Scaling Up: Molecular to Meteorological via Symmetry Breaking and Statistical Multifractality
Abstract
:1. Introduction
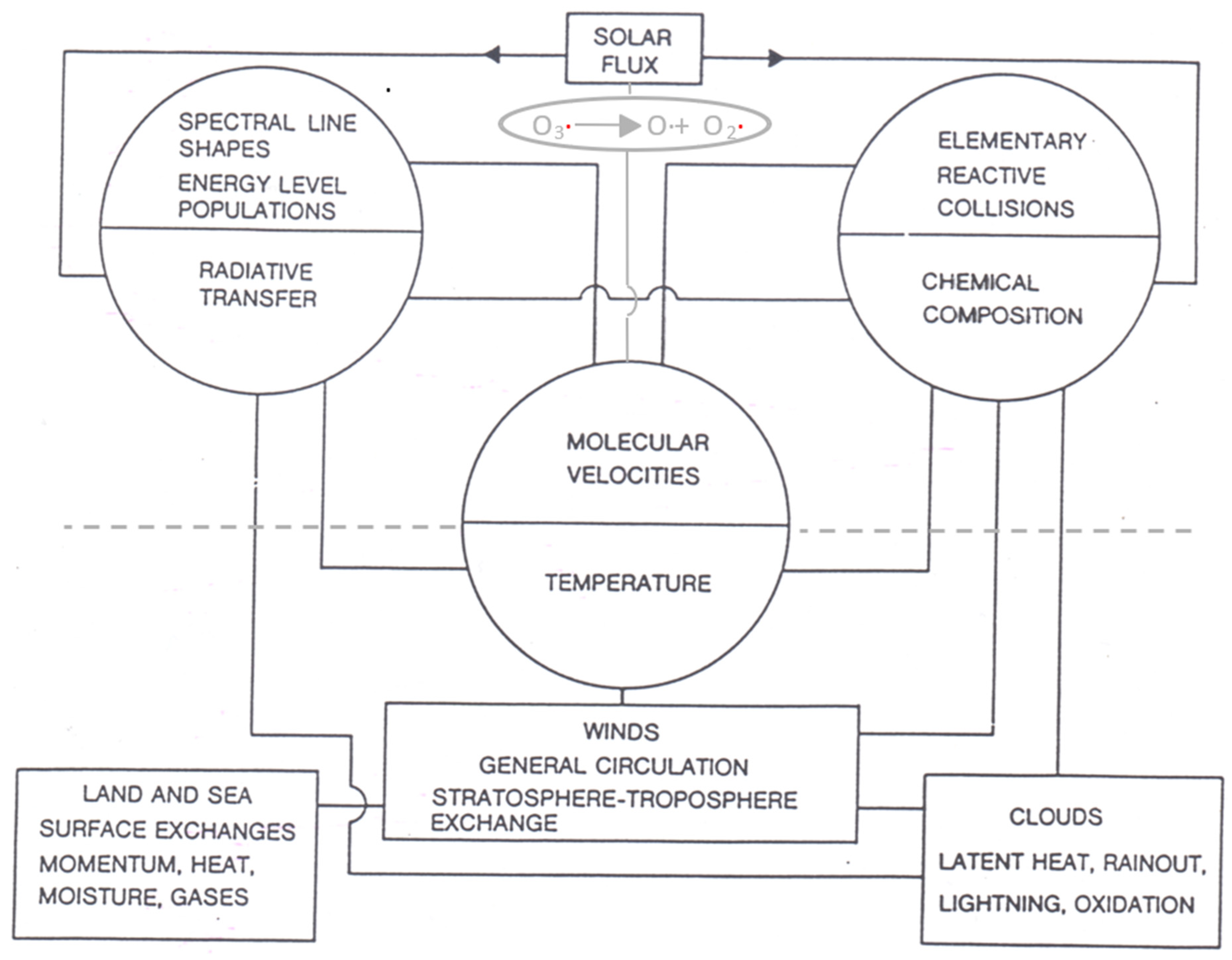
2. Microscopic and Macroscopic Processes
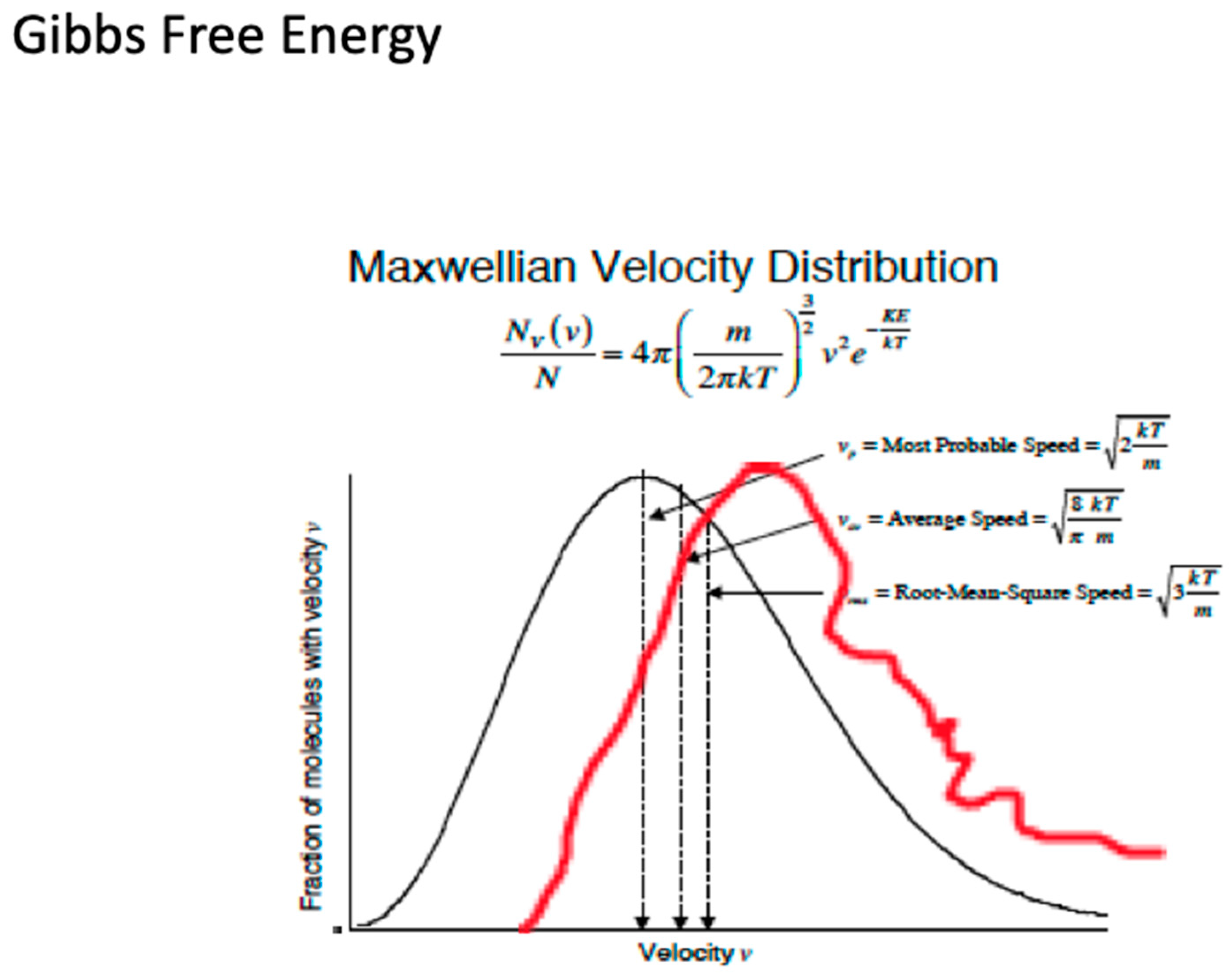
2.1. Persistence of Molecular Velocity after Collision
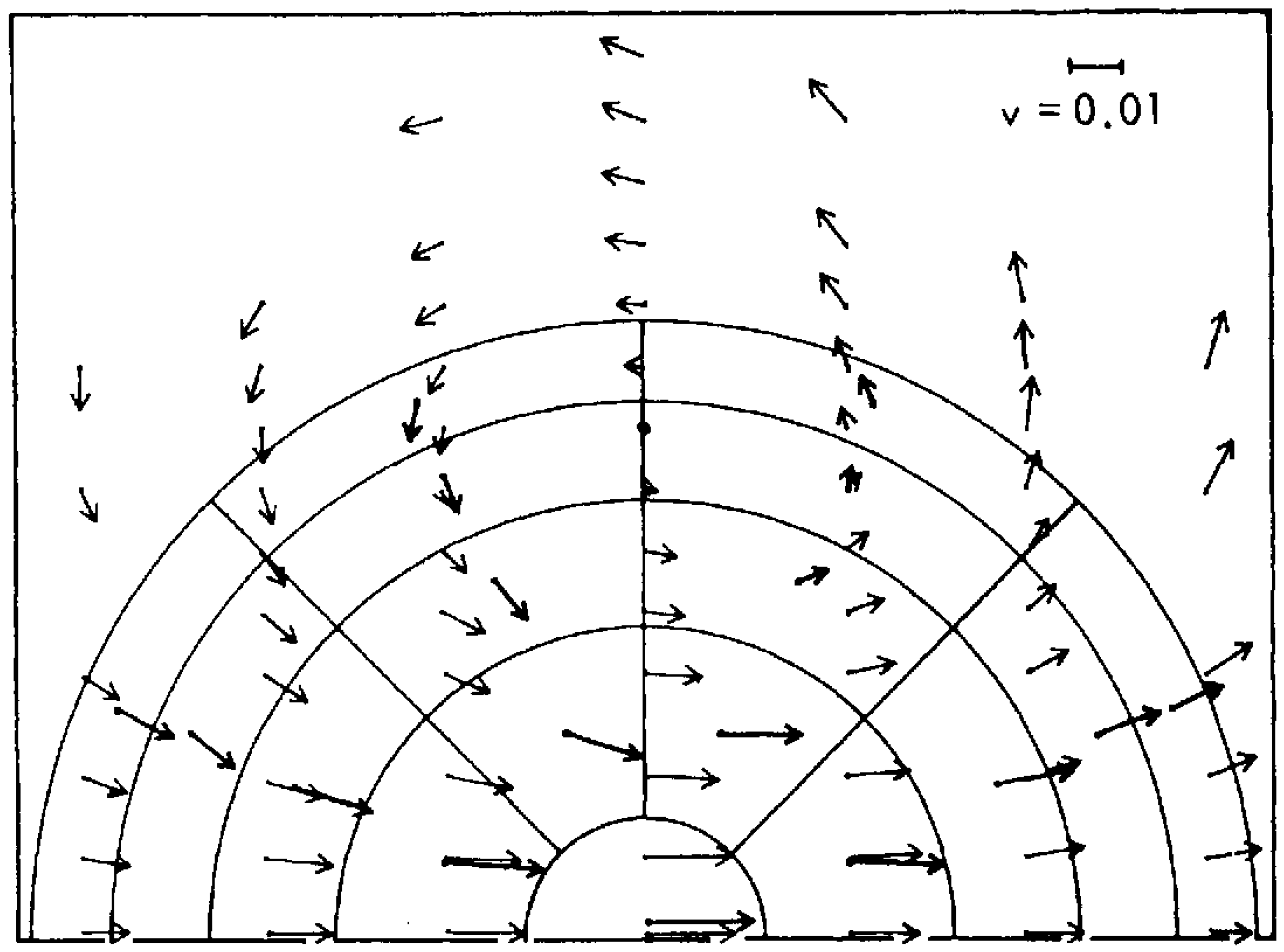
2.2. Emergence of Fluid Flow from a Molecular Population
3. Statistical Multifractality
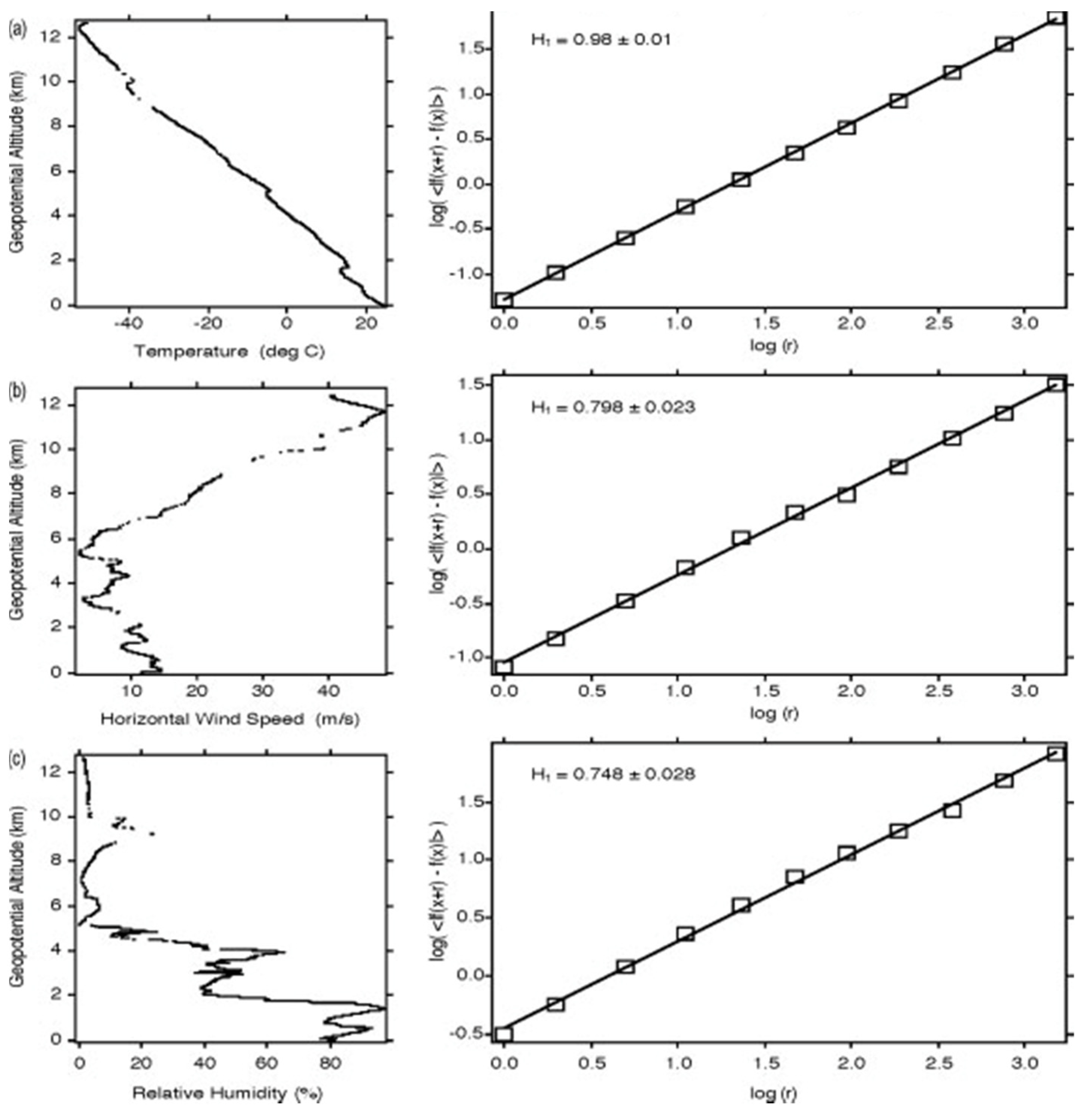
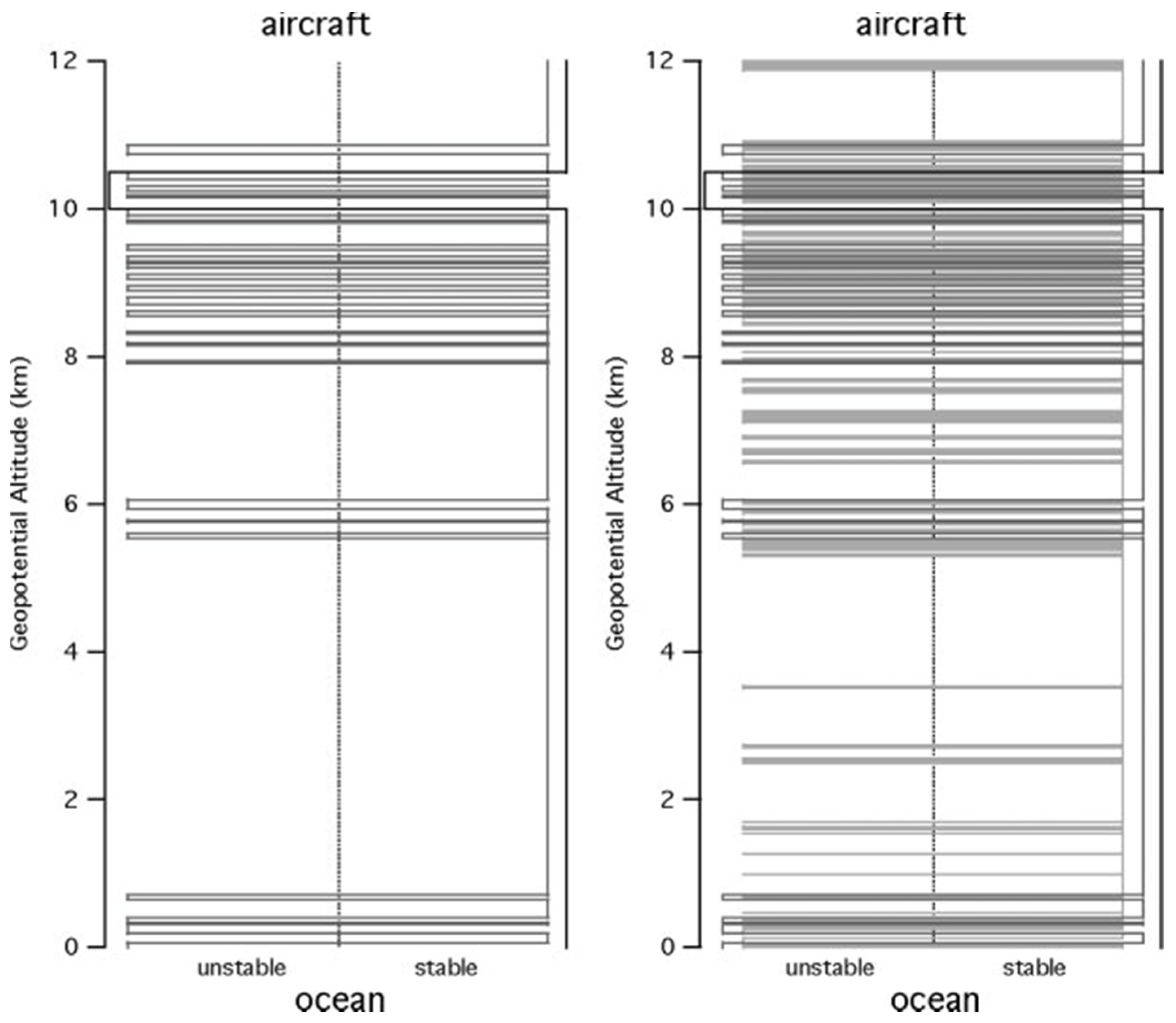
3.1. Vertical Scaling: Horizontal Wind, Temperature and Humidity
3.2. Scaling in Jet Streams
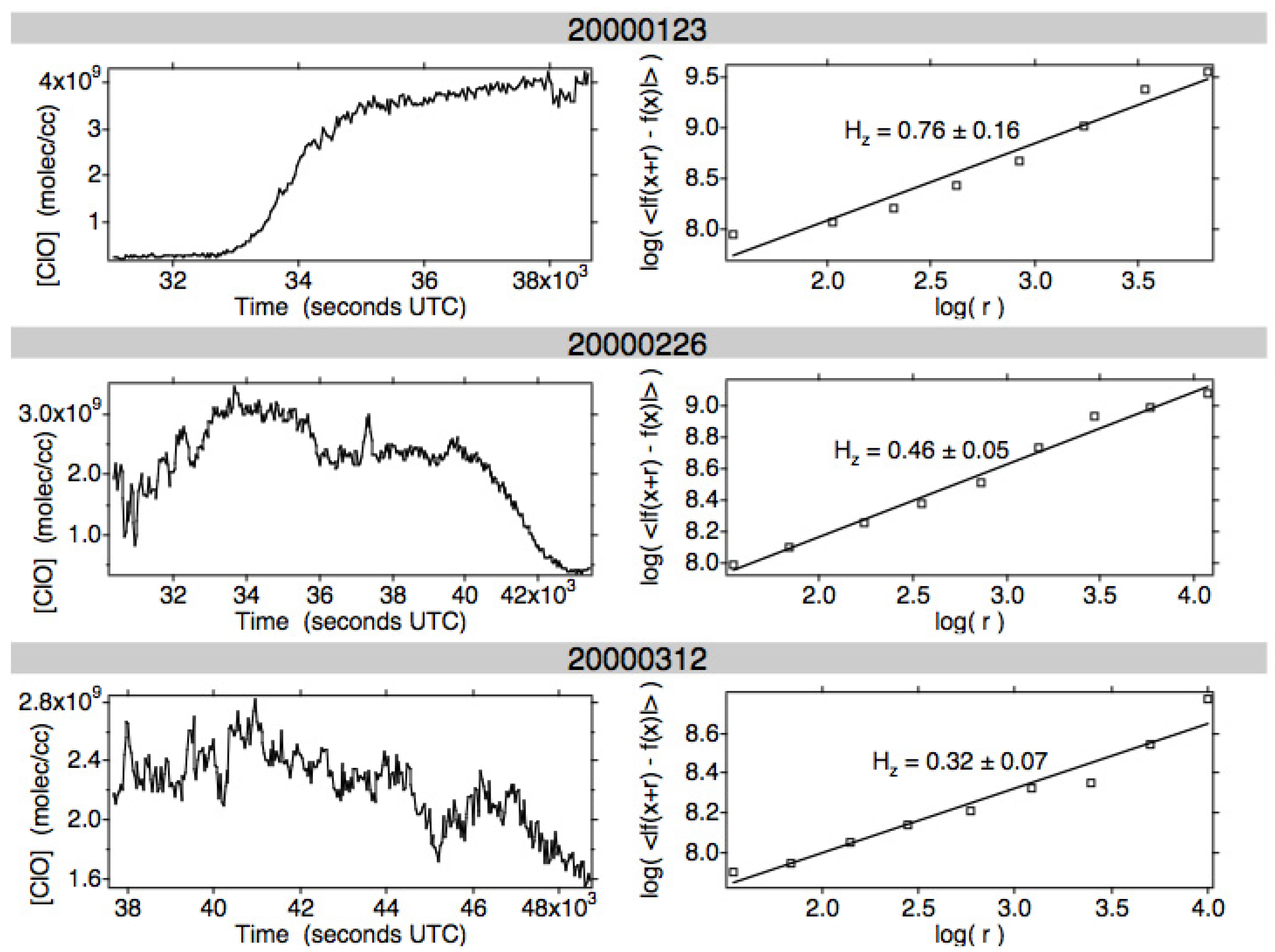
3.3. Molecular and Photochemical Effects
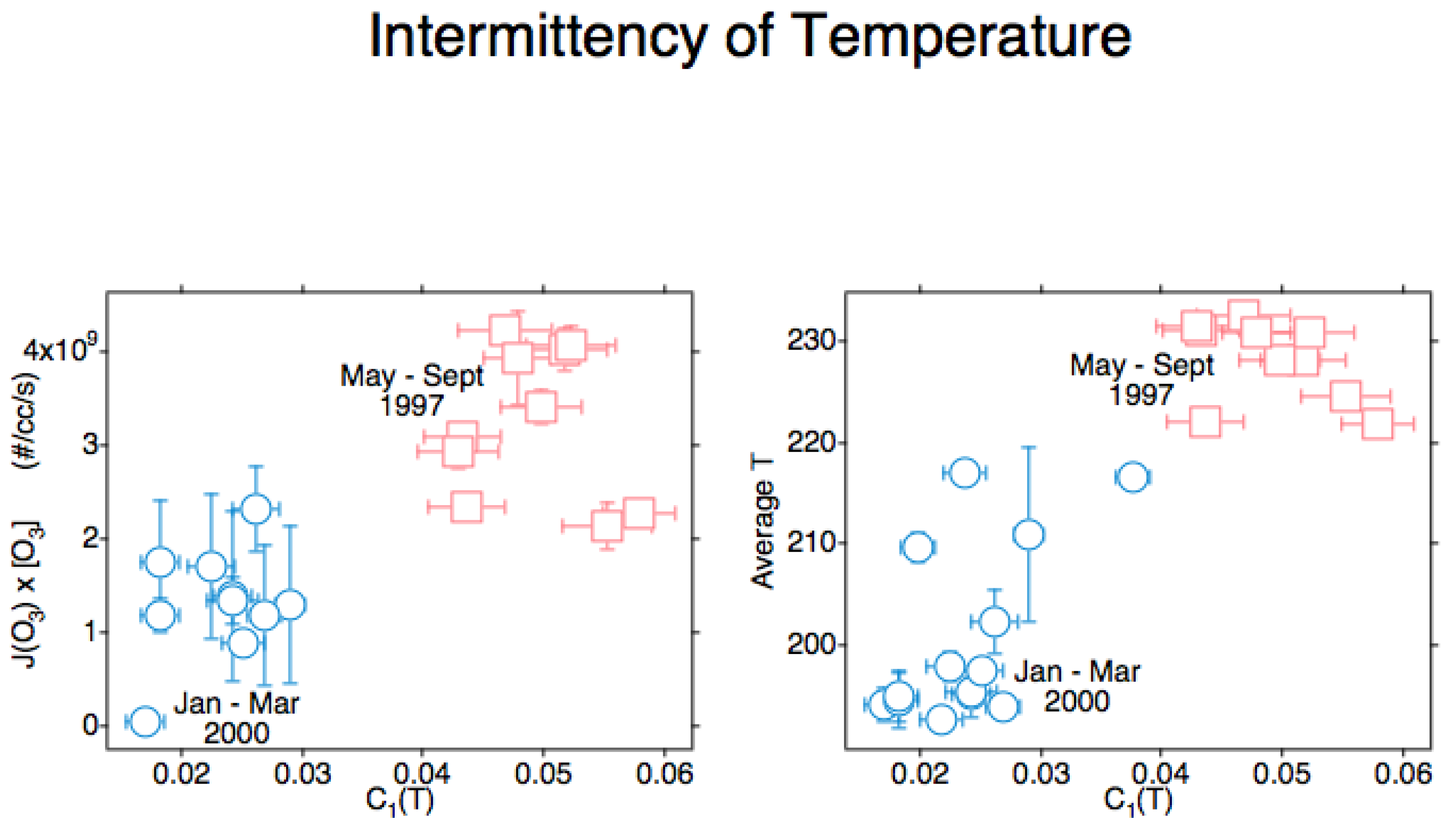
4. The Intermittency of Temperature and Its Correlation with Ozone Photodissociation Rate
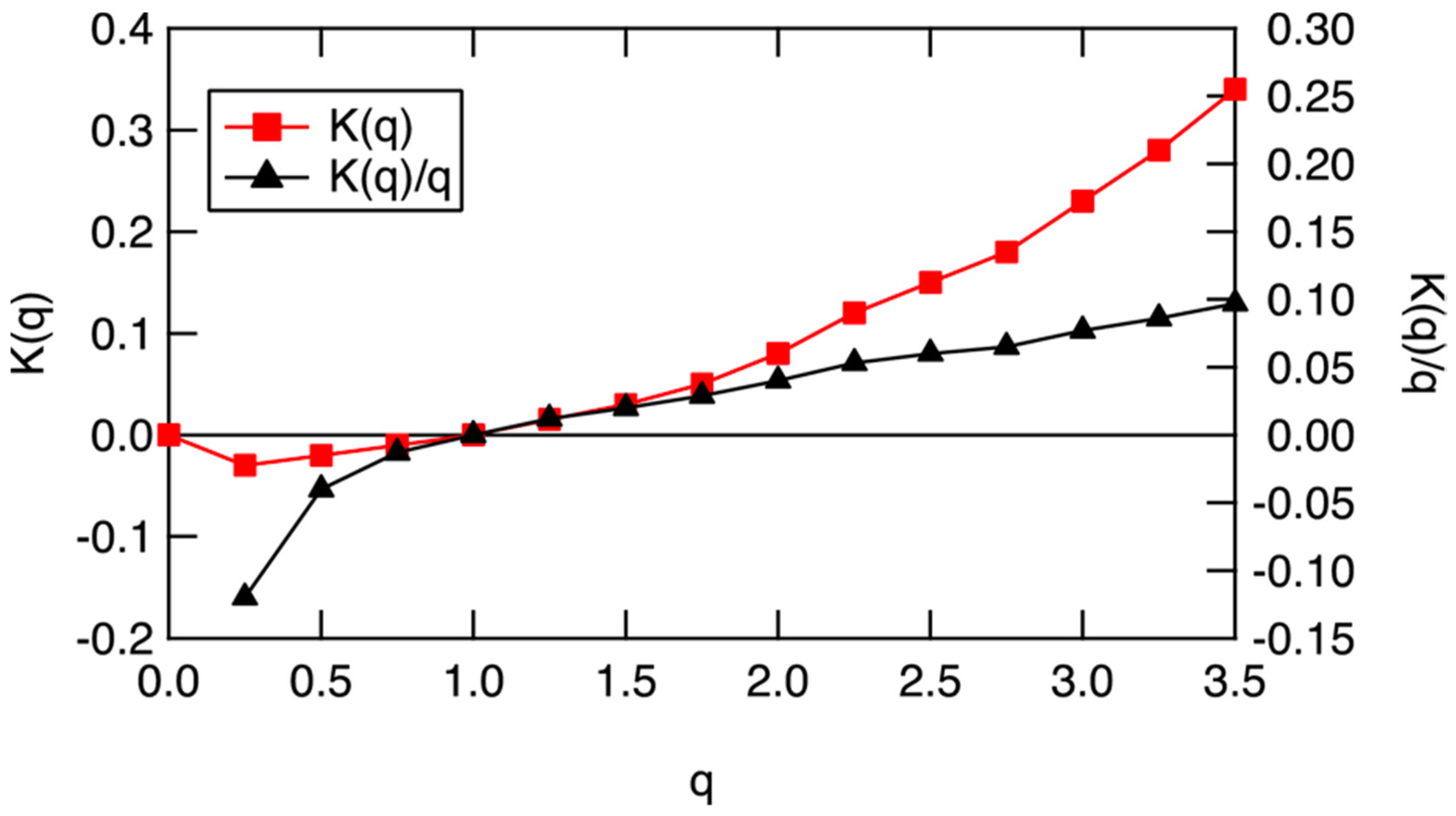
5. Scaling Based Entropy and Gibbs Free Energy
6. Aerosols and Scaling
7. Models and Scaling
7.1. Scaling Issues and Tests for Models
7.2. Scaling and the Cold Bias in Models
7.3. Scaling, Molecules and Maximum Observed Wind Speeds
8. Some Examples
9. Why Turbulence?
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noether, E. Invariante variationsprobleme. In Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen; Vandenhoeck & Ruprecht: Göttingen, Germany, 1918; pp. 235–257. [Google Scholar]
- Landau, L.D. On the theory of phase transformation I&II (in Russian). In Zhurnal Éksperimental’noi i Teoreticheskoi Fiziki 1937; Landau, L.D., ter Haar, D., Eds.; English Translation in Collected Papers; Gordon and Breach: New York, NY, USA, 1965; Volume 11, pp. 26–46, 545. [Google Scholar]
- Anderson, P.W. An approximate quantum theory of the antiferromagnetic ground state. Phys. Rev. 1952, 86, 694–701. [Google Scholar] [CrossRef]
- Higgs, P.W. Broken symmetries and the masses of gauge bosons. Phys. Rev. Lett. 1964, 13, 508–509. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.; Cowling, T.G. The Mathematical Theory of Non-Uniform Gases, 3rd ed.; Chapter 5.5; Cambridge University Press: Cambridge, UK, 1970; pp. 96–99. [Google Scholar]
- Alder, B.J.; Wainwright, T.E. Decay of the velocity autocorrelation function. Phys. Rev. A 1970, 1, 18–21. [Google Scholar] [CrossRef]
- Schertzer, D.; Lovejoy, S. Generalized scale invariance in turbulent phenomena. Physicochem. Hydrodyn. 1985, 6, 623–635. [Google Scholar]
- Schertzer, D.; Lovejoy, S. Physical modeling and analysis of rain and clouds by anisotropic scaling multiplicative processes. J. Geophys. Res. D 1987, 92, 9693–9714. [Google Scholar] [CrossRef]
- Schertzer, D.; Lovejoy, S. (Eds.) Non-Linear Variability in Geophysics: Scaling and Fractals; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Tuck, A.F.; Hovde, S. An examination of stratospheric aircraft data for small-scale variability and fractal character. In Mesoscale Processes in the Stratosphere: Air Pollution Report 69; European Commission: Luxembourg, 1999; pp. 249–254. [Google Scholar]
