High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2
Abstract
1. Introduction
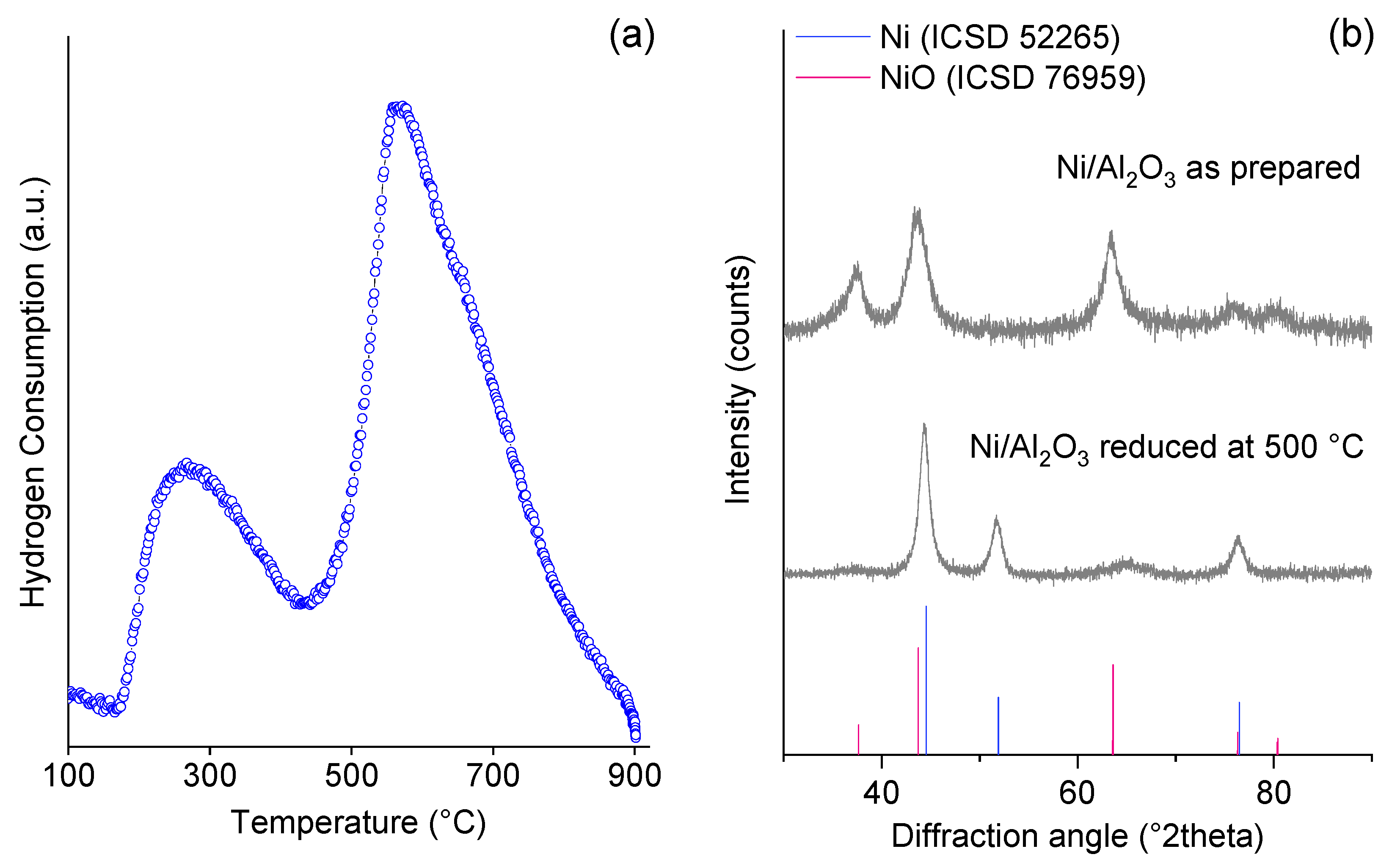
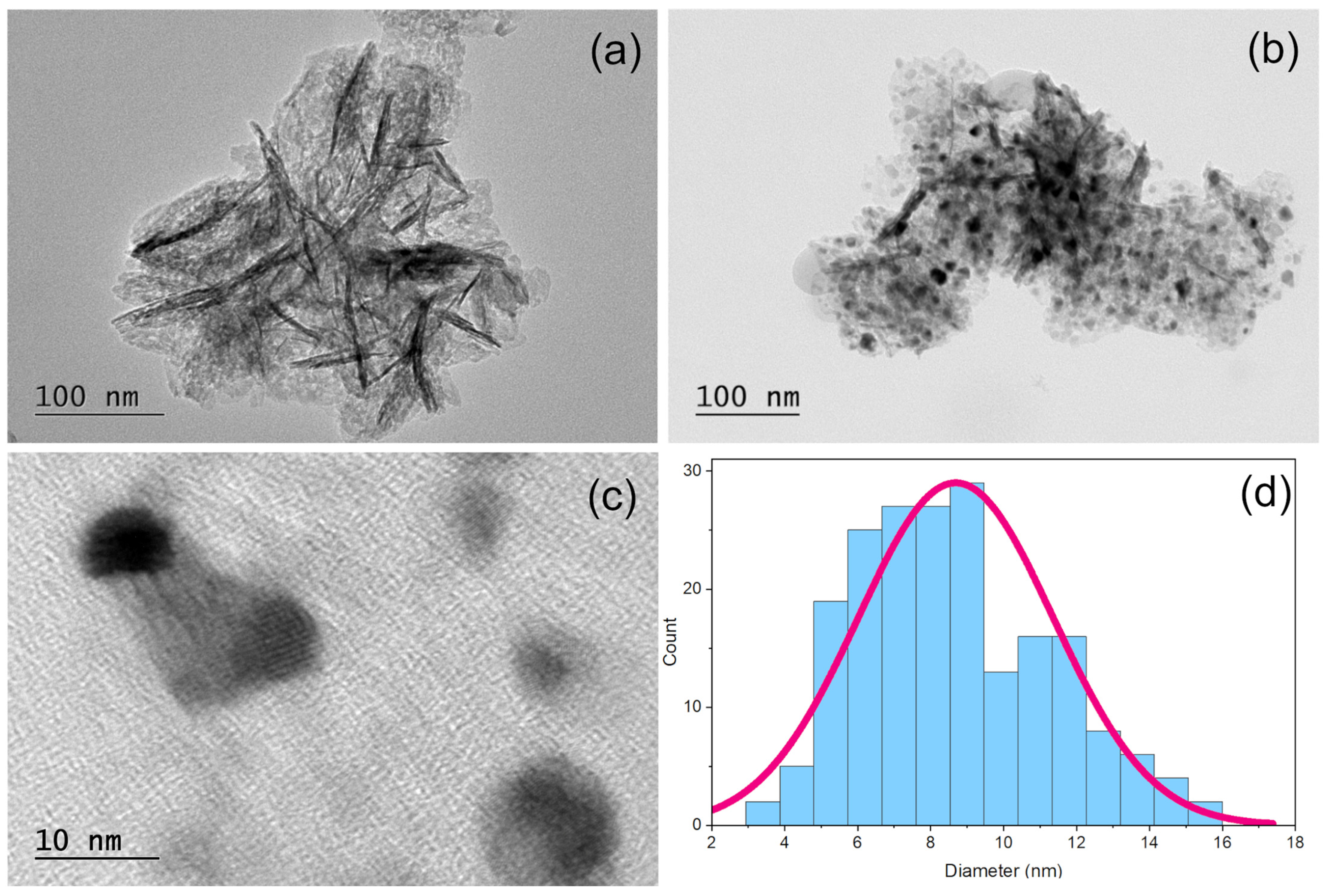
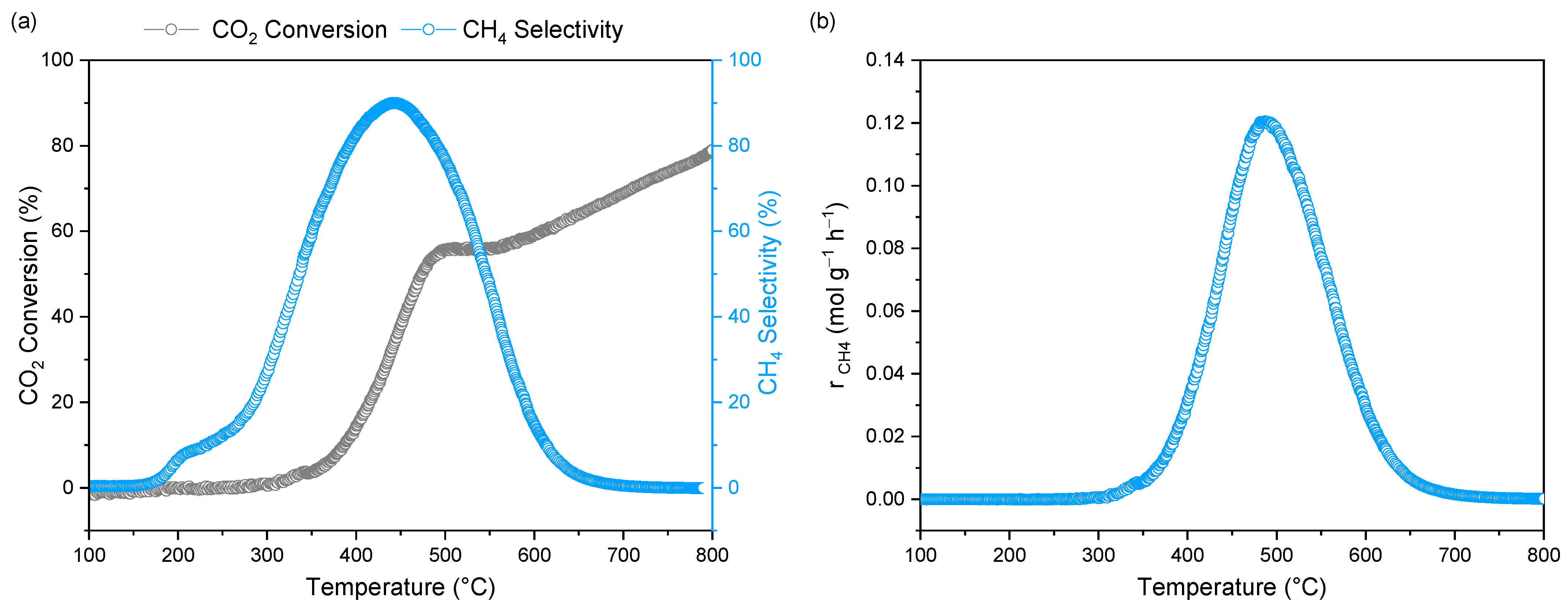
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, H.Ü.; Kimbrough, S.O.; van Dinther, C.; Keles, D. Power-to-gas: Decarbonization of the European electricity system with synthetic methane. Appl. Energy 2022, 323, 119538. [Google Scholar] [CrossRef]
- Genovese, M.; Schlüter, A.; Scionti, E.; Piraino, F.; Corigliano, O.; Fragiacomo, P. Power-to-hydrogen and hydrogen-to-X energy systems for the industry of the future in Europe. Int. J. Hydrogen Energy 2023, 48, 16545–16568. [Google Scholar] [CrossRef]
- De Dokania, S.A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar]
- Nemmour, A.; Inayat, A.; Janajreh, I.; Ghenai, C. Green hydrogen-based E-fuels (E-methane, E-methanol, E-ammonia) to support clean energy transition: A literature review. Int. J. Hydrogen Energy 2023, 48, 29011–29033. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 2022–2027. [Google Scholar]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent Progresses in Constructing the Highly Efficient Ni Based Catalysts with Advanced Low-Temperature Activity Toward CO2 Methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef]
