The Significance of Tablet Internal Structure on Disintegration and Dissolution of Immediate-Release Formulas: A Review
Abstract
1. Introduction
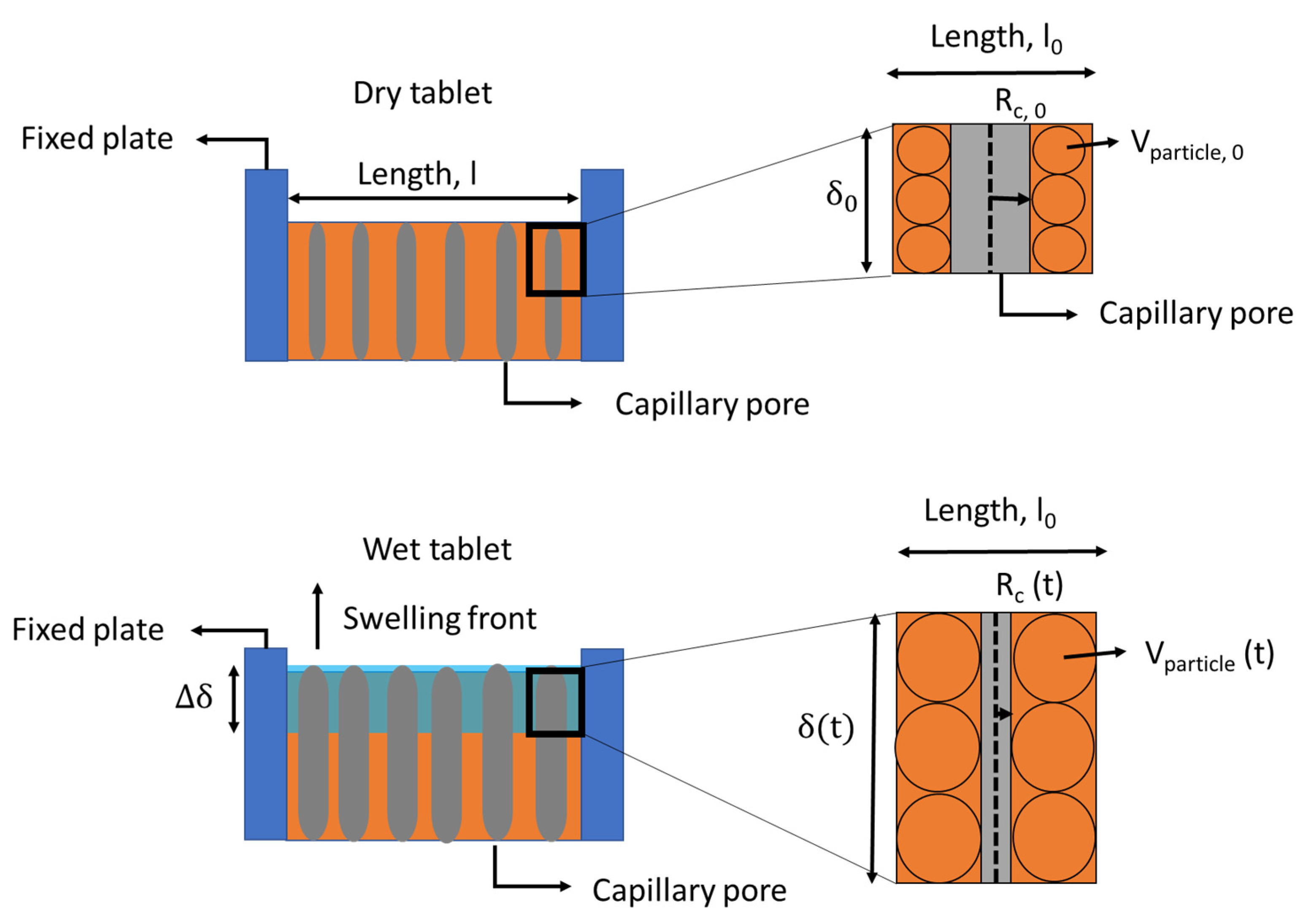
2. Liquid Transport Kinetics
2.1. Vertical Liquid Transport
2.2. Radial Liquid Transport
2.3. Tangential Liquid Transport
2.4. Recent Advancements in Liquid Transport Models
3. Characterizing Release Kinetics of Tablets
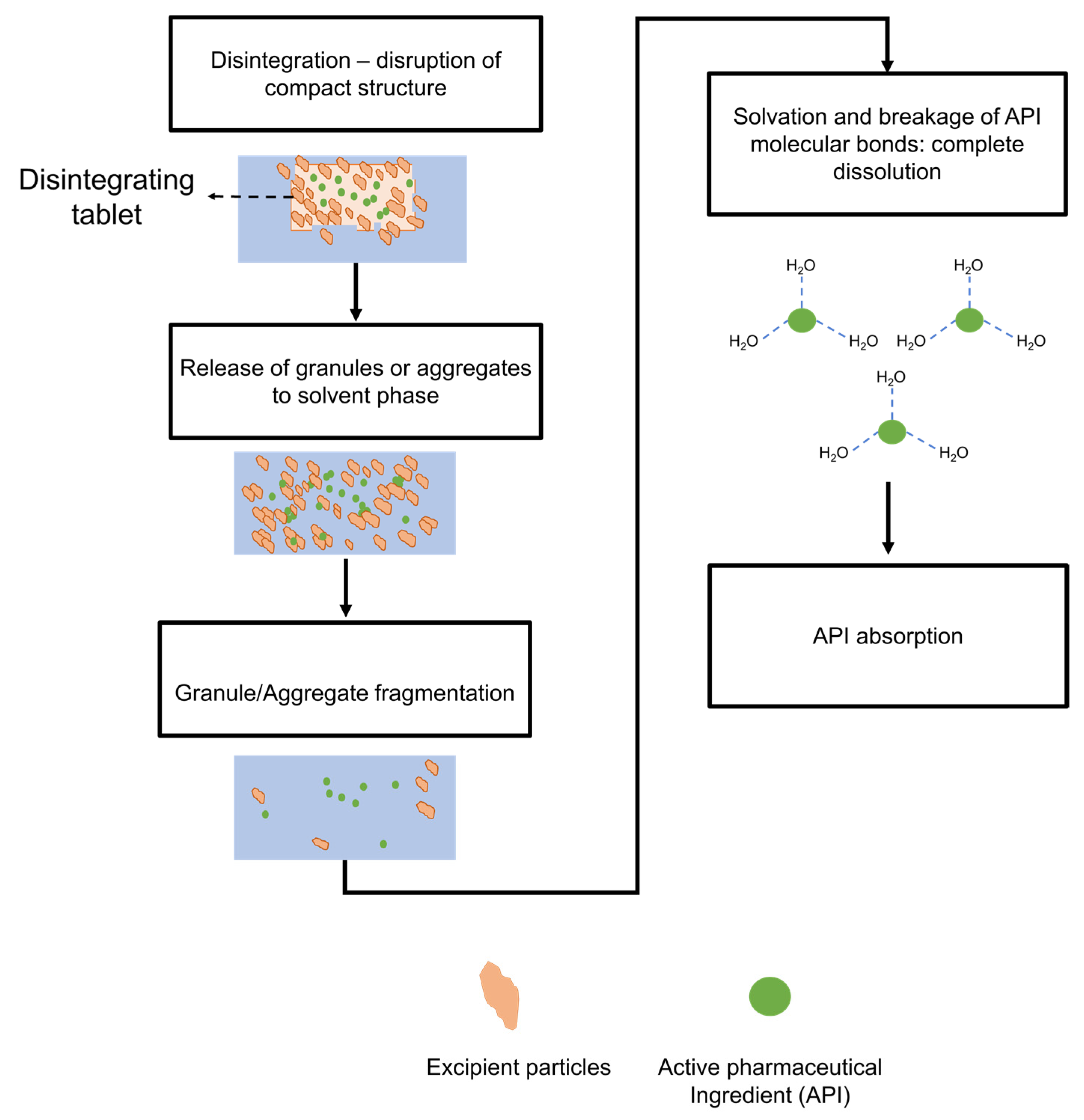
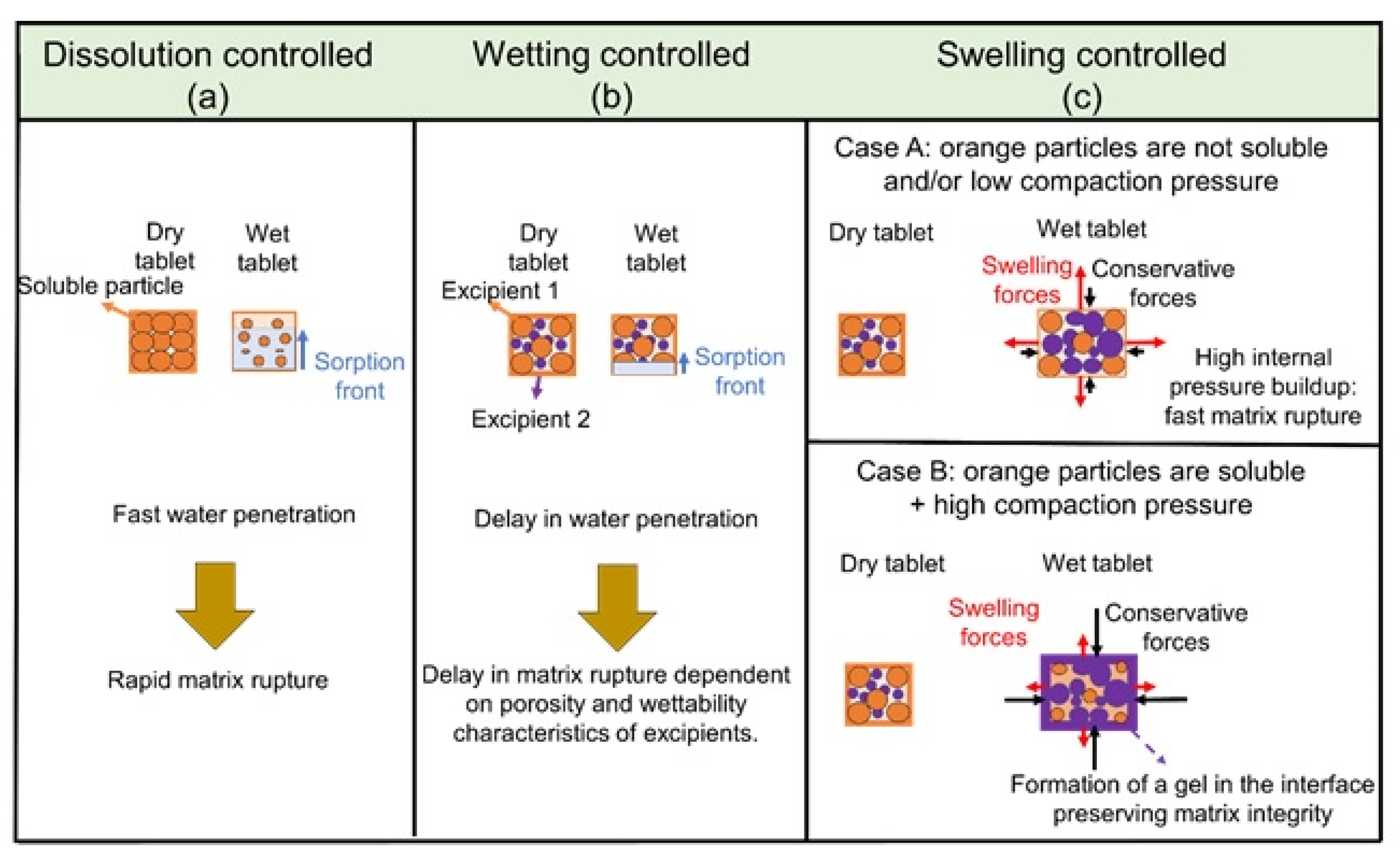
3.1. Mechanisms of Disintegration
3.2. Mechanisms of Dissolution
4. Dependence of Liquid Transport Kinetics on Tablet Microstructure Design
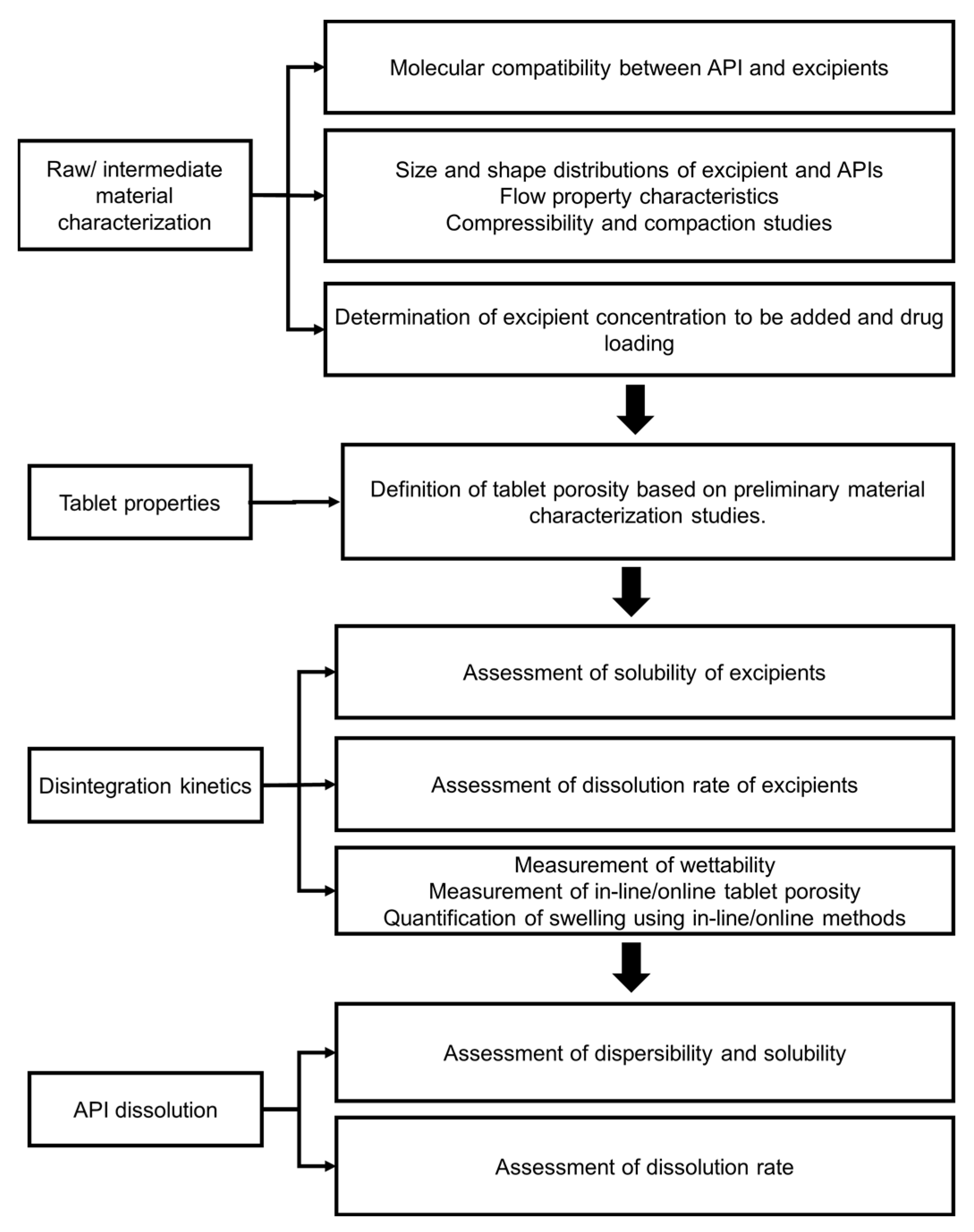
4.1. Effect of Processing on Tablet Microstructure
4.1.1. Intermediate Granule Properties Affected by Dry or Wet Granulation
4.1.2. Uniaxial Compression/Tableting
4.2. Effect of Formulation Design on Disintegration and Dissolution Kinetics
4.2.1. Influence of Porosity
4.2.2. Influence of Excipient Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berardi, A.; Bisharat, L.; Quodbach, J.; Rahim, S.A.; Perinelli, D.R.; Cespi, M. Advancing the understanding of the tablet disintegration phenomenon- An update on recent studies. Int. J. Pharmaceut. 2021, 598, 120390. [Google Scholar] [CrossRef] [PubMed]
- Yekpe, K.; Sbatzoglou, N.; Bataille, B.; Gosselin, R.; Sharkawi, T.; Simard, J.; Cournoyer, A. Predicting the dissolution behavior of pharmaceutical tablets with NIR chemical imaging. Int. J. Pharmaceut. 2015, 486, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.; Webster, G.K.; Bou-Chacra, N.; Lobenberg, R. The significance of disintegration testing in pharmaceutical development. Dissolut. Technol. 2018, 25, 30–38. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, A.J. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef]
- Sun, C.C. Microstructure of tablet- pharmaceutical significance, assessment, engineering. Pharm. Res. 2017, 34, 918–928. [Google Scholar] [CrossRef]
- Yohannes, B.; Gonzalez, M.; Abebe, A.; Sprockel, O.; Cuitino, K. Evolution of the microstructure during the process of consolidation and bonding in soft granular solids. Int. J. Pharmaceut. 2016, 503, 68–77. [Google Scholar] [CrossRef]
- Skelbæk-Pedersen, A.L.; Al-Sharabi, M.; Vilhelmsen, T.K.; Rantanen, J.; Zeitler, J.A. Effect of particle size and deformation behavior on water ingress into tablets. Int. J. Pharmaceut. 2020, 587, 119645. [Google Scholar] [CrossRef]
