Heterologous Production of Acrylic Acid: Current Challenges and Perspectives
Abstract
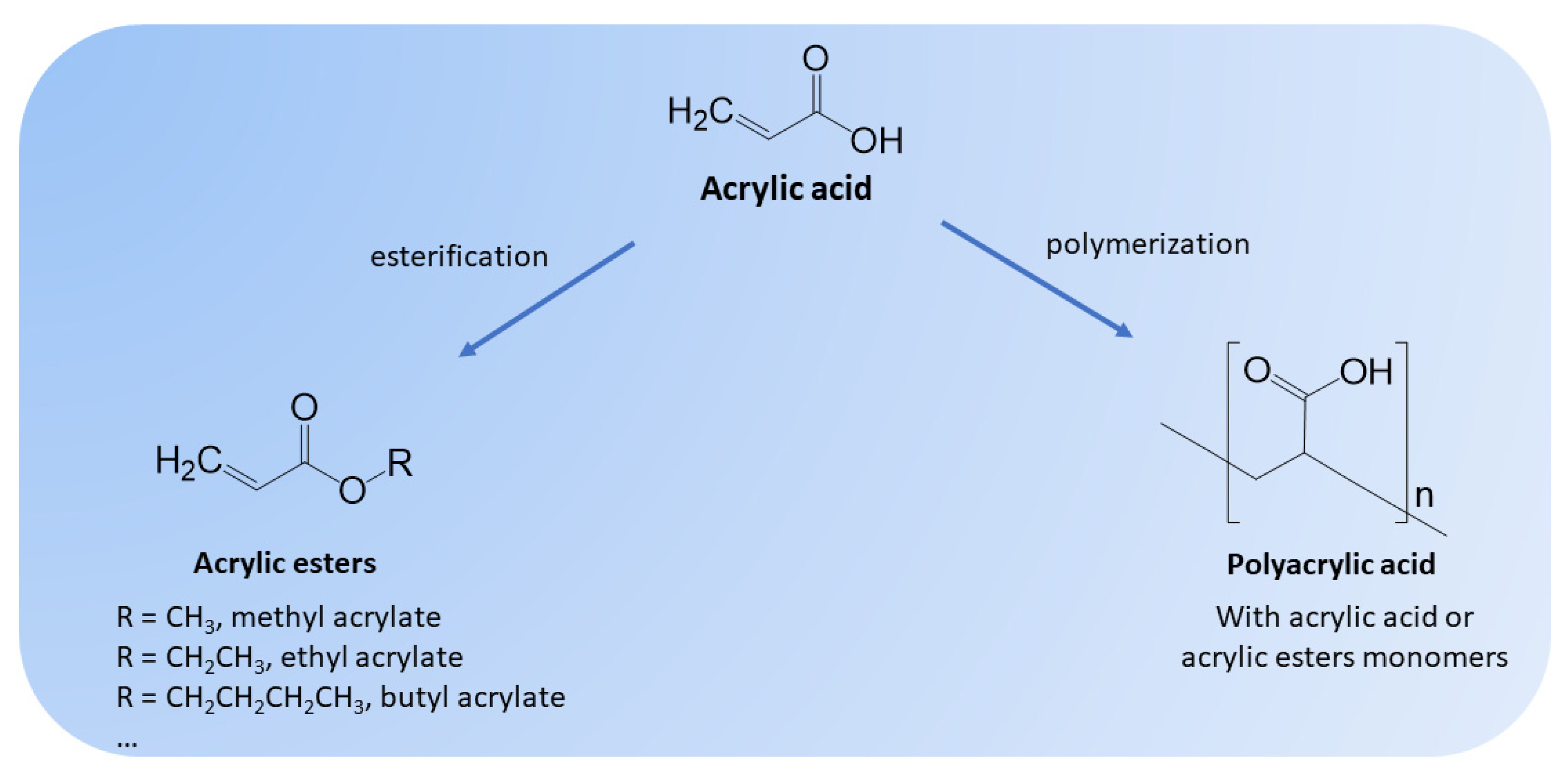
1. Introduction
2. Heterologous Production of 3-HP, a Frequent Intermediary from the AA Pathway
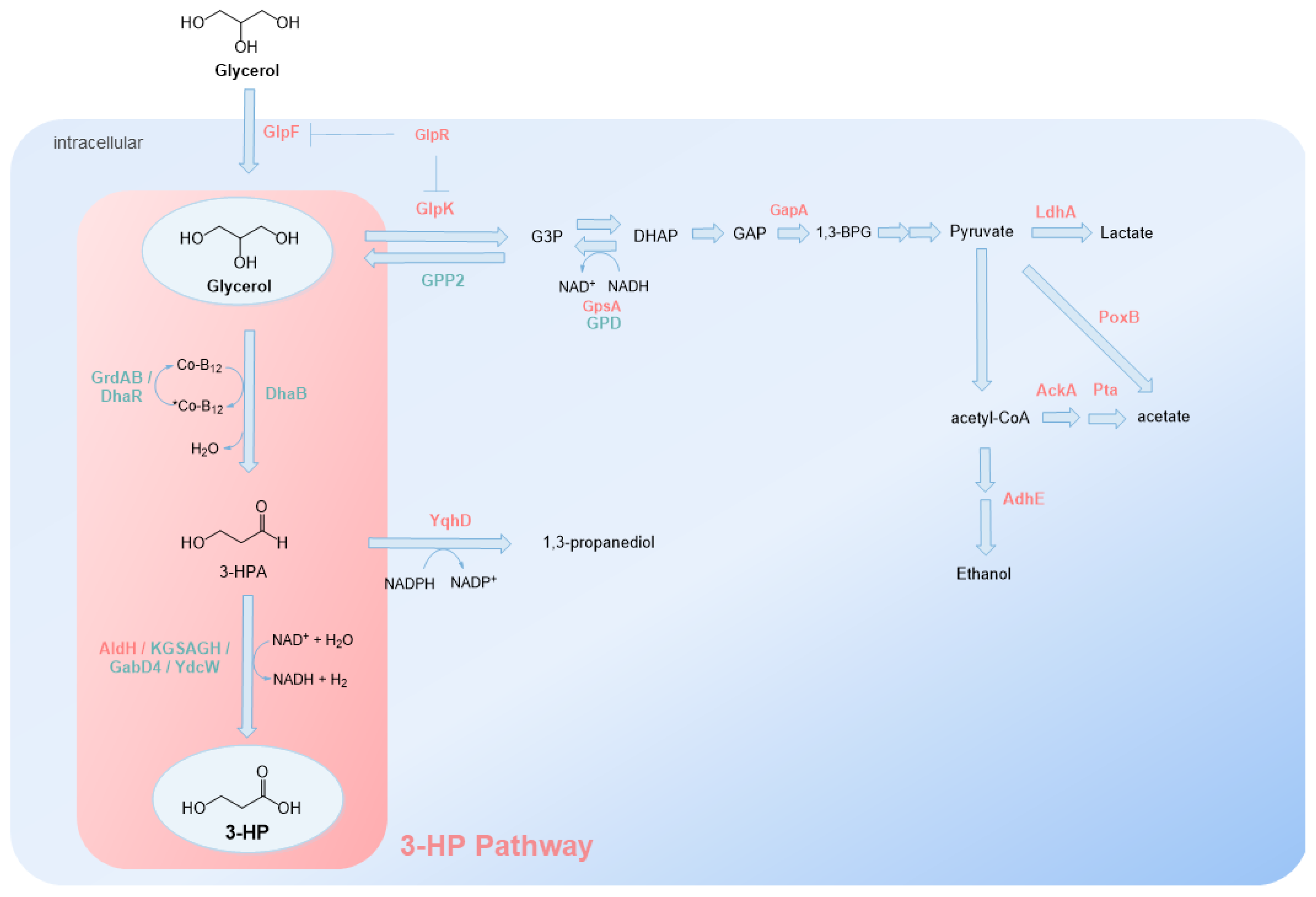
2.1. Glycerol Route
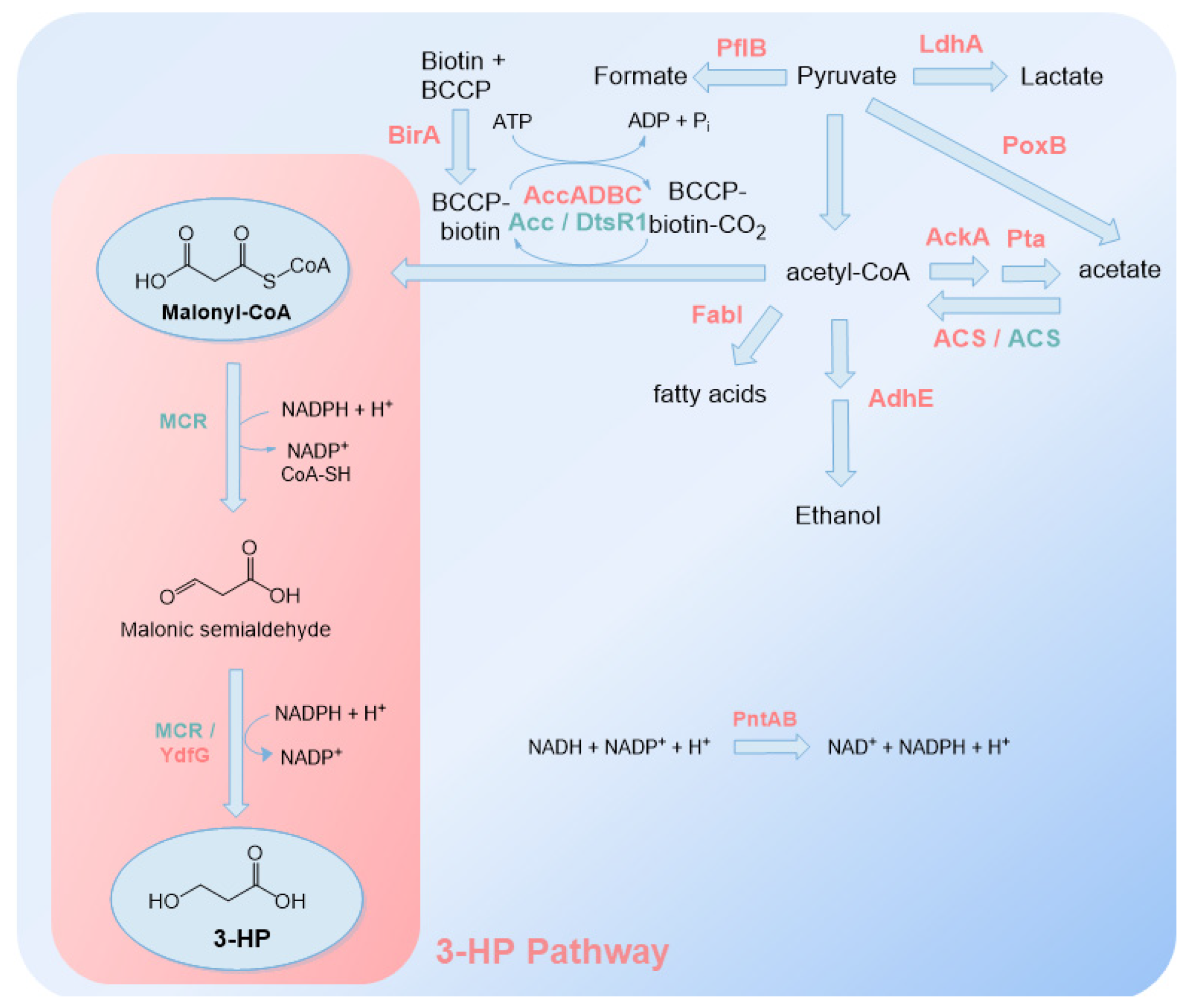
2.2. Malonyl-CoA Route
| Host | Substrate (Concentration) | Pathway Genes Overexpressed in Plasmid | Other Genes Overexpressed in Plasmid | Chassis Modifications | Operational Mode; Fermentation Time | 3-HP Concentration (g/L) 3 | Ref. |
|---|---|---|---|---|---|---|---|
| E. coli BL21 | Glucose (100 mM) | Ca_mcr | Ec_accABCD; Ec_birA; Ec_pntAB | - | Shake-flask (batch); 24 h | 0.193 | [86] |
| E. coli BL21 | Glucose (10 g/L + feed) | Ca_mcr (codon-optimized) | Cg_accBC; Cg_dtsR1 | - | Fed-batch; 36 h | 10.08 | [87] |
| E. coli BL21 | Glucose (20 g/L + feed) | Ca_mcr-c (codon-optimized and mutated) | Ec_accABCD | Ca_mcr-n (3 copies) (codon-optimized) (ΔprpR; ΔmelR; ΔmtlA) | Fed-batch; 72 h | 40.6 | [41] |
| E. coli K12 MG1655 | Glucose (30 g/L + feed) | Ca_mcr (codon-optimized) | Ec_accABCD; Ec_pntAB | ΔldhA; ΔpflB; ΔpoxB; Δpta; ΔackA; ΔmgsA; ΔfabI (at 37 °C) | Fed-batch; 69 h | 49.04 | [43] |
| E. coli BW25113 | Glucose (10 g/L + feed) | Ca_mcr; Ec_ydfG (codon-optimized and mutated) | - | ΔfabI (at 37 °C); ΔldhA; ΔadhE; Δpta; ΔackA; ΔpoxB; ΔpflB; Ec_accABCD mutated; other mutations to increase 3-HP tolerance | Fed-batch; 72 h | 30.0 | [42] |
| S. cerevisiae CEN.PK102-5B | Glucose (22 g/L + feed) | - | - | Ca_mcr; Sc_ALD6; Sc_PDC1; Sc_ACC1 (mutated); Se_ACS; Cla_GAPDH (mutated) (multiple copies) (all genes codon-optimized) | Fed-batch; 100 h | 9.8 | [92] |
| S. pombe A8 | Glucose (20 g/L) and acetate (20 mM) | - | - | Ca_mcr (codon-optimized); cut6p | Shake-flask (batch); 30 h | 7.6 | [93] |
| P. pastoris X-33 | Glycerol (40 g/L + feed) | - | - | Ca_mcr-c; Ca_mcr-n; Yl_ACC; Sc_cPOS5 (all genes codon-optimized) | Fed-batch; 45.5 h | 24.75 | [95] |
2.3. β-Alanine Route
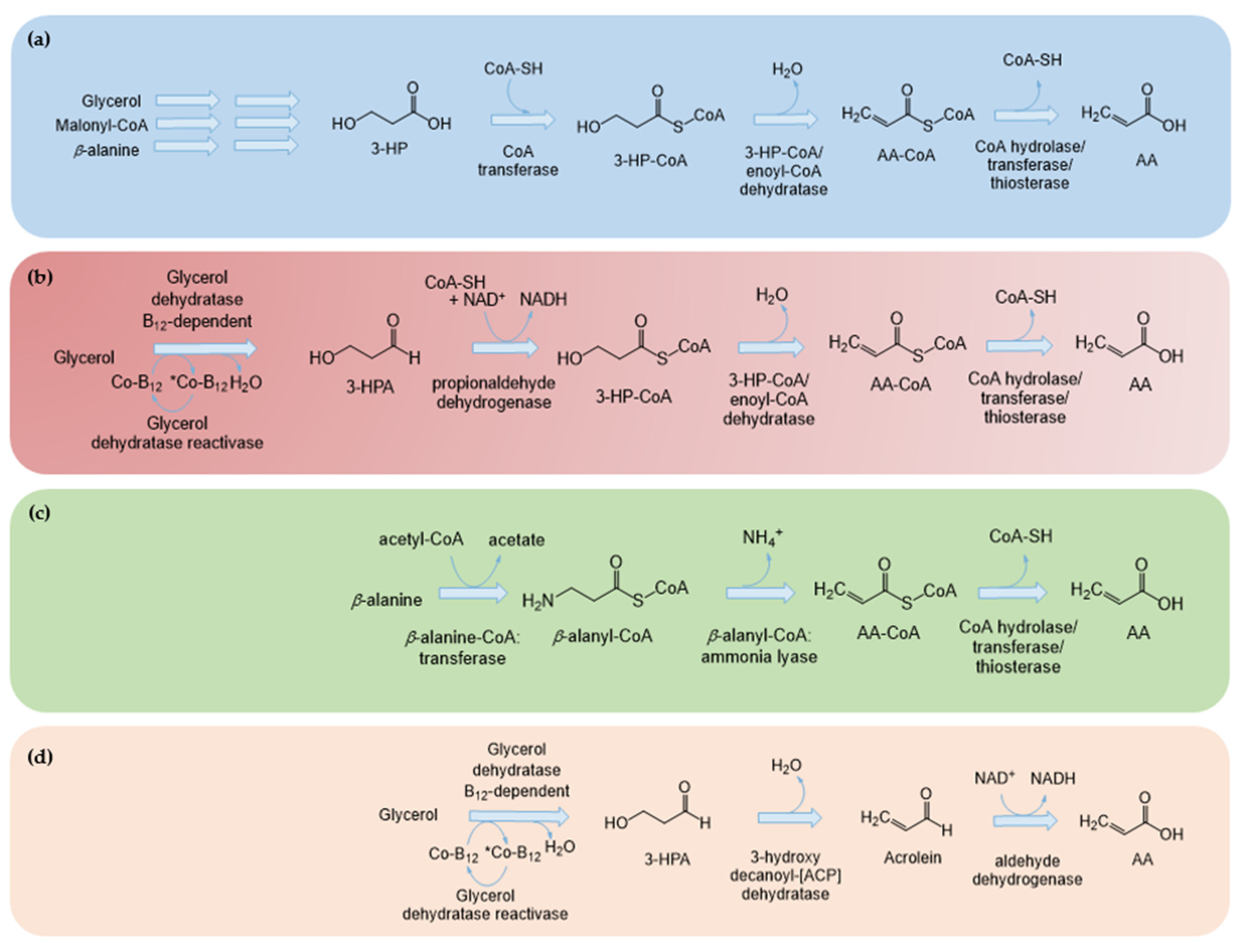
3. Heterologous Production of AA
3.1. Using 3-HP as an Intermediary
3.2. Without 3-HP as an Intermediary
4. Key Points to Optimize AA Heterologous Production
4.1. Testing a B12-Independent Enzyme in the Glycerol Route
4.2. Unravelling More Efficient Enzymes and Pathways
4.3. Improving Chassis Tolerance to AA
5. Concluding Remarks and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Straathof, A.J.J.; Sie, S.; Franco, T.T.; van der Wielen, L.A.M. Feasibility of acrylic acid production by fermentation. Appl. Microbiol. Biotechnol. 2005, 67, 727–734. [Google Scholar] [CrossRef]
- Tong, W.; Xu, Y.; Xian, M.; Niu, W.; Guo, J.; Liu, H.; Zhao, G. Biosynthetic pathway for acrylic acid from glycerol in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 4901–4907. [Google Scholar] [CrossRef]
- Reportsand Data Acrylic Acid Market by Product, by Acrylic Polymer (Acrylic Elastomers, Super Absorbent Polymers, Water Treatment Polymer) and by Application (Surfactants, Organic Chemicals, Adhesives & Sealants, Textiles, Water Treatment, Personal Care Products). Available online: https://www.reportsanddata.com/report-detail/acrylic-acid-market (accessed on 17 March 2022).
- Allied Market Research Acrylic Acid Market by Derivative Type (Acrylic Esters, Acrylic Polymer, and Others), and End-User (Diapers, Surface Coatings Industry, Adhesives and Sealants Industry, Plastic Additives Industry, Water Treatment Industry, Textiles Industry, Surfactants. Available online: https://www.alliedmarketresearch.com/acrylic-acid-market (accessed on 17 March 2022).
