Density Functional Theory Insight into Chemical Vapor Infiltration
Abstract
:1. Introduction
2. Chemical Vapor Infiltration
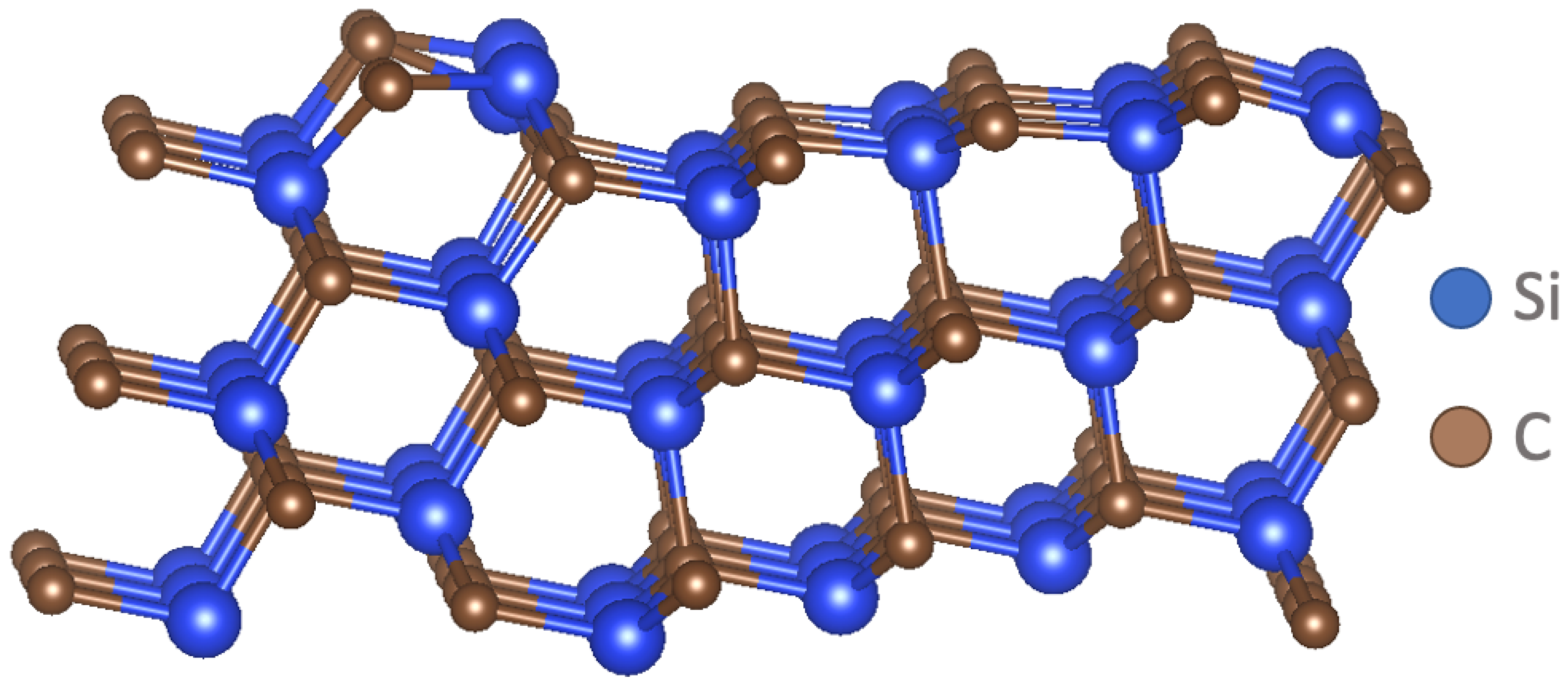
2.1. SiC versus Si Deposition
2.2. Pore Formation
3. Computational Methods
4. Results and Discussion
4.1. Reaction Mechanism
4.2. Methane Deposition
4.3. Summary of Thermodynamics
4.4. Chemical Precursor Suggestions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, Q.; Chen, J.; Ming, W.; Chen, M. Machining of SiC ceramic matrix composites: A review. Chin. J. Aeronaut. 2021, 34, 540–567. [Google Scholar] [CrossRef]
- Nagaraju, H.T.; Nance, J.; Kim, N.H.; Sankar, B.; Subhash, G. Uncertainty quantification in elastic constants of SiCf/SiCm tubular composites using global sensitivity analysis. J. Compos. Mater. 2023, 57, 63–78. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Ivanov, I.G.; Suwannaharn, N.; Hsu, C.-W.; Cora, I.; Pécz, B.; Giannazzo, F.; Sangiovanni, D.G.; Gueorguiev, G.K. MOCVD of AlN on epitaxial graphene at extreme temperatures. CrystEngComm 2021, 23, 385–390. [Google Scholar] [CrossRef]
- Arai, Y.; Inoue, R.; Goto, K.; Kogo, Y. Carbon fiber reinforced ultra-high temperature ceramic matrix composites: A review. Ceram. Int. 2019, 45, 14481–14489. [Google Scholar] [CrossRef]
- Fédou, R.; Langlais, F.; Naslain, R. A Model for the Isothermal Isobaric Chemical Vapor Infiltration (CVI) in a Straight Cylindrical Pore. Application to the CVI of SiC. J. Mater. Synth. Process. 1993, 1, 61–74. [Google Scholar]
- Lu, C.; Cheng, L.; Zhao, C.; Zhang, L.; Xu, Y. Kinetics of chemical vapor deposition of SiC from methyltrichlorosilane and hydrogen. Appl. Surf. Sci. 2009, 255, 7495–7499. [Google Scholar] [CrossRef]
- Vignoles, G. Advances in Composites Manufacturing and Process Design; Woodhead Publishing: Cambridge, UK, 2015; pp. 415–458. [Google Scholar]
- Desenfant, A.; Laduye, G.; Vignoles, G.; Chollon, G. Kinetic and gas-phase study of the chemical vapor deposition of silicon carbide from C2H3SiCl3/H2. J. Ind. Eng. Chem. 2021, 94, 145–158. [Google Scholar] [CrossRef]
- Snead, M.A.; Katoh, Y.; Koyanagi, T.; Singh, G.P. SiC/SiC Cladding Materials Properties Handbook; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2017. [Google Scholar]
- Ge, Y.; Gordon, M.S.; Battaglia, F.; Fox, R.O. Theoretical Study of the Pyrolysis of Methyltrichlorosilane in the Gas Phase. 1. Thermodynamics. J. Phys. Chem. A 2007, 111, 1462–1474. [Google Scholar] [CrossRef]
- Ge, Y.; Gordon, M.S.; Battaglia, F.; Fox, R.O. Theoretical Study of the Pyrolysis of Methyltrichlorosilane in the Gas Phase. 2. Reaction Paths and Transition States. J. Phys. Chem. A 2007, 111, 1475–1486. [Google Scholar] [CrossRef]