- Markl, D.; Wang, P.; Ridway, C.; Karttunen, A.; Chakraborty, M.; Bawuah, P.; Paakkonen, P.; Gane, P.; Ketolainen, J.; Peiponen, K.; et al. Characterization of the pore structure of functionalized calcium carbonate tablets by Terahertz Time-Domain Spectroscopy and X-ray Computed Microtomography. J. Pharm. Sci. 2017, 106, 1586–1595. [Google Scholar] [CrossRef]
- Al-Sharabi, M.; Markl, D.; Dudley, T.; Bawuah, P.; Karttunen, A.; Ridway, C.; Gane, P.; Ketolainen, J.; Peiponen, K.-E.; Rades, T.; et al. Simultaneous investigation of the liquid transport and swelling performance during tablet disintegration. Int. J. Pharmaceut. 2020, 584, 119–380. [Google Scholar] [CrossRef]
- Lee, J.B.; Radu, A.I.; Vontobel, P.; Derome, D.; Carmeliet, J. Absorption of impinging water droplet in porous stones. J. Colloid Interface Sci. 2016, 471, 59–70. [Google Scholar] [CrossRef]
- Vaitukaitis, P.; Maggiolo, D.; Remmelgas, J.; Abrahmsen-Alami, S.; Bernin, D.; Siiskonen, M.; Malmqvist, J.; Sasic, S.; Sardina, G. Water transport and absorption in pharmaceutical tablets- a numerical study. Meccanica 2020, 55, 421–433. [Google Scholar] [CrossRef]
- Brielles, N.; Chantraine, F.; Viana, M.; Chulia, D.; Branlard, P.; Rubinstenn, G.; Lequeux, F.; Mondain-Monval, M. Dissolution of a surfactant-containing active porous material. J. Colloid Interface Sci. 2008, 328, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Forny, L.; Althaus, T.; Dopfer, D.; Niederreiter, G.; Palzer, S. Compaction of food powders: The influence of material properties and process parameters on product structure, strength, and dissolution. Chem. Eng. Sci. 2017, 167, 29–41. [Google Scholar] [CrossRef]
- Donofrio, T.G.; Navaz, H.K.; Markicevic, B.; Mantooth, B.A.; Sumpter, K.B. Experimental and numerical study of spread and sorption of VX sessile droplets into medium grain-size sand. Langmuir 2009, 26, 331723. [Google Scholar] [CrossRef] [PubMed]
- Markicevic, B.; Li, H.; Sikorski, Y.; Zand, A.R.; Sanders, M.; Navaz, H.K. Infiltration time and imprint shape of a sessile droplet imbibing porous medium. J. Colloid Interface Sci. 2009, 336, 698. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.C., Jr.; Griffiths, R.F.; Santos, J.M. Numerical simulation of the impact of liquid droplets on porous surfaces. J. Comput. Phys. 2004, 198, 747. [Google Scholar] [CrossRef]
- Rosenholm, J.B. Liquid spreading on solid surfaces and penetration into porous matrices: Coated and uncoated papers. Adv. Colloid Interface Sci. 2015, 220, 8–53. [Google Scholar] [CrossRef]
