Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review
Abstract
:1. Introduction
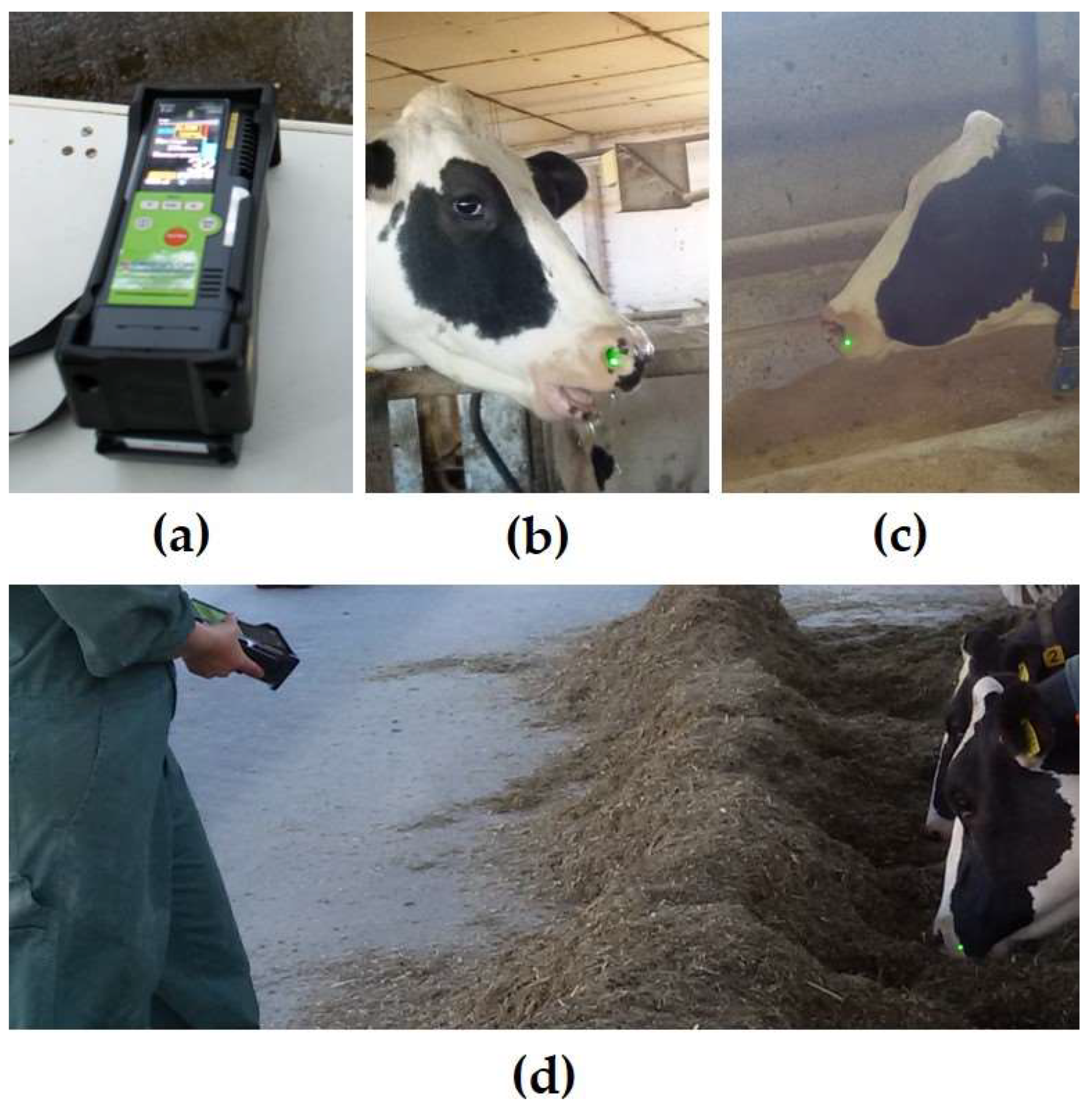
2. The Laser Methane Detector
3. Aspects of the Measurement Protocol
3.1. Distance to the Animal
3.2. Duration of Recording
3.3. Measurement Interval
3.4. Total Number of Repeats per Animal
3.5. Number of Consecutive Days per Measurement
3.6. Time of Day
3.7. Animal Activity
3.8. Pointing Angle
3.9. Location
3.10. Operator
3.11. Device
3.12. Animal Welfare
4. Steps in LMD Data Processing
4.1. Exclusion of Profiles
4.2. Exclusion of Single Data Points
4.3. Accouting for Background CH4 Concentration/Offset
4.4. Accounting for Distance
4.5. Transformation of the CH4 Values
4.6. Separation of Peaks and Troughs
4.7. Separation of Eructation and Respiration
4.8. Reduction of Data to Point Measurements
4.9. Estimation of Daily CH4 Production
5. Comparison with Other Techniques
- Technical evaluations of the accuracy of the sensor and
- Evaluations of the overall measurement and analysis protocol for the LMD.
