1. Introduction
Methane (CH4) is the second most significant greenhouse gas in the atmosphere after carbon dioxide [
1]. Current atmospheric methane emission levels are at their highest in the past 800,000 years [
2], playing a crucial role in the planet’s climate [
3]. Although methane has a relatively short atmospheric lifetime, its global warming potential is over 84 times greater than that of carbon dioxide over a 20-year period [
4,
5]. The urgent need to reduce methane emissions has drawn significant global attention. As of 2024, 158 member states have committed to the Global Methane Pledge, aiming for a 30% reduction in methane emissions by 2030 [
6]. The emphasis on methane reduction stems from its substantial role in reducing near-term warming, positioning it as a highly effective lever for mitigating climate change in the short term [
7]. Despite these efforts, global methane emissions continue to accelerate. From 2019 to 2023, methane concentrations increased by an average of 13.2 parts per billion (ppb) per year, more than double the annual growth rate of 5.1 ppb per year observed between 2009 and 2013 [
8]. This alarming trend underscores the urgency of implementing measures to curb methane emissions and mitigate its outsized impact on global warming. The accelerating increase in methane concentrations necessitates a focused attention on methane mitigation strategies, in combination with carbon reduction, to effectively mitigate global emissions and potentially reverse the global temperature increases in the short term [
9].
1.1. Importance of Reducing Methane Emissions in Livestock
Methane emissions primarily originate from three main sectors: energy, waste, and agriculture [
10]. National mitigation efforts have predominantly concentrated on reducing fugitive methane emissions from the petroleum and natural gas sectors [
11,
12], capturing biogas from agricultural activities and waste management [
13,
14], and minimizing food waste [
15]. However, despite agriculture contributing to 52% of methane emissions in the EU [
16] and 40% globally [
17], efforts to reduce enteric methane emissions from livestock—one of its largest sources—have received limited attention in national methane reduction strategies. This gap highlights the need for more targeted interventions in the livestock sector to complement the ongoing mitigation efforts.
Enteric emissions from ruminant animals contribute 90% of agriculture’s total global methane output [
18], making emissions from beef and dairy production greater than those from the energy or waste sectors. Various strategies have been explored to reduce methane emissions from livestock, including improving the dietary quality, using synthetic or biological feed supplements, manipulating the rumen, genetic selection, enhanced farm management practices, and promoting demand-side behavior changes with various levels of effectiveness and uptake [
19,
20,
21,
22,
23].
Recently, significant funding and research have been directed towards supplementing cattle feed with seaweeds, particularly in countries like Australia. Over AUD 45 million has been allocated in research grants to scale up and commercialize feed supplements aimed at reducing methane emissions from cattle [
24] in Australia. One promising candidate is
Asparagopsis taxiformis, a tropical seaweed native to Australia [
25], which has demonstrated a remarkable 98% reduction in methane emissions with a dosage as low as 0.2% of the cattle’s feed [
26], creating significant interest in academia, government and industry.
This surge in investment and research has generated substantial interest in commercializing seaweed-based feed supplements. Companies such as Future Feed are providing licenses for the production and distribution of
Asparagopsis taxiformis feed supplements, supporting initiatives such as low-methane milk production in Tasmania [
27] and methane-reduced beef in South Australia [
28].
A meta-analysis of 46 strategies for reducing enteric methane emissions highlighted the central role of feed supplements in the Australian beef and dairy industry [
29]. Among these, the seaweed species
Asparagopsis taxiformis has garnered significant attention due to its methane-reducing potential as cattle feed and bioactive compounds, particularly bromoform, which effectively inhibit methane production, improve feed efficiency, and are metabolized rapidly [
30]. Despite the diversity of methane reduction strategies available, Asparagopsis supplementation has been promoted by the Australian agricultural industry as a “triple win” [
31] for governments, farmers, food processors and the food industry in reducing methane emissions, leading to a surge in funding and research dedicated to this singular solution. However, this narrow focus overlooks the need for a farm-based approach and neglects broader environmental, ecological and socio-economic implications.
Currently, the Asparagopsis industry is still in its infancy, constrained by limited knowledge, expertise and collaboration among stakeholders. The proposed Australian National Hatchery Network (NHN) aims to bridge critical gaps in hatchery production, contamination control and cultivation techniques to facilitate industry growth. Even with Federal Government investments in product trials, the lack of a livestock emissions reduction methodology in Australia remains a major obstacle to the widespread adoption of this technology [
32]. However, despite the promising potential of seaweed supplementation, concerns remain about its broader implications, including possible environmental impacts and risks associated with large-scale adoption. These considerations underline the need for further research to ensure that seaweed-based solutions are both sustainable and safe.
1.2. Bromoform Classification
Bromoform, while highly effective in reducing methane emissions, is classified as an ozone-depleting substance and a known carcinogen, raising significant concerns about public health and regulatory compliance [
20,
33]. The high bromoform content in
Asparagopsis taxiformis (
A. taxiformis), which ranges from 1 to 15.8 mg/g of dry weight [
30], positions this seaweed as one of the most promising candidates for curbing livestock methane mitigation. However, this same characteristic presents critical environmental and regulatory challenges, particularly in ensuring its safe and widespread application and sustainable use at scale.
The most abundant halogenated metabolite in
A. taxiformis, bromoform is the key compound responsible for its anti-methanogenic properties [
34]. Known chemically designated as CHBr
3, bromoform is categorized as a very short-lived substance (VSLS) with an atmospheric lifespan of approximately 24 days [
5]. Its environmental impact extends beyond atmospheric breakdown. This compound serves as a natural defense mechanism, enabling marine algae to withstand various physical and chemical stressors [
20]. Notably, marine algae, including
A. taxiformis, are responsible for producing about 70% of global bromoform emissions [
35]. While its rapid breakdown reduces long-term atmospheric impact, concerns remain about its accumulation in animal products, potential effects on food safety and broader environmental risks. Regulatory frameworks, such as those in Australia, impose strict safety standards on bromoform-containing feed additives, limiting their widespread adoption [
36]. Addressing these challenges requires further research into risk mitigation strategies, regulatory approval pathways and sustainable integration into livestock production systems to balance methane reduction benefits with environmental and health risks.
While bromoform is not explicitly listed as a controlled substance under the Montreal Protocol, its classification as an ozone-depleting substance raises concerns about its environmental impact. Given its role in methane reduction and its potential contribution to atmospheric halogen emissions, bromoform remains subject to environmental monitoring and regulatory scrutiny. Understanding the Montreal Protocol’s framework is crucial in assessing how bromoform’s use in methane mitigation aligns with the global efforts to protect the ozone layer and manage greenhouse gas emissions.
1.3. Montreal Protocol
The Montreal Protocol, adopted in 1987, is an internationally agreed framework, designed to phase out ozone-depleting substances (ODSs) such as chlorofluorocarbons (CFCs) which were widely used in refrigeration and air conditioning. These substances release reactive halogen gases, including chlorine and bromine, which contribute to ozone depletion [
37].
The ozone layer, situated in the stratosphere at altitudes of 10 to 15 km above sea level, plays a critical role in shielding life on the Earth by absorbing harmful ultraviolet (UV) radiation, particularly UV-C and UV-B wavelengths. This shielding effect is vital for preventing an increased risk of skin cancer and cataracts in humans, while mitigating the damage to plants, crops and aquatic ecosystems [
38].
The most recent amendment to the Montreal Protocol, the Kigali Amendment, included hydrochlorofluorocarbons (HCFCs) in the list of controlled substances [
39]. Although HCFCs do not directly deplete the ozone layer, their high global warming potential (GWP) prompted this inclusion, signifying a landmark acknowledgment of the interconnectedness of climate change and ozone protection. The amendment mandates the complete phase-out of HCFCs as a banned substance by 2045 [
40].
Around the globe, the Montreal Protocol has been implemented at the national level through various legislative measures. In Australia, it is enforced through the Ozone Protection and Synthetic Greenhouse Gas Management Act 1989 while in the United States, the Clean Air Act Amendments of 1990 serve as the enforcement framework. Similarly, the European Union adheres to the Protocol through EU Regulation EC No. 1005/2008.
Although bromoform (CHBr
3) is not explicitly listed as a controlled substance under the Montreal Protocol or its associated national enforcement laws, it remains subject to environmental regulations and monitoring due to its classification as a hazardous substance. Since 2019, the World Meteorological Organization (WMO) has been tracking CHBr
3 as an atmospheric variable, highlighting its potential impact on the environment [
41].
The carcinogenic potential of bromoform varies and is debated among international health organizations. The International Agency for Research on Cancer (IARC) has classified it as not carcinogenic to humans, citing limited evidence from experimental animal studies [
42]. However, research indicates that bromoform can cause liver and kidney damage and suppress immune responses in animals, though the evidence and the data remain insufficient for definitive conclusions about its risks to human health.
In contrast, the US Environmental Protection Agency (EPA) classifies bromoform as a “probable human carcinogen” (group B2) rather than a “possible human carcinogen”. The EPA’s weight-of-evidence characterization reflects a more cautious approach to assessing bromoform’s potential risks to human health. Researchers have also noted these cancer risks when evaluating strategies to reduce enteric methane emissions, where bromoform is sometimes proposed as a mitigation agent [
23,
30].
2. Research Questions and Aims
In response to the proposed upscaling of Asparagopsis taxiformis production to mitigate enteric methane emissions in Australian and US cattle systems, this study aimed to address the following research questions:
What are the environmental impacts associated with the large-scale production of seaweed for reducing enteric methane emissions?
Does the large-scale production of seaweed for animal feed supplementation for enteric methane reduction align with national and international legal frameworks, particularly Australia’s ozone commitments?
To address these questions, the study established the following research objectives:
To evaluate both the potential environmental and ozone-related impacts of large-scale Asparagopsis taxiformis seaweed production for enteric methane mitigation.
To assess the legal compliance of increased bromoform production, associated with Asparagopsis taxiformis seaweed cultivation, focusing on international regulations and Australia’s legal framework.
3. Methodology
3.1. Systematic Literature Review Approach
A systematic literature review (SLR) was conducted as an important way to understand the current level of knowledge and gaps [
43] using open-source, peer-reviewed publications focusing on the environmental impacts of the supplementation of seaweed, specifically its implications for the ozone layer.
3.2. Data Source and Research Strategy
We used both the ProQuest and Scopus databases for our review. The ProQuest database served as the primary resource for sourcing publications as Scopus returned only 5 entries which overlapped. We continued with ProQuest due to its extensive coverage of interdisciplinary research, including environmental science, policy and agriculture-related studies relevant to this review. Its comprehensive indexing and access to the peer-reviewed literature ensured a robust foundation for analysis. However, we acknowledge that relying solely on ProQuest may exclude relevant studies from other databases. To mitigate this limitation, we applied broad search terms and cross-checked key references to ensure comprehensive coverage of the topic.
The key search terms included:
- -
“bioactive compounds”; “CHBR3”; “bromoform”; “bromochloromethane”; “bromine compounds”; “very short-lived brominated substances”; “seaweed”; “Asparagopsis”; “Asparagopsis taxiformis”; “macroalgae”; and “Asparagopsis armata”
- -
“ruminant feed”; “agricultural feed”; “animal feed”; “supplement” paired with “environmental impact”; “ozone”; “ozone layer”; “production” or “cultivation” or “harvest”; “farm”.
No date limitations were applied, allowing the inclusion of studies up to 31 August 2024. Abstracts were screened by an independent researcher to ensure the relevance to the research objectives and questions.
3.3. Inclusion and Exclusion Criteria
A qualitative SLR approach was adopted, with 30 articles initially identified and screened for relevance based on their titles and abstracts. After removing duplicates and industry studies, publications were considered for inclusion if they met one of the following criteria:
- -
focused on animal feed production, ozone and/or environmental impacts, or the reduction of enteric methane emissions;
- -
provide empirical or policy-related insights.
Articles were excluded if they:
- -
lacked direct relevance to the key themes;
- -
did not provide empirical or policy-related insights;
- -
primarily discussed unrelated sectors.
Any specific reference to international or national ozone legislation was recorded and tabled, and secondary findings were thematically grouped.