- El-Salamony, R.A.; Acharya, K.; Al-Fatesh, A.S.; Osman, A.I.; Alreshaidan, S.B.; Kumar, N.S.; Ahmed, H.; Kumar, R. Enhanced direct methanation of CO2 using Ni-based catalysts supported on ZrO2, CeO2-ZrO2, and La2O3-ZrO2: The effect of support material on the reducible NiO-interacted species and catalytic activity. Mol. Catal. 2023, 547, 113378. [Google Scholar] [CrossRef]
- El-Salamony, R.A.; Al-Fatesh, A.S.; Acharya, K.; Abahussain, A.A.M.; Bagabas, A.; Kumar, N.S.; Ibrahim, A.A.; Khan, W.U.; Kumar, R. Carbon Dioxide Valorization into Methane Using Samarium Oxide-Supported Monometallic and Bimetallic Catalysts. Catalysts 2023, 13, 113. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrogen Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Zhang, L.; Hu, S.; Xiang, J.; Wang, Y.; Xu, L.; Liu, Q.; Zhang, S.; Hu, X. Impacts of nickel loading on properties, catalytic behaviors of Ni/γ–Al2O3 catalysts and the reaction intermediates formed in methanation of CO2. Int. J. Hydrogen Energy 2019, 44, 9291–9306. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4 CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- He, L.; Lin, Q.; Liu, Y.; Huang, Y. Unique catalysis of Ni-Al hydrotalcite derived catalyst in CO2 methanation: Cooperative effect between Ni nanoparticles and a basic support. J. Energy Chem. 2014, 23, 587–592. [Google Scholar] [CrossRef]
- Huynh, H.L.; Yu, Z. CO2 Methanation on Hydrotalcite-Derived Catalysts and Structured Reactors: A Review. Energy Technol. 2020, 8, 1901475. [Google Scholar] [CrossRef]
- Jia, C.; Gao, J.; Dai, Y.; Zhang, J.; Yang, Y.J. The thermodynamics analysis and experimental validation for complicated systems in CO2 hydrogenation process. Energy Chem. 2016, 25, 1027–1037. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.D. Potential of an alumina-supported Ni3Fe catalyst in the methanation of CO2: Impact of alloy formation on activity and stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Synthesis, Characterization, and Activity Pattern of Ni–Al Hydrotalcite Catalysts in CO2 Methanation. Ind. Eng. Chem. Res. 2016, 55, 8299–8308. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.; Shi, L.; Chen, T.; Wan, H.; Wang, P.; Wei, S.; Yao, B.; Zhu, J.; Song, M. Improved performance of Ni/Al2O3 catalyst deriving from the hydrotalcite precursor synthesized on Al2O3 support for dry re-forming of methane. Int. J. Hydrogen Energy 2021, 46, 14301–14310. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Yi, H.; Xue, Q.; Lu, S.; Wu, J.; Wang, Y.; Luo, G. Effect of pore structure on Ni/Al2O3 microsphere catalysts for enhanced CO2 methanation. Fuel 2022, 315, 123262. [Google Scholar] [CrossRef]
- Pieta, I.S.; Lewalska-Graczyk, A.; Kowalik, P.; Antoniak-Jurak, K.; Krysa, M.; Sroka-Bartnicka, A.; Gajek, A.; Lisowski, W.; Mrdenovic, D.; Pieta, P.; et al. CO2 Hydrogenation to Methane over Ni-Catalysts: The Effect of Support and Vanadia Promoting. Catalysts 2021, 11, 433. [Google Scholar] [CrossRef]
- Gao, H.-B.; Qiu, L.-L.; Wu, F.-P.; Xiao, J.; Zhao, Y.-P.; Liang, J.; Bai, Y.-H.; Liu, F.-J.; Cao, J.-P. Highly efficient catalytic hydrogenolysis of lignin model compounds over hydrotalcite-derived Ni/Al2O3 catalysts. Fuel 2023, 337, 127196. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2021, 284, 119082. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Rufford, T.E.; Li, Y.; Zhu, Z. Influence of calcination temperatures of Feitknecht compound precursor on the structure of Ni–Al2O3 catalyst and the corresponding catalytic activity in methane decomposition to hydrogen and carbon nanofibers. Appl. Catal. A Gen. 2009, 362, 1–7. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000; p. 20899. [CrossRef]
- Crist, B.V. Monochromatic XPS Spectra Commercially Pure Binary Oxides; XPS International, LLC: Salem, OR, USA, 2019. [Google Scholar]
- Liu, Z.; Chu, B.; Zhai, X.; Jin, Y.; Cheng, Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor. Fuel 2012, 95, 599–605. [Google Scholar] [CrossRef]
- Hashimoto, N.; Mori, K.; Asahara, K.; Shibata, S.; Jida, H.; Kuwahara, Y.; Yamashita, H. How the Morphology of NiOx-Decorated CeO2 Nanostructures Affects Catalytic Properties in CO2 Methanation. Langmuir 2021, 37, 5376–5384. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Cortés, H.S.; Bailón-García, E.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Active, selective and stable NiO-CeO2 nanoparticles for CO2 methanation. Fuel Proces. Technol. 2021, 212, 106637. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Soares, O.S.G.P.; Pereira, M.F.R.; Kubička, D.; Freire, C. Catalytic Transfer Hydrogenation of Furfural over Co3O4–Al2O3 Hydrotalcite-derived Catalyst. ChemCatChem 2020, 12, 1467–1475. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of lanthanum incorporation method on the performance of nickel-containing hydrotalcite-derived catalysts in CO2 methanation reaction. Catal. Today 2018, 307, 205–211. [Google Scholar] [CrossRef]
- Han, F.; Liu, Q.; Li, D.; Ouyang, J. An emerging and high-performance sepiolite-supported Ni catalyst for low-temperature CO2 methanation: The critical role of hydroxyl groups. J. Environ. Chem. Eng. 2023, 11, 110331. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B 2020, 265, 118538. [Google Scholar] [CrossRef]

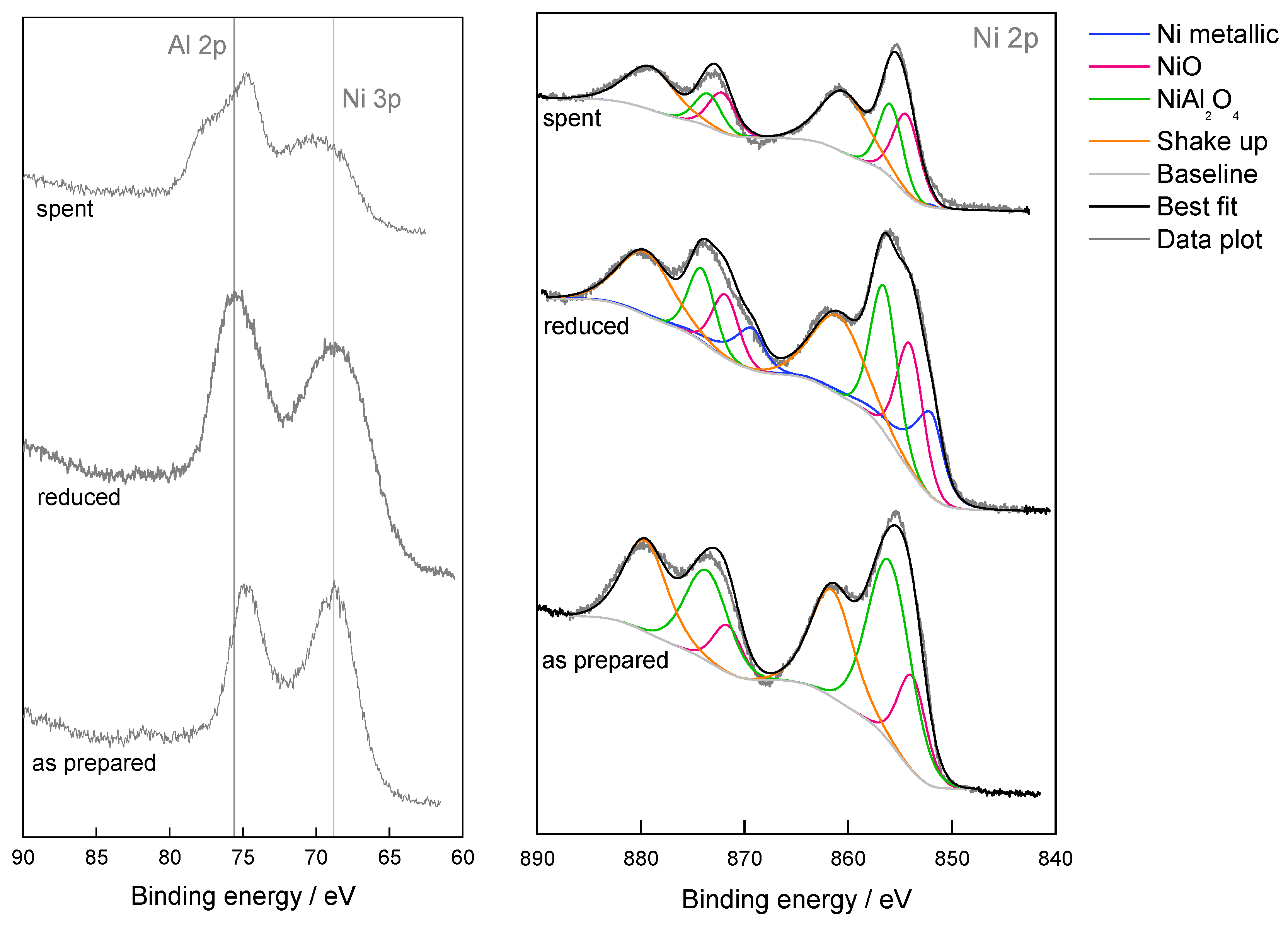
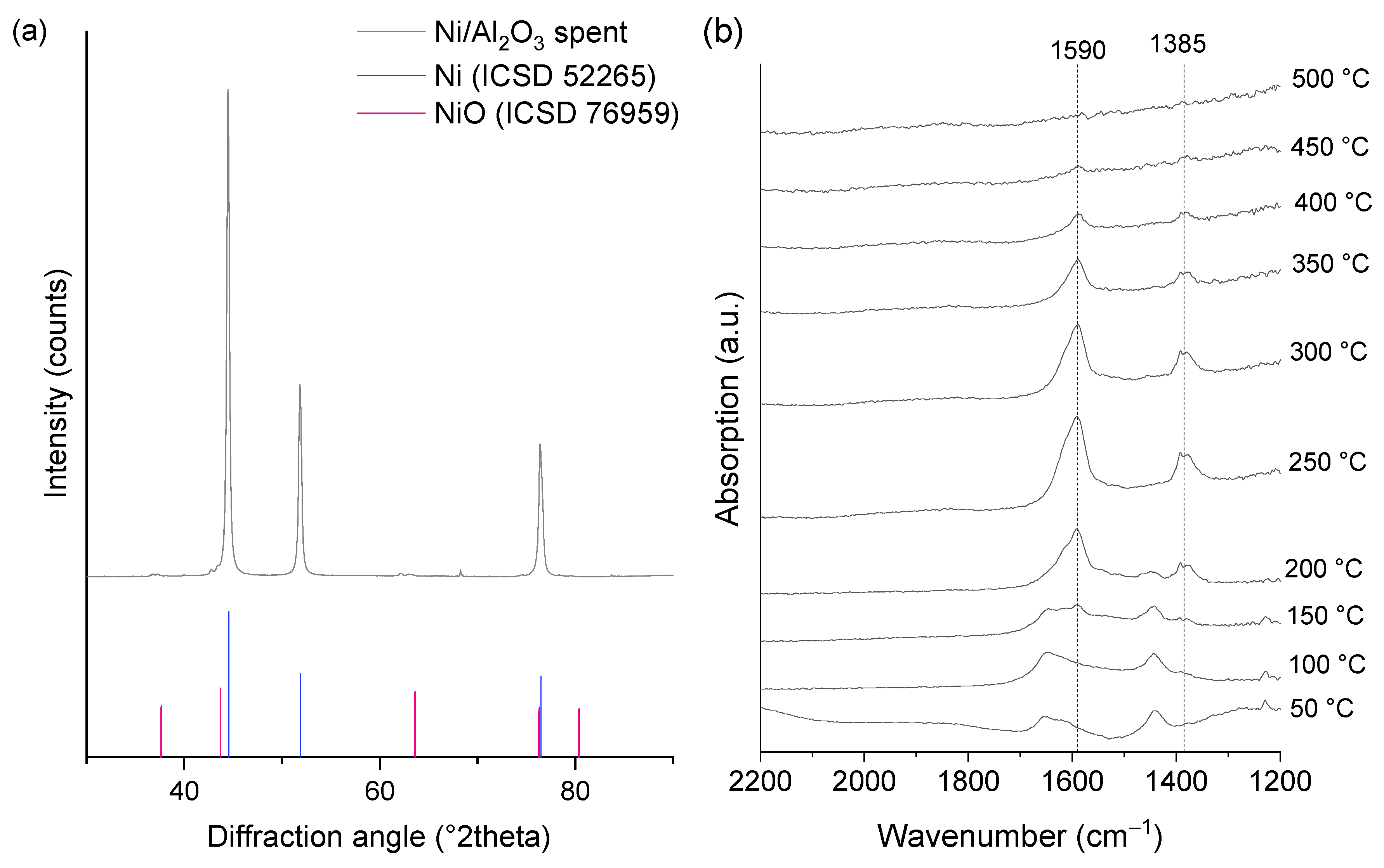
| Ni/Al2O3-HTC | As Prepared | Reduced | Spent |
|---|---|---|---|
| Crystallite size/nm | 3.9 | 7.6 | 42.4 |
| Rp | 1.7% | 1.7% | 0.5% |
| Χ2 | 1.13 | 1.22 | <0.01 |
| NiO | 1.00 | 0.02 | 0.01 |
| Ni metal | - | 0.98 | 0.99 |
| Sample | %C | %H | %N |
|---|---|---|---|
| Ni/Al2O3-HTC as prepared | 0.44 ± 0.04 | 1.29 ± 0.04 | 0.02 ± 0.01 |
| Ni/Al2O3-HTC spent (40 bar) | 0.40 ± 0.06 | 0.24 ± 0.06 | 0.04 ± 0.04 |
| Ni/Al2O3-HTC spent (1 bar) | 0.40 ± 0.06 | 0.59 ± 0.08 | - |