6. Applications
6.1. Genetics
6.2. Nutrition
6.3. Farming Systems and Breeds
6.4. Health and Metabolism
7. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Aim | No 1 | Dis 2 | Dur 3 | Rep 4 | Selected Result(s) | Ref 5 |
|---|---|---|---|---|---|---|
| Comparison of LMD 6 and RC 7 | 20 dairy cows | 1 m | 5 min | 3 | Correlation between daily CH4 g/kg energy corrected milk with LMD and RC: 0.82 | [51] |
| Development of protocol for measurement and data analysis; nutritional study | 2 + 12 dairy cows | 0.6–1.2 m | 2–3 min | 26 | Correlation of daily CH4 production with RC and LMD: 0.55; natural grassland hay not recommended for indoor-fed cows | [18] |
| Relationship of blood serum parameters with CH4 emission | 46 ewes | 1 m | 3 min | - | Zinc, β-hydroxybutyrate, and non-esterified fatty acid blood concentrations had a partially breed-specific significant impact on CH4 emission | [58] |
| Comparison of LMD, RC and MIR 8; | 30 dairy cows | 1 m | 6 min | 12 | No agreement of LMD with RC or MIR | [17] |
| Book chapter reviewing previous work with the LMD | NA 9 | NA | NA | NA | Discussion of advantages and challenges of the LMD | [27] |
| Development of protocol for data analysis | 2 dairy cows | 3 m | 5 min | 15 | Number of peaks best phenotype to discriminate high and low emitters | [42] |
| Comparison of CH4 emissions from different husbandry systems | 448 dairy cows | 1 m | 2 min | 3 | CH4 concentrations were affected by location, breed, and husbandry system along a rural-urban gradient | [19] |
| Genetic analyses | 330 ewes | 1 m | 3 min | - | h2 10 for CH4 concentration 0.00–0.03 | [15] |
| Development of protocol for measurement and data analysis | 4 goats | 2 m | 4–5 min | 60 | No influence of restraint on CH4 concentration; restraint of grazing goats can facilitate LMD measurements | [31] |
| Development of data analysis | 46 dairy cows | 1 m | 5–6 min | 6–8 | Correlation of daily CH4 production with RC, and several LMD phenotypes: up to 0.6; simple phenotypes not outperformed by those considering the time series nature of data | [54] |
| Comparison of LMD and other techniques for CH4 measurement | NA | NA | NA | NA | Review and meta-analysis; sufficient correlation between methods for methods to be combined for international genetic studies | [10] |
| Comparison of LMD and NDIR 11 sniffer | 48 dairy cows | 1 m | 5 min | 6 | Moderate agreement between LMD and NDIR sniffer | [46] |
| Effect of a novel feed additive on CH4 | 30 dairy cows | 1 m | 4 min | - | Feed supplement significantly reduced CH4 concentration (p < 0.05) | [16] |
| Nutritional study | 45 dairy cows | 1 m | - | - | Daily CH4 production and CH4 per kg milk decreased by grass-feeding compared to a total mixed ration | [57] |
| Breed comparison | 24 beef cows | 3 m | 1 min | 72 | No between-breed difference in CH4 output | [32] |
| Genetic analyses | 622 dairy cows | 2 m | 5 min | 1–3 | h2 for CH4 phenotypes 0.05–0.28 | [14] |
| Development of protocol for measurement and data analysis | 4 + 12 goats | 1 m | 2 min | 24 + 63 | Recording interval should be 0.1 s; high variability of CH4 concentration across individual goats and days; LMD able to detect diurnal pattern of CH4 production in goats | [26] |
| Development of data analysis, comparison of LMD, GF 12 and NDIR/FTIR 13 sniffer | 156 dairy cows | 1, 2, 2.5 m | 5 min | 1–6 | Number of CH4 peaks = respiratory rate; similar ranking by LMD, GF and NDIR/FTIR sniffer sensors, regression equation for CH4 g/d from LMD data | [35] |