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Eliot, D.; Lasure, L.; Jones, S. Top Value Added Chemicals from Biomass. Volume 1-Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Trifirò, F. Acrolein and acrylic acid from biomass. Rend. Lincei 2017, 28, 59–67. [Google Scholar] [CrossRef]
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Bhagwat, S.S.; Li, Y.; Cortés-Peña, Y.R.; Brace, E.C.; Martin, T.A.; Zhao, H.; Guest, J.S. Sustainable production of acrylic acid via 3-hydroxypropionic acid from lignocellulosic biomass. ACS Sustain. Chem. Eng. 2021, 9, 16659–16669. [Google Scholar] [CrossRef]
- Dishisha, T.; Pyo, S.-H.; Hatti-Kaul, R. Bio-based 3-hydroxypropionic- and acrylic acid production from biodiesel glycerol via integrated microbial and chemical catalysis. Microb. Cell Fact. 2015, 14, 200. [Google Scholar] [CrossRef]
- Couto, M.R.; Rodrigues, J.L.; Rodrigues, L.R. Cloning, expression and characterization of UDP-glucose dehydrogenases. Life 2021, 11, 1201. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Cardoso, B.B.; Silvério, S.C.; Rodrigues, J.L.; Rodrigues, L.R. Epilactose biosynthesis using recombinant cellobiose 2-epimerase produced by Saccharomyces cerevisiae. ACS Food Sci. Technol. 2021, 1, 1578–1584. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Amorim, C.; Braga, A.; Costa, A.; Rodrigues, J.L.; Silvério, S.; Rodrigues, L.R. Biotech Green Approaches to Unravel the Potential of Residues into Valuable Products. In Green Chemistry for the Sustainable Development of Chemical Industry; Inamuddin, Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 97–150. [Google Scholar]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of caffeic acid from tyrosine in Escherichia coli. Enzyme Microb. Technol. 2015, 71, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Production of curcuminoids from tyrosine by a metabolically engineered Escherichia coli using caffeic acid as an intermediate. Biotechnol. J. 2015, 10, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Rainha, J.; Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable polyphenolic compounds. Life 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.R.; Rodrigues, J.L.; Rodrigues, L.R. Heterologous production of chondroitin. Biotechnol. Rep. 2022, 33, e00710. [Google Scholar] [CrossRef]
- Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Perspectives on the design of microbial cell factories to produce prenylflavonoids. Int. J. Food Microbiol. 2022, 367, 109588. [Google Scholar] [CrossRef] [PubMed]
- Costa, Â.M.A.; Santos, A.O.; Sousa, J.; Rodrigues, J.L.; Gudiña, E.J.; Silvério, S.C.; Rodrigues, L.R. Improved method for the extraction of high-quality DNA from lignocellulosic compost samples for metagenomic studies. Appl. Microbiol. Biotechnol. 2021, 105, 8881–8893. [Google Scholar] [CrossRef]
- Braga, A.; Gomes, D.; Rainha, J.; Amorim, C.; Cardoso, B.B.; Gudiña, E.J.; Silvério, S.C.; Rodrigues, J.L.; Rodrigues, L.R. Zymomonas mobilis as an emerging biotechnological chassis for the production of industrially relevant compounds. Bioresour. Bioprocess. 2021, 8, 128. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Biosynthesis and heterologous production of furanocoumarins: Perspectives and current challenges. Nat. Prod. Rep. 2021, 38, 869–879. [Google Scholar] [CrossRef]
- Gomes, D.; Rainha, J.; Rodrigues, L.R.; Rodrigues, J.L. Yeast synthetic biology approaches for the production of valuable polyphenolic compounds. In Synthetic Biology of Yeasts; Harzevili, F., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 119–156. [Google Scholar]
