Analysis of the Influence of Process and Formulation Properties on the Drying Behavior of Pharmaceutical Granules in a Semi-Continuous Fluid Bed Drying System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Impact of Process Parameters
2.1.2. Impact of API Properties
2.1.3. Generalisation of the Data Set
2.2. Granule Size Measurements
2.3. Residual Moisture Content
2.4. Flowability Test
2.5. Friability Test
3. Results and Discussion
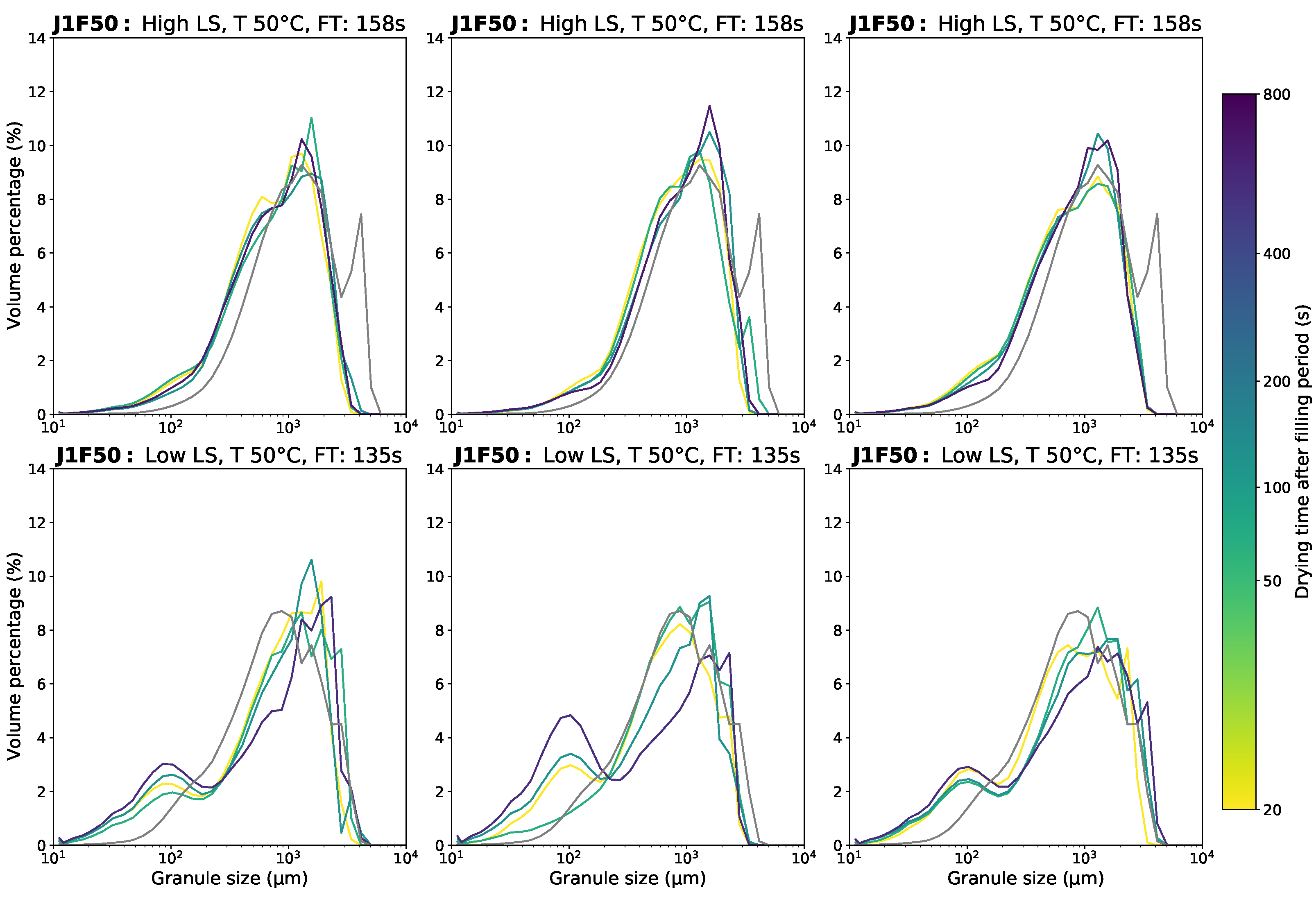
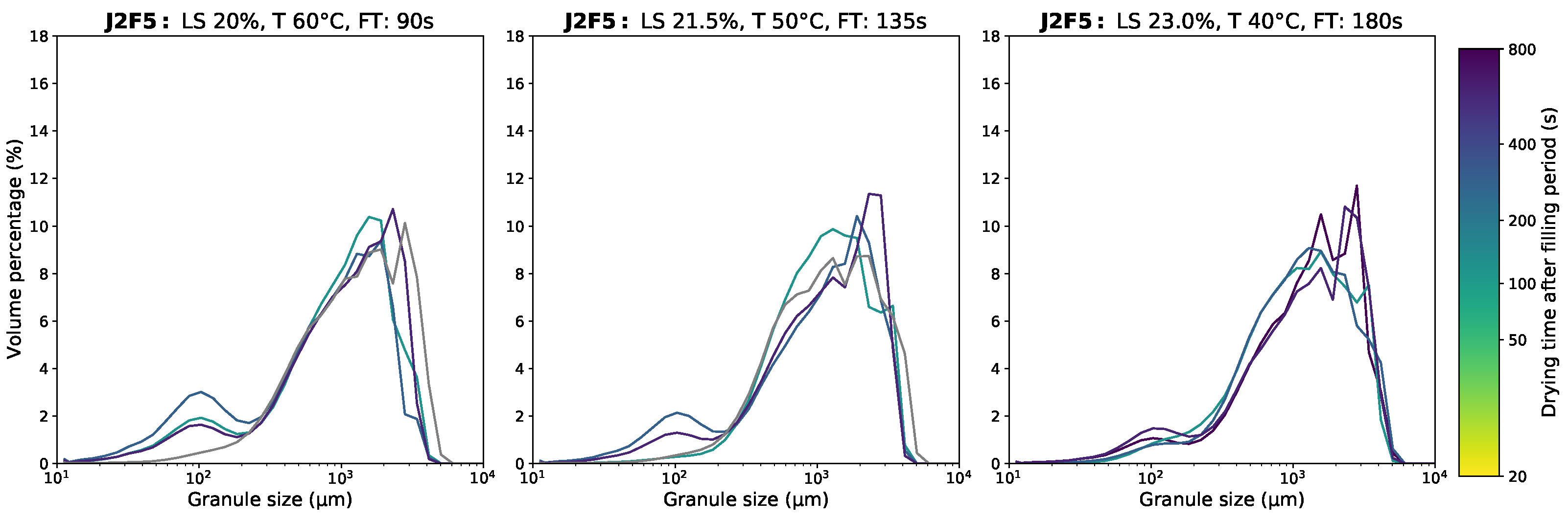
3.1. Granule Size Distribution
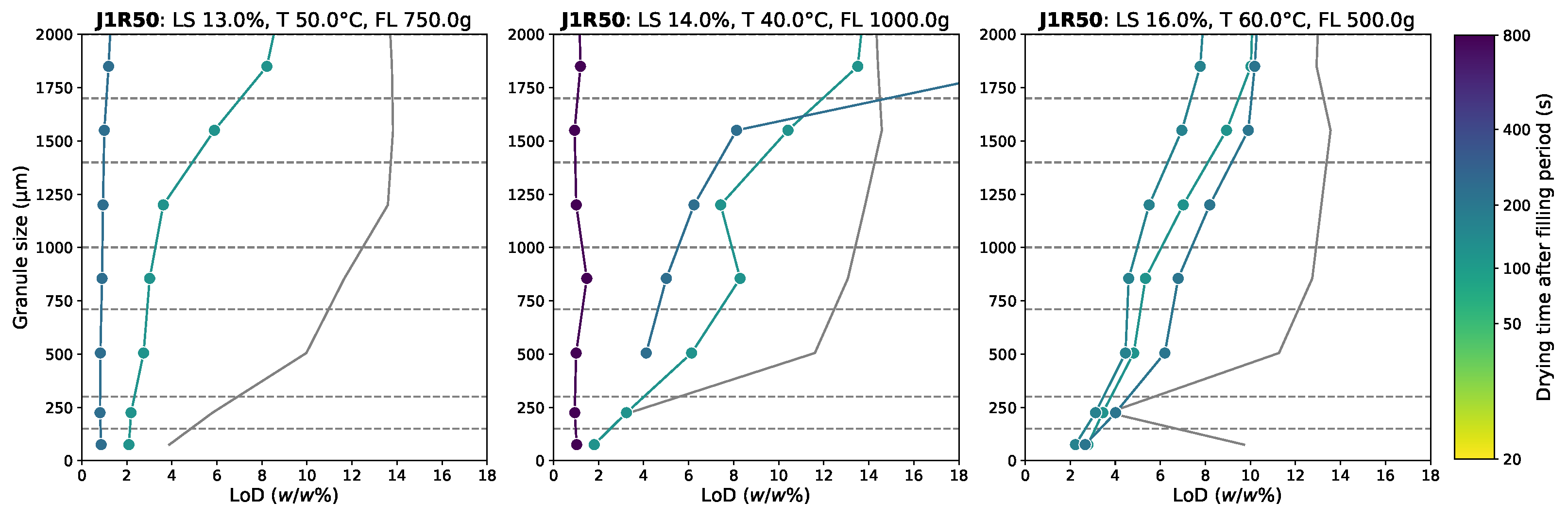
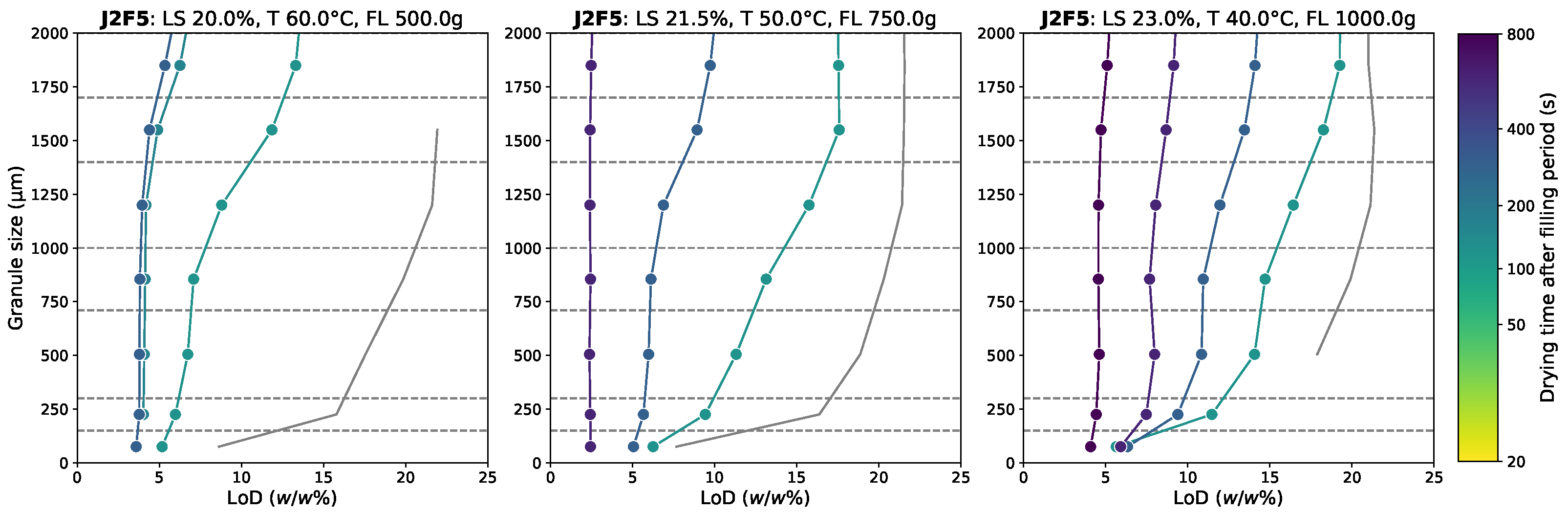
3.2. Residual Moisture Content Distribution
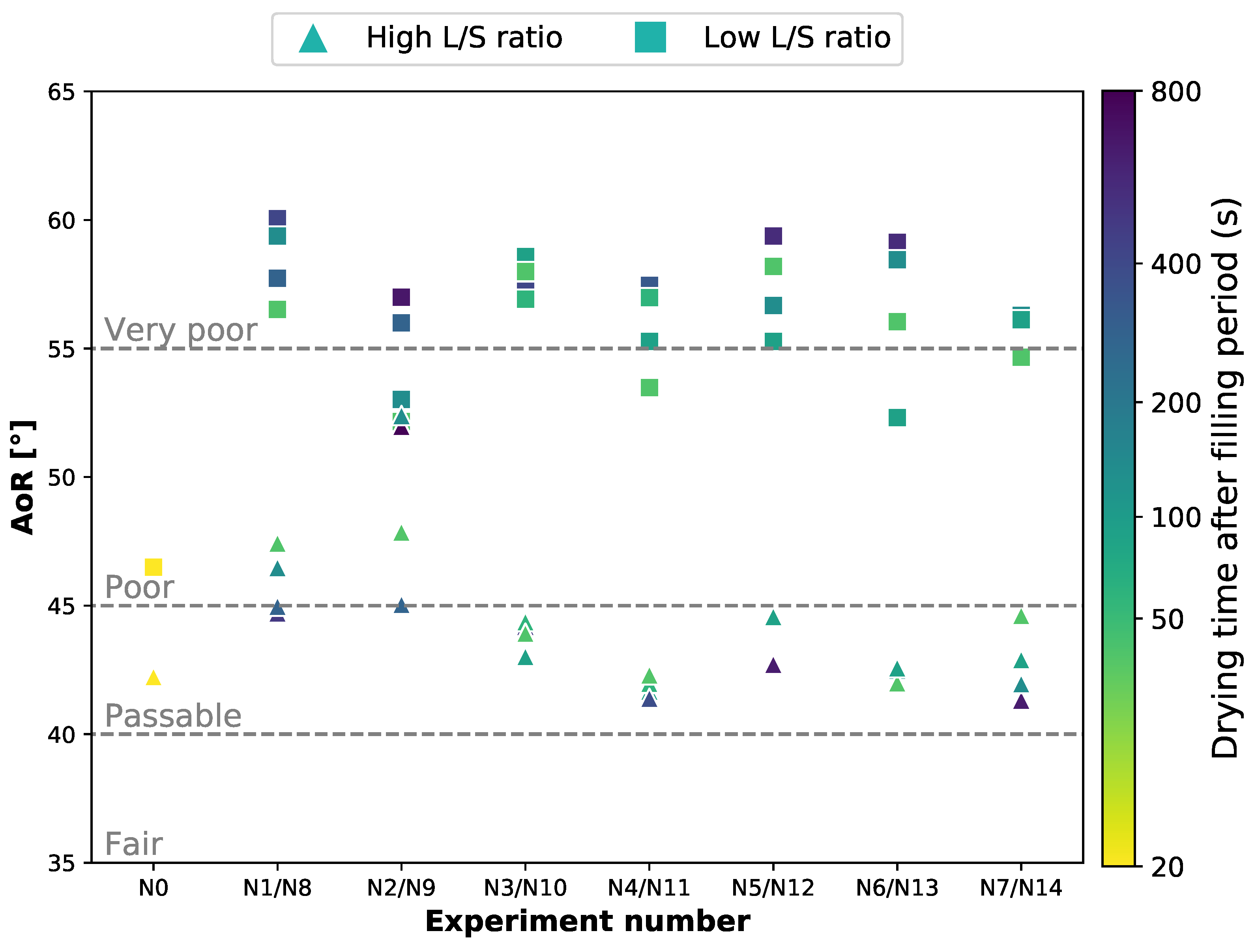
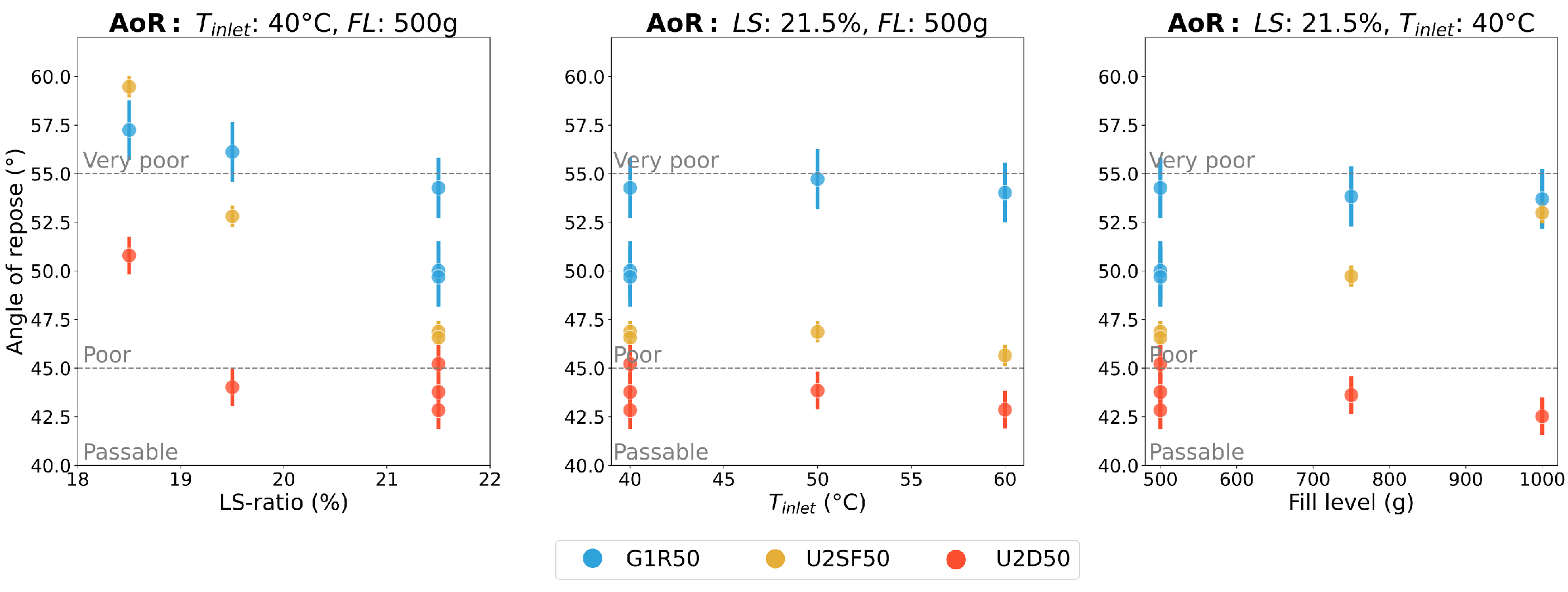
3.3. Flowability
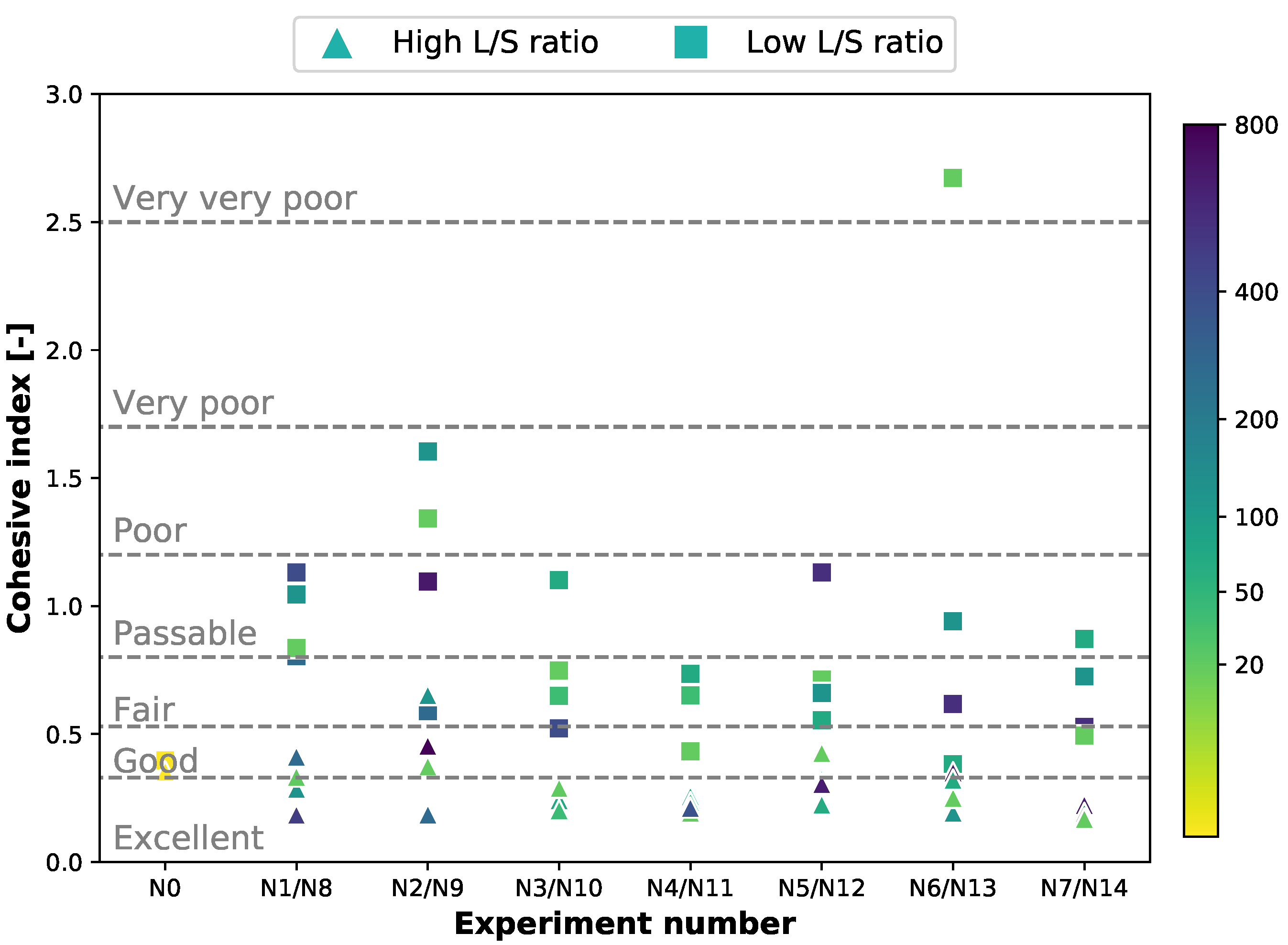
3.4. Friability
3.5. Generalisation of the Data Set
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AoR | Angle of Repose |
| API | active pharmaceutical ingredient |
| CI | static cohesive index |
| Cp | heat capacity |
| CQA | critical quality attribute |
| DoE | design of experiments |
| FBD | fluid bed drying |
| FL | fill level |
| FT | fill time |
| GSD | granule size distribution |
| KPCA | kernel principle component analysis |
| LoD | Loss-on-drying |
| LS | liquid-to-solid |
| MFR | mass flow rate |
| MMD | maximum mean discrepancy |
| QbD | quality-by-design |
| RMC | residual moisture content |
| T | inlet air temperature |
| TSWG | twin-screw wet granulation |
Appendix A
| API | Heat Capacity [-] | Water Solubility [-] | Flowability [-] | d0.1 [-] | d0.5 [-] | d0.9 [-] |
|---|---|---|---|---|---|---|
| J1F | 1.00 | 0.93 | 0.00 | 0.33 | 0.33 | 0.09 |
| U1R | 0.63 | 0.98 | 0.35 | 1.00 | 1.00 | 0.83 |
| U2SF | 0.41 | 0.99 | 0.05 | 0.35 | 0.36 | 0.06 |
| U2R | 0.00 | 0.10 | 0.26 | 0.23 | 0.50 | 0.21 |