| Nutritional study | 18 goats | 1.5 m | 5 min | - | CH4 production affected by energy and tannin levels of feed, and sex | [50] |
| Development of protocol for measurement and data analysis | 71 + 18 dairy cows | 1 m | ~5 min | NA | Optimal recording duration > 3 min, correlation of LMD and RC CH4 average 0.47, regression equation for CH4 g/d from LMD data | [33] |
| Development of protocol for measurement and data analysis | 8 dairy cows | 3 m | 1 min | 40 | The proposed LMD measurement protocol could not be successfully implemented due to local weather conditions (grazing) | [43] |
| Development of protocol for measurement and data analysis | - | 0.4–2.5 m | 5–10 min | NA | Duration of recording should be >2 min; no significant change in CH4 concentration above 0.4 m distance; pointing angle, operator, LMD unit, time of day have significant effect on CH4 concentration; number of peaks is the respiratory rate; prediction equation for total daily CH4 from LMD record | [34] |
| Comparison of LMD and RC, comparison of 2 LMD devices | 4 dairy cows | NA | continuous | NA | Good agreement in concentration in spent air from RC measured with RC and LMD, good agreement between 2 LMD devices | [40] |
| Genetic analyses | 57 dairy cows | 1 m | 1–5 min | Up to 9 | h2 for CH4 concentration 0.05 | [13] |
| Development of protocol for measurement and data analysis | 32 sheep | 1 m | 5–10 min | 9–12 | Correlation of daily CH4 production with RC and LMD: 0.57 | [29] |
| Comparison of Bonsmara, Nguni and Jersey cattle | 12 heifers | 3 m | 1 min | 40 | Differences in CH4 concentration between breeds and feed sources | [30] |
| Development of protocol for measurement and data analysis | 24 ewes; 72 steers | ? | 2 min; 4 min | 5; 3 | Low correlation of CH4 from RC and LMD-model improved by DMI 14; best LMD phenotypes: length of eructation and maximum of respiration CH4 | [38] |
| Review summarizing previous work with the LMD | NA | NA | NA | NA | The LMD is a very recent tool and has potential to measure enteric CH4 production in ruminants, further validation needed | [44] |
| Development of protocol for measurement and data analysis | 2 + 24 dairy cows; 4 sheep | 2.75 m | 5 min | - | LMD has good sensitivity and specificity in detecting high and low CH4 concentrations as compared to RC; cow activity and meteorological factors affect CH4 concentrations | [36] |
| Development of protocol for measurement and data analysis | 72 cross-bred steers | 1 m | 4 min | - | Significant regression of LMD on RC with R2 = 0.27; similar results for mean of all values, all peaks, respiration peaks or eructation peaks | [28] |
| Comparison of LMD and RC | 10 dairy cows | 2.3 m | continuous (12–16 h) | NA | Good agreement in concentration in spent air from RC measured with RC and LMD | [45] |
| Development of protocol and data analysis | 71 dairy cows | 3 m | 15–25 s | 3 | LMD is applicable for cows, data make biological sense | [12] |
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; p. xxi + 115. [Google Scholar]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Fagodiya, R.; Hiloidhari, M.; Dahiya, R.; Kumar, A. Methane production and estimation from livestock husbandry: A mechanistic understanding and emerging mitigation options. Sci. Total Environ. 2020, 709, 136135. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.; Clark, H.; Cowie, A.L.; Emmet-Booth, J.; Gonzalez Fischer, C.; Herrero, M.; Howden, M.; Leahy, S. How necessary and feasible are reductions of methane emissions from livestock to support stringent temperature goals? Philos. Trans. R. Soc. A 2021, 379, 20200452. [Google Scholar] [CrossRef]