- Tuck, A.F.; Hovde, S.J. Fractal behavior of ozone, wind and temperature in the lower stratosphere. Geophys. Res. Lett. 1999, 26, 1271–1274. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Richard, E.C.; Fahey, D.W.; Gao, R.-S. A scaling analysis of ER-2 data in the inner vortex during January-March 2000. J. Geophys. Res. D 2003, 108, 8306. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Gao, R.-S.; Richard, E.C. Law of mass action in the Arctic lower stratospheric polar vortex: ClO scaling and the calculation of ozone loss rates in a turbulent fractal medium. J. Geophys. Res. D 2003, 108, 4451. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Richard, E.C.; Gao, R.-S.; Bui, T.P.; Swartz, W.H.; Lloyd, S.A. Molecular velocity distributions and generalized scale invariance in the turbulent atmosphere. Faraday Discuss. 2005, 130, 181–193. [Google Scholar] [CrossRef]
- Tuck, A.F. Atmospheric Turbulence: A Molecular Dynamics Perspective; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Course of Theoretical Physics, Volume 5, Statistical Physics, 3rd ed.; Part 1, §29; Butterworth-Heinemann: Oxford, UK, 1980. [Google Scholar]
- Lovejoy, S.; Tuck, A.F.; Hovde, S.J.; Schertzer, D. Is isotropic turbulence relevant in the atmosphere? Geophys. Res. Lett. 2007, 34, L15802. [Google Scholar] [CrossRef]
- Lovejoy, S.; Tuck, A.F.; Hovde, S.J.; Schertzer, D. Do stable atmospheric layers exist? Geophys. Res. Lett. 2008, 35, L01802. [Google Scholar] [CrossRef] [Green Version]
- Hovde, S.J.; Tuck, A.F.; Lovejoy, S.; Schertzer, D. Vertical scaling of temperature, wind and humidity fluctuations: Dropsondes from 13 km to the surface of the Pacific Ocean. Int. J. Remote Sens. 2011, 32, 5891–5918. [Google Scholar] [CrossRef]
- Lovejoy, S.; Schertzer, D. The Weather and Climate: Emergent Laws and Multifractal Cascades; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Tuck, A.F. From molecules to meteorology via turbulent scale invariance. Q. J. R. Meteorol. Soc. 2010, 136, 1125–1144, Erratum in Q. J. R. Meteorol. Soc. 2011, 137, 275. [Google Scholar] [CrossRef]
- Tuck, A.F. Proposed empirical entropy and Gibbs energy based on observations of scale invariance in open nonequilibrium systems. J. Phys. Chem. A 2017, 121, 6620–6629. [Google Scholar] [CrossRef] [Green Version]
- Lovejoy, S. Weather, Macroweather and the Climate; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Lovejoy, S.; Tuck, A.F.; Hovde, S.J.; Schertzer, D. The vertical cascade structure of the atmosphere and multifractal dropsonde outages. J. Geophys. Res. D 2009, 114, D07111. [Google Scholar] [CrossRef] [Green Version]
- Pinel, J.; Lovejoy, S.; Schertzer, D.; Tuck, A.F. Joint horizontal-vertical anisotropic scaling, isobaric and isoheight wind statistics from aircraft data. Geophys. Res. Lett. 2012, 39, GL051689. [Google Scholar] [CrossRef] [Green Version]