| G1R | 0.69 | 0.05 | 0.14 | 0.09 | 0.03 | 0.00 |
| J1R | 0.78 | 1.00 | 0.15 | 0.39 | 0.77 | 0.30 |
| J2F | 0.65 | 0.00 | 0.02 | 0.00 | 0.00 | 0.54 |
| J3R | 0.40 | 0.00 | 1.00 | 0.24 | 0.31 | 1.00 |
Appendix B
| EXPN | FORM | LS (% (w/w)) | T (°C) | MFR (kg/h) | FL (kg) | SS (rpm) | t1 (s) | t2 (s) | t3 (s) | t4 (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | J1F50 | 10.60 | 40 | 20 | 0.50 | 675 | 110 | 210 | 360 | 450 |
| N2 | J1F50 | 10.60 | 40 | 20 | 1.00 | 675 | 200 | 300 | 450 | 853 |
| N3 | J1F50 | 10.60 | 60 | 20 | 0.50 | 675 | 110 | 130 | 160 | 500 |
| N4 | J1F50 | 10.60 | 60 | 20 | 1.00 | 675 | 200 | 220 | 250 | 500 |
| N5 | J1F50 | 10.60 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 684 |
| N6 | J1F50 | 10.60 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 684 |
| N7 | J1F50 | 10.60 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 684 |
| N8 | J1F50 | 13.80 | 40 | 17.03 | 0.50 | 809 | 126 | 226 | 376 | 585 |
| N9 | J1F50 | 13.80 | 40 | 17.03 | 1.00 | 809 | 231 | 331 | 481 | 1000 |
| N10 | J1F50 | 13.80 | 60 | 17.03 | 0.50 | 809 | 126 | 146 | 176 | 585 |
| N11 | J1F50 | 13.80 | 60 | 17.03 | 1.00 | 809 | 231 | 251 | 281 | 585 |
| N12 | J1F50 | 13.80 | 50 | 17.03 | 0.75 | 809 | 178 | 228 | 278 | 800 |
| N13 | J1F50 | 13.80 | 50 | 17.03 | 0.75 | 809 | 178 | 228 | 278 | 800 |
| N14 | J1F50 | 13.80 | 50 | 17.03 | 0.75 | 809 | 178 | 228 | 278 | 800 |
| N15 | U1R50 | 6.36 | 40 | 20 | 0.50 | 675 | 110 | 210 | 360 | 450 |
| N16 | U1R50 | 6.36 | 40 | 20 | 1.00 | 675 | 200 | 300 | 450 | 1000 |
| N17 | U1R50 | 6.36 | 60 | 20 | 0.50 | 675 | 110 | 160 | 210 | 450 |
| N18 | U1R50 | 6.36 | 60 | 20 | 1.00 | 675 | 200 | 250 | 300 | 1000 |
| N19 | U1R50 | 6.36 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| N20 | U1R50 | 6.36 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| N21 | U1R50 | 6.36 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| N22 | U1R50 | 9.78 | 40 | 20 | 0.50 | 675 | 110 | 210 | 360 | 450 |