- Zhao, Y.; Nan, X.; Yang, L.; Zheng, S.; Jiang, L.; Xiong, B. A review of enteric methane emission measurement techniques in ruminants. Animals 2020, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, P.R. Method and System for Monitoring and Reducing Ruminant Methane Production. U.S. Patent 7,966,971, 28 June 2011. [Google Scholar]
- Lassen, J.; Løvendahl, P.; Madsen, J. Accuracy of noninvasive breath methane measurements using Fourier transform infrared methods on individual cows. J. Dairy Sci. 2012, 95, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negussie, E.; Lehtinen, J.; Mäntysaari, P.; Bayat, A.R.; Liinamo, A.-E.; Mäntysaari, E.; Lidauer, M. Non-invasive individual methane measurement in dairy cows. Animal 2017, 11, 890–899. [Google Scholar] [CrossRef]
- Johnson, K.; Huyler, M.; Westberg, H.; Lamb, B.; Zimmerman, P. Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ. Sci. Technol. 1994, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Garnsworthy, P.C.; Difford, G.F.; Bell, M.J.; Bayat, A.R.; Huhtanen, P.; Kuhla, B.; Lassen, J.; Peiren, N.; Pszczola, M.; Sorg, D. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals 2019, 9, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L. Measurement Methods to Assess Methane Production of Individual Dairy Cows in a Barn. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2016. [Google Scholar]
- Chagunda, M.G.G.; Ross, D.; Roberts, D.J. On the use of a laser methane detector in dairy cows. Comput. Electron. Agric. 2009, 68, 157–160. [Google Scholar] [CrossRef]
- Pickering, N.K.; Chagunda, M.G.G.; Banos, G.; Mrode, R.; McEwan, J.C.; Wall, E. Genetic parameters for predicted methane production and laser methane detector measurements. J. Anim. Sci. 2015, 93, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Mühlbach, S.; Sorg, D.; Rosner, F.; Kecman, J.; Swalve, H. Genetic analyses for CH4 concentrations in the breath of dairy cows measured on-farm with the Laser Methane Detector. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11–16 February 2018. [Google Scholar]
- Reintke, J.; Brügemann, K.; Yin, T.; Engel, P.; Wagner, H.; Wehrend, A.; König, S. Assessment of methane emission traits in ewes using a laser methane detector: Genetic parameters and impact on lamb weaning performance. Arch. Anim. Breed. 2020, 63, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Vrancken, H.; Suenkel, M.; Hargreaves, P.R.; Chew, L.; Towers, E. Reduction of Enteric Methane Emission in a Commercial Dairy Farm by a Novel Feed Supplement. Open J. Anim. Sci. 2019, 9, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Denninger, T.M.; Schwarm, A.; Dohme-Meier, F.; Münger, A.; Bapst, B.; Wegmann, S.; Grandl, F.; Vanlierde, A.; Sorg, D.; Ortmann, S.; et al. Accuracy of methane emissions predicted from milk mid-infrared spectra and measured by laser methane detectors in Brown Swiss dairy cows. J. Dairy Sci. 2020, 103, 2024–2039. [Google Scholar] [CrossRef]
