The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Brain Metabolism and Neurological Disorders
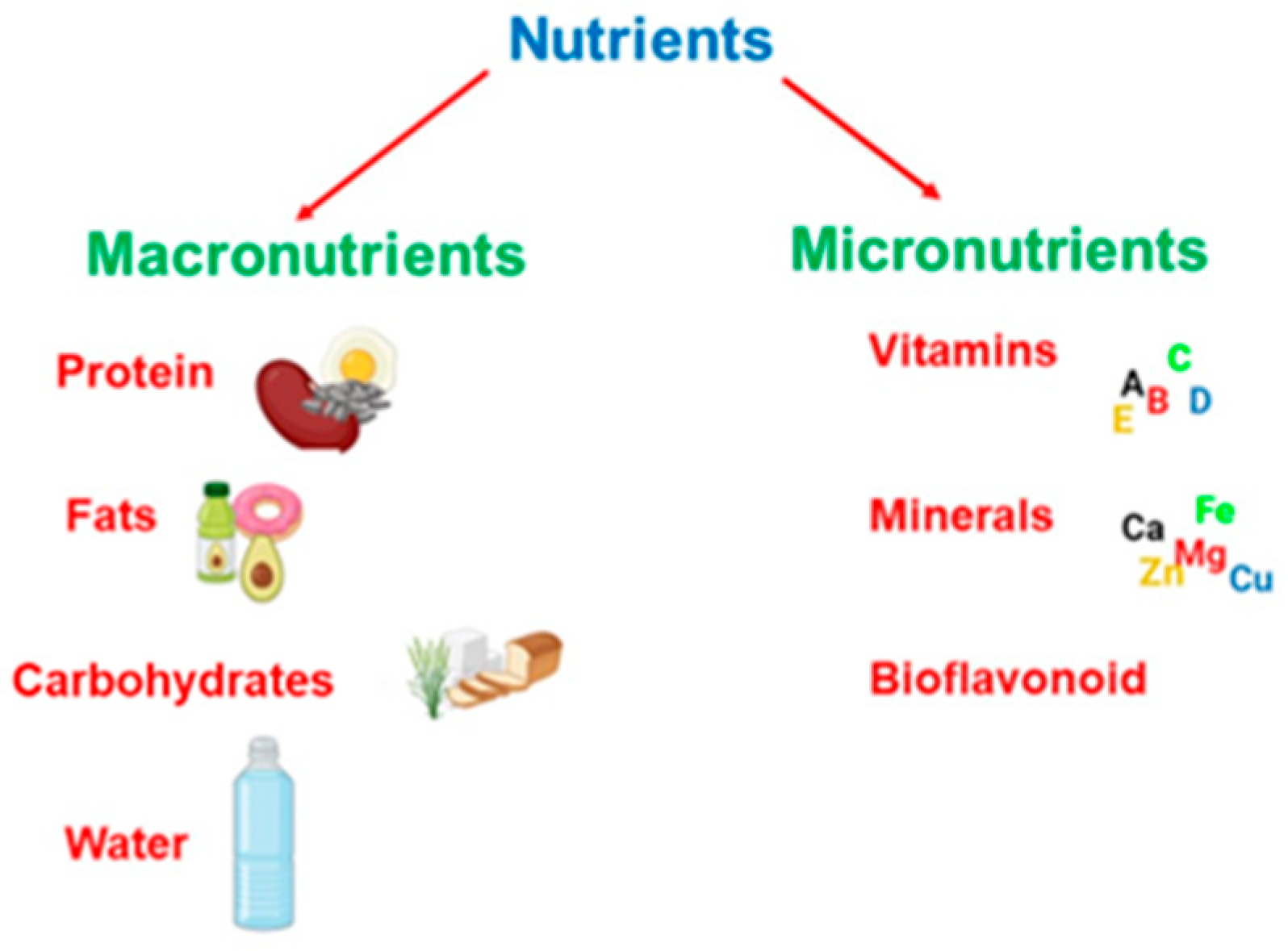
3. The Role of Diet in Brain Health
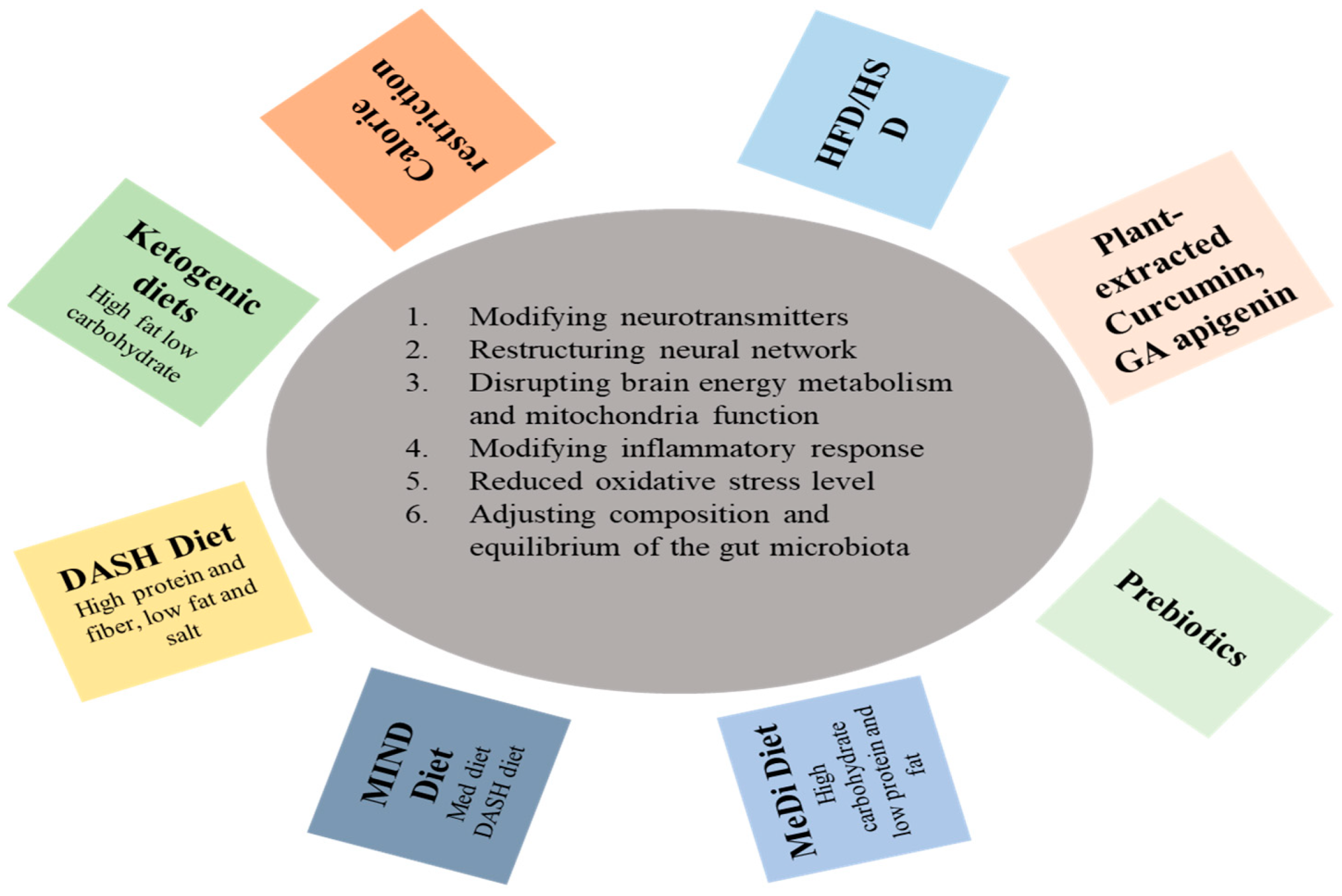
3.1. Dietary Interventions for Brain Metabolism
3.2. Neurological Challenges through Dietary Interventions
| Sr. No. | Dietary Factors | Experimental Study Design/Participants | Results | Study Conclusion | Ref. |
|---|---|---|---|---|---|
| 1. | MIND diet | There is baseline I (7983 participants included) and baseline II (4040 participants included) associated with dietary history used for the population-based Rotterdam study. | A 53% decrease in the incidence of AD was predicted for those in the top tertile of MIND scores. | Lower risk of dementia | [80] |
| 2. | DASH diet | Used 4169 MESA participants and evaluated the association between DASH diet adherence and cognitive functions | Higher nut/legume consumption was linked to improved CASI scores on Exams 5 (p = 0.003) and 6 (p = 0.007) were enhanced. | Improved cognitive performance, with ethnic disparities perhaps existing. | [66] |
| 3. | Omega-3 Fatty Acid | A randomized study including 9 participants, 5 of them assigned to low-fat diets and antioxidants and the other 4 being placebo groups with only a low-fat diet. | Long-chain omega-3 fatty acids in the early stages of AD to prevent or delay cognitive decline. | Involved in the cellular metabolism and multiple sclerosis-linked inflammatory processes. | [68,75] |
| 4. | Polyphenols/Curcumin | In this randomized study, 60–85-year-old individuals with fluency in writing and speaking English, normal vision, and other specified criteria participated. A total of 61 participants, all free from bleeding and metabolic disorders, were enrolled. | Significantly reduced total and LDL cholesterol. | Effects of curcumin on cognition in healthy elderly population of human. | [69] |