| N23 | U1R50 | 9.78 | 40 | 20 | 1.00 | 675 | 200 | 300 | 450 | 1000 |
| N24 | U1R50 | 9.78 | 60 | 20 | 0.50 | 675 | 110 | 160 | 210 | 450 |
| N25 | U1R50 | 9.78 | 60 | 20 | 1.00 | 675 | 200 | 250 | 300 | 1000 |
| N26 | U1R50 | 9.78 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| N27 | U1R50 | 9.78 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| N28 | U1R50 | 9.78 | 50 | 20 | 0.75 | 675 | 155 | 205 | 255 | 755 |
| EXPN | FORM | LS (% (w/w)) | T (°C) | MFR (kg/h) | FL (kg) | SS (rpm) | t1 (s) | t2 (s) | t3 (s) | t4 (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| N29 | U2SF50 | 18.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N30 | U2SF50 | 19.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N31 | U2SF50 | 21.5 | 60 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N32 | U2SF50 | 21.5 | 50 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N33 | U2SF50 | 21.5 | 40 | 20 | 1.00 | 675 | 145 | 270 | 490 | 800 |
| N34 | U2SF50 | 21.5 | 40 | 20 | 0.75 | 675 | 145 | 225 | 445 | 755 |
| N35 | U2SF50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N36 | U2SF50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N37 | U2SF50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N38 | U2R50 | 18.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N39 | U2R50 | 19.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N40 | U2R50 | 21.5 | 60 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N41 | U2R50 | 21.5 | 50 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N42 | U2R50 | 21.5 | 40 | 20 | 1.00 | 675 | 145 | 270 | 490 | 800 |
| N43 | U2R50 | 21.5 | 40 | 20 | 0.75 | 675 | 145 | 225 | 445 | 755 |