| Ni/Al2O3-HTC | %At of Surface Elements | %At of Surface Ni Species | ||||
|---|---|---|---|---|---|---|
| Ni | Al | O | Ni Metallic | NiO | NiAl2O4 | |
| As prepared | 21 | 36 | 43 | - | 26 | 74 |
| Reduced | 13 | 36 | 50 | 31 | 32 | 37 |
| Spent | 11 | 33 | 56 | 1 | 54 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothe, M.L.; Figueredo, A.L.; Borges, L.R.; Ramos, R.; Peixoto, A.F.; Vidinha, P. High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2. Methane 2024, 3, 53-64. https://doi.org/10.3390/methane3010004
Gothe ML, Figueredo AL, Borges LR, Ramos R, Peixoto AF, Vidinha P. High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2. Methane. 2024; 3(1):53-64. https://doi.org/10.3390/methane3010004
Chicago/Turabian StyleGothe, Maitê L., Adolfo L. Figueredo, Laís R. Borges, Ruben Ramos, Andreia F. Peixoto, and Pedro Vidinha. 2024. "High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2" Methane 3, no. 1: 53-64. https://doi.org/10.3390/methane3010004
APA StyleGothe, M. L., Figueredo, A. L., Borges, L. R., Ramos, R., Peixoto, A. F., & Vidinha, P. (2024). High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2. Methane, 3(1), 53-64. https://doi.org/10.3390/methane3010004