- Rainha, J.; Rodrigues, J.L.; Rodrigues, L.R. CRISPR-Cas9: A powerful tool to efficiently engineer Saccharomyces cerevisiae. Life 2021, 11, 13. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Synthetic biology: Perspectives in industrial biotechnology. In Current Developments in Biotechnology and Bioengineering: Foundations of Biotechnology and Bioengineering; Pandey, A., Teixeira, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–269. ISBN 9780444636799. [Google Scholar]
- Nam, T.; Song, Y.; Song, S.; Yun, J.; Lee, J.; Chu, H.; Choi, I. Microorganism Having Novel Acrylic Acid Synthesis Pathway Having Enhanced Activity of Coa Acylating Aldehyde Dehydrogenase and Method of Producing Acrylic Acid Using the Same. U.S. Patent Application No. 14/620,002, 14 January 2016. [Google Scholar]
- Valle, F.; Agard, N.; Noriega, C. Direct Biocatalytic Production of Acrylic Acid and Other Carboxylic Acid Compounds. U.S. Patent Application No. 14/345,495, 20 November 2014. [Google Scholar]
- Chu, H.; Ahn, J.; Nam, T.; Yun, J.; Choi, I.; Song, Y.; Lee, J. Microorganism Having Novel Acrylic Acid Synthesis Pathway and Method of Producing Acrylic Acid by Using the Microorganism. U.S. Patent No. 9,506,089, 29 November 2016. [Google Scholar]
- Lee, S.Y.; Ko, Y.S.; Song, C.W.; Kim, J.W. Recombinant Mutant Microorganisms Having Acrylic Acid Productivity and Method for Producing Acrylic Acid Using Same. European Patent EP3406724A1, 17 January 2017. [Google Scholar]
- Liu, Z.; Liu, T. Production of acrylic acid and propionic acid by constructing a portion of the 3-hydroxypropionate/4-hydroxybutyrate cycle from Metallosphaera sedula in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2016, 43, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.S.; Ahn, J.-H.; Yun, J.; Choi, I.S.; Nam, T.-W.; Cho, K.M. Direct fermentation route for the production of acrylic acid. Metab. Eng. 2015, 32, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.K.; Church, G.M. Genetically encoded sensors enable real-time observation of metabolite production. Proc. Natl. Acad. Sci. USA 2016, 113, 2388–2393. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Kim, J.W.; Chae, T.U.; Song, C.W.; Lee, S.Y. A novel biosynthetic pathway for the production of acrylic acid through β-alanine route in Escherichia coli. ACS Synth. Biol. 2020, 9, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.S.; Kim, Y.S.; Lee, C.M.; Lee, J.H.; Jung, W.S.; Ahn, J.; Song, S.H.; Choi, I.S.; Cho, K.M. Metabolic engineering of 3-hydroxypropionic acid biosynthesis in Escherichia coli. Biotechnol. Bioeng. 2015, 112, 356–364. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, Y.; Chae, T.U.; Lee, S.Y. High-level production of 3-hydroxypropionic acid from glycerol as a sole carbon source using metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2020, 117, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Seol, E.; Sekar, B.S.; Kwon, S.; Lee, J.; Park, S. Metabolic engineering of Klebsiella pneumoniae J2B for co-production of 3-hydroxypropionic acid and 1, 3-propanediol from glycerol: Reduction of acetate and other by-products. Bioresour. Technol. 2017, 244, 1096–1103. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Min, W.-K.; Kim, H.J.; Seo, J.-H. Improved production of 3-hydroxypropionic acid in engineered Escherichia coli by rebalancing heterologous and endogenous synthetic pathways. Bioresour. Technol. 2020, 299, 122600. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ge, X.; Tian, P. High production of 3-hydroxypropionic acid in Klebsiella pneumoniae by systematic optimization of glycerol metabolism. Sci. Rep. 2016, 6, 26932. [Google Scholar] [CrossRef]