- Gillespie, T. The capillary rise of a liquid in a vertical strip of filter paper. J. Colloid Sci. 1959, 14, 123–130. [Google Scholar] [CrossRef]
- Borhan, A.; Rungta, K.K. An experimental study of the radial penetration of liquids in thin porous substrates. J. Colloid Interface Sci. 1993, 158, 403–411. [Google Scholar] [CrossRef]
- Markl, D.; Yassin, S.; Wilson, D.I.; Goodwin, D.J.; Anderson, A.; Zeitler, J.A. Mathematical modelling of liquid transport in swelling pharmaceutical immediate release tablets. Int. J. Pharmaceut. 2017, 526, 1–10. [Google Scholar] [CrossRef]
- Schott, H. Kinetics of swelling of polymers and their gels. J. Pharm. Sci. 1990, 81, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.H.C. Particle Mechanics Approach to Modeling Disintegration of Confined Granular Systems due to Hydration and Swelling. Ph.D. Dissertation, Purdue University, West Lafayete, IN, USA, 2021; pp. 28–69. [Google Scholar]
- Perez-Gandarillas, L.; Mazor, A.; Lecoq, O.; Michrafy, A. Compaction properties of dry granulated powders based on Drucker-Prager Cap model. Powder Technol. 2018, 337, 43–50. [Google Scholar] [CrossRef]
- Wang, Z.; Shah, U.V.; Olusanmi, D.; Narang, A.S.; Hussain, M.A.; Gamble, J.F.; Tobyn, M.J.; Heng, J.Y.Y. Measuring the sticking of mefenamic acid powders on stainless steel surface. Int. J. Pharmaceut. 2015, 496, 407–413. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, F. Micromechanical analysis on the compaction of tetrahedral particles. Chem. Eng. Res. Des. 2018, 136, 10–619. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Chapter 9: Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation: Theory and Practice in a Quality by Design Paradigm, 1st ed.; Narang, A.S., Badawy, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 317–349. [Google Scholar]
- Markl, D.; Strobel, A.; Scholossnikl, R.; Botker, J.; Bawuah, P.; Ridway, C.; Rantanen, J.; Rades, T.R.; Gane, P.; Peiponen, K.; et al. Characterization of pore structures of pharmaceutical tablets: A review. Int. J. Pharmaceut. 2018, 538, 188–214. [Google Scholar] [CrossRef]
- Bawuah, P.; Markl, D.; Turner, A.; Evans, M.; Portieri, A.; Farrell, D.; Lucas, R.; Anderson, A.; Goodwin, D.J.; Zeitler, J.A. A fast and non-destructive Terahertz dissolution assay for immediate release tablets. J. Pharm. Sci. 2020, 110, 2083–2092. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B-Phys. 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Kiortsis, S.; Kachrimanis, K.; Broussali, T.; Malamataris, S. Drug release from tableted wet granulations comprising cellulosic (HPMC or HPC) and hydrophobic component. Eur. J. Pharm. Biopharm. 2005, 59, 73–83. [Google Scholar] [CrossRef]