- Kobayashi, N.; Hou, F.; Tsunekawa, A.; Yan, T.; Tegegne, F.; Tassew, A.; Mekuriaw, Y.; Mekuriaw, S.; Hunegnaw, B.; Mekonnen, W. Laser methane detector-based quantification of methane emissions from indoor-fed Fogera dairy cows. Anim. Biosci. 2021, 34, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Yin, T.; Reichenbach, M.; Bhatta, R.; Malik, P.K.; Schlecht, E.; König, S. Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity. Agriculture 2020, 10, 628. [Google Scholar] [CrossRef]
- Iseki, T. A portable remote methane detector using an InGaAsP DFB laser. Environ. Geol. 2004, 46, 1064–1069. [Google Scholar] [CrossRef]
- Tokyo Gas Engineering Solutions. SA3C50A, LaserMethane Mini-g Operating Manual. Available online: https://www.crowcon.com/wp-content/uploads/2018/12/SA3C50A-Manual.pdf (accessed on 29 November 2021).
- Crowcon Detection Instruments. LaserMethane Mini Specifications. Available online: https://www.crowcon.com/products/portables/lmm-gen-2/#specs (accessed on 26 November 2021).
- Tokyo Gas Engineering Solutions. GasViewer, Laser Methane Linked Software for Android Devices, Instruction Manual. Available online: https://www.crowcon.com/wp-content/uploads/2018/12/GasViwer-Eng-manual.pdf (accessed on 26 November 2021).
- Murray, R.; Bryant, A.; Leng, R. Rates of production of methane in the rumen and large intestine of sheep. Br. J. Nutr. 1976, 36, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, C.; Yan, T.; Wills, D.A.; Murray, S.; Gordon, A.W. Comparison of the sulfur hexafluoride tracer and respiration chamber techniques for estimating methane emissions and correction for rectum methane output from dairy cows. J. Dairy Sci. 2012, 95, 3139–3148. [Google Scholar] [CrossRef]
- Roessler, R.; Chefor, F.; Schlecht, E. Using a portable laser methane detector in goats to assess diurnal, diet- and position-dependent variations in enteric methane emissions. Comput. Electron. Agric. 2018, 150, 110–117. [Google Scholar] [CrossRef]
- Jonker, A.; Chagunda, M.G.G.; Grobler, M.; Waghorn, G.C.; Van Wyngaard, J.D. Hand-held laser methane detector to determine methane plume concentrations from ruminants. In Guideline for Estimating Methane Emissions from Individual Ruminants Using: GreenFeed, Sniffers, Hand-Held Laser Detector and Portable Accumulation Chambers; Jonker, A., Waghorn, G.C., Eds.; MPI Technical Paper; Ministry for Primary Industries: Wellington, New Zealand, 2020; pp. 41–47. [Google Scholar]
- Rooke, J.; Ricci, P.; Duthi, C.; Roehe, R.; Waterhouse, A. Measurements of methane using the laser methane detector are related to total daily methane output in beef cattle. In Emissions of Gas and Dust from Livestock; Hassouna, M., Guingand, N., Eds.; IFIP—Institut Technique du Porc: Paris, France, 2013; pp. 367–370. ISBN 978-2-85969-221-6. [Google Scholar]
- Doran, S. Development of Proxy Indicators of Methane Output by Sheep Using Rapid-Throughput Field and Laboratory Technologies. Ph.D. Thesis, Aberystwyth University, Aberystwyth, UK, 2014. [Google Scholar]
- Grobler, S.; Scholtz, M.; Van Rooyen, H.; Mpayipheli, M.; Neser, F. Methane production in different breeds, grazing different pastures or fed a total mixed ration, as measured by a Laser Methane Detector. S. Afr. J. Anim. Sci. 2014, 44, 12–16. [Google Scholar] [CrossRef]
- Roessler, R.; Schlecht, E. Application of the laser methane detector for measurements in freely grazing goats: Impact on animals’ behaviour and methane emissions. Animal 2021, 15, 100070. [Google Scholar] [CrossRef] [PubMed]