| N44 | U2R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N45 | U2R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N46 | U2R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N47 | G1R50 | 18.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N48 | G1R50 | 19.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N49 | G1R50 | 21.5 | 60 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N50 | G1R50 | 21.5 | 50 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N51 | G1R50 | 21.5 | 40 | 20 | 1.00 | 675 | 145 | 270 | 490 | 800 |
| N52 | G1R50 | 21.5 | 40 | 20 | 0.75 | 675 | 145 | 225 | 445 | 755 |
| N53 | G1R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N54 | G1R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| N55 | G1R50 | 21.5 | 40 | 20 | 0.50 | 675 | 100 | 180 | 400 | - |
| EXPN | FORM | LS (% (w/w)) | T (°C) | MFR (kg/h) | FL (kg) | SS (rpm) | t1 (s) | t2 (s) | t3 (s) | t4 (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| N56 | J1R50 | 16 | 40 | 20 | 1.00 | 675 | 300 | 360 | 420 | 1000 |
| N57 | J1R50 | 14 | 50 | 20 | 0.75 | 675 | 255 | 375 | 555 | - |
| N58 | J1R50 | 12 | 60 | 20 | 0.50 | 675 | 210 | 330 | 510 | - |
| N59 | J2F5 | 23 | 40 | 20 | 1.00 | 675 | 300 | 480 | 780 | 1000 |
| N60 | J2F5 | 21.5 | 50 | 20 | 0.75 | 675 | 255 | 435 | 735 | - |
| N61 | J2F5 | 20 | 60 | 20 | 0.50 | 675 | 210 | 390 | 670 | - |
| N62 | J3R50 | 21 | 40 | 20 | 1.00 | 675 | 300 | 480 | 780 | 1000 |
| N63 | J3R50 | 19 | 50 | 20 | 0.75 | 675 | 255 | 435 | 735 | - |
| N64 | J3R50 | 16 | 60 | 20 | 0.50 | 675 | 210 | 390 | 690 | - |
Appendix C
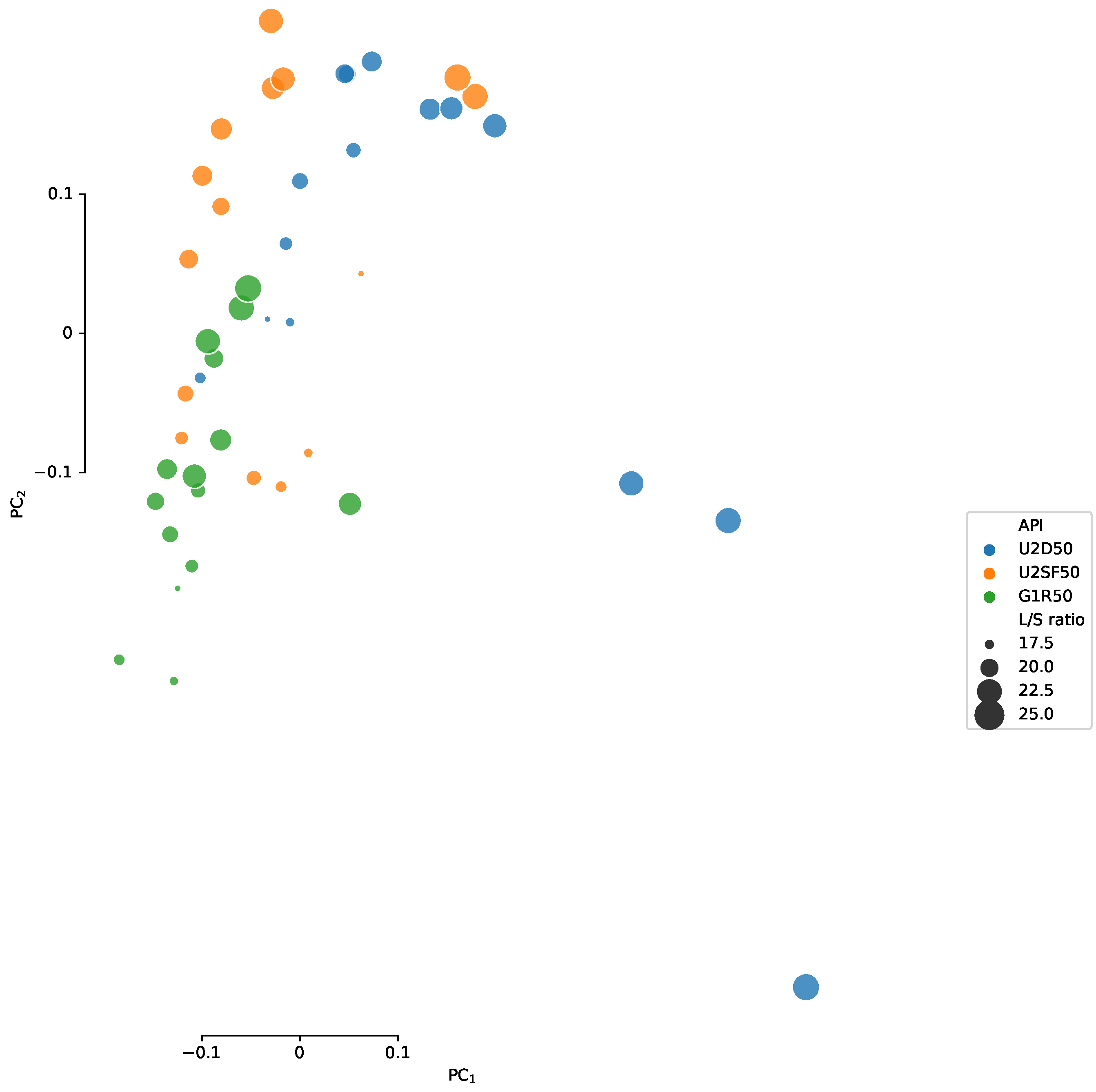

Appendix D
Appendix D.1. Granule Size Data

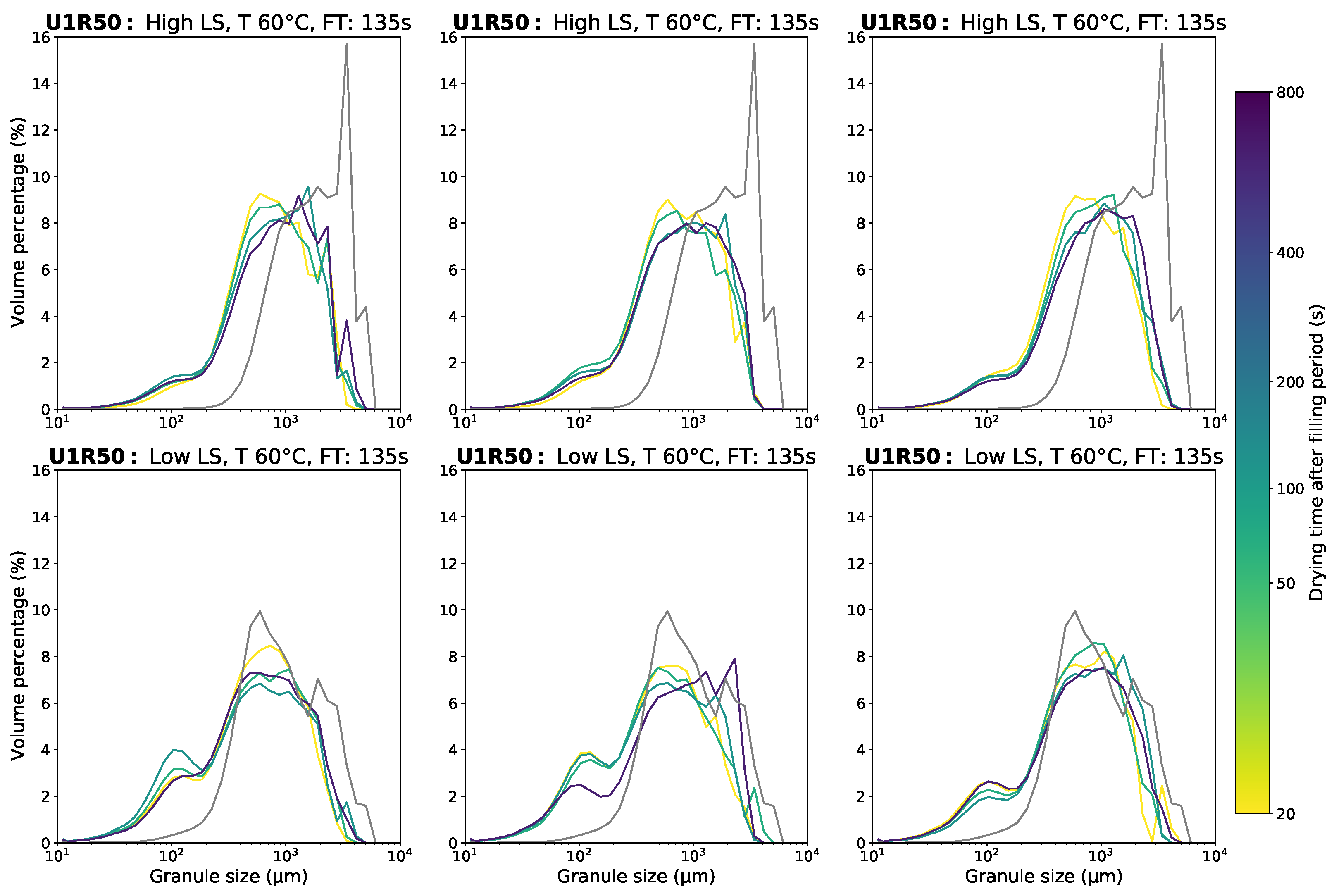
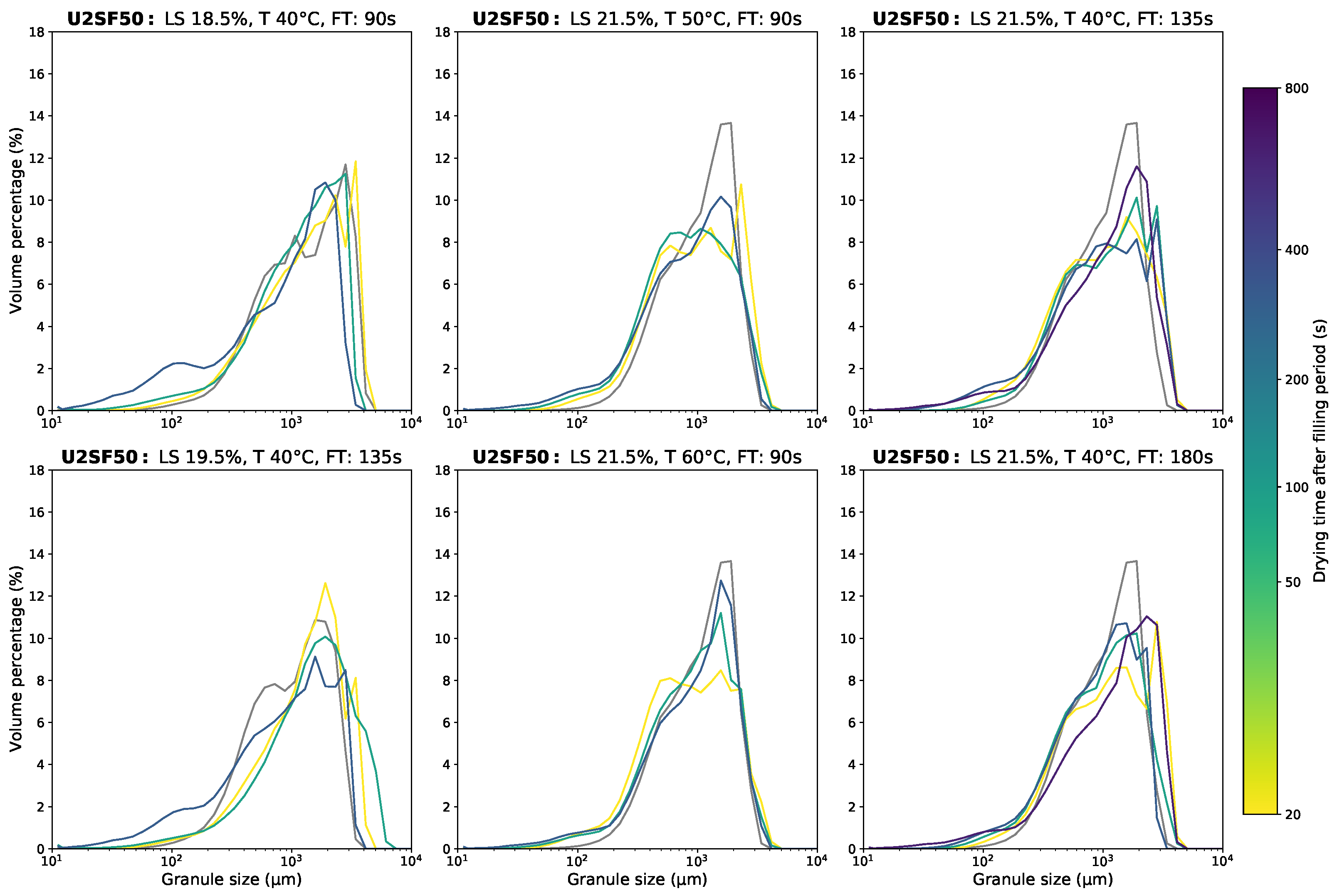

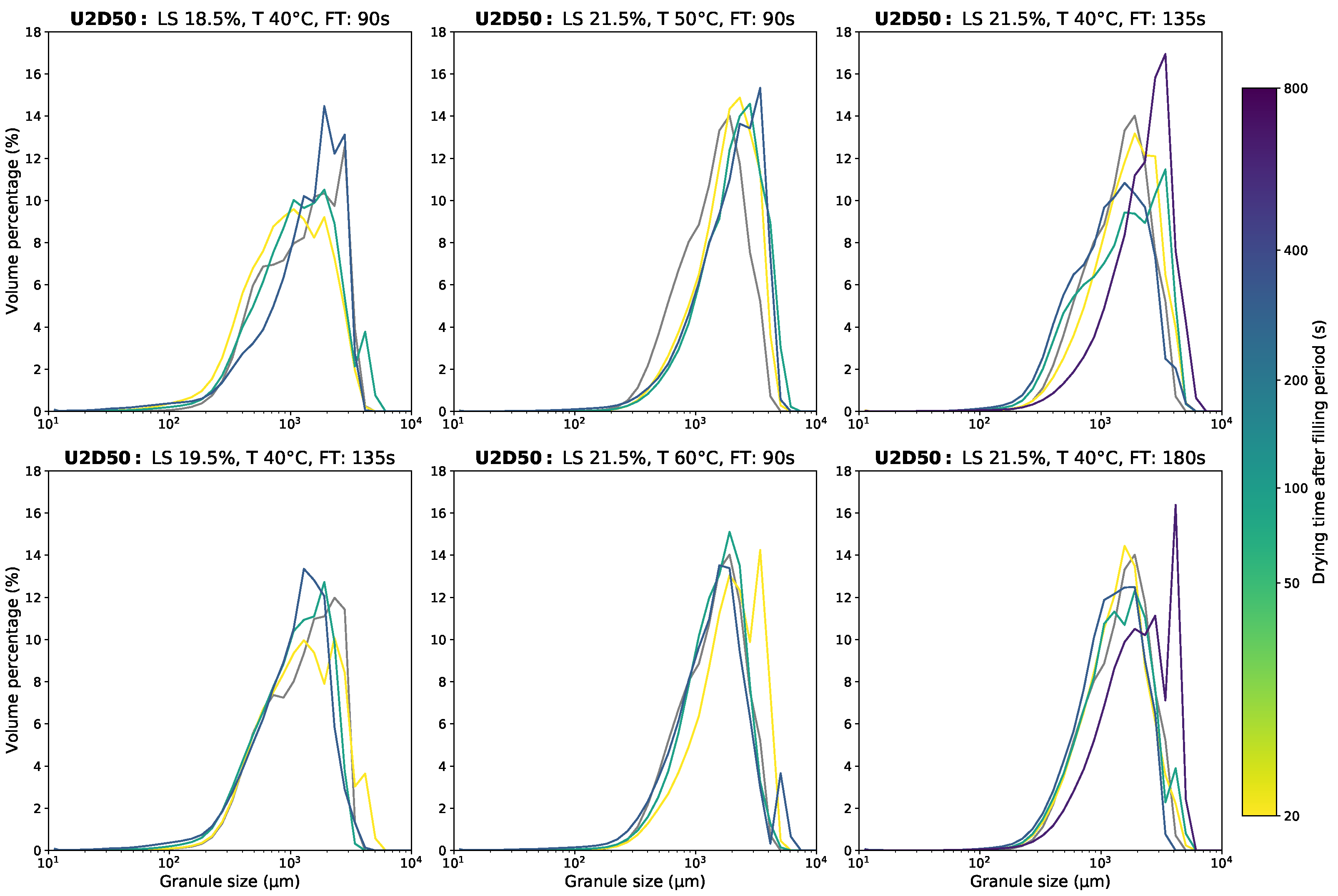
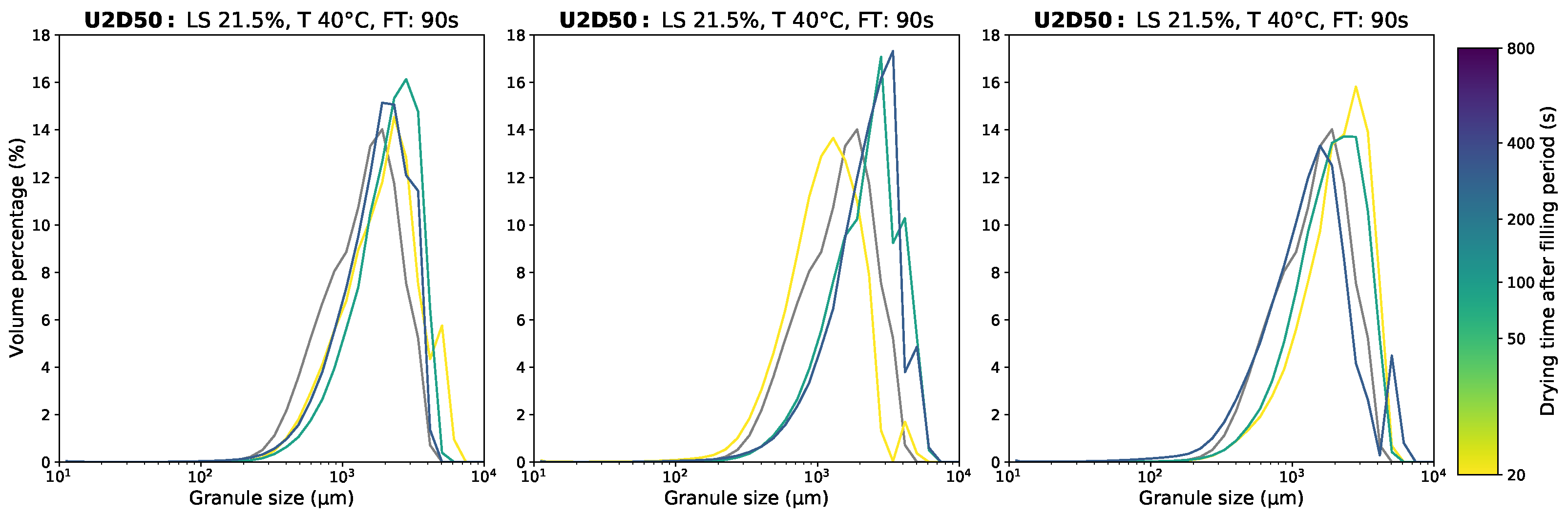
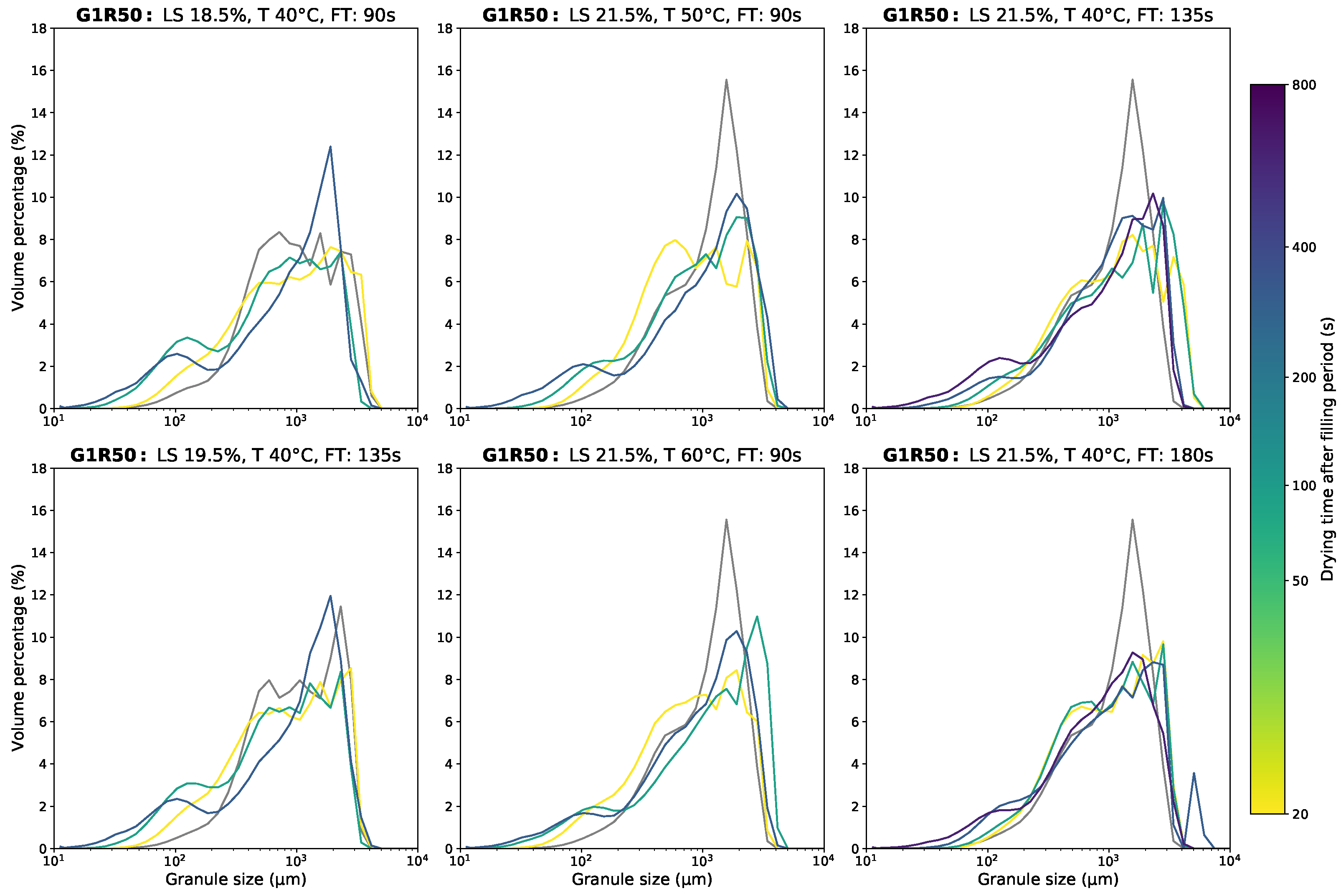
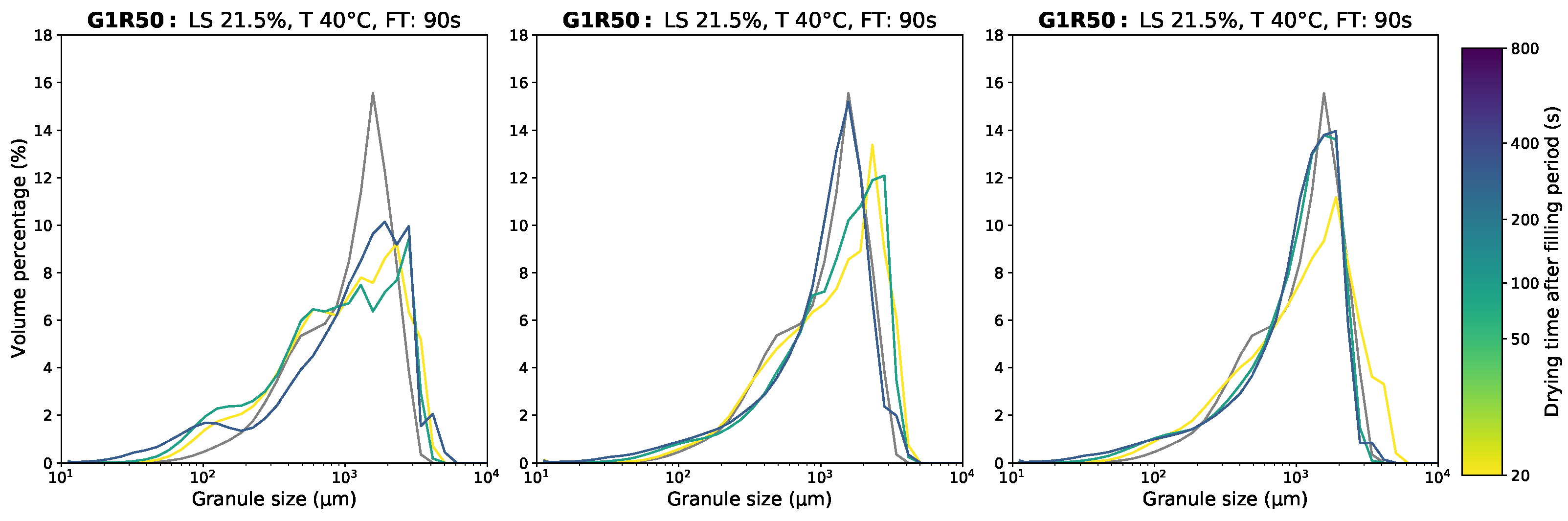
Appendix D.2. Moisture Content Data