- Lis, A.V.; Schneider, K.; Weber, J.; Keasling, J.D.; Jensen, M.K.; Klein, T. Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae. Microb. Cell Fact. 2019, 18, 50. [Google Scholar] [CrossRef]
- Liu, C.; Ding, Y.; Zhang, R.; Liu, H.; Xian, M.; Zhao, G. Functional balance between enzymes in malonyl-CoA pathway for 3-hydroxypropionate biosynthesis. Metab. Eng. 2016, 34, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, L.; Choudhury, A.; Bassalo, M.C.; Garst, A.D.; Tarasava, K.; Gill, R.T. Iterative genome editing of Escherichia coli for 3-hydroxypropionic acid production. Metab. Eng. 2018, 47, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.D.; Gill, R.T.; Lipscomb, T.E.W. Method for Producing 3-Hydroxypropionic Acid and Other Products. U.S. Patent 10,100,342, 27 May 2016. [Google Scholar]
- Park, S.; Shengfang, Z.; Ashok, S.; Seol, E.H.; Ainala, S.K. Promoter System Inducing Expression by 3-Hydroxypropionic Acid and Method for Biological Production of 3-Hydroxypropionic Acid Using Same. U.S. Patent No. 10,808,255, 20 October 2020. [Google Scholar]
- Shim, J.Y.; Park, K.S.; Somasundar, A.; Park, S.H. Production and Separation of 3-Hydroxypropionic Acid. U.S. Patent Application No. 16/858,065, 12 November 2020. [Google Scholar]
- Song, C.W.; Kim, J.W.; Cho, I.J.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of 3-hydroxypropionic acid and malonic acid through β-alanine route. ACS Synth. Biol. 2016, 5, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ma, C.; Xu, L.; Tian, P. Exploiting tandem repetitive promoters for high-level production of 3-hydroxypropionic acid. Appl. Microbiol. Biotechnol. 2019, 103, 4017–4031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zabed, H.M.; Yun, J.; Zhang, G.; Wang, Y.; Qi, X. Notable improvement of 3-hydroxypropionic acid and 1,3-propanediol coproduction using modular coculture engineering and pathway rebalancing. ACS Sustain. Chem. Eng. 2021, 9, 4625–4637. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Wu, Y.; Wu, W.; Zhang, Y.; Liu, D. Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose. Metab. Eng. 2017, 39, 151–158. [Google Scholar] [CrossRef]
- Sankaranarayanan, M.; Eunhee, S.; Kwon, S.; Sunghoon, P. Production of 3-hydroxypropionic acid by balancing the pathway enzymes using synthetic cassette architecture. J. Biotechnol. 2017, 259, 140–147. [Google Scholar] [CrossRef]
- Heo, W.; Kim, J.H.; Kim, S.; Kim, K.H.; Kim, H.J.; Seo, J.-H. Enhanced production of 3-hydroxypropionic acid from glucose and xylose by alleviation of metabolic congestion due to glycerol flux in engineered Escherichia coli. Bioresour. Technol. 2019, 285, 121320. [Google Scholar] [CrossRef]
- Sankaranarayanan, M.; Ashok, S.; Park, S. Production of 3-hydroxypropionic acid from glycerol by acid tolerant Escherichia coli. J. Ind. Microbiol. Biotechnol. 2014, 41, 1039–1050. [Google Scholar] [CrossRef]
- Lim, H.G.; Noh, M.H.; Jeong, J.H.; Park, S.; Jung, G.Y. Optimum rebalancing of the 3-hydroxypropionic acid production pathway from glycerol in Escherichia coli. ACS Synth. Biol. 2016, 5, 1247–1255. [Google Scholar] [CrossRef]
- Novozymes Novozymes, Cargill Continue Bio-Acrylic Acid Partnership as BASF Exits. Available online: https://www.novozymes.com/en/news/news-archive/2015/01/novozymes-cargill-continue-bio-acrylic-acid-partnership-basf-exits (accessed on 13 April 2022).
- Novozymes BASF, Cargill and Novozymes Achieve Milestone in Bio-Based Acrylic Acid Process. Available online: https://www.novozymes.com/en/news/news-archive/2013/07/basf_-cargill-and-novozymes-achieve-milestone (accessed on 13 April 2022).