- Tuck, A.F. Turbulence: Vertical shear of the horizontal wind, jet streams, symmetry breaking, scale invariance and Gibbs free energy. Atmosphere 2021, 12, 1414. [Google Scholar] [CrossRef]
- Lovejoy, S.; Tuck, A.F.; Schertzer, D.; Hovde, S.J. Reinterpreting aircraft measurements in anisotropic scaling turbulence. Atmos. Chem. Phys. 2009, 9, 5007–5025. [Google Scholar] [CrossRef] [Green Version]
- Tuck, A.F.; Baumgardner, D.; Chan, K.R.; Dye, J.E.; Elkins, J.W.; Hovde, S.J.; Kelly, K.K.; Loewenstein, M.; Margitan, J.J.; May, R.D.; et al. The Brewer-Dobson circulation in the light of high altitude in situ aircraft observations. Q. J. R. Meteorol. Soc. 1997, 123, 1–69. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Kelly, K.K.; Mahoney, M.J.; Proffitt, M.H.; Richard, E.C.; Thompson, T.L. Exchange between the upper tropical troposphere and the lower stratosphere studied with aircraft observations. J. Geophys. Res. 2003, 108, 4734. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Bui, T.P. Scale invariance in jet streams: ER-2 data around the lower-stratospheric polar night vortex. Q. J. R. Meteorol. Soc. 2004, 130, 2423–2444. [Google Scholar] [CrossRef]
- McElroy, C.T. A spectroradiometer for the measurement of direct and scattered solar irradiance from on-board the NASA high altitude research aircraft. Geophys. Res. Lett. 1995, 22, 1361–1364. [Google Scholar] [CrossRef]
- Tuck, A.F. Theoretical chemistry and the calculation of the atmospheric state. Atmosphere 2021, 12, 727. [Google Scholar] [CrossRef]
- Kelly, K.K.; Tuck, A.F.; Murphy, D.M.; Proffitt, M.H.; Fahey, D.W.; Jones, R.L.; McKenna, D.S.; Loewenstein, M.; Podolske, J.R.; Strahan, S.S.; et al. Dehydration in the lower Antarctic stratosphere during late winter and early spring, 1987. J. Geophys. Res. 1989, 94, 11317–11357. [Google Scholar] [CrossRef]
- Kelly, K.K.; Tuck, A.F.; Davies, T. Wintertime asymmetry of upper tropospheric water betweeen the Northern and Southern Hemispheres. Nature 1991, 353, 244–247. [Google Scholar] [CrossRef]
- Fahey, D.W.; Kelly, K.K.; Ferry, G.V.; Poole, L.R.; Wilson, J.C.; Murphy, D.M.; Chan, K.R. In situ measurements of total reactive nitrogen, total water vapor and aerosol in a polar stratospheric cloud in the Antarctic. J. Geophys. Res. 1989, 94, 11299–11315. [Google Scholar] [CrossRef]
- Hübler, G.; Fahey, D.W.; Kelly, K.K.; Montzka, D.D.; Carroll, M.A.; Tuck, A.F.; Heidt, L.E.; Pollock, W.H.; Gregory, G.L.; Vedder, J.F. Redistribution of reactive odd nitrogen in the Arctic lower stratosphere. Geophys. Res. Lett. 1990, 17, 385–388. [Google Scholar] [CrossRef]
- Proffitt, M.H.; Steinkamp, M.J.; Powell, J.A.; McLaughlin, R.J.; Mills, O.A.; Schmeltekopf, A.L.; Thompson, T.L.; Tuck, A.F.; Tyler, T.; Winkler, R.H.; et al. In situ ozone measurements within the 1987 Antarctic ozone hole from a high-altitude ER-2 aircraft. J. Geophys. Res. 1989, 94, 16547–16555. [Google Scholar] [CrossRef]