3.4. Data Extraction and Analysis
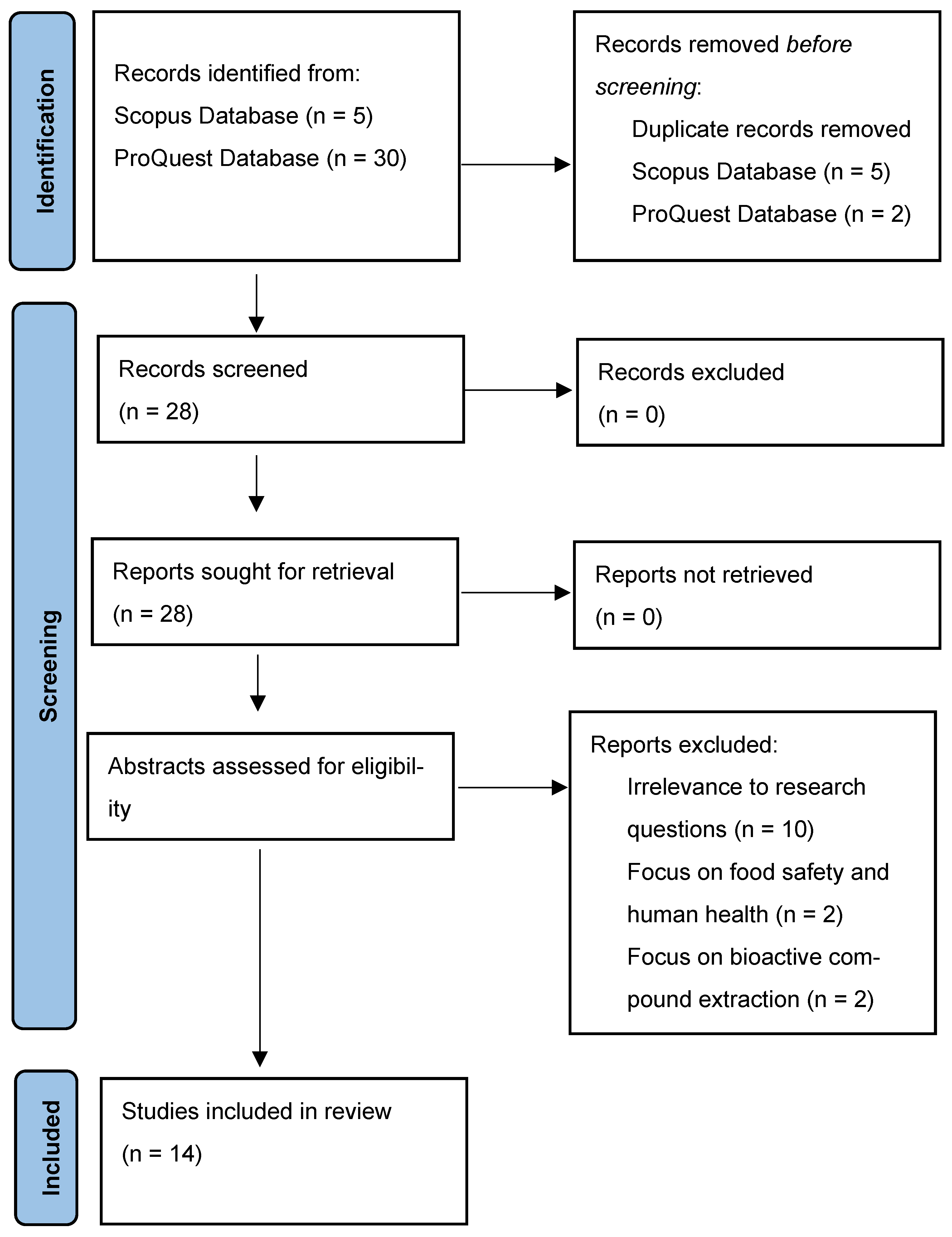
Out of the 30 articles, 14 met the inclusion criteria, specifically addressing ozone and environmental impacts. These selected articles were evaluated further following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (
https://www.prisma-statement.org/prisma-2020-flow-diagram accessed on 5 September 2024), which involved a structured process of identification, screening, eligibility assessment and final inclusion.
Figure 1 illustrates this process, which ensures transparency and methodological rigor in the study selection and review process.
4. Results
There were some major primary results from the SLR in relation to the environmental and ozone impacts of seaweed production. Only two studies in the SLR specifically addressed bromoform emissions in the context of seaweed production for enteric methane reduction. They are reported below first, followed by secondary findings related to the bromoform concentration variability, degradation of bromoform concentrations and challenges in the commercialization and distribution of seaweed feed supplements.
4.1. Environmental and Ozone Impacts of Seaweed Production
The SLR resulted in identifying a very limited number of studies which assessed the impact of seaweed production on ozone layer depletion, specifically due to the production of bromoform. It also identified some gaps.
4.1.1. Bromoform Emissions
Jia et al. [
44] projected a 0.48% increase in global ozone depletion due to bromoform (CHBr
3) emissions from the industrial-scale production of
Asparagopsis taxiformis. This projection was based on medium-range weather forecasts and an emission rate of 27.1 mg CHBr
3 per gram of dry seaweed. Although emissions varied seasonally and by cultivation site, production exceeding one million tonnes of seaweed was deemed unlikely to significantly affect the ozone layer. This study was funded by the Commonwealth Scientific and Industrial Research Organization (CSIRO), an Australian government agency actively engaged in the commercialization of seaweed production for enteric methane reduction, raising potential concerns about the objectivity of the findings evaluating CHBr
3’s contribution to the global atmospheric bromine budget and its role in stratospheric ozone depletion, on the assumption of biomass production to supply 50% of feedlot and dairy cattle in Australia. With a 30% global adoption rate at 0.4% of the dry matter intake (DMI), it was estimated that 1.04 million tonnes of CHBr
3 emissions would be produced annually.
Romero et al. [
45] conducted the only other study on bromoform emissions, examining its degradation in an in vitro batch culture of
Asparagopsis taxiformis. The study included a seaweed industry chief executive officer (CEO) as an author, which may raise concerns about perceived bias and the independence of the research focused on the environmental implications of bromoform release, particularly in terms of its potential contribution to atmospheric pollution. At varying concentrations of 0.4, 2, 10 and 50 micromoles per liter, bromoform degraded by 90% within three hours, regardless of the forage diet. This degradation process led to the conversion of bromoform into dibromomethane, another brominated compound that also has an environmental significance. Dibromomethane, like bromoform, can contribute to ozone depletion in the stratosphere by releasing bromine, a potent ozone-depleting substance [
46]. The rapid degradation of bromoform within the culture suggests that the environmental fate of these compounds is influenced by the conditions of the cultivation process, and their release into the atmosphere could have broader implications for global ozone health, highlighting the regulatory concerns of producing methane-reducing seaweed.
The review of the literature reveals a notable gap in addressing international ozone protection legislation in the context of methane-reducing seaweed production. Only two studies explicitly referenced the Montreal Protocol, which regulates substances with ozone-depleting potential, and one study referred to national legislation in Australia (
Table 1). McGurrin et al. [
47] referenced Tomkins et al.’s study [
48] which studied bromochloromethane (BCM), a substance controlled under the Protocol, extracted from seaweed for cattle feed supplementation. Similarly, Lileikis et al. [
22] emphasized the strict regulation or prohibition of halogenated hydrocarbons like BCM due to their ozone-depleting properties. However, despite the known volatility of halocarbons in a study on
Asparagopsis taxiformis, Norskov et al. [
25] did not address the Montreal Protocol or any other international regulatory standards in their assessment of 18 seaweed species, including
Asparagopsis taxiformis. This oversight highlights a critical gap in understanding the environmental regulatory context of seaweed cultivation for methane reduction. On a national level, only one study, conducted by Abbot et al. [
20], mentioned Australian legislation related to ozone protection. The study highlighted issues of non-compliance with the Ozone Protection and Synthetic Greenhouse Gas Management Act, which prohibits the manufacturing, export and import of ozone-depleting substances like bromochloromethane. This lack of attention to international and national regulatory frameworks underscores the need for further research to ensure that methane-reducing seaweed production does not inadvertently contribute to ozone depletion (through BCM).
4.1.2. Environmental Impact Findings
Of the fourteen articles reviewed, six addressed the environmental and sustainability challenges associated with upscaling seaweed production for reducing enteric methane emissions. These challenges included issues related to the overall sustainability of the proposed industry, upstream emissions, the invasive nature of certain seaweed species and impacts on both animal health and marine ecosystems, as summarized in
Table 2.
The resource requirements for producing seaweed were highlighted as a significant risk arising from upscaling Asparagopsis industries. Life cycle assessments (LCAs) were deemed critical to evaluating the production impacts of growing, harvesting, drying, shipping and storing seaweed supplements [
47,
49,
50]. For example, in a meta-analysis of nine feed strategies, Pepeta et al. [
50] reported an average 35.5% decline in methane yields (g/kg DMI) and a 3.75% loss of average daily gain (ADG) when supplementing with
Asparagopsis taxiformis, with large variations in methane reduction potentially due to fiber content in the feed, such as concentrated feed. They recommended conducting life cycle assessments (LCAs) or other modeling approaches to capture the farm-scale production impacts, assess net greenhouse gas emissions and evaluate the long-term sustainability of combining multiple methane reduction strategies. McGurrin et al. [
47] supported this call in a review of the anti-methanogenic potential of seaweeds, particularly highlighting the processing challenges during the post-harvest stage, where drying the biomass is required within hours of harvesting. Their study demonstrated that maintaining bromoform’s potency for up to 12 weeks using an oil emulsion could extend its effectiveness, but would require approximately 7400 L of vegetable oil per day to store seaweed supplementation at 0.5% of the dry matter intake (DMI) for a typical 350-head cattle farm—representing a significant challenge for post-harvest efficiency. Similarly, Bačėninaitė et al. [
49] reviewed the strategies to reduce methane emissions and highlighted LCA studies on terrestrial seaweed production entailing a high salt requirement to promote growth, accounting for up to 48% of the 9.2 kg CO2e generated per kilogram of seaweed, depending on the choice of salt source. They noted the upstream effects of resource depletion, marine eutrophication and water usage as a result, but also highlighted the possibility of seaweed cultivation as a tool for circular resource management. Without such assessments of the resource requirements, scaling up seaweed production could risk offsetting the methane reduction benefits achieved in ruminant livestock [
22].
The scale of seaweed required for global livestock supplementation was highlighted by both Ahmed et al. [
51] and McGurrin et al. [
47]. Ahmed et al. [
51] described the required quantities as “tremendous”, estimating that 50–100 g of seaweed per head per day would necessitate substantial fresh algae harvesting, particularly given a 90% drying rate, and raises significant biodiversity and ecosystem concerns. The proliferation of seaweed agriculture could block sunlight for seabed species and co-opt nutrients from other marine organisms [
47,
49,
52]. One study observed a 10% decrease in pH levels after introducing seaweed agriculture in Indonesian waters [
20], raising concerns about acidification’s impact on vulnerable species. Furthermore, producing
Asparagopsis taxiformis, an invasive species in Spain [
52] and in the Mediterranean [
53], poses a biosecurity threat with the potential to spread globally from native habitats via maritime traffic, ballast water and oyster trading [
52].
Animal health concerns were also noted, particularly regarding ruminal mucosa damage after supplementation with seaweed for enteric methane reduction in sheep [
22,
47,
49,
51] and cows [
22]. A meta-analysis of 110 studies, which included data from 14 publications on
Asparagopsis taxiformis and other seaweeds used for methane reduction, revealed a statistically significant reduction in the average daily weight gain (−3.75%) after seaweed supplementation, a finding not observed with other dietary strategies in the meta-analysis [
50]. This raises additional concerns about the long-term viability of seaweed supplementation in livestock diets due to animal and environmental health.
4.2. Secondary Findings
Given the limited data on legislative compliance with ozone protection regulations, the systematic literature review identified several thematic findings concerning the production of seaweed for enteric methane reduction. These themes included the variability in bromoform concentrations, the rapid degradation of bromoform, and the challenges associated with the processing, harvesting and compound extraction of seaweed. Additionally, the review highlighted the implications of seaweed supplementation for animal health.