- Lynch, M.D.; Gill, R.T.; Lipscomb, T.E.W. Methods for Producing 3-Hydroxypropionic Acid and Other Products. U.S. Patent 8883464B2, 18 February 2014. [Google Scholar]
- Lynch, M.D.; Gill, R.T.; Lipscomb, T.E.W. Method for Producing 3-Hydroxypropionic Acid and Other Products. U.S. Patent 9388419B2, 12 July 2016. [Google Scholar]
- Kumar, V.; Ashok, S.; Park, S. Recent advances in biological production of 3-hydroxypropionic acid. Biotechnol. Adv. 2013, 31, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Garai-Ibabe, G.; Ibarburu, I.; Berregi, I.; Claisse, O.; Lonvaud-Funel, A.; Irastorza, A.; Dueñas, M. Glycerol metabolism and bitterness producing lactic acid bacteria in cidermaking. Int. J. Food Microbiol. 2008, 121, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Zhang, Y.; Guo, Q.; Yun, J.; Yang, M.; Zhang, G.; Qi, X. Co-biosynthesis of 3-hydroxypropionic acid and 1,3-propanediol by a newly isolated Lactobacillus reuteri strain during whole cell biotransformation of glycerol. J. Clean. Prod. 2019, 226, 432–442. [Google Scholar] [CrossRef]
- Ansede, J.H.; Pellechia, P.J.; Yoch, D.C. Metabolism of acrylate to β-hydroxypropionate and its role in dimethylsulfoniopropionate lyase induction by a salt marsh sediment bacterium, Alcaligenes faecalis M3A. Appl. Environ. Microbiol. 1999, 65, 5075–5081. [Google Scholar] [CrossRef] [PubMed]
- Berg, I.A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Quiroga-Sánchez, D.L.; Zhang, X.; Chang, Y.; Luo, H. Coupled synthetic pathways improve the production of 3-hydroxypropionic acid in recombinant Escherichia coli strains. Biotechnol. Notes 2022, 3, 25–31. [Google Scholar] [CrossRef]
- Zhao, P.; Tian, P. Biosynthesis pathways and strategies for improving 3-hydroxypropionic acid production in bacteria. World J. Microbiol. Biotechnol. 2021, 37, 117. [Google Scholar] [CrossRef]
- Tong, T.; Tao, Z.; Chen, X.; Gao, C.; Liu, H.; Wang, X.; Liu, G.-Q.; Liu, L. A biosynthesis pathway for 3-hydroxypropionic acid production in genetically engineered Saccharomyces cerevisiae. Green Chem. 2021, 23, 4502–4509. [Google Scholar] [CrossRef]
- de Fouchécour, F.; Sánchez-Castañeda, A.-K.; Saulou-Bérion, C.; Spinnler, H.É. Process engineering for microbial production of 3-hydroxypropionic acid. Biotechnol. Adv. 2018, 36, 1207–1222. [Google Scholar] [CrossRef]
- Mohan Raj, S.; Rathnasingh, C.; Jung, W.-C.; Park, S. Effect of process parameters on 3-hydroxypropionic acid production from glycerol using a recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 84, 649–657. [Google Scholar] [CrossRef]
- Rathnasingh, C.; Raj, S.M.; Jo, J.-E.; Park, S. Development and evaluation of efficient recombinant Escherichia coli strains for the production of 3-hydroxypropionic acid from glycerol. Biotechnol. Bioeng. 2009, 104, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kang, J.H.; Chu, H.S.; Choi, I.S.; Cho, K.M. Elevated production of 3-hydroxypropionic acid by metabolic engineering of the glycerol metabolism in Escherichia coli. Metab. Eng. 2014, 23, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Tsuruno, K.; Honjo, H.; Hanai, T. Enhancement of 3-hydroxypropionic acid production from glycerol by using a metabolic toggle switch. Microb. Cell Fact. 2015, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Yeh, I.; Sung, L.; Wu, M.; Chao, Y.; Ng, I.; Hu, Y. Enhanced integration of large DNA into E. coli chromosome by CRISPR/Cas9. Biotechnol. Bioeng. 2017, 114, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.M.; Rathnasingh, C.; Jo, J.-E.; Park, S. Production of 3-hydroxypropionic acid from glycerol by a novel recombinant Escherichia coli BL21 strain. Process Biochem. 2008, 43, 1440–1446. [Google Scholar] [CrossRef]
- Kwak, S.; Park, Y.-C.; Seo, J.-H. Biosynthesis of 3-hydroxypropionic acid from glycerol in recombinant Escherichia coli expressing Lactobacillus brevis dhaB and dhaR gene clusters and E. coli K-12 aldH. Bioresour. Technol. 2013, 135, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Xiong, T.; Qin, H.; Wu, H.; Liu, Z.; Zheng, Y. 3-Hydroxypropionic acid production by recombinant Escherichia coli ZJU-3HP01 using glycerol–glucose dual-substrate fermentative strategy. Biotechnol. Appl. Biochem. 2017, 64, 572–578. [Google Scholar] [CrossRef]
- Market Data Forecast Biodiesel Market—By Feedstock Type (Vegetable Oils, Animal Fats, Brown Grease/Trap Grease), Application (Fuel, Power Generation) & by Region (North America, Latin America, Europe, Asia Pacific, Middle East & Africa)—Global Industry Analysis, Size. Available online: https://www.marketdataforecast.com/market-reports/biodiesel-market (accessed on 21 March 2022).
- IndexBox The global Biodiesel Market Retains Robust Growth Despite the Pandemic and Low Oil Prices. Available online: https://www.globaltrademag.com/the-global-biodiesel-market-retains-robust-growth-despite-the-pandemic-and-low-oil-prices/ (accessed on 21 March 2022).
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, U.J.; Nam, N.H.; Choi, S.J.; Nasir, A.; Lee, S.-G.; Kim, K.J.; Jung, G.Y.; Choi, S.; Shim, J.Y.; et al. Engineering an aldehyde dehydrogenase toward its substrates, 3-hydroxypropanal and NAD+, for enhancing the production of 3-hydroxypropionic acid. Sci. Rep. 2017, 7, 17155. [Google Scholar] [CrossRef]
- Wei, X.; Meng, X.; Chen, Y.; Wei, Y.; Du, L.; Huang, R. Cloning, expression, and characterization of coenzyme-B12-dependent diol dehydratase from Lactobacillus diolivorans. Biotechnol. Lett. 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Liu, D. Regulation of 3-hydroxypropionaldehyde accumulation in Klebsiella pneumoniae by overexpression of dhaT and dhaD genes. Enzyme Microb. Technol. 2009, 45, 305–309. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, W.; Tian, P. Development of cyclic AMP receptor protein-based artificial transcription factor for intensifying gene expression. Appl. Microbiol. Biotechnol. 2018, 102, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.-K.; Park, Y.-C.; Seo, J.-H. Enhanced production of 3-hydroxypropionic acid from glycerol by modulation of glycerol metabolism in recombinant Escherichia coli. Bioresour. Technol. 2014, 156, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-Y.; Lee, J.-W.; Min, W.-K.; Park, Y.-C.; Seo, J.-H. Simultaneous conversion of glucose and xylose to 3-hydroxypropionic acid in engineered Escherichia coli by modulation of sugar transport and glycerol synthesis. Bioresour. Technol. 2015, 198, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Ferreira, D.; Rodrigues, L.R. Synthetic biology strategies towards the development of new bioinspired technologies for medical applications. In Bioinspired Materials for Medical Applications; Mohapatra, S., Ranjan, S., Dasgupta, N., Mishra, R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 451–497. ISBN 9780081007464. [Google Scholar]