- Catellani, P.; Predella, P.; Bellotti, A.; Colombo, P. Tablet water uptake and disintegration force measurements. Int. J. Pharmaceut. 1989, 51, 63–66. [Google Scholar] [CrossRef]
- Yin, X.; Li, L.; Gu, X.; Wang, H.; Wu, L.; Qin, W.; Xiao, T.; York, P.; Zhang, P.; Mao, S. Dynamic structure model of polyelectrolyte complex based controlled-release matrix tablets visualized by synchrotron radiation micro-computed tomography. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 116, 111137. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Guo, Z.; Wu, L.; Chen, F.; Matas, M.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Quantification of swelling and erosion in the controlled release of a poorly water-soluble drug using synchrotron X-ray computed microtomography. The AAPS J. 2013, 15, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Reuhs, B.L.; Simsek, S.N. Nuclear Magnetic Resonance. In Food Analysis, 5th ed.; Nielsen, S.S., Ed.; Springer: Berlin/Heidelberg, Germany; Purdue University: West Lafayette, IN, USA, 2010. [Google Scholar]
- Tritt-Goc, J.; Pislewski, N. Magnetic resonance imaging study of the swelling kinetics of hydroxypropyl methylcellulose (HPMC) in water. J. Control. Release. 2002, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Borompichaichartkul, C.; Moran, G.; Srzednicki, G.; Price, W.S. Nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) studies of corn at subzero temperatures. J. Food Eng. 2005, 69, 199–205. [Google Scholar] [CrossRef]
- Antonio, M.; Maggio, R.M. Assessment of mefenamic acid polymorphs in commercial tablets using chemometric coupled to MIR and NIR spectroscopies. Prediction of dissolution performance. J. Pharm. Biomed. Anal. 2018, 149, 603–611. [Google Scholar] [CrossRef]
- Abe, H.; Otsuka, M. Effects of lubricant-mixing time on prolongation of dissolution time and its prediction by measuring near infrared spectra from tablets. Drug Dev. Ind. Pharm. 2012, 38, 412–419. [Google Scholar] [CrossRef]