| 5. | Antioxidants | In this population-based study, 16,010 women participated based on educational qualifications and other parameters like health status. | Neuronal damage, AD progression, and oxidative stress production/aggregation | Dietary intake of flavonoids, especially derived from berries, appears to reduce cognitive decline in older adults. | [81] |
| 6. | Carbohydrates | In this study, APP/PS1 double mutant transgenic mice express human amyloid beta precursor protein containing K595N/M596L Swedish mutations. | Simple carbohydrates impair cognition and increase risk of AD | Regulating the consumption of sugary beverages may be an effective way to curtail the risk of developing AD. | [82] |
| 7. | Polyphenols/Resveratrol | A total of 119 participants were included and were randomized to resveratrol 500 mg orally once daily (with a dose escalation by 500 mg increments every 13 weeks, ending with 1000 mg twice daily). | Exploration of the capacity for mitigating the decline in mini-mental status evaluation scores in individuals diagnosed with AD. | Resveratrol can reduce or modulate neuroinflammation and induce adaptive immunity. | [83] |
| 8. | Polyphenols/Glycyrhizic flavone | In this double-blinded trial study, 128 patients participated and were assessed for eligibility criteria. | Enhancement of the Parkinson’s assessment scale in diagnosed individuals. | Glycyrrhizic flavone could improve the symptoms of PD in patients without serious adverse side effects. | [84] |
| 9. | Alkaloids/Trigonelline | 40 patients participated, with inclusion criteria consisting of PD, ages from 18–70 years, and being on stable doses of L-DOPA with carbidopa. | Improvements in UPDRS for diagnosed neurologic patients. | Fenugreek seed has great potential as an adjuvant to L-DOPA therapy. | [85] |