- Ge, Y.; Gordon, M.S.; Battaglia, F.; Fox, R.O. Theoretical Study of the Pyrolysis of Methyltrichlorosilane in the Gas Phase. 3. Reaction Rate Constant Calculations. J. Phys. Chem. A 2010, 114, 2384–2392. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Kee, R.J. A Model of Silicon Carbide Chemical Vapor Deposition. J. Electrochem. Soc. 1991, 138, 841. [Google Scholar] [CrossRef]
- Sukkaew, P.; Danielsson, O.; Kordina, O.; Janzen, E.; Ojamae, L. Ab Initio Study of Growth Mechanism of 4H–SiC: Adsorption and Surface Reaction of C2H2, C2H4, CH4, and CH3. J. Phys. Chem. C 2017, 121, 1249–1256. [Google Scholar] [CrossRef]
- Sukkaew, P.; Kalered, E.; Janzen, E.; Kordina, O.; Danielsson, O.; Ojamae, L. Growth mechanism of SiC chemical vapor deposition: Adsorption and surface reactions of active Si species. J. Phys. Chem. C 2018, 122, 648–661. [Google Scholar] [CrossRef]
- Danielsson, O.; Karlsson, M.; Sukkaew, P.; Pedersen, H.; Ojamae, L. A Systematic Method for Predictive In Silico Chemical Vapor Deposition. J. Phys. Chem. C 2020, 124, 7725–7736. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Faccio, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Discovering atomistic pathways for supply of metal atoms from methyl-based precursors to graphene surface. Phys. Chem. Chem. Phys. 2023, 25, 829–837. [Google Scholar] [CrossRef]
- Hussey, A.; De Meyere, R.; Deck, C.; Armstrong, D.; Zayachuk, Y. Statistically sound application of fiber push-out method for the study of locally non-uniform interfacial properties of SiC-SiC fiber composites. J. Eur. Ceram. Soc. 2020, 40, 1052–1056. [Google Scholar] [CrossRef]
- Cha, C.M.; Liliedahl, D.; Sankaran, R.; Ramanuj, V. Microstructural and infiltration properties of woven preforms during chemical vapor infiltration. J. Am. Ceram. Soc. 2022, 105, 4595–4607. [Google Scholar] [CrossRef]
- Petroski, K.; Poges, S.; Monteleone, C.; Grady, J.; Bhatt, R.; Suib, S. Rapid Chemical Vapor Infiltration of Silicon Carbide Minicomposites at Atmospheric Pressure. ACS Appl. Mater. Interfaces 2018, 10, 4986–4992. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Wellendorff, J.; Lundgaard, K.T.; Møgelhøj, A.; Petzold, V.; Landis, D.D.; Nørskov, J.K.; Bligaard, T.; Jacobsen, K.W. Density functionals for surface science: Exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 2012, 85, 235149. [Google Scholar] [CrossRef]
- Canavan, J.; Giewont, K.; Kyriakidou, E.A.; Walker, E.A. Feasibility of Methane Oxidation on SSZ-13 Bridged Pd2Ox Sites: A Theoretical Study. J. Phys. Chem. C 2022, 126, 17123–17134. [Google Scholar] [CrossRef]
- Jafari, M.; Zimmerman, P.M. Reliable and efficient reaction path and transition state finding for surface reactions with the growing string method. J. Comput. Chem. 2017, 38, 645–658. [Google Scholar] [CrossRef]