- Pawar, P.; Wang, Y.; Keyvan, G.; Callegari, G.; Cuitino, A.; Muzzio, F. Enabling real time release testing by NIR prediction of dissolution of tablets made by continuous direct compression (CDC). Int. J. Pharmaceut. 2016, 512, 96–107. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Li, J.; Jiang, S.; Wang, Y.; Zhang, X.; Ding, J.; Yu, T.; Mao, S. Insights into the mechanisms of chitosan–anionic polymers-based matrix tablets for extended drug release. Int. J. Pharmaceut. 2014, 476, 253–265. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Bar-Shalom, D.; Mu, H.; Larsen, S.W.; Jensen, H.; Ostergaard, J. Towards functional characterization of excipients for oral solid dosage forms using UV-vis imaging. Liberation, release and dissolution. J. Pharm. Biomed. Anal. 2021, 194, 113789. [Google Scholar] [CrossRef]
- Ward, A.; Walton, K.; Mawla, N.; Kaialy, W.; Liu, L.; Timmins, P.; Conway, B.R.; Asare-Addo, K. Development of a novel method utilizing dissolution imaging for the measurement of swelling behaviour in hydrophilic matrices. Int. J. Pharmaceut: X. 2019, 1, 100013. [Google Scholar]
- Ward, A.; Walton, K.; Stoycheva, S.; Wallis, M.; Adebisi, A.; Nep, E.; Ngwuluka, N.C.; Shaboun, S.; Smith, A.M.; Conway, B.R.; et al. The use of visible and UV dissolution imaging for the assessment of propranolol hydrochloride in liquisolid compacts of sesamum radiatum gum. J. Drug Deliv. Sci. Technol. 2020, 56, 101511. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Basit, A.W. Solid oral dosage forms. In The science and Practice of Pharmacy, 23th rd.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 333–358. [Google Scholar]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Corrigan, O.I. Mechanisms of dissolution of fast release solid dispersions. Drug Dev. Ind. Pharm. 1985, 11, 697–724. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharmaceut. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Bhutani, U.; Basu, T.; Majumdar, S. Oral drug delivery: Conventional to long acting new-age designs. Eur. J. Pharm. Biopharm. 2021, 162, 23–42. [Google Scholar] [CrossRef]
- Gupta, A.; Hunt, R.L.; Shah, R.B.; Sayeed, V.A.; Khan, M.A. Disintegration of highly soluble immediate release tablets: A surrogate for dissolution. AAPS PharmSciTech. 2009, 10, 495–499. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Dissolution in IUPAC Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientifc Publications: Oxford, UK, 1997; Available online: https://goldbook.iupac.org/D01806.html (accessed on 8 January 2023).
- IUPAC. Solvation in IUPAC Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientifc Publications: Oxford, UK, 1997; Available online: https://goldbook.iupac.org/S05747.html (accessed on 8 January 2023).