4.2.1. Bromoform Concentration Variability
The variability of bromoform concentrations within
Asparagopsis taxiformis is influenced by multiple environmental and biological factors. Lileikis et al. [
22] reported that bromoform levels fluctuate based on factors such as habitat, species, strain, light intensity, lifecycle stage, water temperature and nutrient availability [
26,
54]. Cotas et al. [
55] also noted that these environmental variables directly affect the development of bioactive compounds and the quality of the final product. Tavares et al. [
56] noted the significant variation in the chemical composition of seaweed products used in the healthcare industry, emphasizing the importance of raw source analysis through spectrometry to ensure the product quality. This variability is further demonstrated by the differing bromoform concentrations reported in various studies. Ahmed et al. [
51] measured the levels of 953 µg/g dry matter (DM) in
A. taxiformis, while concentrations in California and Queensland were found to be 2305 µg/g DM and 6650 µg/g DM, respectively. De Bhowmick and Hayes [
57] further indicated that factors such as photosynthetic activity, seawater pH and location-specific environmental factors play crucial roles in CHBr
3 emissions. Notably,
A. taxiformis exhibits a wide range of bromoform emissions, ranging from 3.4 to 43 mg/g of dry weight [
44].
Abbott et al. [
20] reported that bromoform emissions for red seaweeds like
Asparagopsis taxiformis ranged from 43 to 1256 ng/g/h, while
Asparagopsis armata exhibited a range from 0.71 to 4960 pmol/g/h (approximately 861.5 ng/g/h). In contrast, other seaweed species, such as brown and green seaweeds, show considerably less variability in bromoform emissions. For instance, Abbott et al. [
20] found that brown seaweeds like
Laminaria digitata and
Macrocystis pyrifera emitted 49.7 ng/g/h and 125 ng/g/h, respectively, while green seaweeds, such as
Ulva intestinalis, exhibited even lower emissions, ranging from 87 to 192 ng/g/h. This substantial variability highlights the lack of consistency in bromoform concentrations and emphasizes the need to consider environmental factors and specific seaweed cultivars when assessing bromoform emissions and their potential environmental impacts.
4.2.2. Degradation of Bromoform Concentrations
The SLR revealed a significant concern regarding the rapid degradation of bromoform both within the rumen and following the harvesting of seaweed. In the rumen, Romero et al. [
45] found that bromoform’s effectiveness diminishes significantly, with degradation into dibromomethane resulting in a 90% reduction in bromoform within three hours. Attempts to reduce the degradation were researched by Lileikis et al. [
22] who reported that freeze drying
Asparagopsis taxiformis reduced bromoform concentrations by 84% under lighted conditions and by 75% in dark environments; however, Cotas et al. [
55] advocate for freeze drying as the preferred method for extracting the highest yield of bromoform while preserving the integrity of biomolecules. Tavares et al. [
56] emphasized the lack of understanding regarding the impacts of processing methods, such as heat and pressure, as most studies analyze seaweed based on the fresh weight. Abbott et al. [
20] and De Bhowmick and Hayes [
57] both highlighted the uncertainty surrounding the duration of methane reduction achieved through bromoform supplementation. They suggested that the effects may be temporary, with the longevity of the reduced methane emissions remaining unknown. Furthermore, McGurrin et al. [
47] examined various post-harvesting methods and found that not rinsing seaweed during processing was the most effective way to inhibit enteric methane emissions, compared to freeze drying or drying. However, a study by Stefenoni et al. [
58], cited by McGurrin et al. [
47], found no reduction in methane emissions after 56 days of in vitro supplementation with
A. taxiformis. Collectively, these findings highlight the challenges of relying on bromoform’s efficacy for sustained methane reduction in livestock beyond the short term.
4.2.3. Challenges in the Commercialization and Distribution of Seaweed Feed Supplements
The research highlighted the significant challenges associated with processing, harvesting and cultivation, delivery and profitability of seaweed production to reduce enteric methane in livestock.
Harvesting and Cultivation Issues
The harvesting of seaweed to produce cattle feed supplements presents several significant challenges as found in the SLR. The seasonal nature of seaweed crops further complicates this process, requiring detailed technoeconomic analyses to evaluate the feasibility of large-scale production [
20]. Additionally, land-based seaweed farming requires substantial financial investments with efficient drying processes being crucial to prevent decay during post-harvest processing [
47]. Furthermore, inland seaweed production consumes considerable energy due to the requirements for tropical conditions, salt and adequate filtration for productive growth [
22] while offshore large-scale seaweed cultivation can disrupt the existing marine environmental balances, potentially harming local ecosystems [
47]. Lastly, concerns regarding sustainability and technical feasibility must be addressed, as emphasized by Nilsson and Martin’s bioeconomic model [
59], which highlights the need for careful planning in land-based cultivation systems as further emphasized by McGurrin et al. [
47]. Together, harvesting and cultivation issues highlight the complexities involved in the production of seaweed feed supplements to reduce enteric methane.
Processing Issues
The processing issues in the production of seaweed feed supplements present significant challenges that could affect their commercialization and efficacy. Lileikis et al. [
22] emphasized the difficulties associated with legally registering pure compounds for commercial use due to their ozone depletion potential. They also underscored the need for improved processing methods to enhance the efficiency and feasibility of distributing seaweed supplements.
Improper processing techniques can also exacerbate the environmental concerns, as they may increase the bromoform flux into the atmosphere, thereby elevating its potential contribution to ozone depletion [
22]. Notably, the bromoform content in seaweed decreases substantially over time, suggesting that alternative preservation methods beyond freeze drying are necessary to maintain its efficacy and minimize the environmental impact [
22]. An 84% decrease in bromoform concentration was found after four months of storage which highlights a major processing challenge for seaweed supplementation [
47]. Abbot et al. [
20] confirm that the processing method is a current knowledge gap with many options to research such as a capsule or injectable form, or via feed pellets, feed solutions, fresh seaweed, or incorporation into water. It is unknown what is the most effective way to process seaweed. Collectively, the processing challenges must be addressed to ensure the successful commercialization and sustainability of scaled-up seaweed feed supplements for ruminants.
Feed Delivery Choices
Effective delivery methods for seaweed supplements are critical for their successful integration into the food and drug industries, requiring a thorough focus on both the extraction processes and administration techniques. Cotas et al. [
55] emphasized the importance of compound extraction and purification, as these processes significantly influence the quality and efficacy of seaweed products. However, a considerable knowledge gap remains regarding the optimal delivery mechanisms for feed supplementation. These methods could include capsules, injections, boluses, pelletized feed, fresh applications or incorporation through the water supply [
20]. This lack of clarity complicates the predictions about the feasibility, effectiveness and practicality of these approaches in real-world applications [
20]. Additionally, the use of oil-based storage methods presents sustainability concerns, as a substantial volume of vegetable oil for preservation is needed, such as 100 ml of vegetable oil per 120 g of seaweed biomass [
60], raising questions about the environmental impact and economic viability of such practices [
50]. Moreover, the current non-standardized dosages for seaweed supplements raise questions about the consistency and effectiveness, indicating that further research is needed to establish uniform processing standards [
47]. Addressing these delivery issues is essential for enhancing the commercial success and environmental sustainability of seaweed supplements in agricultural and health-related applications.
Economic Issues
The economic viability of producing seaweed feed supplements faces significant challenges due to the wide variability in the production costs and profit margins. For instance, the cost of cultivating seaweed varieties can range from USD 155 per tonne to USD 16,640 per tonne of dry weight, depending on the location and production methods used [
57]. This substantial disparity makes it difficult to assess the profitability and scalability of seaweed as a commercial feed supplement. In particular, the commercial upscaling of
Asparagopsis taxiformis, is often deemed economically “infeasible” [
51] primarily due to the large quantities required to achieve effective methane mitigation, with supplementation rates ranging between 50 and 100 g of dry weight per day per animal [
51,
61]. Furthermore, the existing economic analyses of seaweed production in Australia lack a comprehensive assessment of the key cost factors. Whilst some calculations point towards a net positive value benefit of USD 7.5 per kg, critical variables such as raw material expenses, labor costs, downstream processing and supply chain logistics, which are essential for determining the true economic feasibility of large-scale seaweed production are yet to be clearly detailed [
57]. Consequently, the absence of detailed economic models poses a barrier to understanding the commercial potential of seaweed feed supplements.
Alternative Bioactive Compounds’ Potential
The alternative bioactive compounds present in various seaweeds have the potential to reduce methane emissions through mechanisms other than bromoform, expanding the scope for different seaweed species to be utilized in methane mitigation strategies. Compounds such as peptides, bacteriocins, phlorotannins, lipids, carbohydrates, alkaloids and saponins have been identified as having potential methane-reducing properties [
20]. These compounds work through a variety of pathways, such as inhibiting methanogenic archaea or altering the rumen microbial environment, thus reducing the methane production. This opens up the possibility for species beyond
Asparagopsis taxiformis to be explored for their bioactive properties. For example, species that are less variable in bromoform concentrations or that naturally contain higher levels of these alternative compounds may offer more stable and scalable options for methane reduction in livestock feed. The exploration of these bioactive compounds could pave the way for the development of new feed additives derived from a wider range of seaweed species, offering more flexibility and potentially fewer environmental concerns related to bromoform emissions [
47].
5. Discussion
This systematic literature review explored the environmental implications of scaling up seaweed production for enteric methane-reducing feed supplements. The review underscores the significant gap in addressing ozone regulations, with only 3 out of 14 environmental impact articles referencing international or national legislative frameworks. This is concerning given that Tomkins et al. [
48] identified regulatory restrictions on bromochloromethane (BCM), a compound banned under the Australian Ozone Protection and Synthetic Greenhouse Gas Management Act and strictly regulated by the Montreal Protocol.
While BCM and bromoform (CHBr
3) are distinct chemical compounds, both contain bromine (Br), a key very short-lived atmospheric substance linked to ozone depletion potential [
62]. For example, methyl bromide (CH
3Br), also known as bromomethane, is classified as a controlled substance under the Montreal Protocol due to its ozone depletion potential (ODP) of 0.6. The production and use of both methyl bromide and bromochloromethane are tightly regulated and, according to the Montreal Protocol Handbook, should not exceed the stipulated levels following their global phase-out in 2015 [
63].
Bromine-containing compounds such as bromoform and methyl bromide are tracked as halocarbons by the Intergovernmental Panel on Climate Change (IPCC) and WMO because of their contributions as short-lived substances [
41,
64]. However, due to its relatively short atmospheric lifetime, bromoform is not classified as a banned substance under the Montreal Protocol.
5.1. Bromoform
Bromoform is recognized as one of the most prevalent very short-lived substances (VSLSs) in the atmosphere, predominantly produced by macroalgae and phytoplankton [
5,
65]. A comprehensive study using gas and liquid chromatography to analyze the metabolic profiles of various seaweeds found that among the 18 varieties examined,
Asparagopsis taxiformis was the highest producer of halomethanes (a type of halocarbon), generating 4200 µg/g dry weight (DW) of bromoform alongside six other volatile halocarbons [
25]. Although bromoform is typically destroyed in the lower atmosphere through chemical reactions, approximately 25% (5 ppt of the total ~20 ppt) of the bromine loading in the stratosphere originates from naturally brominated VSLSs like bromoform [
66]. On the other hand, the increase in brominated VSLS emissions due to the enhanced vertical transport in tropical areas may be counterbalanced by a corresponding decrease in inorganic bromine in the troposphere [
67].
Bromoform, the predominant bromine-containing compound found in
Asparagopsis taxiformis, comprises halogenated compounds of both bromine and chlorine atoms [
25]. These compounds, while not explicitly included in atmospheric ozone calculations, share common characteristics with those in chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs) and methyl bromide [
64]. Moreover, research has shown that
Asparagopsis taxiformis was the only seaweed species among those tested to contain both bromine and chlorine atoms [
25], which are acknowledged ozone-depleting substances. Chlorine and bromine atoms are of particular concern because reactive Cl and Br atoms can catalytically destroy ozone, with each atom potentially degrading thousands of ozone molecules before exiting the stratosphere [
38]. Once ultraviolet (UV) light chemically converts bromoform into reactive chlorine and bromine atoms in the stratosphere, even a minor amount of these atoms can have a disproportionately large impact on ozone depletion. For example, if 25% of the emissions are produced by supplying 30% of global cattle feed with
Asparagopsis taxiformis [
44], then approx. 250,000 tonnes of bromoform would reach the ozone layer with the power of up to 6.4 times more potent than that of CFC-11 [
68].
5.1.1. Bromoform’s Lifespan
Bromoform (CHBr
3) is a halogenated compound consisting of one carbon atom bonded to three bromine atoms and one hydrogen atom and is classified as an ozone-depleting very short-lived substance (VSLS) due to its short atmospheric lifespan of approximately 24 days [
5]. Such VSLSs are defined as halogenated substances with atmospheric lifespans of less than 6 months and are not regulated under the Montreal Protocol [
5]. Long-lived ozone-depleting substances (ODSs) that produce tropospheric chlorine and bromine have been on the decline since 2014. However, this decrease has been partially offset by the increases in methyl chloride and VSLSs [
66] such as bromoform.