- Kalantari, A.; Chen, T.; Ji, B.; Stancik, I.A.; Ravikumar, V.; Franjevic, D.; Saulou-Bérion, C.; Goelzer, A.; Mijakovic, I. Conversion of glycerol to 3-hydroxypropanoic acid by genetically engineered Bacillus subtilis. Front. Microbiol. 2017, 8, 638. [Google Scholar] [CrossRef]
- Rathnasingh, C.; Raj, S.M.; Lee, Y.; Catherine, C.; Ashok, S.; Park, S. Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J. Biotechnol. 2012, 157, 633–640. [Google Scholar] [CrossRef]
- Cheng, Z.; Jiang, J.; Wu, H.; Li, Z.; Ye, Q. Enhanced production of 3-hydroxypropionic acid from glucose via malonyl-CoA pathway by engineered Escherichia coli. Bioresour. Technol. 2016, 200, 897–904. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of curcuminoids. Microbiol. Mol. Biol. Rev. 2015, 79, 39–60. [Google Scholar] [CrossRef]
- Rainha, J.; Rodrigues, J.L.; Faria, C.; Rodrigues, L.R. Curcumin biosynthesis from ferulic acid by engineered Saccharomyces cerevisiae. Biotechnol. J. 2021, 17, 2100400. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Gomes, D.; Rodrigues, L.R. A combinatorial approach to optimize the production of curcuminoids from tyrosine in Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 59. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Xian, M.; Ding, Y.; Zhao, G. Dissection of malonyl-coenzyme A reductase of Chloroflexus aurantiacus results in enzyme activity improvement. PLoS ONE 2013, 8, e75554. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Jensen, N.B.; Schneider, K.; Czarnotta, E.; Özdemir, E.; Klein, T.; Maury, J.; Ebert, B.E.; Christensen, H.B.; Chen, Y. Engineering and systems-level analysis of Saccharomyces cerevisiae for production of 3-hydroxypropionic acid via malonyl-CoA reductase-dependent pathway. Microb. Cell Fact. 2016, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Suyama, A.; Higuchi, Y.; Urushihara, M.; Maeda, Y.; Takegawa, K. Production of 3-hydroxypropionic acid via the malonyl-CoA pathway using recombinant fission yeast strains. J. Biosci. Bioeng. 2017, 124, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bao, J.; Kim, I.-K.; Siewers, V.; Nielsen, J. Coupled incremental precursor and co-factor supply improves 3-hydroxypropionic acid production in Saccharomyces cerevisiae. Metab. Eng. 2014, 22, 104–109. [Google Scholar] [CrossRef]
- Fina, A.; Brêda, G.C.; Pérez-Trujillo, M.; Freire, D.M.G.; Almeida, R.V.; Albiol, J.; Ferrer, P. Benchmarking recombinant Pichia pastoris for 3-hydroxypropionic acid production from glycerol. Microb. Biotechnol. 2021, 14, 1671–1682. [Google Scholar] [CrossRef]
- Jensen, N.; Borodina, I.; Chen, Y.; Maury, J.; Kildegaard, K.; Forster, J.; Nielsen, J. Microbial Production of 3-Hydroxypropionic Acid. Patent WO 2014/198831 A1, 12 June 2014. [Google Scholar]
- Çelik, E.; Ozbay, N.; Oktar, N.; Çalık, P. Use of biodiesel byproduct crude glycerol as the carbon source for fermentation processes by recombinant Pichia pastoris. Ind. Eng. Chem. Res. 2008, 47, 2985–2990. [Google Scholar] [CrossRef]
- Borodina, I.; Kildegaard, K.R.; Jensen, N.B.; Blicher, T.H.; Maury, J.; Sherstyk, S.; Schneider, K.; Lamosa, P.; Herrgård, M.J.; Rosenstand, I. Establishing a synthetic pathway for high-level production of 3-hydroxypropionic acid in Saccharomyces cerevisiae via β-alanine. Metab. Eng. 2015, 27, 57–64. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Wang, Z.; Chen, Y.; Nielsen, J.; Borodina, I. Production of 3-hydroxypropionic acid from glucose and xylose by metabolically engineered Saccharomyces cerevisiae. Metab. Eng. Commun. 2015, 2, 132–136. [Google Scholar] [CrossRef]
- Liao, H.; Gokarn, R.; Gort, S.; Jessen, J.; Selifonova, O.V. Production of 3-Hydroxypropionic Acid Using Beta-Alanine/Pyruvate Aminotransferase. U.S. Patent 8,124,388 B2, 28 February 2012. [Google Scholar]
- Jessen, H.; Rush, B.; Huryta, J.; Mastel, B.; Berry, A.; Yaver, D.; Catlett, M.; Barnhardt, M. Compositions and Methods for 3-Hydroxypropionic Acid Production. Patent WO 2012/074818 A2, 7 June 2012. [Google Scholar]
- Liao, H.; Gokarn, R.; Gort, S.; Jessen, H.; Selifonova, O. Production of 3-Hydroxypropionic Acid Using Beta-Alanine/Pyruvate Aminotransferase. Patent WO 2005/118719 A2, 15 December 2005. [Google Scholar]
- Barnhart, M.; Negrete-Raymond, A.; Frias, J.; Barbier, G.; Catlett, M. 3-Hydroxypropionic Acid Production by Recombinant Yeasts Expressing an Insect Aspartate 1-Decarboxylase. Patent WO 2015/017721 A1, 5 February 2015. [Google Scholar]
- Oliveira, A.; Rodrigues, J.; Ferreira, E.; Rodrigues, L.; Dias, O. A kinetic model of the central carbon metabolism for acrylic acid production in Escherichia coli. PLoS Comput. Biol. 2021, 17, e1008704. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Ro, K.-S.; Xie, J.; Wei, D. Discovery of a novel acrylic acid formation pathway in Gluconobacter oxydans and its application in biosynthesis of acrylic acid from glycerol. Process Biochem. 2022, 118, 182–189. [Google Scholar] [CrossRef]
- Ishii, M.; Chuakrut, S.; Arai, H.; Igarashi, Y. Occurrence, biochemistry and possible biotechnological application of the 3-hydroxypropionate cycle. Appl. Microbiol. Biotechnol. 2004, 64, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Alber, B.E.; Fuchs, G. Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J. Biol. Chem. 2002, 277, 12137–12143. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, C.; Ding, Y.; Sun, C.; Zhang, R.; Xian, M.; Zhao, G. Development of genetically stable Escherichia coli strains for poly(3-hydroxypropionate) production. PLoS ONE 2014, 9, e97845. [Google Scholar] [CrossRef] [PubMed]
- Asao, M.; Alber, B.E. Acrylyl-coenzyme A reductase, an enzyme involved in the assimilation of 3-hydroxypropionate by Rhodobacter sphaeroides. J. Bacteriol. 2013, 195, 4716–4725. [Google Scholar] [CrossRef][Green Version]
- Arya, A.S.; Lee, S.A.; Eiteman, M.A. Differential sensitivities of the growth of Escherichia coli to acrylate under aerobic and anaerobic conditions and its effect on product formation. Biotechnol. Lett. 2013, 35, 1839–1843. [Google Scholar] [CrossRef]
- Noh, M.H.; Lim, H.G.; Moon, D.; Park, S.; Jung, G.Y. Auxotrophic selection strategy for improved production of coenzyme B12 in Escherichia coli. iScience 2020, 23, 100890. [Google Scholar] [CrossRef]
- Fang, H.; Li, D.; Kang, J.; Jiang, P.; Sun, J.; Zhang, D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018, 9, 4917. [Google Scholar] [CrossRef]
- Yun, J.; Zabed, H.M.; Zhang, Y.; Parvez, A.; Zhang, G.; Qi, X. Co-fermentation of glycerol and glucose by a co-culture system of engineered Escherichia coli strains for 1, 3-propanediol production without vitamin B12 supplementation. Bioresour. Technol. 2021, 319, 124218. [Google Scholar] [CrossRef]