- Seager, R.J.; Acevedo, A.J.; Spill, F.; Zaman, M.H. Solid dissolution in a fluid solvent is characterized by the interplay of surface area-dependent diffusion and physical fragmentation. Sci. Rep. Nat. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, H.; Rohera, B.D. Fundamentals of powder compression. I. The compactibility and compressibility of pharmaceutical powders. Pharm. Res. 1986, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Sofronis, P. Modeling the interaction between densification mechanisms in powder compaction. Int. J. Solids Struct. 2001, 38, 7899–7918. [Google Scholar] [CrossRef]
- Nordström, J.; Persson, A.S.; Lazorova, L.; Frenning, G.; Alderborn, G. The degree of compression of spherical granular solids controls the evolution of microstructure and bond probability during compaction. Int. J. Pharmaceut. 2013, 442, 3–12. [Google Scholar] [CrossRef]
- Nicklasson, F.; Johansson, B.; Alderborn, G. Tabletting behaviour of pellets of a series of porosities—A comparison between pellets of two different compositions. Eur. J. Pharm. Sci. 1999, 8, 11–17. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Reklaitis, G.V.; Venkatasubramanian, V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: Process dynamics and control framework. J. Pharmaceut. Innov. 2010, 5, 14–23. [Google Scholar] [CrossRef]
- Gupta, A.; Peck, G.E.; Miller, R.W.; Morris, K.R. Real-time near-infrared monitoring of content uniformity, moisture content, compact density, tensile strength, and Young’s modulus of roller compacted powder blends. J. Pharmaceut. Sci. 2005, 94, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.C.; Winstead, D.; Zavaliangos, A. Understanding variation in roller compaction through finite element-based process modeling. Comp. Chem. Eng. 2010, 34, 1058–1071. [Google Scholar] [CrossRef]
- Maharjan, R.; Jeong, S.H. High shear seeded granulation: Its preparation mechanism, formulation, process, evaluation, and mathematical simulation. Powder Tech. 2020, 366, 667–688. [Google Scholar] [CrossRef]
- Johansen, A.; Schaefer, T. Effects of interactions between powder particle size and binder viscosity on agglomerate growth mechanisms in a high shear mixer. Eur. J. Pharm. Sci. 2001, 12, 297–309. [Google Scholar] [CrossRef]
- de Koster, S.A.L.; Pitt, K.; Litster, J.D.; Smith, R.M. High-shear granulation: An investigation into the granule consolidation and layering mechanism. Powder Tech. 2019, 355, 514–525. [Google Scholar] [CrossRef]
- Iveson, S.M.; Litster, J.D.; Hapgood, K.; Ennis, B.J. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Tech. 2001, 117, 3–39. [Google Scholar] [CrossRef]
- Pradhan, S. Quality by Design in Twin Screw Granulation; Purdue University: West Lafayette, IN, USA, 2017. [Google Scholar]
- Hoffman, T.; Rieck, C.; Buck, A.; Peglow, M.; Tsotsas, E. Influence of granule porosity during fluid bed spray granulation. Proced. Eng. 2015, 102, 458–467. [Google Scholar] [CrossRef]
- Diez, E.; Meyer, K.; Buck, A.; Tsotsas, E.; Heinrich, S. Influence of process conditions on the product properties in a continuous fluidized bed spray granulation process. Chem. Eng. Res. Des. 2018, 139, 104–115. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of solids—On the contact of rigid elastic solids and on hardness. Misc. Pap. 1896, 146–183. [Google Scholar]
- Van Veen, B.; Van der, V.; Maarschalk, K.; Bolhuis, G.; Zuurman, K.; Frijlink, H. Tensile strength of tablets containing two materials with a different compaction behaviour. Int. J. Pharmaceut. 2000, 203, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.G.; Klose, K.; Kleinebudde, P. Comparison of different dry binders for roll compaction/dry granulation. Pharm. Dev. Technol. 2007, 12, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Arndt, O.-R.; Kleinebudde, P. Towards a better understanding of dry binder functionality. Int. J. Pharmaceut. 2018, 552, 258–264. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Ford, J.L.; Rubinstein, M.H. Studies on the interaction between water and hydroxypropyl methylcellulose. J. Pharm. Sci. 1997, 86, 608–615. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer properties and characterization. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice, 2nd ed.; Qiu, Y., Zhang, G.G.Z., Porter, W.R., Chen, Y., Liu, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 181–223. [Google Scholar]
- Wang, J.; Flanagan, D.R. Fundamentals of dissolution. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Qiu, Y., Zhang, G.G.Z., Mantri, R.V., Chen, Y., Yu, L., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 309–318. [Google Scholar]
- Maclean, N.; Walsh, E.; Soundaranathan, M.; Khadra, I.; Mann, J.; Williams, H.; Markl, D. Exploring the performance-controlling tablet disintegration mechanisms for direct compression formulations. Int. J. Pharmaceut. 2021, 599, 120221. [Google Scholar] [CrossRef]
- Rubinstein, M.H.; Birch, M. The effect of excipient solubility on the in-vitro and in-vivo properties of bendrofluazide tablets 5 mg. Drug Dev. Ind. Pharm. 1977, 3, 439–450. [Google Scholar] [CrossRef]
- Markl, D.; Warman, W.; Dumarey, M.; Bergman, E.; Folestad, S.; Shi, Z.; Manley, L.F.; Goodwin, D.J.; Zeitler, J.A. Review of real time release testing of pharmaceutical tablets: State of the art, challenges and future perspectives. Int. J. Pharmaceut. 2020, 580, 119353. [Google Scholar] [CrossRef]
- Johnson, J.; Wang, L.; Gordon, M.S.; Chowhan, Z.T. Effect of formulation solubility and hygroscopicity on disintegrant efficiency in tablets prepared by wet granulation, in terms of dissolution. J. Pharm. Sci. 1991, 80, 469–471. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. Quality by Design applied development of immediate-release rabeprazole sodium dry-coated tablet. Pharmaceut. 2021, 13, 259. [Google Scholar] [CrossRef]
- Oh, G.-H.; Park, J.-H.; Shin, H.-W.; Kim, J.-E.; Park, Y.-J. Quality-by-design approach for the development of telmisartan potassium tablets. Drug Dev. Ind. Pharm. 2018, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.M.; Rohera, B.D. An integrated, quality by design (QbD) approach for design, development and optimization of orally disintegrating tablet formulation of carbamazepine. Pharm. Dev. Technol. 2017, 22, 889–903. [Google Scholar] [CrossRef] [PubMed]

| Methods | Description | References |
|---|---|---|
| Measuring water uptake Swelling force measurement | Water uptake can be measured based on changes in weight gained by the tablet using a microbalance. The swelling force is measured by fixing the tablet in a cage attached with a load cell, which records the force displacement over time. | Dees (1980) as cited in Markl and Zeitler [4,31] |