Due to the short atmospheric life span of bromoform, its transport to altitudes of 10–15 km, where the stratosphere and ozone layer are located, is generally unlikely under most conditions [
38], yet biogenic sources of VSLSs and methyl bromine now account for more than half of the annual bromine input into the stratosphere [
66]. Although VSLSs generally do not possess an official ozone depletion potential (ODP) under the Montreal Protocol due to their limited lifespans and tendency to degrade before reaching the stratosphere [
66], exceptions have been documented. For instance, the transport of bromoform in tropical regions during the summer can extend its lifespan by 10–15% due to transportation via Brewer–Dobson circulation [
69], which strengthens and extends as a response to climate change [
70] with an observed 1.7% increase per decade between 1980 and 2018 [
71].
As a result, brominated VSLSs contribute approximately 5 ± 2 ppt to the stratospheric bromine load [
69,
72] and are significantly more efficient at depleting ozone—about 60 times more—than chlorine [
69]. The stratosphere currently contains approximately 20 ppt of bromine, with 5 ppt attributed to naturally brominated VSLSs such as bromoform [
5,
72]. In certain polar regions, higher concentrations of bromine have been observed [
73].
Efforts have been made to apply long-lived ODS ODP values to VSLSs; see
Table 3. Pisso et al. [
74] developed a trajectory-based method for calculating VSLS ODP values that differentiates and combines the separate chemistry, transport, and chemical reactions and behavior found in the troposphere and in the stratosphere. This methodology [
74] was applied by Jia et al. [
44] and Tegtmeier et al. [
68] with minimum ODP values of bromoform ranging from 0 to 1 and the maximum ranging from 0.5 to 6.4. Papanastasiou et al. [
69] conducted an assessment of the UV absorption spectrum and atmospheric lifetime of bromoform, using Brioude et al.’s ODP modeling [
75], identifying seasonally dependent ODP values in the Indian subcontinent: 0.34 in the winter, 0.72 in the spring, 0.23 in the autumn, and as high as 10 during the summer [
69]. In 2007, the WMO found an ODP of 0.18 per bromine atom assuming a similar molecular weight to that of CFC-11 and a bromine alpha factor of 60 [
76]. These findings suggest that bromoform’s ODP in tropical regions during the summer is comparable to that of banned substances such as Methyl Bromide (CH
3Br), which has an ODP of 0.66 [
77], and chlorobromomethane (CH2BrCl), with an ODP of 0.12 [
77] when factoring in the relevant lifespan. A comparison of regulated ODP substances to unregulated bromoform is shown in
Figure 2.
5.1.2. Uncertainty in Bromine Emissions
The uncertainty surrounding brominated very short-lived substances (VSLSs) stems from multiple factors, including the variability in oceanic production, sea–air fluxes, atmospheric lifetimes and transport pathways, all of which are sensitive to the changing environmental conditions [
5]. Shifts in irradiance and temperature can alter the halocarbon production by macroalgae [
65], while climate change and ocean acidification impact macroalgal distribution and diversity in species-specific ways which complicates VSLS predictions. Factors such as an increasing sea surface temperature and wind speed may enhance natural sea–air VSLS fluxes, assuming that VSLS mixing ratios are constant [
79], while anthropogenic sources like power plant emissions of bromoform could add to the future totals [
80]. Climate-driven impacts in the oxidizing capacity of the troposphere and changes in troposphere–stratosphere convective transport may further contribute to the unpredictability of brominated VSLS emissions and their impact on the stratospheric halogen budget [
81]. The uncertainty can be addressed by quantifying the ODP using ODP-weighted emissions which factor in the temporal variability and stratospheric chlorine levels based on historical data and modeling by Tegtmeier et al. [
82].
5.2. Techno-Feasibility Challenges
Even though the use of seaweed-based ingredients to mitigate enteric methane emissions in livestock has gained attention, the wider literature aligns with the challenges of feasibility of scaling up seaweed production to meet industry needs. It presents significant challenges relating to the technical, economic and environmental considerations associated with scaled-up seaweed aquaculture [
32].
Abbott et al. [
20] argue that there is a “lack of evidence that using seaweed-based ingredients to reduce CH4 emissions will be competitive, feasible, and technically and economically successful”, emphasizing the need for bioeconomic modeling to ensure that the technical capabilities are financially viable. Establishing a new seaweed aquaculture industry requires significant government financial support of approximately EUR 30 to 40 million per seaweed farm in Ireland, demonstrating that scaling up seaweed production would not be economically feasible without a conducive financial environment provided by the government [
83].
Glasson et al. [
30] analyzed the toxicological risks of bromoform in ruminant animals supplemented with Asparagopsis, finding that bromoform is not accumulated in milk, tissue or fat, and is rapidly excreted via the feces. However, its ongoing inclusion in diet is needed as a methane mitigation strategy with minimum dosages and intervals is yet to be determined. They highlighted the need for further large-scale animal feed trials before commercial implementation due to the uncertainties surrounding the quantification of bromoform pathways after cattle ingestion and the complexity of microbial interactions in the rumen.
Bačėninaitė et al. [
49] question the sustainability of algae cultivation for enteric methane purposes, pointing to the potential limitations and emphasize the importance of conducting a life cycle assessment (LCA) to holistically evaluate the environmental impact of growing, harvesting, drying and transporting seaweed biomass to determine whether enteric methane reduction benefits outweigh greenhouse gas emissions produced during production [
50]. Nilsson et al. [
59] conducted a cradle-to-gate life cycle assessment of Asparagopsis cultivated in open raceways in Sweden, finding that the inoculum tank stage accounted for 65% of total GHG emissions. Of these emissions, 75% came from the 24 kg of salt required per kilogram of dried seaweed (at a 90% drying rate), while the provision of 318 MJ of heat contributed the remaining 25%. The study suggested that emissions could be reduced by using sea salt to maintain the salinity at an optimal rate of 30 g per liter of water and utilizing thermal energy to heat the tanks. Duarte et al. [
84] call for the spatial planning of seaweed aquaculture to minimize the life cycle CO
2 emissions through the co-location of farming and processing, with supplementation from renewable energy where possible, and could be linked with offshore windfarms [
49]. Careful consideration of the site is supported by García-Poza et al. [
85], who emphasize the need for detailed site planning to manage the risks, including ensuring that the water quality is free from contaminants, selecting an appropriate type of aquaculture for the site, assessing the environmental pressures to prevent negative impacts, and thoroughly evaluating the socio-economic impact.
McGurrin et al. [
47] call for further research into the commercial upscaling of seaweed to address the cultivation and processing challenges before widespread industry adoption. They emphasize the need to standardize species, dosage and processing steps for different farming systems, noting that while
Asparagopsis taxiformis dominates the current research, dosages range widely from 0.2% to 25%, and other species may also be suitable, which is echoed by Abbot et al. [
20] who advocate for full bioeconomic modeling to assess the industrial-scale feasibility, due to a current lack of evidence in regard to the competitiveness, feasibility and technicality in using seaweed-based ingredients to reduce methane emissions.
5.2.1. Production Feasibility
Relying on supplementation with seaweed to reduce enteric methane emissions as a sole strategy appears to be impractical. Vijn et al. [
61] claim that the quantities required to supplement the global herd of 1.4 billion cattle would surpass half the current global seaweed production currently at 32.4 million tonnes [
86], making a new industry focused solely on enteric methane reduction with seaweed infeasible. McCauley et al. [
87] calculated that a typical dairy farm of 350 cows, supplemented with 0.5% daily dry matter, would require 265 kg of fresh algae per day, assuming a 90% reduction in weight after drying. Ahmed et al. [
51] further stress the substantial amounts needed, with supplementation ranging from 50 to 100 g per cow per day, yet feasibility studies on the costs of scaling up seaweed production are still lacking [
61]. Institutional and operational uncertainties, as well as harvesting and processing bottlenecks, have been identified in the upscaling of seaweed production in Ireland [
83], highlighting the opportunity for farmers to explore supplementing feed with other additives, with options such as 8% biochar and nitrates, 0.1% citral extract [
88], 4.4% rapeseed oil [
89], 50% oat concentrate [
90], 9.4% blue lupini seed meal [
91], or up to 45% wheat [
92] in dry matter intake. These alternatives, while reducing enteric methane emissions to a lesser extent than Asparagopsis supplementation, still present viable methane mitigation options. Alternatively, industry and policy measures have proven to be the most effective strategies for reducing enteric methane overall, as demonstrated in a study analyzing 46 enteric methane reduction strategies in Australia [
29]. The key measures include updating the national breeding indexes to incorporate a greenhouse gas subindex, where methane production is negatively weighted in the breeding goals [
93], and converting farmland to native habitat [
94].
5.2.2. Offshore Production Challenges
Jia et al. (2022) [
44] assumed that 80% of seaweed cultivated in Australia would be grown offshore, predominately near Darwin, to supplement half of the Australian beef and dairy sectors at 0.4%
Asparagopsis taxiformis. The offshore production challenges include the susceptibility to rising sea temperatures [
95], pollution [
96] and the variability in the bromoform content due to the location and season [
97]. Offshore cultivation presents its own challenges, including the unsuitability of locations and vulnerability to the environmental conditions [
84], particularly in areas like Darwin and north-western Australia, which face high storm surge and strong wind risks [
80]. While seaweed farming technologies are evolving globally on an annual basis [
95], the technological advances in seaweed production in Asian countries—particularly China, which accounts for 60% of global algae production [
96]—have driven industrial-scale innovations. These advancements include industrialized seedling raising, large-scale offshore cultivation, mechanized harvesting and a fully integrated supply chain encompassing product processing and sales [
96]. The sector increasingly leverages general automation and sensor-based real-time data systems [
98]. Also, the seaweed industry is progressively integrating expertise from diverse research fields, including computational fluid dynamics, mechanical and chemical engineering, informatics, electrotechnical engineering and biological sciences [
85]. Satellite data, combined with site observations, help identify the optimal locations for large-scale commercial operations, using multi-temporal and spatial measurements to prevent algal blooms in China [
98]. Multidisciplinary analysis conducted by collaborating engineers and biologists to design resilient structures capable of withstanding harsh oceanic conditions [
99,
100,
101] can reduce the production challenges of offshore seaweed cultivation.
Offshore seaweed aquaculture, while offering benefits like the lack of fertilizers and pesticides [
55], remains vulnerable to heavy metal accumulation and potential ecosystem imbalances [
47]. Despite most seaweeds being easy to cultivate in nutrient-rich waters with ample sunlight [
56,
102], labor shortages [
103], particularly in high-income countries, can persist as a challenge despite the potential for scaled-up trials and economic feasibility evidenced through the existing companies cultivating seaweed for enteric methane reduction such as GH4Global in Australia [
20].
5.2.3. Onshore Production Challenges
McGurrin et al. [
47] underscore the substantial costs involved with land-based cultivation systems needed to replicate the optimal growing conditions, supported by Nilsson’s and Martin’s [
59] LCA study, which found that the current small-scale production faces technical and logistical hurdles that must be addressed before large-scale transitions. Producing seaweed onshore demands extensive investment for optimized growing environments [
47,
104]. Post-harvest processing [
105], photosynthetic activity [
106], pH levels in seawater [
107], desiccation, oxidative stress, tissue age and damage [
107] further complicate the production. However, salt sources for cultivation tanks could potentially increase emissions and compromise the methane reduction efforts, as seen in studies from Sweden [
13]. Technological advances in the wider cultivation of seaweed species onshore have enabled the precise control of the environmental conditions, leading to greater product standardization and improved growth rates. These advancements include accurate nutrient supplementation [
108], photoperiod control through shading or LED lighting of grow tanks [
109], the manipulation of pH and CO
2 levels [
85], the regulation of the salinity [
108], and the use of computational fluid dynamics (CFD) to model the fluid flow and biochemical reactions within the production sites [
110]. Despite these innovations, the high costs associated with infrastructure, maintenance and land availability render onshore production unprofitable for most commercial seaweed products [
108], including Asparagopsis. Notably, the concerns surrounding the large-scale processing of Asparagopsis might actually increase CO
2 emissions despite reducing enteric methane, due to the high-temperature, salt and filtration requirements, and the improper processing of harvests can also heighten bromoform emissions [
22].