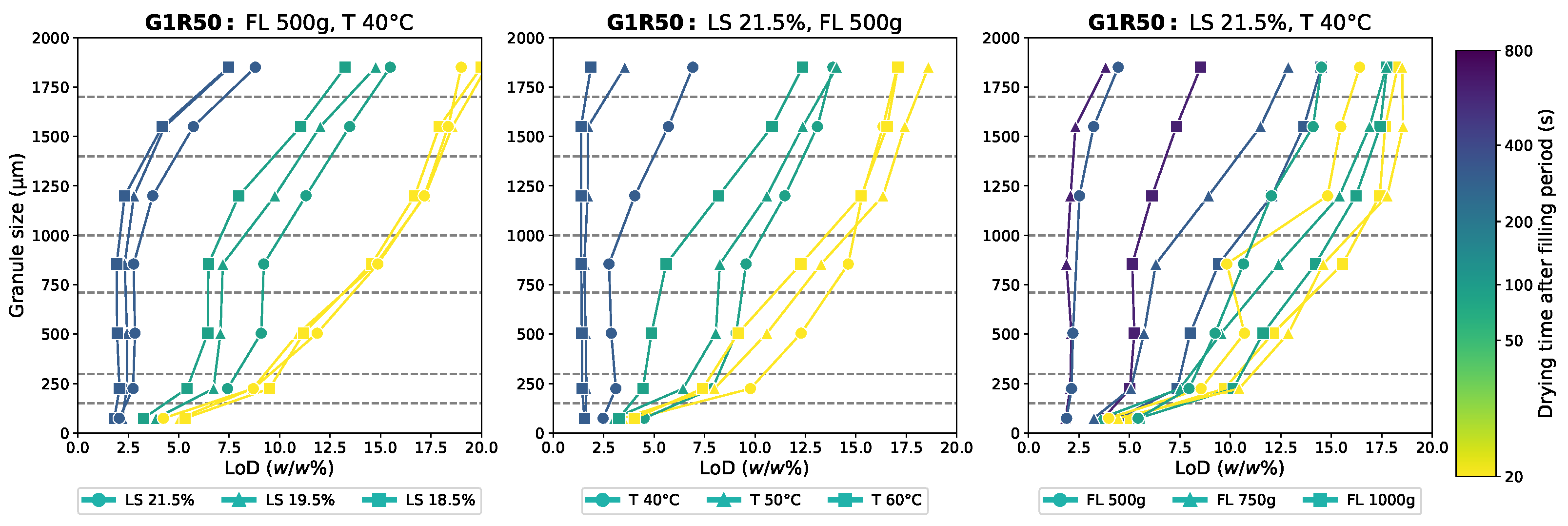

Appendix D.3. Static Cohesion Index

References
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Dall Agnol, R.; The Manufacturing Classification System (MCS) Working Group. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, F.; Boulanger, E.; Pilcer, G. Sampling and diversion strategy for twin-screw granulation lines using batch statistical process monitoring. Eur. J. Pharm. Sci. 2022, 171, 106126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Iveson, S.M.; Litster, J.D.; Hapgood, K.; Ennis, B.J. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Technol. 2001, 117, 3–39. [Google Scholar] [CrossRef]
- Cheong, Y.S.; Mangwandi, C.; Fu, J.; Adams, M.J.; Hounslow, M.J.; Salman, A.D. Chapter 26 A Mechanistic Description of Granule Deformation and Breakage. In Particle Breakage; Salman, A.D., Ghadiri, M., Hounslow, M.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; Volume 12, pp. 1055–1120. [Google Scholar] [CrossRef]
- Fonteyne, M.; Correia, A.; De Plecker, S.; Vercruysse, J.; Ilić, I.; Zhou, Q.; Vervaet, C.; Remon, J.P.; Onofre, F.; Bulone, V.; et al. Impact of microcrystalline cellulose material attributes: A case study on continuous twin screw granulation. Int. J. Pharm. 2015, 478, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Moll, K.P.; Krumme, M.; Kleinebudde, P. Impact of fill-level in twin-screw granulation on critical quality attributes of granules and tablets. Eur. J. Pharm. Biopharm. 2017, 115, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Díaz, D.C.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef]
- Li, H.; Thompson, M.; O’donnell, K. Understanding wet granulation in the kneading block of twin screw extruders. Chem. Eng. Sci. 2014, 113, 11–21. [Google Scholar] [CrossRef]