| Terahertz pulsed imaging (TPI) | TPI measures the difference in the refractive index of dry material and liquid, resulting in the reflection of the terahertz pulse at the solid–liquid interface. The change in refractive index as a function of time records the liquid penetration through the porous matrix. The pore size, shape, and compact density can also be assessed using this technique. The swelling rate can be determined from the shift in reflectance. | [8] |
| Erodibility | Erosion of porous tablets can be evaluated by weighing the tablets upon drying and removing excess liquid. | [32] |
| X-ray microtomography (XRµT) | Based on the principle of voxelization, 2-D X-ray images are reconstructed and converted into 3-D images. Pore volume, size distributions, degree of anisotropy, and the distinction between open and closed pores can also be computed with this technique. The porosity distributions as a function of dissolution time can be measured offline by freeze-drying samples before the analysis. The pore size and density can be plugged into the capillary transport models to map the liquid distribution. The swelling ratio can also be computed using the changes in volume, measured using XRµT, and the change in weight. | [33,34] |
| Nuclear magnetic resonance (NMR) | The NMR technique can be used to map the movement of water in a solid matrix and calculate the diffusion coefficient by studying the decrease in the solid’s NMR spectra signal and the increase in the liquid’s NMR spectra. NMR imaging instruments can be used to map the swelling kinetics and diffusion behavior of water in dry compacted tablets. | [35,36] |
| Magnetic resonance imaging (MRI) | In this technique, 2D or 3D images are obtained by placing a sample into a magnet and varying the field strength and the frequency of pulses over time and space. The result of the analysis represents a distribution of the sample protons at different phases and frequencies. The free induction decay can be analyzed using multidimensional Fourier transformation to produce spatial slices of the sample. The images can present information, for example, about the water distribution patterns in solid samples and the changes in granular microstructures as a function of the water ingress. | [36,37] |
| Near Infrared Spectroscopy (NIR) | NIR is used as an in-line process analytical tool to estimate the number of compounds in a formulation, the level of strain recovery, the amount and particle size of formulation constituents, the ratio between polymorphic forms, and compression force in direct compression experiments. This is a robust technique that provides information for the prediction of the disintegration and dissolution kinetics. | [2,38,39,40,41,42,43] |
| Texture analyzer | Force displacement profiles can be directly measured using the penetration of a probe within a compact. The force profile is directly proportional to the strength of the tablets. A disadvantage of this technique over NRI, microCT, and NMR-MRI is the lack of detail on the porosity distribution and swelling kinetics. | [40] |
| Visible and UV-dissolution imaging | This technique is widely used in intrinsic dissolution tests and transport studies and has recently been used to investigate drug liberation mechanisms and excipient properties, such as swelling kinetics. The reduced imaging area restricts its use to small-size tablets. | [41,42,43] |
| Type of Oral Dosage Form | Description |
|---|---|
| Orally disintegrating tablets | Offer rapid disintegration within the oral cavity in the presence of salivary fluid. They are generally produced using freeze-drying or loose compression and recently using 3-D printing technology to improve dosage content. These tablets can be used as immediate-release agents. |
| Lozenges | Require slow dissolution in the mouth, typically dedicated to the relief of cough, sore throat problems, mild anesthesia, or antiseptics. These tablets can be used as extended-release agents. |
| Layered tablets | Multi-layered tablets containing multiple layers on top of each other and press-coated tablets whereby a core tablet is enclosed with a shell tablet. These tablets can be used in extended- and delayed-release applications. |
| Effervescent tablets | Intended to release carbon dioxide upon immersion in water, which prompts its disintegration. The chemical reaction to produce CO2 is a result of the interaction between carbonate or bicarbonate salt and a weak acid in the presence of water. Upon ingestion, the gastric pH levels enable rapid drug absorption from the upper small intestine. These immediate-release formulas are often manufactured using direct compression or with granulation followed by compaction. |
| Buccal or sublingual | The mechanism of drug absorption is by positioning the tablet into the internal mucosa of the cheek (buccal) or under the tongue. The drug is absorbed through systemic circulation. The surface area of these tablets is usually small, ensuring good adhesion properties. Gums or bioadhesives can be added to improve adherence. These tablets also possess higher porosity for fast disintegration and absorption of the drug and are, therefore, classified as immediate-release products. Polymers with a melting temperature close to body temperature can also be added to the formula to trigger rapid glassy–rubbery transition and, hence, fasten drug dispersion. |
| Formulation/Process Design | Design Characteristics |
|---|---|
| Roll hydraulic pressure (kPa) | High roll pressures achieved by adjusting the gap between the rollers can increase the interparticulate bonding and accelerate material densification, consequently generating ribbons with superior relative densities (low porosity). By evaluating the interaction between the input parameters using the Johanson’s rolling theory coupled with mass balance in the nip region, Hsu et al. [57] stated that the roll pressure exhibits the most significant effect on the ribbon porosity. Roll pressure can also influence granule properties, including particle size and porosity |
| Roll gap | Roll gap defines the thickness of ribbons and can influence the porosity of ribbons, but this parameter is highly correlated with roll hydraulic pressure, roll speed, feed, and tamp auger speeds. Therefore, caution should be exerted when manipulating this variable to ensure proper consideration of the effect of hydraulic pressure and roll speed on the roll gap. |