5.2.4. Supply Chain Logistical Challenges
The insights from the healthcare and medicinal industry underscore the sensitivity of seaweed species to environmental factors, which affect bioactive components like bromoform highlighting the potential supply chain issues. This sensitivity can directly influence the final product’s quality and potency, as described by Cotas et al. [
55] and Tavares et al. [
56]. Timely transport and drying within hours of harvesting are necessary to prevent decay, despite being costly due to the high energy consumption. This approach, however, extends the shelf life and enhances the storage potential [
83].
5.2.5. Maintaining Bromoform’s Potency Challenges
The purification of compounds is described as “the most important and vital point in seaweed exploitation” [
55], with freeze drying cited as the optimal method to maintain the integrity of bioactive molecules [
111], such as bromoform. Nonetheless, studies have shown that oil-steeped
Asparagopsis armata can yield comparable bromoform levels and the associated enteric methane reduction effects [
112,
113]. In contrast, McGurrin et al. [
47] explored various post-harvest methods, such as rinsing, freeze drying and drying, finding that not rinsing the seaweed preserved higher bromoform levels, which corresponded to the greater inhibition of CH4 emissions. Indeed, Tavares et al. [
56] emphasize the limited understanding of how processing impacts seaweed’s bioactive properties, noting that most analyses are conducted on fresh biomass and often exclude the effects of heat and pressure from drying and pelletization. This gap in knowledge has led to calls for standardized certification processes to ensure that nutritional profiles are verified through bacteriological assays [
114] and may need application in the enteric methane reduction sector.
5.2.6. Financial Feasibility
Li et al. [
97] also discuss the financial concerns, highlighting that near-shore and offshore production may reduce the costs but faces competition from other uses, such as urban development, recreation and fishing. The processing costs are significant; for instance, 100 g of vegetable oil is needed for every 120 g of biomass to maintain bromoform potency for up to 12 weeks [
60]. DeBhowmick et al. [
57] illustrate the economic potential by showing that carrageenan, a popular seaweed used for thickening purposes in cooking produced by seaweed farms, costs USD 1400 per tonne to produce, while the final product can be valued at USD 10,500. Similar profitability ratios apply to other products such as alginate and agar [
57]. With the limited data available on the scaled-up production costs of Asparagopsis, financial estimates for the capital required to expand production and establish a supply chain to supplement 2.5 million cattle in Australian feedlot systems range from AUD 132 million to AUD 1.06 billion per hectare [
115]. This estimate assumes that 100% of feedlot cattle are supplemented at 0.38% dry matter (DM), requiring 10,625 hectares of seaweed cultivation based on a conservative yield of 2.5 t/ha. Regardless of the wide range of projected costs—driven by hatchery expenses for commercialization and post-harvest processing via freeze drying or oil immersion—the estimates provided account for only 3% of the Australian cattle sector in feedlots. These estimates also consider the potential expansion to supply global markets in the US and EU, raising concerns about maintaining the potency and shelf life across long-haul supply chains.
The production costs for
Asparagopsis taxiformis range from USD 155 to USD 16,630 per tonne of dry matter, depending on the cultivation system and labor expenses [
116]. A recent estimate from the Faroe Islands in the North Sea placed the costs at approximately EUR 1850 [
117]. Costs can be offset by integrating offshore cultivation with other industries, such as wind energy production, which can reduce costs by up to 10% [
118]. However, a case study in the Australian red meat sector that calculated a USD 7.5 per kilo production cost for
Asparagopsis taxiformis [
119], was challenged due to the lack of raw material, labor or supply chain expenses detailed [
57]. With the Australian minimum wage ranking the highest globally [
120] in 2020 at AUD 24.10 [
121], it is unlikely that production costs will be low in Australia. A 2019 study reported USD 2010 per tonne whilst undertaking a life cycle assessment for seaweed biorefinery production with approximately 44% of the costs associated with drying biomass [
122]. Additionally, as an inherently seasonal crop, seaweed requires significant upfront capital and a large, short-term workforce, creating labor shortages [
103].
5.2.7. Broader Approach Needed
A multidisciplinary approach, as advocated by Garcia-Poza et al. [
85], is essential to address the limited research into the abiotic factors affecting seaweed aquaculture. This includes computational fluid dynamics to optimize growth through the understanding of water and nutrient flow, mechanical engineering for weather resilience, chemical engineering for managing the pH and temperature, and biological sciences to advance seaweed aquaculture to Industry 4.0 standards.
5.3. Environmental Impacts of Seaweed Production
The environmental impacts of seaweed production relate to biodiversity risks and reciprocal impacts between the climate and ozone. Furthermore, aquaculture is vulnerable to climate change and can be impacted by UV radiation.
5.3.1. Biodiversity Risks
Asparagopsis taxiformis is on the “black list” of marine invasive species in the EU [
53]. The expansion of Asparagopsis cultivation brings significant biodiversity concerns, primarily due to the risk of invasive species dispersal through maritime traffic and ballast water, as discussed by Pinteus et al. [
123]. These invasive species can rapidly colonize new ecosystems, where the absence of natural predators poses a substantial threat to the native biodiversity [
124]. For instance, researchers in Italy observed that the introduction of
Asparagopsis taxiformis into native algal forests in the Mediterranean Sea could have severe ecological consequences, altering the abundance, richness and diversity of epifaunal organisms, leading to the fragmentation and isolation of native algae patches, and exacerbating the biodiversity loss [
125]. In a study of six intertidal rock pools in Portugal,
Asparagopsis armata—a species with bromoform content and methane reduction potential similar to
Asparagopsis taxiformis and considered invasive in Europe—was manually removed from three pools to assess its impact on the local macroalgae and macroinvertebrate community assemblages. After ten months, the results showed a statistically significant effect on the abundance and taxonomic composition of native macroalgae communities by reducing the biomass in rockpools in the spring and autumn [
126].
The biodiversity risks are particularly pronounced due to the unique reproductive strategies of
Asparagopsis taxiformis. This species reproduces through floating gametophytes that attach to various substrates via creeping stolons and through asexual fragmentation, enhancing its ability to colonize new habitats [
127] and sprawl into new areas [
128]. This rapid spread is further facilitated by its strong natural defenses and the absence of natural predators, heightening the potential for ecological disruption [
52,
129]. A review of over 407 introduced seaweed species found that hull fouling and aquaculture require focused management, particularly for Asparagopsis varieties, which are among the most widespread and well-documented seaweed invaders globally. Their growth is facilitated by native biota, and they are unpalatable to herbivores, which may otherwise help control their spread [
130]. An Italian study on the impact of invasive
Asparagopsis taxiformis on native
Ericaria brachycarpa along the rocky shorelines of Sicily found that
A. taxiformis hosted only 12 unique taxa of mollusks, amphipods and annelids, compared to 46 and 38 taxa in homogeneous or mixed
E. brachycarpa stands [
125]. This represents a 6- to 10-fold loss in epifauna, with the potential for significant negative cascading impacts on benthic ecosystems [
125].
Large-scale seaweed farming may also influence the broader ecosystem dynamics. For example, Indonesian seaweed farming increased by 33% in the last decade, contributing to decreased ocean pH levels, which can affect marine biogeochemical cycles and stratospheric chemistry, including the ozone layer [
131,
132]. These interconnected impacts highlight the importance of balancing methane reduction benefits with potential ecosystem trade-offs.
5.3.2. Impact of Ozone on the Climate
The link between ozone and climate change is well known to be due to halocarbons, including bromoform, in addition to regulated chlorofluorocarbons (CFCs), accounting for approximately 13% of the total radiative forcing of all greenhouse gases from 1750 to 2000 [
64]. The Kigali amendment to the Montreal Protocol now expands controlled substances to include the phasing down of 18 hydrofluorocarbon (HFC) gases due to global warming potentials between 12 and 4000 [
39].
Polvani et al. [
133] found that the Montreal Protocol successfully mitigated the acceleration of the Brewer–Dobson circulation, a critical Earth system mechanism that distributes ozone and other atmospheric gases globally. This circulation influences the climate through modifications in stratospheric temperatures and global circulation patterns.
Klobas et al. [
134] analyzed various IPCC climate scenarios to assess the impact of climate change on the concentrations and ratios of bromine and chlorine molecules in the extra-polar stratosphere. The study indicated that increasing methane emissions to 7 ppmv could enhance the bromine alpha factor (a measure of bromine’s relative effectiveness in ozone depletion) to approximately 87, depending on the latitude and season. This contrasts with the global average bromine alpha factor of 60 to 65, suggesting a potential 39.2% increase in the ozone depletion potential of bromine compared to chlorine. This finding highlights the importance of reducing methane emissions through non-bromine-related strategies. Additionally, rising methane emissions and changes in stratospheric temperatures that affect halogen reactivity, along with shifts in atmospheric circulation, further drive the enhanced efficiency of ozone depletion [
134].
Leedham et al. [
132] emphasize that the projected growth in seaweed aquaculture may contribute to significant increases in halocarbon emissions. This expansion could exacerbate greenhouse gas concentrations and pose additional environmental challenges.
5.3.3. Impact of the Climate on Ozone
The rising sea temperatures and climate-driven changes in circulation patterns increase ozone depletion. Zepp et al. [
135] explored the connections between solar UV radiation and climate change, revealing that rising global temperatures can boost halogen production in aquatic environments, which in turn interacts with and depletes stratospheric ozone. This view is supported by Tang et al. [
136], who noted that the changes in atmospheric circulation, including the stratosphere–troposphere exchange and shifts in the hydrological cycle, are significantly influenced by increased temperatures and humidity.
Furthermore, Deckert and Dameris [
137] demonstrated that the higher sea surface temperatures contribute to the movement of bromine monoxide (BrO) from the troposphere to the stratosphere, exacerbating stratospheric ozone depletion. This is facilitated by the alterations in atmospheric circulation, as also highlighted by Tang et al. [
136]. The interconnected relationship between climate change, ozone levels, and their mutual effects on one another has been well documented. Tang et al. discuss that if greenhouse gas emissions continue to rise, the tropospheric ozone concentrations are likely to increase over the next 20 to 40 years, particularly in low- to mid-latitude regions [
136].
5.3.4. Aquaculture’s Vulnerability to Climate Change
Deteriorating conditions for seaweed farming due to climate change are observed [
138], noting that rising sea temperatures expand the habitat range for overgrazing predators and lead to more extreme weather events. These changes compromise the viability of seaweed aquaculture and affect the overall productivity of marine farming operations, depending on the variety chosen.
Consequently, Ballaré et al. [
139] studied the effects of UV radiation on terrestrial ecosystems and discovered that climate change-related phenomena, such as rising temperatures and melting sea ice, reduce the reflective capacity of snow and ice. This change increases the exposure of ecosystems to UV radiation, potentially impairing the resilience of drought-affected plants when exposed to UV-B radiation [
140].
On the other hand, warmer waters can enhance carbon sequestration through increased phytoplankton activity. Ross et al. [
141] noted that shifts in dominant grazers, from krill to salps, play a significant role in this process. Salps feed on the burgeoning phytoplankton populations and produce pellet-like feces that sink to the ocean floor, effectively sequestering carbon for thousands of years [
142]. This natural mechanism could partially offset some of the adverse effects of climate change on marine ecosystems, although its implications for aquaculture remain complex.
Macroalgae also play a role in aerosol formation through the production of ultra-fine particles, contributing to the development of cloud condensation nuclei. These nuclei can influence cloud properties, such as reflectivity, longevity and the balance of atmospheric radiation [
132].
5.3.5. Increased UV Impacts
Increased UV radiation risks the ability of drought-stressed vegetation to survive and produce yields as expected. The reduced rainfall in arid regions forces plants to allocate energy toward repair and maintenance, limiting the resources available for UV protection. Species already existing at their physiological limits could see diminished survivability when taking into account the biological cost of UV-B repair or protection [
139]. This phenomenon has been demonstrated in the research on crops such as soybeans [
143] and cotton [
144], which showed reduced productivity under heightened UV-B radiation. These effects are exacerbated under high carbon emission scenarios [
145], with increased sensitivity noted in response to UV-B [
146]. On a molecular scale, elevated ambient UV-B levels can reduce yields in sensitive crops, compromising the profitability due to increased deoxyribonucleic acid (DNA) damage in leaf tissue [
147].