- Vercruysse, J.; Burggraeve, A.; Fonteyne, M.; Cappuyns, P.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int. J. Pharm. 2015, 479, 171–180. [Google Scholar] [CrossRef]
- Djuric, D.; Kleinebudde, P. Impact of screw elements on continuous granulation with a twin-screw extruder. J. Pharm. Sci. 2008, 97, 4934–4942. [Google Scholar] [CrossRef]
- De Leersnyder, F.; Vanhoorne, V.; Bekaert, H.; Vercruysse, J.; Ghijs, M.; Bostijn, N.; Verstraeten, M.; Cappuyns, P.; Van Assche, I.; Vander Heyden, Y.; et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: Influence of process parameters and wet granule transfer. Eur. J. Pharm. Sci. 2018, 115, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, A.; Ghijs, M.; Portier, C.; Djuric, D.; Funke, A.; Vervaet, C.; De Beer, T. The influence of equipment design and process parameters on granule breakage in a semi-continuous fluid bed dryer after continuous twin-screw wet granulation. Pharmaceutics 2021, 13, 293. [Google Scholar] [CrossRef]
- Ghijs, M.; Schäfer, E.; Kumar, A.; Cappuyns, P.; Van Assche, I.; De Leersnyder, F.; Vanhoorne, V.; De Beer, T.; Nopens, I. Modeling of semicontinuous fluid bed drying of pharmaceutical granules with respect to granule size. J. Pharm. Sci. 2019, 108, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Fonteyne, M.; Soares, S.; Vercruysse, J.; Peeters, E.; Burggraeve, A.; Vervaet, C.; Remon, J.P.; Sandler, N.; De Beer, T. Prediction of quality attributes of continuously produced granules using complementary pat tools. Eur. J. Pharm. Biopharm. 2012, 82, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Barrera Jiménez, A.A.; Van Hauwermeiren, D.; Peeters, M.; De Beer, T.; Nopens, I. Improvement of a 1D Population Balance Model for Twin-Screw Wet Granulation by Using Identifiability Analysis. Pharmaceutics 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Van Hauwermeiren, D. On the Simulation of Particle Size Distributions in Continuous Pharmaceutical Wet Granulation. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2020. [Google Scholar]