- Mapfumo, L.; Grobler, S.; Mupangwa, J.; Scholtz, M.; Muchenje, V. Enteric methane output from selected herds of beef cattle raised under extensive arid rangelands. Pastoralism 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bruder, T.; Rouillé, B.; Yan, T.; Chagunda, M.G.G. Towards a robust protocol for enteric methane measurements using a hand held Laser Methane Detector® in Ruminants. ICAR Tech. Ser. 2017, 22, 27–33. [Google Scholar]
- Sorg, D.; Mühlbach, S.; Kecman, J.; Swalve, H. Protocol for measuring CH4 concentrations with the laser methane detector in the breath of cows. In Proceedings of the 68th Annual Meeting of the European Federation of Animal Science, Tallinn, Estonia, 28 August–1 September 2017; p. 200. [Google Scholar]
- Sorg, D.; Difford, G.F.; Mühlbach, S.; Kuhla, B.; Swalve, H.H.; Lassen, J.; Strabel, T.; Pszczola, M. Comparison of a laser methane detector with the GreenFeed and two breath analysers for on-farm measurements of methane emissions from dairy cows. Comput. Electron. Agric. 2018, 153, 285–294. [Google Scholar] [CrossRef]
- Chagunda, M.G.G.; Ross, D.; Rooke, J.; Yan, T.; Douglas, J.-L.; Poret, L.; McEwan, N.R.; Teeranavattanakul, P.; Roberts, D.J. Measurement of enteric methane from ruminants using a hand-held laser methane detector. Acta Agr. Scand. A-An. 2013, 63, 68–75. [Google Scholar] [CrossRef]
- Falconer, D.; Mackay, T.F. Introduction to Quantitative Genetics, 4th ed.; Longman Group Ltd.: Harlow, UK, 1996. [Google Scholar]
- Ricci, P.; Chagunda, M.G.G.; Rooke, J.; Houdijk, J.G.M.; Duthie, C.-A.; Hyslop, J.; Roehe, R.; Waterhouse, A. Evaluation of the laser methane detector to estimate methane emissions from ewes and steers. J. Anim. Sci. 2014, 92, 5239–5250. [Google Scholar] [CrossRef]
- Della, M.R.; Jonker, A.; Waghorn, G. A review of technical variations and protocols used to measure methane emissions from ruminants using respiration chambers, SF6 tracer technique and GreenFeed, to facilitate global integration of published data. Anim. Feed Sci. Technol. 2021, 279, 115018. [Google Scholar] [CrossRef]
- Sorg, D.; Mühlbach, S.; Rosner, F.; Kuhla, B.; Derno, M.; Meese, S.; Schwarm, A.; Kreuzer, M.; Swalve, H. The agreement between two next-generation laser methane detectors and respiration chamber facilities in recording methane concentrations in the spent air produced by dairy cows. Comput. Electron. Agric. 2017, 143, 262–272. [Google Scholar] [CrossRef]
- Bell, M.J.; Craigon, J.; Saunders, N.; Goodman, J.R.; Garnsworthy, P.C. Does the diurnal pattern of enteric methane emissions from dairy cows change over time? Animal 2018, 12, 2065–2070. [Google Scholar] [CrossRef]
- Niero, G.; Cendron, F.; Penasa, M.; De Marchi, M.; Cozzi, G.; Cassandro, M. Repeatability and Reproducibility of Measures of Bovine Methane Emissions Recorded using a Laser Detector. Animals 2020, 10, 606. [Google Scholar] [CrossRef] [Green Version]
- Van Wyngaard, J.; Du Toit, C.; Meeske, R.; Erasmus, L.; Goemans, Z. Laser methane detector: Not a practical measurement tool for grazing dairy systems. In Proceedings of the South African Society for Animal Science, Port Elizabeth, South Africa, 18–21 September 2017; pp. 244–245. [Google Scholar]
- Chagunda, M.G.G. Opportunities and challenges in the use of the Laser Methane Detector to monitor enteric methane emissions from ruminants. Animal 2013, 7 (Suppl. S2), 394–400. [Google Scholar] [CrossRef] [Green Version]