- Loewenstein, M.; Podolske, J.R.; Chan, K.R.; Strahan, S.E. Nitrous oxide as a dynamical tracer in the 1987 Airborne Antarctic Ozone Experiment. J. Geophys. Res. 1989, 94, 11589–11598. [Google Scholar] [CrossRef]
- Proffitt, M.H.; Fahey, D.W.; Kelly, K.K.; Tuck, A.F. High-latitude ozone loss outside the Antarctic ozone hole. Nature 1989, 342, 233–237. [Google Scholar] [CrossRef]
- Anderson, J.G.; Brune, W.H.; Proffitt, M.H. Ozone destruction by chlorine radicals within the Antarctic vortex: The spatial and temporal evolution of ClO-O3 anticorrelation based on in situ ER-2 data. J. Geophys. Res. 1989, 94, 11465–11479. [Google Scholar] [CrossRef]
- Brune, W.H.; Toohey, D.W.; Anderson, J.G.; Chan, K.R. In situ observations of ClO in the Arctic stratosphere: ER-2 aircraft results from 590N to 800N latitude. Geophys. Res. Lett. 1990, 17, 505–508. [Google Scholar] [CrossRef]
- Tuck, A.F. Scale invariant turbulence and Gibbs free energy in the atmosphere. In Entropy and Exergy in Renewable Energy; Wang, L.-S., Ed.; Chapter 5; IntechOpen: London, UK, 2021. [Google Scholar]
- Tuck, A.F. Summary of atmospheric chemistry observations from the Antarctic and Arctic aircraft campaigns. SPIE Remote Sens. Atmos. Chem. 1991, 1491, 252–272. [Google Scholar]
- Yu, P.; Murphy, D.M.; Portmann, R.W.; Toon, O.B.; Froyd, K.D.; Rollins, R.W.; Gao, R.-S.; Rosenlof, K.H. Radiative forcing from anthropogenic sulfur and organic emissions reaching the lower stratosphere. Geophys. Res. Lett. 2016, 43, 9361–9367. [Google Scholar] [CrossRef]
- Prather, K.A.; Marr, L.C.; Schooley, R.T.; McDiarmid, M.A.; Wilson, M.E.; Milton, D.K. Airborne transmission of SARS-CoV-2. Science 2020, 370, 303–304. [Google Scholar]
- Cox, R.A.; Ammann, M.; Crowley, J.N.; Griffiths, P.T.; Herrmann, H.; Hoffmann, E.H.; Jenkin, M.E.; McNeill, V.F.; Mellouki, A.; Penkett, C.J.; et al. Opinion: The germicidal effect of ambient air (open-air factor) revisited. Atmos. Chem. Phys. 2021, 21, 13011–13018. [Google Scholar] [CrossRef]
- Tuck, A.F. Gibbs free energy and reaction rate acceleration in and on microdroplets. Entropy 2019, 21, 1044. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, D.J.; Tuck, A.F.; Vaida, V. Spontaneous fission of atmospheric aerosol particles. Phys. Chem. Chem. Phys. 2001, 3, 5270–5273. [Google Scholar] [CrossRef]
- Donaldson, D.J.; Vaida, V. The influence of organic films at the air-aqueous boundary on atmospheric processes. Chem. Revs. 2006, 106, 1445–1461. [Google Scholar] [CrossRef]
- Kappes, K.J.; Deal, A.M.; Jesperson, M.F.; Blair, S.L.; Doussin, J.-F.; Cazanau, M.; Pangui, E.; Hopper, B.N.; Johnson, M.S.; Vaida, V. Chemistry and photochemistry of pyruvic acid at the air-water interface. J. Phys. Chem. A 2021, 125, 1036–1049. [Google Scholar] [CrossRef]