- Smola, A.; Gretton, A.; Song, L.; Schölkopf, B. A Hilbert space embedding for distributions. In Proceedings of the International Conference on Algorithmic Learning Theory, San Diego, CA, USA, 13–15 June 2007; pp. 13–31. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Badawy, S.; Pandey, P. Design, development, and scale-up of the high-shear wet granulation process. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2017; pp. 749–776. [Google Scholar]
- De Simone, V.; Caccavo, D.; Dalmoro, A.; Lamberti, G.; d’Amore, M.; Barba, A.A. Inside the phenomenological aspects of wet granulation: Role of process parameters. In Granularity in Materials Science; InTech: London, UK, 2018; pp. 63–84. [Google Scholar]
- Zuurman, K.; Riepma, K.; Bolhuis, G.; Vromans, H.; Lerk, C. The relationship between bulk density and compactibility of lactose granulations. Int. J. Pharm. 1994, 102, 1–9. [Google Scholar] [CrossRef]
- Granutools™. Small Doses Analysis Using Granuheap™. Available online: https://www.granutools.com/en/news/92_small-doses-analysis-using-granuheap (accessed on 20 May 2021).
- Kudo, Y.; Yasuda, M.; Matsusaka, S. Effect of particle size distribution on flowability of granulated lactose. Adv. Powder Technol. 2020, 31, 121–127. [Google Scholar] [CrossRef]
- Vandevivere, L.; Denduyver, P.; Portier, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Influence of binder attributes on binder effectiveness in a continuous twin screw wet granulation process via wet and dry binder addition. Int. J. Pharm. 2020, 585, 119466. [Google Scholar] [CrossRef]
- Kotamarthy, L.; Metta, N.; Ramachandran, R. Understanding the Effect of Granulation and Milling Process Parameters on the Quality Attributes of Milled Granules. Processes 2020, 8, 683. [Google Scholar] [CrossRef]