An increase in UV-A radiation reaching the Earth can enhance carbon emissions, further accelerating climate change by promoting greater decomposition of leaf and plant litter. This process releases carbon into the atmosphere and is exacerbated by reduced cloud cover and canopy density, which enhance the photodegradation of plant litter [
139].
6. Limitations
This study acknowledges several limitations. One key challenge is the scarcity of qualitative data on the impacts of large-scale seaweed farming for enteric methane reduction. While insights from the existing studies from other sectors contribute valuable perspectives, the limited availability of in-depth studies restricts the possibility of a more comprehensive analysis. Another limitation is the potential regional variability in cultivation impacts, which is not explicitly explored in this review. Factors such as climatic conditions, marine ecosystems and regulatory environments could influence the outcomes of large-scale seaweed production, warranting further investigation across different geographic contexts.
Regarding the data sources, this review relied on the ProQuest database, which, while comprehensive in covering interdisciplinary research, may not provide a direct comparison with other academic databases more specialized in agriculture, aquaculture, or environmental sciences (e.g., Web of Science). This could limit the breadth of sources included, affecting the generalizability of the findings.
7. Implications for Policy and Practice
As the literature highlights, there is uncertainty surrounding the scaling up of seaweed production to reduce enteric methane. The following recommendations are put forward for the livestock industry.
Conduct a comprehensive Techno-Economic Analysis (TEA) by applying a costing model similar to that used for biofuel production in the EU conducted by Veipa et al. [
148]. This analysis should employ a stakeholder-based approach, in which each production stage is costed, considering indicators such as capital and production costs, raw material consumption, energy flow and income from seaweed production. The analysis should be conducted under various scenarios to assess the feasibility of scaling up seaweed production for enteric methane reduction upon feedback from key stakeholders, including farmers, policymakers, environmental groups and industry representatives who should be involved in defining and evaluating these scenarios.
Conduct a comprehensive life cycle assessment (LCA) in accordance with ISO 14040:2006, which can be integrated with the Techno-Economic Analysis (TEA). This LCA should evaluate both the upstream and downstream environmental impacts of scaling up macroalgae production to reduce enteric methane emissions. Building upon the environmental assessment conducted by Nilsson and Martin [
61], the LCA should specifically assess the net methane reduction benefits, accounting for the upstream emissions generated during cultivation, processing, harvesting and storage.
The LCA should encompass a wide range of environmental impact categories, including ecosystem quality and natural resource indicators such as climate change, ozone depletion, acidification, eutrophication and the scarcity of mineral and fossil resources. Additionally, it should include human health indicators, particularly focusing on the toxicity of bromoform and biodiversity indexes such as the species evenness index or Shannon-Wiener Diversity Index to estimate the impact of the epiphytic nature of Asparagopsis on surrounding ecosystems.
Implement certification standards for bromoform supplementation to ensure the consistent dosage and potency of products. The literature highlights a range of factors affecting potencies from within different plants of the same species under similar growing conditions. It is recommended to develop testing protocols to determine the potency of bromoform and resultant methane reduction capability after harvesting, drying and pelletizing. A transparent certification process will build trust in suppliers, producers and regulators.
8. Conclusions
This study examined the environmental and legal implications of scaling up
Asparagopsis taxiformis production for enteric methane mitigation in livestock systems. The findings highlight the significant environmental challenges associated with large-scale seaweed cultivation. Biodiversity risks are driven by the invasive reproductive strategies of
Asparagopsis taxiformis, while potential disruptions to marine ecosystems, including water quality impacts and habitat alterations, remain underexplored in certain locations. Bromoform emissions, although compliant with the Montréal Protocol, pose a significant ozone depletion potential (ODP) in certain circumstances [
69]. Evidence suggests that under certain conditions, bromoform released during production and harvesting could reach the stratosphere, contributing to ozone depletion [
69]. Climate change further intensifies these impacts, creating a feedback loop between ozone depletion and increasing global temperatures.
The designation of bromoform emissions as biogenic complicates their scrutiny under international and national frameworks, raising transparency and accountability concerns for this emerging industry. Additionally, technological feasibility assessments indicate significant barriers to scaling up Asparagopsis farming, including financial constraints, supply chain challenges and uncertainties about production scalability and environmental sustainability.
In light of these findings, this study highlights the necessity for alternative methane-reduction strategies that avoid the environmental risks associated with large-scale Asparagopsis production. Promising avenues include exploring non-bromine and non-chlorine-based seaweeds, selective breeding for low-emission livestock, land restoration practices and shifts in consumer dietary patterns. A comprehensive techno-economic analysis and life cycle assessment are recommended for future research to evaluate the economic feasibility, upstream and downstream impacts, and environmental trade-offs of large-scale Asparagopsis farming. Furthermore, the development of transparent certification standards for bromoform potency and methane-reduction capabilities can help address the variability in seaweed efficacy and build trust among stakeholders.
Ultimately, while Asparagopsis offers a potential as a methane mitigation tool, its implementation must align with environmental sustainability and international legal commitments. This requires addressing the current knowledge gaps and exploring less impactful solutions to ensure a balanced approach to methane reduction in livestock systems.
Author Contributions
Conceptualization, M.K., D.B. and D.M.; methodology, M.K. and D.B.; formal analysis, M.K. and D.B.; investigation, M.K.; writing—original draft preparation, M.K.; writing—review and editing, M.K., D.B. and D.M.; visualization, M.K.; supervision, D.B. and D.M.; project administration, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the reported results are presented in the text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aan den Toorn, S.I.; Worrell, E.; van den Broek, M.A. How much can combinations of measures reduce methane and nitrous oxide emissions from European livestock husbandry and feed cultivation? J. Clean. Prod. 2021, 304, 127138. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). AR6 Synthesis Report: Climate Change. 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 6 April 2025).
- Almeida, A.K.; Hegarty, R.S. Managing Livestock to Reduce Methane Emissions: Assessment of Strategies for Abatement of Enteric Methane; NSW Department of Primary Industries: Orange, NSW, Australia, 2021.
- Allen, M.R.; Shine, K.P.; Fuglestvedt, J.S.; Millar, R.J.; Cain, M.; Frame, D.J.; Macey, A.H. A solution to the misrepresentations of CO2-equivalent emissions of short-lived climate pollutants under ambitious mitigation. Npj Clim. Atmos. Sci. 2018, 1, 16. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Chapter 1: Update on Ozone-Depleting Substances (ODSs) and Other Gases of Interest to the Montreal Protocol. In Scientific Assessment of Ozone Depletion GAW Report No. 278; WMO: Geneva, Switzerland, 2022; p. 509. [Google Scholar]
- Climate & Clean Air Coalition Secretariat. Global Methane Pledge: Fast Action on Methane to Keep a 1.5 Degree Future in Reach. Available online: https://www.globalmethanepledge.org/ (accessed on 6 April 2025).
- United Nations Environment Programme; Climate and Clean Air Coalition. Global Methane Assesment: Benefits and Costs of Mitigating Methane Emissions; United Nations Environment Progamme: Nairobi, Nairobi County, Kenya, 2021. [Google Scholar]
- Global Monitoring Laboratory. The NOAA Annual Greenhouse Gas Index (AGGI). Available online: https://gml.noaa.gov/aggi/aggi.html (accessed on 6 April 2025).
- Perez-Dominguez, I.; del Prado, A.; Mittenzwei, K.; Hristov, J.; Frank, S.; Tabeau, A.; Witzke, P.; Havlik, P.; van Meijl, H.; Lynch, J.; et al. Short- and long-term warming effects of methane may affect the cost-effectiveness of mitigation policies and benefits of low-meat diets. Nat. Food 2021, 2, 970–980. [Google Scholar] [CrossRef]
- Climate and Clean Air Coalition. The Imperative for Methane Action. Available online: https://www.globalmethanepledge.org/imperative-methane-action (accessed on 6 October 2023).
- United States Environmental Protection Agency. Methane Emissions Reduction Progam. Available online: https://www.epa.gov/inflation-reduction-act/methane-emissions-reduction-program (accessed on 6 April 2025).
- Environment and Climate Change Canada. Faster and Further: Canada’s Methane Strategy; Environment and Climate Change Canada: Gatineau, QC, Canada, 2022.
- Ministry of Environment. Sweden’s Methane Action Plan; Government of Sweden: Stockholm, Sweden, 2022.
- Ministry of the Environment. Iceland’s Methane Roadmap; Government of Iceland: Reykjavík, Iceland, 2023.
- Republic of Korea. Overview of Republic of Korea’s 2030 MethaneEmissions Reduction Roadmap; Climate and Clean Air Coalition: Paris, France, 2023. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee, and the Committee of the Regions. An EU Strategy to Reduce Methane Emissions; European Commission: Luxembourg, 2020. [Google Scholar]
- European Space Agency. The 2024 Global Methane Budget Reveals Alarming Trends; European Space Agency: Paris, France, 2024. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD) and Food and Agriculture Organization of the United Nations (FAO). OECD-FAO Agricultural Outlook 2022–2031; OECD/FAO: Paris, France, 2022. [Google Scholar]
- Beauchemin, K.A.; Ungerfeld, E.M.; Abdalla, A.L.; Alvarez, C.; Arndt, C.; Becquet, P.; Benchaar, C.; Berndt, A.; Mauricio, R.M.; McAllister, T.A.; et al. Invited review: Current enteric methane mitigation options. J. Dairy Sci. 2022, 105, 9297–9326. [Google Scholar] [CrossRef]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Grondahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Krizsan, S.J.; Kirwan, S.F.; et al. Seaweed and Seaweed Bioactives for Mitigation of Enteric Methane: Challenges and Opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef]
- Almeida, A.K.; Cowley, F.C.; Hegarty, R.S. A regional-scale assessment of nutritional-system strategies for abatement of enteric methane from grazing livestock. Anim. Prod. Sci. 2023, 63, 1461–1472. [Google Scholar] [CrossRef]
- Lileikis, T.; Nainienė, R.; Bliznikas, S.; Uchockis, V. Dietary Ruminant Enteric Methane Mitigation Strategies: Current Findings, Potential Risks and Applicability. Animals 2023, 13, 2586. [Google Scholar] [CrossRef]
- Arndt, C.; Hristov, A.N.; Price, W.J.; McClelland, S.C.; Pelaez, A.M.; Cueva, S.F.; Oh, J.; Dijkstra, J.; Bannink, A.; Bayat, A.R.; et al. Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5 °C target by 2030 but not 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2111294119. [Google Scholar] [CrossRef]
- Department of Climate Change, Energy, the Environment and Water. Reducing Methane from Livestock. Available online: https://www.dcceew.gov.au/climate-change/emissions-reduction/agricultural-land-sectors/livestock (accessed on 6 April 2025).
- Nørskov, N.P.; Bruhn, A.; Cole, A.; Nielsen, M.O. Targeted and untargeted metabolic profiling to discover bioactive compounds in seaweeds and hemp using gas and liquid chromatography-mass spectrometry. Metabolites 2021, 11, 259. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Ashgrove Cheese. Eco-Milk. Available online: https://www.ashgrovecheese.com.au/pages/climate-friendly-milk?srsltid=AfmBOoqsNrHzm2xlRwpV2HgWkHtW3gkNQcAeq-ySgHOKt6Nr9Fo7wyG1 (accessed on 6 April 2025).
- Fogden, A. First methane-reduced beef to be exported. Queensland Country Life, 2 August 2024. [Google Scholar]
- Kelliher, M.; Bogueva, D.; Marinova, D. Meta-Analysis and Ranking of the Most Effective Methane Reduction Strategies for Australia’s Beef and Dairy Sector. Climate 2024, 12, 50. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Ellis, N.R.; Tschakert, P. Triple-wins as pathways to transformation? A critical review. Geoforum 2019, 103, 167–170. [Google Scholar] [CrossRef]
- Australian Sustainable Seaweed Alliance. Asparagopsis R&D Review; Australian Sustainable Seaweed Association: Canberra, Australia, 2023. [Google Scholar]
- United States Environmental Protection Agency (EPA). Bromoform; Bromoform 75-25-2; EPA: Washington, DC, USA, 2016.
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Liss, P.S. On temperate sources of bromoform and other reactive organic bromine gases. J. Geophys. Res. Atmos. 2000, 105, 20539–20547. [Google Scholar] [CrossRef]
- Vaskoska, R.S. Raising a Need for a Risk Assessment of Bromoform Transferred from Feed to Food; Food Legal: Melbourne, VIC, Australia, 2021. [Google Scholar]
- United Nations Environment Programme. Country Data-All Ratifications. Available online: https://ozone.unep.org/all-ratifications (accessed on 6 April 2025).