- Deal, A.M.; Rapf, R.J.; Vaida, V. Water-air interfaces as as environments to address the water paradox in prebiotic chemistry; a physical chemistry perspective. J. Phys. Chem. A 2021, 125, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Tuck, A.F.; Donaldson, D.J.; Hitchman, M.H.; Richard, E.C.; Tervahattu, H.; Vaida, V.; Wilson, J.C. On geoengineering with sulphate aerosols in the tropical upper troposphere and lower stratosphere. Clim. Chang. 2008, 90, 315–331. [Google Scholar] [CrossRef]
- Palmer, T.N. A nonlinear perspective on model error: A proposal for nonlocal stochastic-dynamical paramaterization in weather and climate prediction. Q. J. R. Meteorol. Soc. 2001, 127, 279–304. [Google Scholar]
- Schertzer, D.; Tchiguirinskaia, I.; Lovejoy, S.; Tuck, A.F. Quasi-geostrophic turbulence and generalized scale invariance, a theoretical reply. Atmos. Chem. Phys. 2012, 12, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Stolle, J.; Lovejoy, S.; Schertzer, D. The stochastic multiplicative structure of deterministic numerical models of the atmosphere. Nonlinear Processes Geophys. 2009, 16, 607–621. [Google Scholar] [CrossRef] [Green Version]
- Kahn, B.H.; Teixeira, J.; Fetzer, E.J.; Gettelman, A.; Hristova-Veleva, S.M.; Huang, X.; Kochanski, A.K.; Köhler, M.; Krueger, S.K.; Wood, R.; et al. Temperature and water vapor variance scaling in global models: Comparisons to satellite and aircraft data. J. Atmos. Sci. 2012, 68, 2156–2168. [Google Scholar] [CrossRef] [Green Version]
- Hicke, J.; Tuck, A.; Rosenlof, K. MM5 simulations of a stratospheric gravity wave observed during the WB57F Aerosol Mission. In Mesoscale Processes in the Stratosphere: Air Pollution Report 69; Carslaw, K.S., Amanatidis, G.T., Eds.; EUR 18912 EN; European Commission: Luxembourg, 1999; ISBN 92-828-4629-6. [Google Scholar]
- Tuck, A.F.; Davies, T.; Hovde, S.J.; Noguer-Alba, M.; Fahey, D.W.; Kawa, S.R.; Kelly, K.K.; Murphy, D.M.; Proffitt, M.H.; Margitan, J.J.; et al. Polar stratospheric cloud-processed air and potential vorticity in the northern hemisphere lower stratosphere at mid-latitudes during winter. J. Geophys. Res. D 1992, 97, 7883–7904. [Google Scholar] [CrossRef]
- Tuck, A.F. Use of ECMWF products in stratospheric measurement campaigns. In Workshop Proceedings, Stratosphere and Numerical Weather Prediction; ECMWF: Reading, UK, 1993; pp. 73–105. [Google Scholar]
- Marlton, G.; Charlton-Perez, A.; Harrison, G.; Polichtchouk, I.; Hauchecorne, A.; Keckhut, P.; Wing, R.; Leblanc, T.; Steinbrecht, W. Using a network of temperature lidars to identify temperature biases in the upper stratosphere in ECMWF reanalyses. Atmos. Chem. Phys. 2021, 21, 6079–6092. [Google Scholar] [CrossRef]
- Forecasters’ Reference Book, 2nd ed; Section 1.8.4; Meteorological Office: Exeter, UK, 1993; ISBN 0 86180 306X.