- Raynaud, C.; Sarçabal, P.; Meynial-Salles, I.; Croux, C.; Soucaille, P. Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Proc. Natl. Acad. Sci. USA 2003, 100, 5010–5015. [Google Scholar] [CrossRef]
- Tang, X.; Tan, Y.; Zhu, H.; Zhao, K.; Shen, W. Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 1628–1634. [Google Scholar] [CrossRef]
- O’Brien, J.R.; Raynaud, C.; Croux, C.; Girbal, L.; Soucaille, P.; Lanzilotta, W.N. Insight into the mechanism of the B12-independent glycerol dehydratase from Clostridium butyricum: Preliminary biochemical and structural characterization. Biochemistry 2004, 43, 4635–4645. [Google Scholar] [CrossRef] [PubMed]
- Saint-Amans, S.; Girbal, L.; Andrade, J.; Ahrens, K.; Soucaille, P. Regulation of carbon and electron flow in Clostridium butyricum VPI 3266 grown on glucose-glycerol mixtures. J. Bacteriol. 2001, 183, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, W.; Chistoserdov, A.; Bajpai, R.K. Glycerol dehydratases: Biochemical structures, catalytic mechanisms, and industrial applications in 1,3-propanediol production by naturally occurring and genetically engineered bacterial strains. Appl. Biochem. Biotechnol. 2016, 179, 1073–1100. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Jeong, W.-S.; Choi, I.-S. Microbial Conversion of Glycerol. Patent KR20150025897A, 11 March 2015. [Google Scholar]
- Kim, N.-J.; Choi, J.H.; Kim, Y.C.; Lee, J.; Lee, S.Y.; Chang, H.N.; Lee, P.C. Development of anaerobically inducible nar promoter expression vectors for the expression of recombinant proteins in Escherichia coli. J. Biotechnol. 2011, 151, 102–107. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Sousa, M.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Selection of Escherichia coli heat shock promoters toward their application as stress probes. J. Biotechnol. 2014, 188, 61–71. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Couto, M.R.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.; Rodrigues, L.R. Hydroxycinnamic acids and curcumin production in engineered Escherichia coli using heat shock promoters. Biochem. Eng. J. 2017, 125, 41–49. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Potential applications of the Escherichia coli heat shock response in synthetic biology. Trends Biotechnol. 2018, 36, 186–198. [Google Scholar] [CrossRef]
- Halperin, S.O.; Tou, C.J.; Wong, E.B.; Modavi, C.; Schaffer, D.V.; Dueber, J.E. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window. Nature 2018, 560, 248–252. [Google Scholar] [CrossRef]
- Schweiger, G.; Buckel, W. Identification of acrylate, the product of the dehydration of (R)-lactate catalysed by cell-free extracts from Clostridium propionicum. FEBS Lett. 1985, 185, 253–256. [Google Scholar] [CrossRef]
- Seeliger, S.; Janssen, P.H.; Schink, B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 2002, 211, 65–70. [Google Scholar] [CrossRef]
- Akedo, M.; Cooney, C.L.; Sinskey, A.J. Direct demonstration of lactate–acrylate interconversion in Clostridium propionicum. Bio/Technol. 1983, 1, 791–794. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, D.; Liu, X.; Nie, Z.; Quiroga-Sánchez, D.L.; Chang, Y. Production of 3-hydroxypropionic acid via the propionyl-CoA pathway using recombinant Escherichia coli strains. PLoS ONE 2016, 11, e0156286. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cai, J.; Zhu, L.; Zhu, X.; Huang, L.; Xu, Z.; Cen, P. Toxic effects of acrylic acid on Clostridium propionicum and isolation of acrylic acid-tolerant mutants for production of acrylic acid. Eng. Life Sci. 2012, 12, 567–573. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Hallström, B.M.; Blicher, T.H.; Sonnenschein, N.; Jensen, N.B.; Sherstyk, S.; Harrison, S.J.; Maury, J.; Herrgard, M.; Juncker, A. Evolution reveals a glutathione-dependent mechanism of 3-hydroxypropionic acid detoxification. In Proceedings of the Metabolic Engineering X: Biological Design and Synthesis, Vancouver, BC, Canada, 15–19 June 2014. [Google Scholar]
- Masip, L.; Veeravalli, K.; Georgiou, G. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 2006, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Hallström, B.M.; Blicher, T.H.; Sonnenschein, N.; Jensen, N.B.; Sherstyk, S.; Harrison, S.J.; Maury, J.; Herrgård, M.J.; Juncker, A.S.; et al. Evolution reveals a glutathione-dependent mechanism of 3-hydroxypropionic acid tolerance. Metab. Eng. 2014, 26, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Stack, V.T. Toxicity of α,β-unsaturated carbonyl compounds to microorganisms. Ind. Eng. Chem. 1957, 49, 913–917. [Google Scholar] [CrossRef]
- Warnecke, T.E.; Lynch, M.D.; Lipscomb, M.L.; Gill, R.T. Identification of a 21 amino acid peptide conferring 3-hydroxypropionic acid stress-tolerance to Escherichia coli. Biotechnol. Bioeng. 2012, 109, 1347–1352. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Z.; Tong, W.; Ding, Y.; Liu, B.; Shi, Y.; Wang, J.; Sun, S.; Liu, M.; Wang, Y.; et al. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat. Commun. 2020, 11, 1496. [Google Scholar] [CrossRef]
- Chemarin, F.; Moussa, M.; Chadni, M.; Pollet, B.; Lieben, P.; Allais, F.; Trelea, I.C.; Athès, V. New insights in reactive extraction mechanisms of organic acids: An experimental approach for 3-hydroxypropionic acid extraction with tri-n-octylamine. Sep. Purif. Technol. 2017, 179, 523–532. [Google Scholar] [CrossRef]
- Nagengast, J.; Hahn, S.; Taccardi, N.; Kehrer, M.; Kadar, J.; Collias, D.; Dziezok, P.; Wasserscheid, P.; Albert, J. Highly selective synthesis of acrylic acid from lactide in the liquid phase. ChemSusChem 2018, 11, 2936–2943. [Google Scholar] [CrossRef]
- İlalan, İ.; İnci, İ.; Baylan, N. Comparison of strongly and weakly basic anionic resins as adsorbent for acrylic acid removal. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Kar, A.; Bagde, A.; Athankar, K.K.; Wasewar, K.L.; Shende, D.Z. Reactive extraction of acrylic acid with tri-n-butyl phosphate in natural oils. J. Chem. Technol. Biotechnol. 2017, 92, 2825–2834. [Google Scholar] [CrossRef]
- Baylan, N. Imidazolium-based ionic liquids for acrylic acid separation from water by bulk liquid membrane and extraction methods: A comparison study. J. Chem. Eng. Data 2020, 65, 3121–3129. [Google Scholar] [CrossRef]
- Liu, R.; Liang, L.; Jiang, M.; Ma, J.; Chen, K.; Jia, H.; Wei, P.; Ouyang, P. Effects of redox potential control on succinic acid production by engineered Escherichia coli under anaerobic conditions. Process Biochem. 2014, 49, 740–744. [Google Scholar] [CrossRef]



| Host | Substrate (Concentration) | Pathway Genes Overexpressed in Plasmid | Other Genes Overexpressed in Plasmid | Chassis Modification | Operational Mode; Fermentation Time | 3-HP Concentration (g/L) 4 | Ref. |
