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Thomas, K.A.; Kay, J.F.; Jarcho, M. Hydroxyapatite-Coated Titanium for Orthopedic Implant Applications. Clin. Orthop. Relat. Res. 1988, 232, 225–243. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; Ding, C. Bond Strength of Plasma-Sprayed Hydroxyapatite/Ti Composite Coatings. Biomaterials 2000, 21, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tian, G.; Zhi, M.; Liu, Z.; Du, Y.; Lu, X.; Li, M.; Bai, J.; Li, X.; Deng, J.; et al. Functionalized PLGA Microsphere Loaded with Fusion Peptide for Therapy of Bone Defects. ACS Biomater. Sci. Eng. 2024, 10, 2463–2476. [Google Scholar] [CrossRef]
- Bell, B.F.; Schuler, M.; Tosatti, S.; Textor, M.; Schwartz, Z.; Boyan, B.D. Osteoblast Response to Titanium Surfaces Functionalized with Extracellular Matrix Peptide Biomimetics: Osteoblast Response to Ligand-Functionalized Titanium. Clin. Oral. Implant. Res. 2011, 22, 865–872. [Google Scholar] [CrossRef]
- Chausse, V.; Mas-Moruno, C.; Martin-Gómez, H.; Pino, M.; Díaz-Ricart, M.; Escolar, G.; Ginebra, M.-P.; Pegueroles, M. Functionalization of 3D Printed Polymeric Bioresorbable Stents with a Dual Cell-Adhesive Peptidic Platform Combining RGDS and YIGSR Sequences. Biomater. Sci. 2023, 11, 4602–4615. [Google Scholar] [CrossRef]
| Material | Sequence | Refs. |
|---|---|---|
| Ti | RKLPDA | [42,43,44,45,46,47,48] |
| RKLPDAPGMHTW | [42,49,50] | |
| RPRENRGRERGL | [48,50,51,52,53,54,55] | |
| SRPNGYGGSESS | [50,51,55] | |
| HAYKQPVLSTPF | [51] | |
| CGHTHYHAVRTQT | [56,57] | |
| ATWVSPY | [48,58] | |
| Ti-6Al-4V | SVSVGMKPSPRP | [59] |
| WDPPTLKRPVSP | [59] | |
| SHKHPVTPRFFVVESK | [60] | |
| NiTi | NHHMMPAWNVKH | [61] |
| SUS 316 | MTWDPSLASPRS | [62] |
| SUS 316L | VQHNTKYSVVIR | [63] |
| CoCr alloy | TSNLWRYDRLTM | [61] |
| CoCrMo alloy | QHKYTPIHEGRW | [64,65] |
| Au | WALRRSIRRQSY | [66] |
| WAGAKRLVLRRE | [66] | |
| Pt | PTSTGQA | [67] |
| PtW | LTPHKHHKHLHA | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migita, S. Solid-Binding Peptide for Enhancing Biocompatibility of Metallic Biomaterials. SynBio 2024, 2, 329-343. https://doi.org/10.3390/synbio2040020
Migita S. Solid-Binding Peptide for Enhancing Biocompatibility of Metallic Biomaterials. SynBio. 2024; 2(4):329-343. https://doi.org/10.3390/synbio2040020
Chicago/Turabian StyleMigita, Satoshi. 2024. "Solid-Binding Peptide for Enhancing Biocompatibility of Metallic Biomaterials" SynBio 2, no. 4: 329-343. https://doi.org/10.3390/synbio2040020
APA StyleMigita, S. (2024). Solid-Binding Peptide for Enhancing Biocompatibility of Metallic Biomaterials. SynBio, 2(4), 329-343. https://doi.org/10.3390/synbio2040020