- World Meteorological Organization; United Nations Environment Programme; National Oceanic and Atmospheric Administration; National Aeronautics and Space Administration; European Commission. Twenty Questions and Answers About the Ozone Layer: 2018 Update; World Meterological Organization: Geneva, Switzerland, 2019. [Google Scholar]
- United Nations Environment Programme. Frequently Asked Questions Relating to the Kigali Amendmentto the Montreal Protocol. Available online: https://ozone.unep.org/sites/default/files/2020-01/FAQs_Kigali_Amendment.pdf (accessed on 6 April 2025).
- Birmpili, T. Montreal Protocol at 30: The governance structure, the evolution, and the Kigali Amendment. Comptes Rendus Geosci. 2018, 350, 425–431. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). ChBr3 Bromoform. Available online: http://codes.wmo.int/wmdr/ObservedVariableAtmosphere/282 (accessed on 6 April 2025).
- International Agency for Research on Cancer. Bromoform; World Health Organisation IARC Publications: Geneva, Switzerland, 1991. [Google Scholar]
- Carrera-Rivera, A.; Ochoa, W.; Larrinaga, F.; Lasa, G. How-to conduct a systematic literature review: A quick guide for computer science research. MethodsX 2022, 9, 101895. [Google Scholar] [CrossRef]
- Jia, Y.; Quack, B.; Kinley, R.D.; Pisso, I.; Tegtmeier, S. Potential environmental impact of bromoform from Asparagopsis farming in Australia. Atmos. Chem. Phys. 2022, 22, 7631–7646. [Google Scholar] [CrossRef]
- Romero, P.; Belanche, A.; Jiménez, E.; Hueso, R.; Ramos-Morales, E.; Joan King, S.; Kebreab, E.; Yáñez-Ruiz, D.R. Rumen microbial degradation of bromoform from red seaweed (Asparagopsis taxiformis) and the impact on rumen fermentation and methanogenic archaea. J. Anim. Sci. Biotechnol. 2023, 14, 1–15. [Google Scholar] [CrossRef]
- Dunse, B.; Derek, N.; Fraser, P.J.; Krummel, P. Australian and Global Emissions of Ozone Depleting Substances; Department of Agriculture, Water and the Environment Melbourne Australia: Canberra, ACT, Australia, 2021. [Google Scholar]
- McGurrin, A.; Maguire, J.; Tiwari, B.K.; Garcia-Vaquero, M. Anti-methanogenic potential of seaweeds and seaweed-derived compounds in ruminant feed: Current perspectives, risks and future prospects. J. Anim. Sci. Biotechnol. 2023, 14, 145. [Google Scholar] [CrossRef]
- Tomkins, N.; Colegate, S.; Hunter, R. A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Anim. Prod. Sci. 2009, 49, 1053–1058. [Google Scholar] [CrossRef]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global Warming and Dairy Cattle: How to Control and Reduce Methane Emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Pepeta, B.N.; Hassen, A.; Tesfamariam, E.H. Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis. Animals 2024, 14, 763. [Google Scholar] [CrossRef]
- Ahmed, E.; Suzuki, K.; Nishida, T. Micro- and Macro-Algae Combination as a Novel Alternative Ruminant Feed with Methane-Mitigation Potential. Animals 2023, 13, 796. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature (IUCN). Marine Alien Invasive Species Strategy for the MedPAN Network; IUCN: Malaga, Spain, 2012. [Google Scholar]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Cotas, J.; Lomartire, S.; Gonçalves, A.M.M.; Pereira, L. From Ocean to Medicine: Harnessing Seaweed’s Potential for Drug Development. Int. J. Mol. Sci. 2024, 25, 797. [Google Scholar] [CrossRef]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae Food Products as a Healthcare Solution. Mar. Drugs 2023, 21, 578. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. Potential of Seaweeds to Mitigate Production of Greenhouse Gases during Production of Ruminant Proteins. Glob. Chall. 2023, 7, 2200145. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef]
- Nilsson, J.; Martin, M. Exploratory environmental assessment of large-scale cultivation of seaweed used to reduce enteric methane emissions. Sustain. Prod. Consum. 2022, 30, 413–423. [Google Scholar] [CrossRef]
- Magnusson, M.; Vucko, M.J.; Neoh, T.L.; de Nys, R. Using oil immersion to deliver a naturally-derived, stable bromoform product from the red seaweed Asparagopsis taxiformis. Algal Res. 2020, 51, 102065. [Google Scholar] [CrossRef]
- Vijn, S.; Compart, D.P.; Dutta, N.; Foukis, A.; Hess, M.; Hristov, A.N.; Kalscheur, K.F.; Kebreab, E.; Nuzhdin, S.V.; Price, N.N.; et al. Key Considerations for the Use of Seaweed to Reduce Enteric Methane Emissions From Cattle. Front. Vet. Sci. 2020, 7, 597430. [Google Scholar] [CrossRef]
- Quack, B.; Wallace, D.W.R. Air-sea flux of bromoform: Controls, rates, and implications. Glob. Biogeochem. Cycles 2003, 17, 1023. [Google Scholar] [CrossRef]
- The Secretariat for the Vienna Convention for the Protection of the Ozone Layer and for the Montreal Protocol on Substances that Deplete the Ozone Layer. Handbook for the Montreal Protocolon Substances that Deplete the Ozone Layer; United Nations Environment Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) and Technology anjd Economic Assessment Panel (TEAP). Safeguarding the Ozone Layer and the Global Climate System; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Keng, F.S.-L.; Phang, S.-M.; Abd Rahman, N.; Yeong, H.-Y.; Malin, G.; Leedham Elvidge, E.; Sturges, W. Halocarbon emissions by selected tropical seaweeds exposed to different temperatures. Phytochemistry 2021, 190, 112869. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Executive Summary. Scientific Assessment of Ozone Depletion 2018; WMO: Geneva, Switzerland, 2018. [Google Scholar]
- Falk, S.; Sinnhuber, B.M.; Krysztofiak, G.; Jöckel, P.; Graf, P.; Lennartz, S.T. Brominated VSLS and their influence on ozone under a changing climate. Atmos. Chem. Phys. 2017, 17, 11313–11329. [Google Scholar] [CrossRef]
- Tegtmeier, S.; Krüger, K.; Quack, B.; Atlas, E.L.; Pisso, I.; Stohl, A.; Yang, X. Emission and transport of bromocarbons: From the West Pacific ocean into the stratosphere. Atmos. Chem. Phys. 2012, 12, 10633–10648. [Google Scholar] [CrossRef]
- Papanastasiou, D.K.; McKeen, S.A.; Burkholder, J.B. The very short-lived ozone depleting substance CHBr3 (bromoform): Revised UV absorption spectrum, atmospheric lifetime and ozone depletion potential. Atmos. Chem. Phys. 2014, 14, 3017–3025. [Google Scholar] [CrossRef]
- McLandress, C.; Shepherd, T.G. Simulated Anthropogenic Changes in the Brewer–Dobson Circulation, Including Its Extension to High Latitudes. J. Clim. 2009, 22, 1516–1540. [Google Scholar] [CrossRef]
- Fu, Q.; Solomon, S.; Pahlavan, H.A.; Lin, P. Observed changes in Brewer-Dobson circulation for 1980–2018. Environ. Res. Lett. 2019, 14, 114026. [Google Scholar] [CrossRef]
- Aschmann, J.; Sinnhuber, B.M. Contribution of very short-lived substances to stratospheric bromine loading: Uncertainties and constraints. Atmos. Chem. Phys. 2013, 13, 1203–1219. [Google Scholar] [CrossRef]
- Rotermund, M.K.; Bense, V.; Chipperfield, M.P.; Engel, A.; Grooß, J.U.; Hoor, P.; Hüneke, T.; Keber, T.; Kluge, F.; Schreiner, B.; et al. Organic and inorganic bromine measurements around the extratropical tropopause and lowermost stratosphere: Insights into the transport pathways and total bromine. Atmos. Chem. Phys. 2021, 21, 15375–15407. [Google Scholar] [CrossRef]
- Pisso, I.; Haynes, P.H.; Law, K.S. Emission location dependent ozone depletion potentials for very short-lived halogenated species. Atmos. Chem. Phys. 2010, 10, 12025–12036. [Google Scholar] [CrossRef]
- Brioude, J.; Portmann, R.W.; Daniel, J.S.; Cooper, O.R.; Frost, G.J.; Rosenlof, K.H.; Granier, C.; Ravishankara, A.R.; Montzka, S.A.; Stohl, A. Variations in ozone depletion potentials of very short-lived substances with season and emission region. Geophys. Res. Lett. 2010, 37, 19. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Scientific Assessment of Ozone Depletion: 2006; WMO: Geneva, Switzerland, 2007. [Google Scholar]
- World Meteorlogical Organisation (WMO). Scientific Assessment of Ozone Depletion: 2010, Global Ozone Research and Monitoring Project-Report No. 52; WMO: Geneva, Switzerland, 2011. [Google Scholar]
- Wuebbles, D.; Patten, K.; Johnson, M.; Kotamarthi, V. New methodology for Ozone Depletion Potentials of short-lived compounds: N-Propyl bromide as an example. J. Geophys. Res. 2001, 106, 14551–14572. [Google Scholar] [CrossRef]
- Ziska, F.; Quack, B.; Abrahamsson, K.; Archer, S.D.; Atlas, E.; Bell, T.; Butler, J.H.; Carpenter, L.J.; Jones, C.E.; Harris, N.R.P.; et al. Global sea-to-air flux climatology for bromoform, dibromomethane and methyl iodide. Atmos. Chem. Phys. 2013, 13, 8915–8934. [Google Scholar] [CrossRef]
- Maas, J.; Tegtmeier, S.; Jia, Y.; Quack, B.; Durgadoo, J.V.; Biastoch, A. Simulations of anthropogenic bromoform indicate high emissions at the coast of East Asia. Atmos. Chem. Phys. 2021, 21, 4103–4121. [Google Scholar] [CrossRef]
- Dessens, O.; Zeng, G.; Warwick, N.; Pyle, J. Short-lived bromine compounds in the lower stratosphere; impact of climate change on ozone. Atmos. Sci. Lett. 2009, 10, 201–206. [Google Scholar] [CrossRef]
- Tegtmeier, S.; Ziska, F.; Pisso, I.; Quack, B.; Velders, G.J.M.; Yang, X.; Krüger, K. Oceanic bromine emissions weighted by their ozone depletion potential. Atmos. Chem. Phys. Discuss. 2015, 15, 14643–14684. [Google Scholar] [CrossRef]
- Cerca, M.; Sosa, A.; Murphy, F. Responsible supply systems for macroalgae: Upscaling seaweed cultivation in Ireland. Aquaculture 2023, 563, 738996. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Thinking About the Future of Food Safety—A Foresight Report; FAO: Rome, Italy, 2022. [Google Scholar]
- McCauley, J.I.; Labeeuw, L.; Jaramillo-Madrid, A.C.; Nguyen, L.N.; Nghiem, L.D.; Chaves, A.V.; Ralph, P.J. Management of enteric methanogenesis in ruminants by algal-derived feed additives. Curr. Pollut. Rep. 2020, 6, 188–205. [Google Scholar] [CrossRef]
- Parra, A.S.; Mora-Delgado, J. Emission factors estimated from enteric methane of dairy cattle in Andean zone using the IPCC Tier-2 methodology. Agrofor. Syst. 2019, 93, 783–791. [Google Scholar] [CrossRef]
- Ramin, M.; Chagas, J.C.; Smidt, H.; Exposito, R.G.; Krizsan, S.J. Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil. Animals 2021, 11, 1322. [Google Scholar] [CrossRef]
- Fant, P.; Leskinen, H.; Ramin, M.; Huhtanen, P. Effects of replacement of barley with oats on milk fatty acid composition in dairy cows fed grass silage-based diets. J. Dairy Sci. 2023, 106, 2347–2360. [Google Scholar] [CrossRef]
- Bryszak, M.; Szumacher-Strabel, M.; Huang, H.; Pawlak, P.; Lechniak, D.; Kołodziejski, P.; Yanza, Y.R.; Patra, A.; Varadyova, Z.; Adam, C. Lupinus angustifolius seed meal supplemented to dairy cow diet improves fatty acid composition in milk and mitigates methane production. Anim. Feed. Sci. Technol. 2020, 267, 114590. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Deighton, M.H.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Jacobs, J.L.; Hill, J.; Wales, W.J. Effects of feeding wheat or corn and of rumen fistulation on milk production and methane emissions of dairy cows. Anim. Prod. Sci. 2019, 59, 891–905. [Google Scholar] [CrossRef]