|---|---|---|---|---|---|---|---|
| E. coli BL21 | Glycerol (500 mM) or (500 mM + feed) | Kp_dhaB; Ec_aldH | - | - | Shake-flask; 48 h Fed-batch; 72 h | 4.4 31 | [67] |
| E. coli BL21 | Glycerol (500 mM + feed) | Kp_dhaB; Kp_gdrAB; Ec_aldH/Ab_kgsadh | - | - | Fed-batch; 80 h | 38.7 | [68] |
| E. coli BL21 Star | Glucose (20 g/L + feed) and glycerol (feed) | Lb_dhaB; Lb_dhaR; Ec_aldH | - | - | 2-Step fed-batch; 60 h | 14.3 | [73] |
| E. coli BL21 Star | Glucose (20 g/L + feed) and glycerol (feed) | Lb_dhaB; Lb_dhaR; Ec_aldH | - | ΔglpK; ΔyqhD | 2-Step fed-batch; 48 h | 57.3 | [82] |
| E. coli W | Glycerol (25 mM + feed) | Kp_dhaB; Kp_gdrAB; Ab_kgsadh | - | - | Fed-batch; 48 h | 41.5 | [52] |
| E. coli W3110 | Glycerol (40 g/L + feed) | Kp_dhaB; Kp_gdrAB; Ec_aldH | Ec_glpF | Δpta; ΔackA; ΔyqhD; ΔglpR | Fed-batch; 35 h | 42.1 | [69] |
| E. coli W3110 | Glucose (40 g/L) and glycerol (80 g/L + feed) | Kp_dhaB; Kp_gdrAB; mutant Cn_gabD4 | - | Δpta; ΔackA; ΔyqhD | Fed-batch; 40 h | 71.9 | [35] |
| E. coli BL21 Star | Glucose (13 g/L + feed) and xylose (7 g/L + feed) | Lb_dhaB; Lb_dhaR; Pa_aldH | Sc_gpp2; Sc_gpd; Ec_xylR | ΔglpK; ΔyqhD; ΔptsG | Fed-batch; 70 h | 29.4 | [83] |
| E. coli BW25113 | Glycerol (200 mM) | Kp_dhaB; Kp_gdrAB; Ab_kgsadh | Ec_gapA (toggle switch) | ΔgapA; ΔyqhD | Shake-flask | 6.0 | [70] |
| E. coli W | Glucose and glycerol (uncertain) | Kp_dhaB; Kp_gdrAB; Ab_khgsadh (UTR opt 3) | - | Δpta; ΔackA; ΔyqhD | Fed-batch; 30 h | 40.51 | [53] |
| E. coli BL21 | Glucose (20 g/L + feed) and glycerol (30 g/L + feed) | Kp_dhaB; Kp_gdrAB; Ab_kgsadh | - | - | Fed-batch; 50 h | 17.2 | [74] |
| E. coli W | Glucose (20 mM) and glycerol (200 mM + feed) | Kp_dhaB; Kp_gdrAB; Ab_kgsadh (UTR opt 3) | - | - | Fed-batch; 50 h | 56.4 | [50] |
| E. coli BL21 Star | Glucose (13 g/L + feed) and xylose (7 g/L + feed) | Lb_dhaB; Lb_dhaR; Pa_aldH | Ec_galP; Sc_gpp2; Ec_xylR; Ec_gpsA | ΔglpK; ΔyqhD; ΔptsG | Fed-batch; 70 h | 37.6 | [51] |
| E. coli W3110 | Glycerol (40 g/L + feed) or Crude glycerol (40 g/L + feed) | Kp_dhaB; Kp_gdrAB; Kp_ydcW | - | ΔlacI | Fed-batch; 48 h | 76.2 61.0 | [36] |
| E. coli BL21 Star | Glucose (13 g/L + feed) and xylose (7 g/L + feed) | Lb_dhaB; Lb_dhaR; Pa_aldH | Ec_galP; Sc_gpp2; Sc_gpd; Ec_xylR | ΔglpK; ΔyqhD; ΔptsG; ΔpuuR; PaldH replacement | Fed-batch; ±92 h | 53.7 | [38] |
| C. glutamicum | Glucose (50 g/L) or glucose (25 g/L) and xylose (25 g/L) | Cn_gabD4; Kp_pduCDEGH | Sc_gpd; Sc_gpp2 | Ec_xylA; Ec_xylB; Cg_araE; ΔldhA; Δpta; ΔackA; ΔptsH; ΔglpK; ΔiolR; ΔpoxB; Cg_gapA mutation; Pglk and PiolT1 replacement | Fed-batch; 72 h | 62.6 54.8 | [49] |
| P. denitrificans | Glycerol (100 mM + feed) or crude glycerol (100 mM + feed) | Kp_dhaB; Kp_gdrAB Ab_kgsadh (mutated) | - | Δ3hpdh; Δ3hibdh; PB12 pathway replacement | Fed-batch; 48 h | up to 102 65.0 | [45] |
| K. pneumoniae DSM 2026 | Glycerol (25 g/L + feed) | Kp_puuC | - | Δpta; ΔldhA | Fed-batch; 72 h | 83.8 | [39] |
| K. pneumoniae J2B | Glycerol (100 mM + feed) | Kp_dhaB; Kp_gdrAB; Ab_kgsadh | - | ΔldhA; ΔfrdA; ΔadhE | Fed-batch; 48 h | 43.0 | [37] |
| K. pneumoniae DSM 2026 | Glycerol (40 g/L + feed) | Kp_puuC (expressed by tandem repetitive tac promoter) | - | - | Fed-batch; 98 h | 102.61 | [47] |
| L. reuteri FXZ014 + E. coli BL21 | Glycerol (20 g/L + feed) | Cn_gabD4; Lr_pduQ (in E. coli) | - | - | Fed-batch; 24 h | 125.93 | [48] |
| Host | Substrate (Concentration) | Pathway Genes Overexpressed in Plasmid | Other Genes Overexpressed in Plasmid | Chassis Modifications | Operational Mode; Fermentation Time | 3-HP Concentration (g/L) 3 | Ref. |
|---|---|---|---|---|---|---|---|
| S. cerevisiae CEN.PK102-5B | Glucose (44 g/L + feed) | - | - | Bc_bapat; Ec_ydfG; Sc_PYC1; Sc_PYC2; Sc_AAT2; Tc_panD (multiple copies) | Fed-batch; 80 h | 13.7 | [98] |
| S. cerevisiae CEN.PK 113-7D | Xylose (20 g/L + feed) | - | - | Bc_bapat; Ec_ydfG; Sc_PYC1; Sc_PYC2; Tc_panD; Ps_xyl1; Ps_xyl2; Ps_xyl3 | Fed-batch; 120 h | 7.37 | [99] |
| S. cerevisiae CEN.PK102-5B | Glucose (10 g/L + feed) (phosphorus limitation) | - | - | Bc_bapat; Ec_ydfG; Sc_PYC1; Sc_PYC2; Sc_AAT2; Tc_panD (multiple copies) | Fed-batch (exponential feeding ramp); 70 h | 25 | [40] |
| E. coli W3110 | Glucose (20 g/L + feed) | Pa_pa0132; Ec_ydfG | Cg_panD; Ec_aspA; Ec_ppc | ΔfumAC; ΔfumB; ΔiclR; PsdhC and PaspA replacement | Fed-batch; 49 h | 31.1 | [46] |
| Host | Substrate (Concentration) | Route | Pathway Genes Overexpressed in Plasmid | Other Genes Overexpressed in Plasmid | Chassis Modifications | Operational Mode; Fermentation Time | Concentration (mg/L) 3 | Ref. |
|---|---|---|---|---|---|---|---|---|
| E. coli W3110 | Glucose (unknown) | Glycerol (via 3-HP) | Cn_ydiF; Af_aflv_0566; Ec_yciA | Ec_gpsA; Sc_gpp2 | Kp_dhaB; Kp_gdrAB; Cn_gabD4; Δpta; ΔackA; ΔyqhD; | Shake-flask (batch); 15 h | 120 | [32] |
| E. coli DH5α | Glucose (50 mM) | Malonyl-CoA (via 3-HP) | Ca_mcr; Ca_pcs (truncated); Ab_ach | - | - | 96-well plate (batch); 12 h (w/cerulenin supply) | 4.32 | [33] |
| E. coli K12 MG1655 | Glucose (20 g/L + feed) | Malonyl-CoA (via 3-HP) | Ms_mcr (codon-optimized); Ms_msr; Ms_3hpcs; Ms_3hpcd; Ec_sucCD | - | ΔpoxB; Δpta; ΔackA | Fed-batch; 24 h | 13.28 | [31] |
| E. coli BL21 | Glycerol (20 g/L) | Glycerol (not via 3-HP) | Ca_pcs (truncated, mutated); St_pduP | - | Kp_gdrAB; Kp_dhaB; ΔprpR | Shake-flask (batch); 48 h | 37.7 | [2] |
| E. coli K12 MG1655 | Glycerol (30 g/L) and glucose (1 g/L) | Glycerol (not via 3-HP) | Ip_dhaB; Ip_gdrAB; Lr_pduP; Me_MELS_1449; Ec_yciA | Δpta; ΔackA; ΔyqhD | Fermenter (batch); 48 h | 44 | [27] | |
| E. coli W3110 | Glucose (10 g/L + feed) | β-alanine (not via 3-HP) | Cp_act; Cp_acl2; Ec_yciA | Cg_panD; Ec_aspA | ΔyhdH; ΔfumAC; ΔfumB; ΔiclR; Pacs and Pasp replacement | Shake-flask (batch); n/d Fed-batch; 57.5 h | 60 237 | [34] |
| E. coli Bl21 | Glycerol (10 g/L) | Glycerol (not via 3-HP) | Kp_dhaB; Go_3had | - | - | Shake-flask (batch); 32 h | 144 | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, J.L. Heterologous Production of Acrylic Acid: Current Challenges and Perspectives. SynBio 2023, 1, 3-32. https://doi.org/10.3390/synbio1010002
Rodrigues JL. Heterologous Production of Acrylic Acid: Current Challenges and Perspectives. SynBio. 2023; 1(1):3-32. https://doi.org/10.3390/synbio1010002
Chicago/Turabian StyleRodrigues, Joana L. 2023. "Heterologous Production of Acrylic Acid: Current Challenges and Perspectives" SynBio 1, no. 1: 3-32. https://doi.org/10.3390/synbio1010002
APA StyleRodrigues, J. L. (2023). Heterologous Production of Acrylic Acid: Current Challenges and Perspectives. SynBio, 1(1), 3-32. https://doi.org/10.3390/synbio1010002