- Zimmerman, P.M. Single-ended transition state finding with the growing string method. J. Comput. Chem. 2015, 36, 601–611. [Google Scholar] [CrossRef]
- Walker, E.; Ammal, S.C.; Terejanu, G.A.; Heyden, A. Uncertainty quantification framework applied to the water–gas shift reaction over Pt-based catalysts. J. Phys. Chem. C 2016, 120, 10328–10339. [Google Scholar] [CrossRef]
- Medford, A.J.; Wellendorff, J.; Vojvodic, A.; Studt, F.; Abild-Pedersen, F.; Jacobsen, K.W.; Bligaard, T.; Nørskov, J.K. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 2014, 345, 197–200. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, W.-J.; Choi, D.J.; Park, J.Y. Deposition of compositionally graded SiC/C layers on C–C composites by low pressure chemical vapor deposition. J. Nucl. Mater. 2002, 307–311, 1084–1087. [Google Scholar] [CrossRef]
- Loumagne, F.; Langlais, F.; Naslain, R.; Schamm, S.; Dorignac, D.; Sévely, J. Physicochemical properties of SiC-based ceramics deposited by low pressure chemical vapor deposition from CH3SiCl3H2. Thin Solid Films 1995, 254, 75–82. [Google Scholar] [CrossRef]
- Papasouliotis, G.D.; Sotirchos, S.V. Experimental study of atmospheric pressure chemical vapor deposition of silicon carbide from methyltrichlorosilane. J. Mater. Res. 1999, 14, 3397–3409. [Google Scholar] [CrossRef]
- Lespiaux, D.; Langlais, F.; Naslain, R.; Schamm, S.; Sevely, J. Chlorine and oxygen inhibition effects in the deposition of SiC-based ceramics from the Si C H Cl system. J. Eur. Ceram. Soc. 1995, 15, 81–88. [Google Scholar] [CrossRef]







| Reaction Equation | Reaction Energy (eV) | Standard Deviation (eV) |
|---|---|---|
| 3.344 | 0.296 | |
| −0.185 | 0.339 | |
| 3.278 | 0.269 | |
| −0.251 | 0.266 | |
| −1.228 | 0.170 | |
| −13.893 | 1.309 | |
| −22.409 | 1.480 | |
| −16.020 | 1.463 | |
| −24.536 | 1.586 | |
| −8.947 | 1.389 | |
| −17.463 | 1.568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, E.A.; Marziale, J.J.; Chen, J. Density Functional Theory Insight into Chemical Vapor Infiltration. Methane 2023, 2, 415-425. https://doi.org/10.3390/methane2040028
Walker EA, Marziale JJ, Chen J. Density Functional Theory Insight into Chemical Vapor Infiltration. Methane. 2023; 2(4):415-425. https://doi.org/10.3390/methane2040028
Chicago/Turabian StyleWalker, Eric A., Joseph J. Marziale, and James Chen. 2023. "Density Functional Theory Insight into Chemical Vapor Infiltration" Methane 2, no. 4: 415-425. https://doi.org/10.3390/methane2040028
APA StyleWalker, E. A., Marziale, J. J., & Chen, J. (2023). Density Functional Theory Insight into Chemical Vapor Infiltration. Methane, 2(4), 415-425. https://doi.org/10.3390/methane2040028