- Chagunda, M.G.G.; Yan, T. Do methane measurements from a laser detector and an indirect open-circuit respiration calorimetric chamber agree sufficiently closely? Anim. Feed Sci. Technol. 2011, 165, 8–14. [Google Scholar] [CrossRef]
- Rey, J.; Atxaerandio, R.; Ruiz, R.; Ugarte, E.; González-Recio, O.; Garcia-Rodriguez, A.; Goiri, I. Comparison between non-invasive methane measurement techniques in cattle. Animals 2019, 9, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Boston, MA, USA, 1977. [Google Scholar]
- Meinert, T.R.; Norman, H.D. Merit of Outliers for Milk Yield as Indicators of Accuracy of Genetic Evaluations of Sires. J. Dairy Sci. 1998, 81, 2951–2955. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment, 5th ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Washaya, S.; Mupangwa, J.; Muchenje, V. Chemical composition of Lablab purpureus and Vigna unguiculata and their subsequent effects on methane production in Xhosa lop-eared goats. S. Afr. J. Anim. Sci. 2018, 48, 445–458. [Google Scholar] [CrossRef]
- Kecman, J.; Heinicke, J.; Grütter, L.; Kuhla, B.; Swalve, H.H. Methane emissions from dairy cows measured using laser methane detector and respiration chambers. In Proceedings of the 72nd Annual Meeting of the European Federation of Animal Science, Davos, Switzerland, 30 August–3 September 2021; p. 123. [Google Scholar]
- Derno, M.; Elsner, H.G.; Paetow, E.A.; Scholze, H.; Schweigel, M. Technical note: A new facility for continuous respiration measurements in lactating cows. J. Dairy Sci. 2009, 92, 2804–2808. [Google Scholar] [CrossRef] [PubMed]
- Llonch, P.; Troy, S.M.; Duthie, C.-A.; Somarriba, M.; Rooke, J.; Haskell, M.J.; Roehe, R.; Turner, S.P. Changes in feed intake during isolation stress in respiration chambers may impact methane emissions assessment. Anim. Prod. Sci. 2016, 58, 1011–1016. [Google Scholar] [CrossRef] [Green Version]
- Brocklehurst, S.; Hargreaves, P.; March, M. Novel statistical methods to improve analysis of laser methane detector data. Proc. Br. Soc. Anim. Sci. Adv. Anim. Biosci. 2019, 10, 1–284. [Google Scholar] [CrossRef] [Green Version]
- de Haas, Y.; Veerkamp, R.F.; de Jong, G.; Aldridge, M.N. Selective breeding as a mitigation tool for methane emissions from dairy cattle. Animal 2021, 15, 100294. [Google Scholar] [CrossRef]
- Lassen, J.; Difford, G.F. Genetic and genomic selection as a methane mitigation strategy in dairy cattle. Animal 2020, 14, s473–s483. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.; Chagunda, M.; Roberts, D.; Lee, M. A comparison of milk yields and methane production from three contrasting high-yielding dairy cattle feeding regimes: Cut-and-carry, partial grazing and total mixed ration. Grass Forage Sci. 2018, 73, 789–797. [Google Scholar] [CrossRef]
- Reintke, J.; Brügemann, K.; Yin, T.; Wagner, H.; Wehrend, A.; Müller, A.; König, S. Associations between minerals and metabolic indicators in maternal blood pre-and postpartum with ewe body condition, methane emissions, and lamb body weight development. Animal 2021, 15, 100034. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Koerkamp, P.W.G.; Ogink, N. Uncertainty assessment of the breath methane concentration method to determine methane production of dairy cows. J. Dairy Sci. 2018, 101, 1554–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Method | Purchase Cost | Running Cost | Labour | Repeatability | Behaviour Alteration | Throughput |
|---|---|---|---|---|---|---|