- Nitert, B.J.; Hounslow, M.J. Moisture content distribution in semibatch drying processes, part II. Falling particle drying rate. AIChE J. 2012, 58, 3708–3717. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandeputte, T.; Ghijs, M.; Peeters, M.; De Man, A.; Van Hauwermeiren, D.; Dos Santos Schultz, E.; Vigh, T.; Stauffer, F.; Nopens, I.; De Beer, T. Analysis of the Influence of Process and Formulation Properties on the Drying Behavior of Pharmaceutical Granules in a Semi-Continuous Fluid Bed Drying System. Powders 2023, 2, 232-258. https://doi.org/10.3390/powders2020016
Vandeputte T, Ghijs M, Peeters M, De Man A, Van Hauwermeiren D, Dos Santos Schultz E, Vigh T, Stauffer F, Nopens I, De Beer T. Analysis of the Influence of Process and Formulation Properties on the Drying Behavior of Pharmaceutical Granules in a Semi-Continuous Fluid Bed Drying System. Powders. 2023; 2(2):232-258. https://doi.org/10.3390/powders2020016
Chicago/Turabian StyleVandeputte, Tuur, Michael Ghijs, Michiel Peeters, Alexander De Man, Daan Van Hauwermeiren, Eduardo Dos Santos Schultz, Tamas Vigh, Fanny Stauffer, Ingmar Nopens, and Thomas De Beer. 2023. "Analysis of the Influence of Process and Formulation Properties on the Drying Behavior of Pharmaceutical Granules in a Semi-Continuous Fluid Bed Drying System" Powders 2, no. 2: 232-258. https://doi.org/10.3390/powders2020016
APA StyleVandeputte, T., Ghijs, M., Peeters, M., De Man, A., Van Hauwermeiren, D., Dos Santos Schultz, E., Vigh, T., Stauffer, F., Nopens, I., & De Beer, T. (2023). Analysis of the Influence of Process and Formulation Properties on the Drying Behavior of Pharmaceutical Granules in a Semi-Continuous Fluid Bed Drying System. Powders, 2(2), 232-258. https://doi.org/10.3390/powders2020016