| Roll speed (rpm) | A reduction in roll speed can produce ribbons with low porosity, depending on the formulation, because of a rise in contact time of the densifying material within the nip region [57,58]. |
| Feed speed (rpm) | The design of the control system should include only one of the speeds as the manipulated variable to maintain a constant ratio between feed speed and roll speed. If this ratio is not constant, then slippage and burning of the powder material between the rollers can lead to product degradation. |
| Granulator sieve types | Granulator screens are selected to determine granule size. Milling occurs clockwise and counterclockwise in oscillating screens. Meanwhile, milling occurs only in one direction in rotating screens. In general, both screen types generate comparable particle size distributions. |
| Granulator speed (rpm) | An increase in granulator speed can reduce granule particle size. The effect of granulation speed on granule porosity depends on the ribbon relative density. |
| Granulator type (−) | There are two main modes of granulators: abrasive fracture and impact fracture. Both methods produce granules with similar particle size distributions and granule strength. |
| Formulation/Process Design | Design Characteristics |
|---|---|
| Feed powder porosity (−) | Porous powders ensure more consolidation in both high and low shear techniques. While less porous powders produce less consolidation and, therefore, more porous granules. For instance, high porosity of primary particles (e.g., MCC) result in enhanced liquid absorption. The stored liquid content is squeezed out of the powder mass upon shearing, enabling granule consolidation [59]. |
| Liquid to solid (L/S ratio) (−) | High values increase deformability, which varies with formulation (e.g., if the binder is more hydrophilic, less solvent is necessary to promote the binder distribution in the high shear granulation processes). Low values decrease deformability and, therefore, produce granules with higher porosity [60]. |
| Binder viscosity (Pa s) | The viscosity of a binder dictates the strength and size distributions of the resulting granules. Studies unveiled thresholds of viscosity at which granule growth was favored, and an opposite effect was observed after surpassing a critical value [61]. Granulation at low viscosities is controlled by layering growth, while granule coalescence is predominant at high viscosities [62]. |
| Surface tension (N/m) | Ivenson et al. [63] found that a decrease in binder surface tension increases the consolidation rate due to a reduction in capillary suction resisting particle dilation. They also showed that a decrease in particle size or an increase in binder viscosity can lower the consolidation rate. |
| Binder wettability (°) | Defines better interaction between the binder and primary particles; better liquid distribution endows more consolidation in both high and low shear wet granulation. |
| Velocity profile (rpm, m/s) | In high shear mixing processes, a decrease in speed generates less densification and liquid distribution. Meanwhile, an increase in speed can generate more granule consolidation. Seeded granules are also formed more rapidly with a high Stokes deformation number [63]. |
| Powder feed rate (kg/s)/Residence time (s) | In high shear mixing processes, this parameter has varying outputs in the literature. |
| Screw geometry in TSG | Higher granule consolidation in twin screw granulators using kneading elements. However, kneading elements may also cause more granule breakage in size ranges between 2 and 3 mm [64]. |
| Formulation/Process Design | Design Characteristics |
|---|---|
| Distance from the nozzle in FBG (m) | In FBG, particles closer to the nozzle position produce more agglomerates [65]. |
| Bed temperature in FBG (°C) | Higher bed temperatures disrupt the liquid bridge as the binder solidification rate may reduce significantly, especially above binder melting temperature. However, adjustment of the air inlet temperature and binder flow rate can improve granule consolidation because of the effect of these two parameters on the drying potential [65,66]. |
| Binder flow rate in FBG (kg/s) | Aggregation is improved with an increase in binder spray rate [66]. |
| Binder droplet size in FBG (m) | Larger droplet size improves wettability and, therefore, the probability of particle coalescence or aggregation [66]. Better liquid distribution improves granule consolidation and growth. |
| Atomizing pressure in FBG (bar) | Lower atomizing pressure reduces consolidation rate; droplet size is negatively correlated with atomizer pressure [66]. |
| Riser dimension (m) | In a Wurster fluid bed with nozzle positioned at the bottom of the bed, smaller riser diameter implies narrower particle size distributions. This is because of stable flow across the bed, which reduces the gap between the residence time of smaller and larger particles [65]. |
| Drying potential | Slow drying rates result in more porous granules using fluid bed spray granulation (coating deposited onto seed particle’s surfaces). A balance between air inlet temperature, velocity profile, air humidity, and binder flow rate need to be considered to obtain suitable granule consolidation but still yield granules with controlled porosity [65,66]. |
| Powder feed rate (kg/s)/Residence time (s) | In fluid bed granulation, reduced residence time seems to decrease volume solid fraction [65,66]. |
| Velocity profile (m/s) | Fluid bed granulation studies show that high velocity profiles can cause particle breakage [66]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jange, C.G.; Wassgren, C.R.; Ambrose, K. The Significance of Tablet Internal Structure on Disintegration and Dissolution of Immediate-Release Formulas: A Review. Powders 2023, 2, 99-123. https://doi.org/10.3390/powders2010008
Jange CG, Wassgren CR, Ambrose K. The Significance of Tablet Internal Structure on Disintegration and Dissolution of Immediate-Release Formulas: A Review. Powders. 2023; 2(1):99-123. https://doi.org/10.3390/powders2010008
Chicago/Turabian StyleJange, Camila G., Carl R. Wassgren, and Kingsly Ambrose. 2023. "The Significance of Tablet Internal Structure on Disintegration and Dissolution of Immediate-Release Formulas: A Review" Powders 2, no. 1: 99-123. https://doi.org/10.3390/powders2010008
APA StyleJange, C. G., Wassgren, C. R., & Ambrose, K. (2023). The Significance of Tablet Internal Structure on Disintegration and Dissolution of Immediate-Release Formulas: A Review. Powders, 2(1), 99-123. https://doi.org/10.3390/powders2010008