- Richardson, C.M.; Amer, P.R.; Quinton, C.; Crowley, J.; Hely, F.S.; van den Berg, I.; Pryce, J.E. Reducing greenhouse gas emissions through genetic selection in the Australian dairy industry. J. Dairy Sci. 2022, 105, 4272–4288. [Google Scholar] [CrossRef]
- Iram, N.; Kavehei, E.; Maher, D.T.; Bunn, S.E.; Rezaei Rashti, M.; Farahani, B.S.; Adame, M.F. Soil greenhouse gas fluxes from tropical coastal wetlands and alternative agricultural land uses. Biogeosciences 2021, 18, 5085–5096. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2024-Blue Transformation in Action; FAO: Rome, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar]
- Li, X.; Norman, H.; Kinley, R.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2016, 58, 681–688. [Google Scholar] [CrossRef]
- Xing, Q.; An, D.; Zheng, X.; Wei, Z.; Wang, X.; Li, L.; Tian, L.; Chen, J. Monitoring seaweed aquaculture in the Yellow Sea with multiple sensors for managing the disaster of macroalgal blooms. Remote Sens. Environ. 2019, 231, 111279. [Google Scholar] [CrossRef]
- Bak, U.G.; Gregersen, Ó.; Infante, J. Technical challenges for offshore cultivation of kelp species: Lessons learned and future directions. Bot. Mar. 2020, 63, 341–353. [Google Scholar] [CrossRef]
- Azevedo, I.C.; Duarte, P.M.; Marinho, G.S.; Neumann, F.; Sousa-Pinto, I. Growth of Saccharina latissima (Laminariales, Phaeophyceae) cultivated offshore under exposed conditions. Phycologia 2019, 58, 504–515. [Google Scholar] [CrossRef]
- Mantri, V.A.; Ashok, K.S.; Saminathan, K.R.; Rajasankar, J.; Harikrishna, P. Concept of triangular raft design: Achieving higher yield in Gracilaria edulis. Aquac. Eng. 2015, 69, 1–6. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; Bernan Distribution: Lanham, MD, USA, 2021. [Google Scholar]
- Adams, J.; Ross, A.; Anastasakis, K.; Hodgson, E.; Gallagher, J.; Jones, J.; Donnison, I. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Pandey, D.; Mansouryar, M.; Novoa-Garrido, M.; Naess, G.; Viswanath, K.; Hansen, H.; Nielsen, M.; Khanal, P. Nutritional and Anti-Methanogenic Potentials of Macroalgae for Ruminants; Burleigh Dodds Science Publishing: London, UK, 2021. [Google Scholar]
- Lim, Y.K.; Keng, F.; Phang, S.-M.; Sturges, W.; Malin, G.; Abd Rahman, N. Effect of irradiance on the emission of short-lived halocarbons from three common tropical marine microalgae. PeerJ 2019, 7, e6758. [Google Scholar] [CrossRef]
- Mithoo-Singh, P.K.; Keng, F.S.-L.; Phang, S.-M.; Elvidge, E.C.L.; Sturges, W.T.; Malin, G.; Abd Rahman, N. Halocarbon emissions by selected tropical seaweeds: Species-specific and compound-specific responses under changing pH. PeerJ 2017, 5, e2918. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, E.; Freile-Pelegrín, Y.; Robledo, D. Nutraceutical assessment of Solieria filiformis and Gracilaria cornea (Rhodophyta) under light quality modulation in culture. J. Appl. Phycol. 2020, 32, 2363–2373. [Google Scholar] [CrossRef]
- Davison, A.V.; Piedrahita, R.H. Temperature modeling of a land-based aquaculture system for the production of Gracilaria pacifica: Possible system modifications to conserve heat and extend the growing season. Aquac. Eng. 2015, 66, 1–10. [Google Scholar] [CrossRef]
- Monteiro, P.; Cotas, J.; Pacheco, D.; Figueirinha, A.; Da Silva, G.J.; Pereira, L.; Gonçalves, A. Seaweed as Food: How to Guarantee Their Quality? In Sustainable Global Resources of Seaweeds; Springer: Cham, Switzerland, 2022; pp. 309–321. [Google Scholar]
- Alvarez-Hess, P.S.; Jacobs, J.L.; Kinley, R.D.; Roque, B.M.; Neachtain, A.S.O.; Chandra, S.; Williams, S.R.O. Twice daily feeding of canola oil steeped with Asparagopsis armata reduced methane emissions of lactating dairy cows. Anim. Feed. Sci. Technol. 2023, 297, 115579. [Google Scholar] [CrossRef]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Cascais, M.; Monteiro, P.; Pacheco, D.; Cotas, J.; Pereira, L.; Marques, J.C.; Gonçalves, A.M.M. Effects of Heat Treatment Processes: Health Benefits and Risks to the Consumer. Appl. Sci. 2021, 11, 8740. [Google Scholar] [CrossRef]
- AgriFutures Australia. Scoping Study of the Capital Requirements for Commercial Production of Asparagopsis for Methane Reduction in Cattle; Commonwealth Bank: Sydney, NSW, Australia, 2022. [Google Scholar]
- van den Burg, S.W.; Dagevos, H.; Helmes, R. Towards sustainable European seaweed value chains: A triple P perspective. ICES J. Mar. Sci. 2021, 78, 443–450. [Google Scholar] [CrossRef]
- Bak, U.G.; Mols-Mortensen, A.; Gregersen, O. Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Röckmann, C.; Lagerveld, S.; Stavenuiter, J. Operation and maintenance costs of offshore wind farms and potential multi-use platforms in the Dutch North Sea. In Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene; Springer: Cham, Switzerland, 2017; pp. 97–113. [Google Scholar]
- Davison, T.M.; Black, J.L.; Moss, J.F. Red meat-an essential partner to reduce global greenhouse gas emissions. Anim. Front. 2020, 10, 14–21. [Google Scholar] [CrossRef]
- World Population Review. Minimum Wage by Country. 2024. Available online: https://worldpopulationreview.com/country-rankings/minimum-wage-by-country (accessed on 6 April 2025).
- Fair Work Ombudsman. Minimum Wages. Available online: https://www.fairwork.gov.au/tools-and-resources/fact-sheets/minimum-workplace-entitlements/minimum-wages (accessed on 6 April 2025).
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity-the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.P.; D’Agostaro, R.; Milazzo, M.; Badalamenti, F.; Musco, L.; Mikac, B.; Lo Brutto, S.; Chemello, R. The invasive seaweed Asparagopsis taxiformis erodes the habitat structure and biodiversity of native algal forests in the Mediterranean Sea. Mar. Environ. Res. 2022, 173, 105515. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M. The effects of the invasive seaweed Asparagopsis armata on native rock pool communities: Evidences from experimental exclusion. Ecol. Indic. 2021, 125, 107463. [Google Scholar] [CrossRef]
- AgriFutures Australia. Optimising Cultivation Conditions for Asparagopsis Taxiformis Gametophytes; AgriFutures Australia: Wagga Wagga, NSW, Australia, 2024. [Google Scholar]
- Andreakis, N. Molecular Phylogeny, Phylogeography and Population Genetics of the Red Seaweed Genus Asparagopsis; Open University (United Kingdom): Milton Keynes, UK, 2006. [Google Scholar]
- Statton, J. Asparagopsis Taxiformis Hatchery and Cultivation Manual; AgriFutures Australia: Wagga Wagga, NSW, Australia, 2024. [Google Scholar]
- Williams, S.; Smith, J. A Global Review of the Distribution, Taxonomy, and Impacts of Introduced Seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- Mata, L.; Gaspar, H.; Santos, R. Carbon/Nutrient Balance in Relation to Biomass Production and Halogenated Compound Content in The Red Alga Asparagopsis Taxiformis (Bonnemaisoniaceae). J. Phycol. 2012, 48, 248–253. [Google Scholar] [CrossRef]
- Leedham, E.C.; Hughes, C.; Keng, F.S.L.; Phang, S.M.; Malin, G.; Sturges, W.T. Emission of atmospherically significant halocarbons by naturally occurring and farmed tropical macroalgae. Biogeosciences 2013, 10, 3615–3633. [Google Scholar] [CrossRef]
- Polvani, L.M.; Abalos, M.; Garcia, R.; Kinnison, D.; Randel, W.J. Significant weakening of Brewer-Dobson circulation trends over the 21st century as a consequence of the Montreal Protocol. Geophys. Res. Lett. 2018, 45, 401–409. [Google Scholar] [CrossRef]
- Klobas, J.E.; Weisenstein, D.K.; Salawitch, R.J.; Wilmouth, D.M. Reformulating the bromine alpha factor and equivalent effective stratospheric chlorine (EESC): Evolution of ozone destruction rates of bromine and chlorine in future climate scenarios. Atmos. Chem. Phys. 2020, 20, 9459–9471. [Google Scholar] [CrossRef]
- Zepp, R.; Erickson, D.; Paul, N.; Sulzberger, B. Effects of solar UV radiation and climate change on biogeochemical cycling: Interactions and feedbacks. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2011, 10, 261–279. [Google Scholar] [CrossRef]
- Tang, X.; Wilson, S.; Solomon, K.; Shao, M.; Madronich, S. Changes in air quality and tropospheric composition due to depletion of stratospheric ozone and interactions with climate. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2011, 10, 280–291. [Google Scholar] [CrossRef]
- Deckert, R.; Dameris, M. From ocean to stratosphere. Science 2008, 322, 53–55. [Google Scholar] [CrossRef]
- Smith, D. Invading Fish Threat to Kelp Forests; Univeristy of New South Wales: Sydney, NSW, Australia, 2017. [Google Scholar]
- Ballaré, C.L.; Caldwell, M.M.; Flint, S.D.; Robinson, S.A.; Bornman, J.F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 226–241. [Google Scholar] [CrossRef]
- Poulson, M.E.; Boeger, M.R.; Donahue, R.A. Response of photosynthesis to high light and drought for Arabidopsis thaliana grown under a UV-B enhanced light regime. Photosynth. Res. 2006, 90, 79–90. [Google Scholar] [CrossRef]
- Ross, R.M.; Quetin, L.B.; Martinson, D.G.; Iannuzzi, R.A.; Stammerjohn, S.E.; Smith, R.C. Palmer LTER: Patterns of distribution of five dominant zooplankton species in the epipelagic zone west of the Antarctic Peninsula, 1993–2004. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2086–2105. [Google Scholar] [CrossRef]
- Häder, D.-P.; Helbling, E.W.; Williamson, C.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 242–260. [Google Scholar] [CrossRef]
- Koti, S.; Reddy, K.R.; Kakani, V.; Zhao, D.; Gao, W. Effects of carbon dioxide, temperature and ultraviolet-B radiation and their interactions on soybean (Glycine max L.) growth and development. Environ. Exp. Bot. 2007, 60, 1–10. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kakanl, V.G.; Zhao, D.; Kotl, S.; Gao, W. Interactive Effects of Ultraviolet-B Radiation and Temperature on Cotton Physiology, Growth, Development and Hyperspectral Reflectance. Photochem. Photobiol. 2004, 79, 416–427. [Google Scholar]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Mohammed, A.R.; Read, J.J.; Gao, W. Leaf and canopy photosynthetic characteristics of cotton (Gossypium hirsutum) under elevated CO2 concentration and UV-B radiation. J. Plant Physiol. 2004, 161, 581–590. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.; Kakani, V.; Read, J.; Sullivan, J. Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlled environmental conditions. Plant Cell Environ. 2003, 26, 771–782. [Google Scholar] [CrossRef]
- Mazza, C.; Battista, D.; Zima, A.; Szwarcberg-Bracchitta, M.; Giordano, C.; Acevedo, A.; Scopel, A.; Ballare, C. The effects of solar ultraviolet-B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses. Plant Cell Environ. 1999, 22, 61–70. [Google Scholar] [CrossRef]
- Veipa, A.; Kirsanovs, V.; Barisa, A. Techno-Economic Analysis of Biofuel Production Plants Producing Biofuels Using Fisher Tropsch Synthesis. Environ. Clim. Technol. 2020, 24, 373–387. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).