- Toumi, R.; Syroka, J.; Barnes, C.; Lewis, P. Robust non-Gaussian statistics and long-range correlation of total ozone. Atmos. Sci. Lett. 2001, 2, 94–103. [Google Scholar] [CrossRef]
- Varotsos, C. Power-law correlations in column ozone over Antarctica. Int. J. Remote Sens. 2005, 26, 3333–3342. [Google Scholar] [CrossRef]
- Varotsos, C.; Tzanis, C. A new tool for the study of the ozone hole over Antarctica. Atmos. Environ. 2018, 133, 569–577. [Google Scholar] [CrossRef]
- Varotsos, C.A.; Lovejoy, S.; Sarlis, N.V.; Tzanis, C.G.; Efstathiou, M.V. On the scaling of the solar incident flux. Atmos. Chem. Phys. 2015, 15, 7301–7306. [Google Scholar] [CrossRef] [Green Version]
- Heisenberg, W. Zur statistischen theorie der turbulenz. Z. Fur Phys. 1948, 124, 628–657. [Google Scholar] [CrossRef]
- Eady, E.T. The cause of the general circulation of the atmosphere. In Centenary Proceedings of the Royal Meteorological Society; Royal Meteorological Society: London, UK, 1950; pp. 156–172. [Google Scholar]
- Eady, E.T.; Sawyer, J.S. Dynamics of flow patterns in extratropical regions. Q. J. R. Meteorol. Soc. 1951, 77, 531–551, discussion 316. [Google Scholar] [CrossRef]
- Kleidon, A. Thermodynamics of the Earth System; Chapter 6; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]

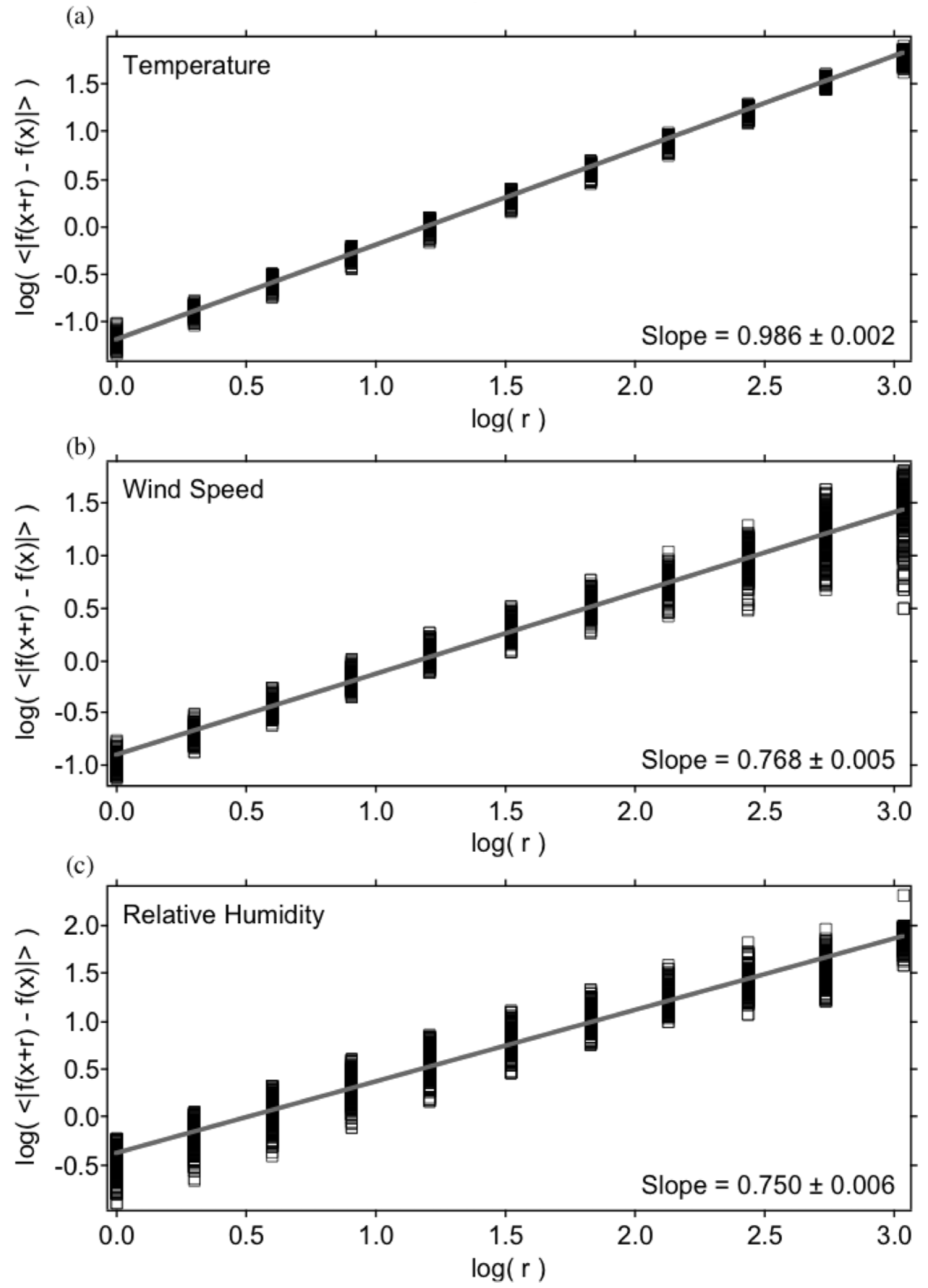
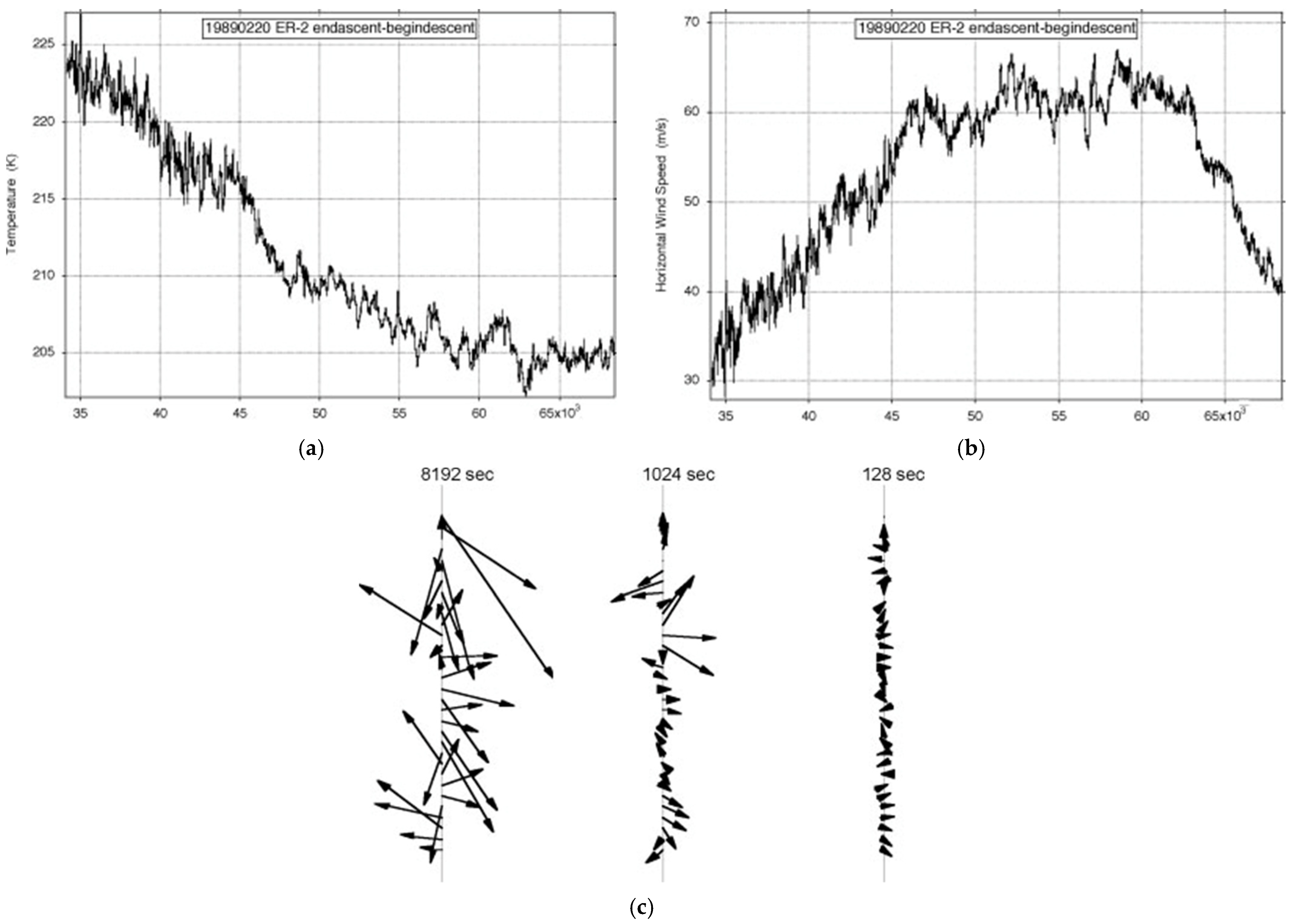

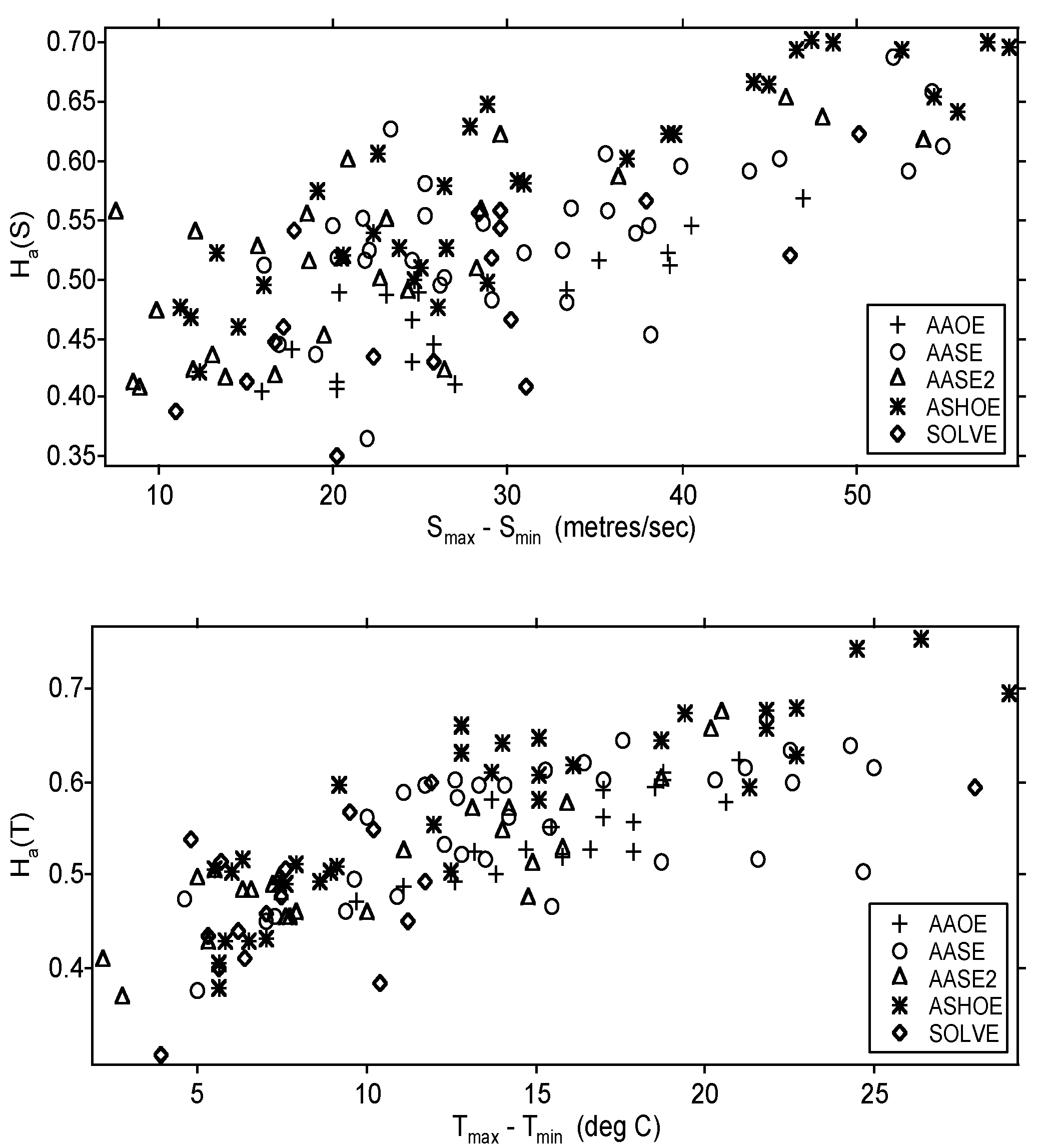
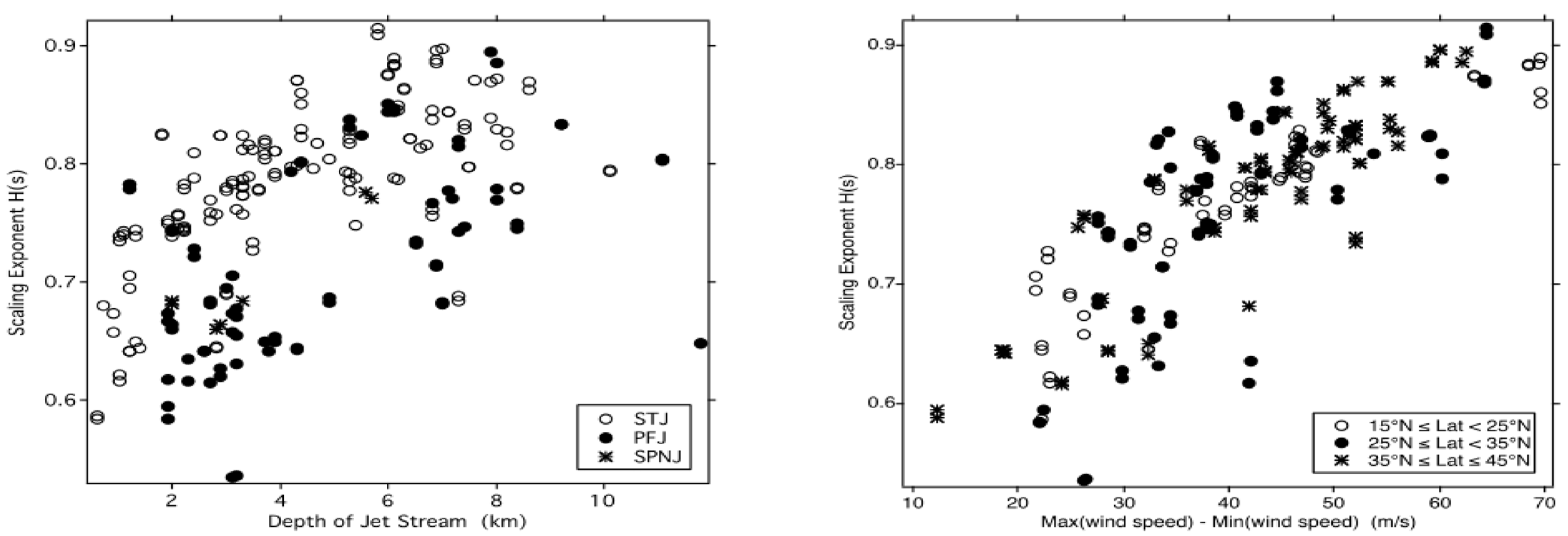
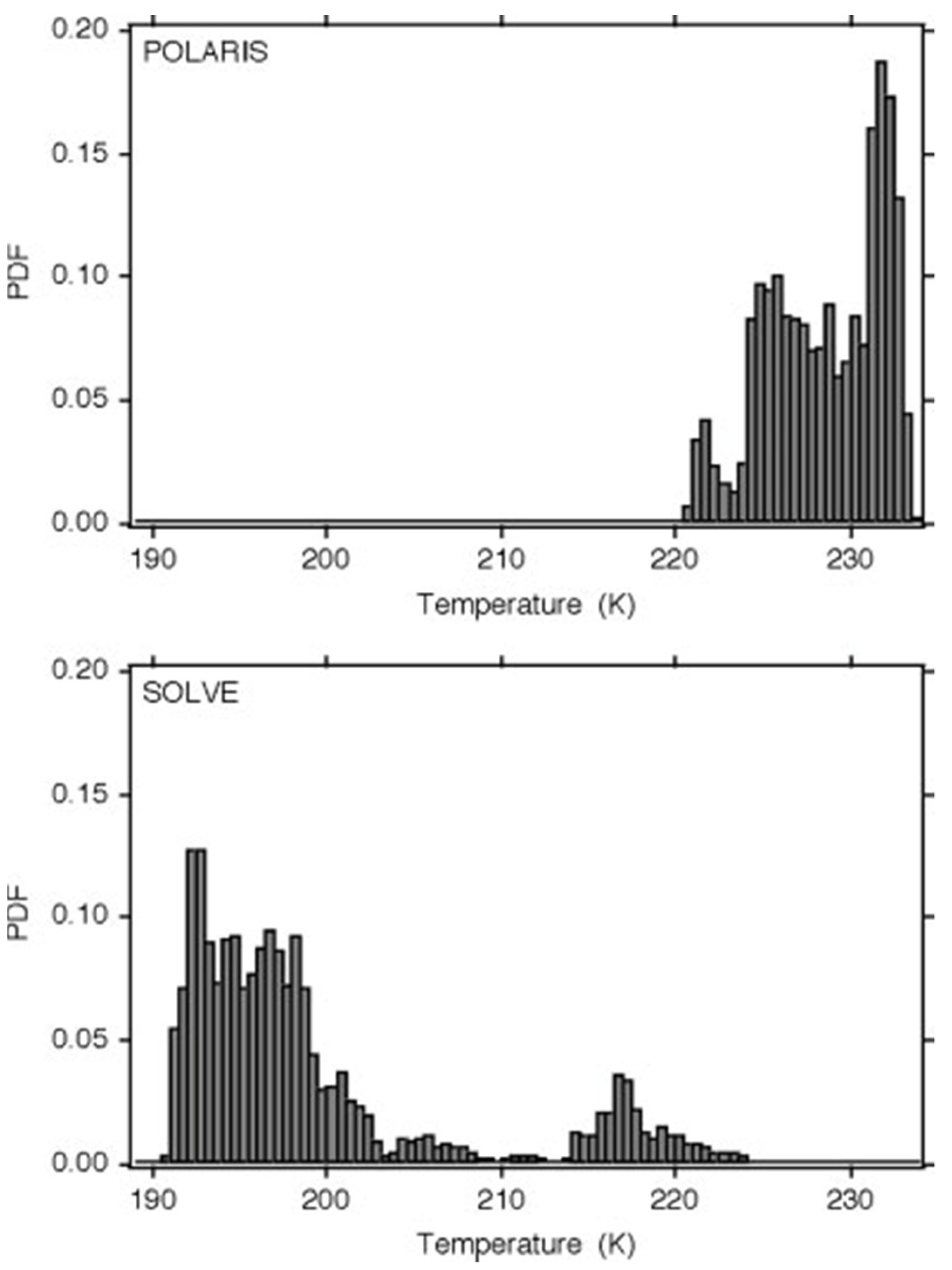
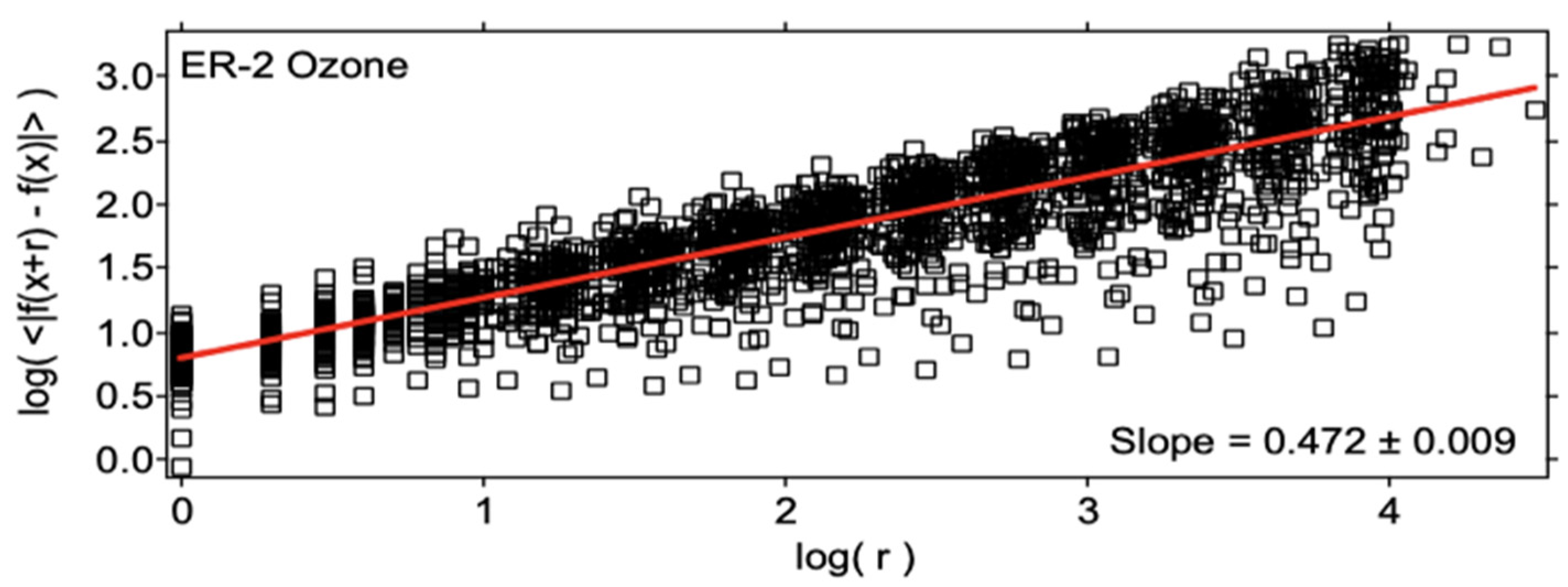
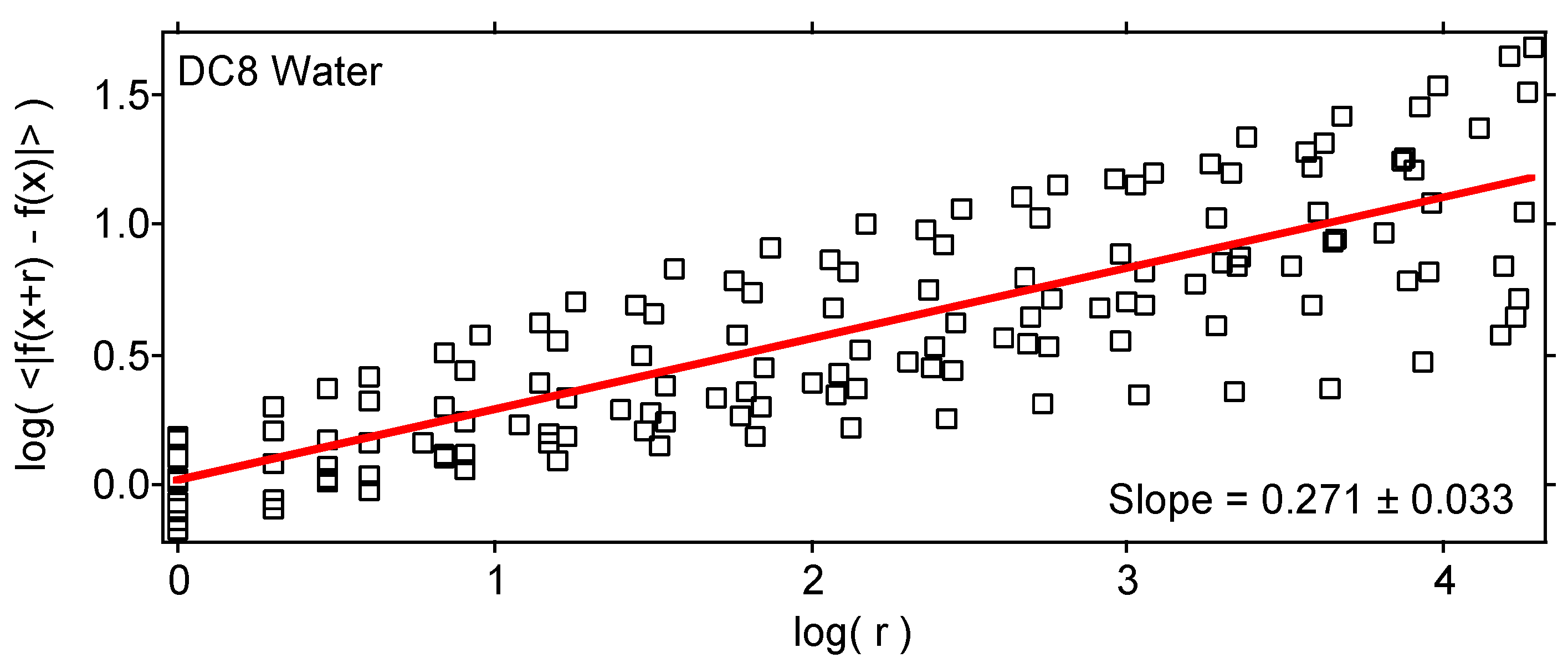
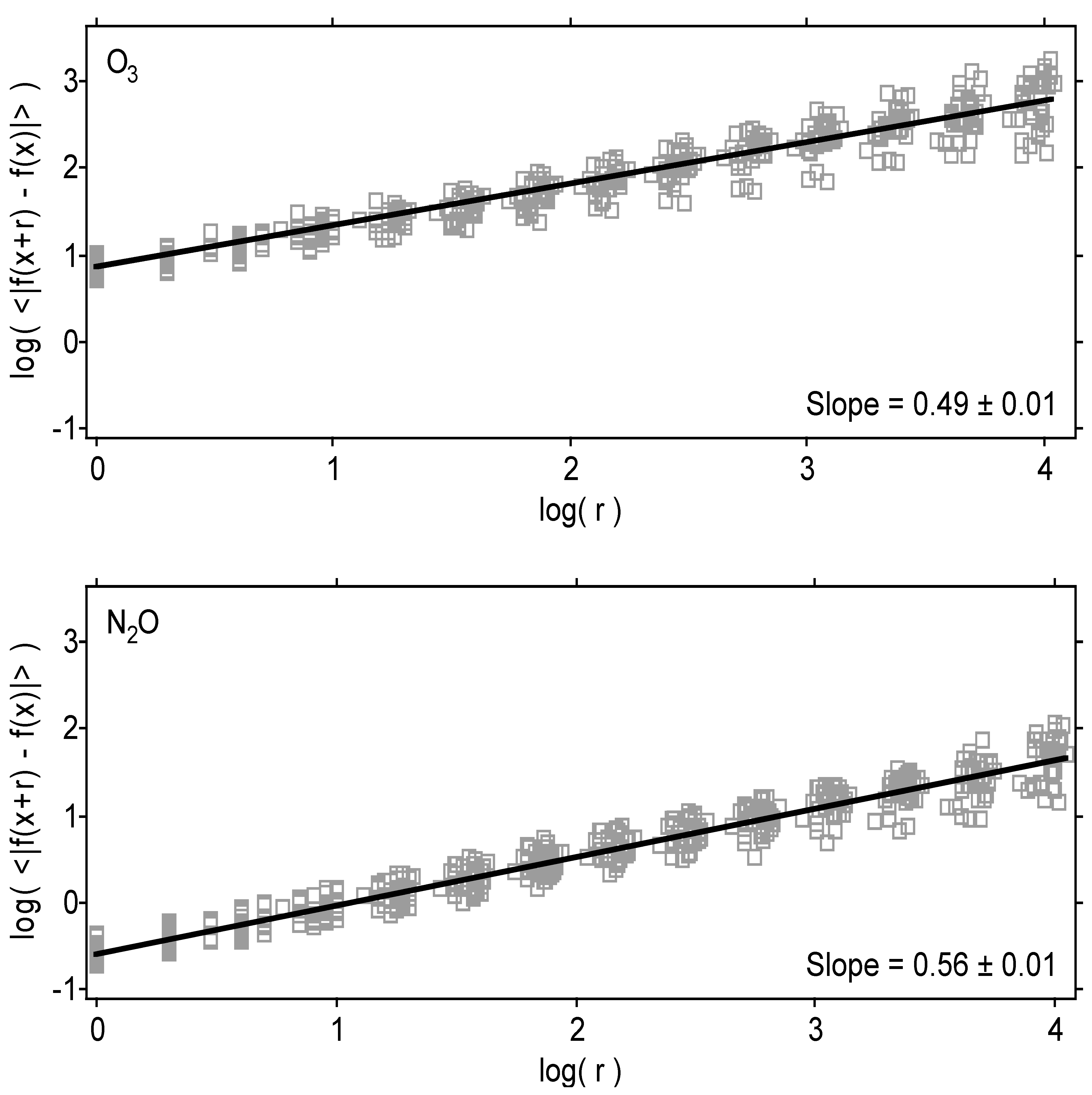

| Variable | Statistical Thermodynamics | Scaling Equivalent |
|---|---|---|
| Temperature | T | 1/qkBoltzmann |
| Partition function | f | e−K(q) |
| Energy | E | γ |
| Entropy | −S(E) | c(γ) |
| Gibbs free energy | −G | K(q)/q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuck, A.F. Scaling Up: Molecular to Meteorological via Symmetry Breaking and Statistical Multifractality. Meteorology 2022, 1, 4-28. https://doi.org/10.3390/meteorology1010003
Tuck AF. Scaling Up: Molecular to Meteorological via Symmetry Breaking and Statistical Multifractality. Meteorology. 2022; 1(1):4-28. https://doi.org/10.3390/meteorology1010003
Chicago/Turabian StyleTuck, Adrian F. 2022. "Scaling Up: Molecular to Meteorological via Symmetry Breaking and Statistical Multifractality" Meteorology 1, no. 1: 4-28. https://doi.org/10.3390/meteorology1010003
APA StyleTuck, A. F. (2022). Scaling Up: Molecular to Meteorological via Symmetry Breaking and Statistical Multifractality. Meteorology, 1(1), 4-28. https://doi.org/10.3390/meteorology1010003