| Respiration chamber | High | High | High | High | High | Low |
| SF6 tracer gas technique | Medium | High | High | Medium | Medium | Medium |
| Breath analysers (“sniffers”) | Low | Low | Low | Medium | None | High |
| GreenFeed | Medium | Medium | Low | Medium | Low | Medium |
| Laser methane detector | Low | Low | High | Low | Low–medium | Medium |
| Variable | Minimum Observed | Reference (Example) | Maximum Observed | Reference (Example) |
|---|---|---|---|---|
| Distance to animal | 1 m | [28] | 3 m | [12] |
| Duration of recording | 15 s | [12] | 10 min | [29] |
| Measurement interval | 0.1 s | [26] | 5 s | [30] |
| Repeats per day | 1 | [14] | 6 | [31] |
| Repeats per animal | 1 | [14] | 72 (cow); 63 (goat) | [26,32] |
| Consecutive days | 1 | [14] | 10 | [30] |
| Category | Point Measurement | Explanation 1 |
|---|---|---|
| All values | Mean | Breath CH4 concentration including re-inhaled and exhaled eructation and background CH4 concentration during inhalation |
| Number | Not meaningful – number is pre-defined by measuring interval (e.g., 2 per s) | |
| Maximum | Highest single CH4 concentration | |
| Sum | Cumulative CH4 concentration including background | |
| Peaks | Mean | Breath CH4 concentration including re-inhaled and exhaled eructation CH4 without background |
| Number | Proxy for breath frequency (not for CH4 emission) | |
| Sum | Cumulative breath CH4 concentration without background | |
| Respiration peaks 2 | Mean | Breath CH4 concentration without eructation |
| Number | Breath frequency but without times of eructation—physiologically not meaningful | |
| Maximum | Highest non-eructation CH4 peak | |
| Sum | Cumulative breath CH4 concentration without eructation and background | |
| Time | Duration of respiration | |
| Percentage | Of respiration peaks from all peaks in a recording—can be used to validate physiological plausibility of the data | |
| Eructation peaks | Mean | Breath CH4 concentration from eructation only |
| Number | Breath frequency during eructation—physiologically not meaningful | |
| Maximum | Highest single CH4 concentration (same as for “all values”) | |
| Sum | Cumulative breath CH4 concentration from eructation only | |
| Time | Duration of eructation | |
| Percentage | Of eructation peaks from all peaks in a recording—can be used to validate physiological plausibility of the data | |
| Eructation events | Mean of the maxima | Series of the highest CH4 concentrations |
| Number | Eructation frequency—not a real CH4 phenotype but can be used to validate physiological plausibility of the data | |
| Maximum | Highest single CH4 concentration (same as for “all values”) | |
| Sum of the maxima | Cumulative breath CH4 concentration from the series of the highest CH4 concentrations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorg, D. Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review. Methane 2022, 1, 38-57. https://doi.org/10.3390/methane1010004
Sorg D. Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review. Methane. 2022; 1(1):38-57. https://doi.org/10.3390/methane1010004
Chicago/Turabian StyleSorg, Diana. 2022. "Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review" Methane 1, no. 1: 38-57. https://doi.org/10.3390/methane1010004
APA StyleSorg, D. (2022). Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review. Methane, 1(1), 38-57. https://doi.org/10.3390/methane1010004