| 10. | Amino acid/Branched-chain amino acid | In this randomized study, patients with clinically proven cirrhosis of different etiologies were included at 30–70 years, body weight between 60–80 kg and a lack of BCAA treatment. | Enhanced cognitive function in cirrhosis-associated hepatic encephalopathy (HE). | Supplementations of diets with oral BCAA are better than casein for mental health management. | [86] |
| 11. | α-linolenic acid | In this study, male offspring mice were reared on the same low or adequate alpha linoleninc acid diet till 4 months of age. | There is an increase in the level of docosahexaenoic acid (DHA) in the brain | Increasing the brain DHA can reduce neuroinflammation and improve functional recovery after TBI. | [87] |
3.3. Nutritional Interventions to Metabolic Disorders
| Sr. No. | Dietary Factors | Metabolic Disease | Experimental Study Design/Participants | Study Conclusion | Reference |
|---|---|---|---|---|---|
| 1. | Caloric restriction | Diabetes | In this study, male B6C3F1 hybrid mice were used, randomized into control or restricted groups at two months of age, and fed 87 kcal/week. | Calorie-restricted animals are metabolically distinct. | [94] |
| 2. | Resveratrol and pterostilbene | Obesity | This study used 6-week-old male Wistar rats and divided them into four groups, i.e., control, high-fat high sugar group, resveratrol-treated group, and pterostilbene-treated group with different concentrations of appropriate conditions. | Adipokines, NOV/CC3 seem to be involved in the weight changes observed in adipose tissue under obesogenic feeding conditions. | [95,96] |
| 3. | Plant-based diet | Diabetes | Used baseline that followed 2918 participants; nonsmoking, nonalcohol, etc., for a median of 5 years with 183 incident diabetes cases. | A vegetarian diet may protect against diabetes. | [98] |
| 4. | Sodium | Hypertension | 370 adults, 70% being women, associated with 72% hypertension. | A high-sodium diet was associated with hypertension and insulin resistance. | [100] |
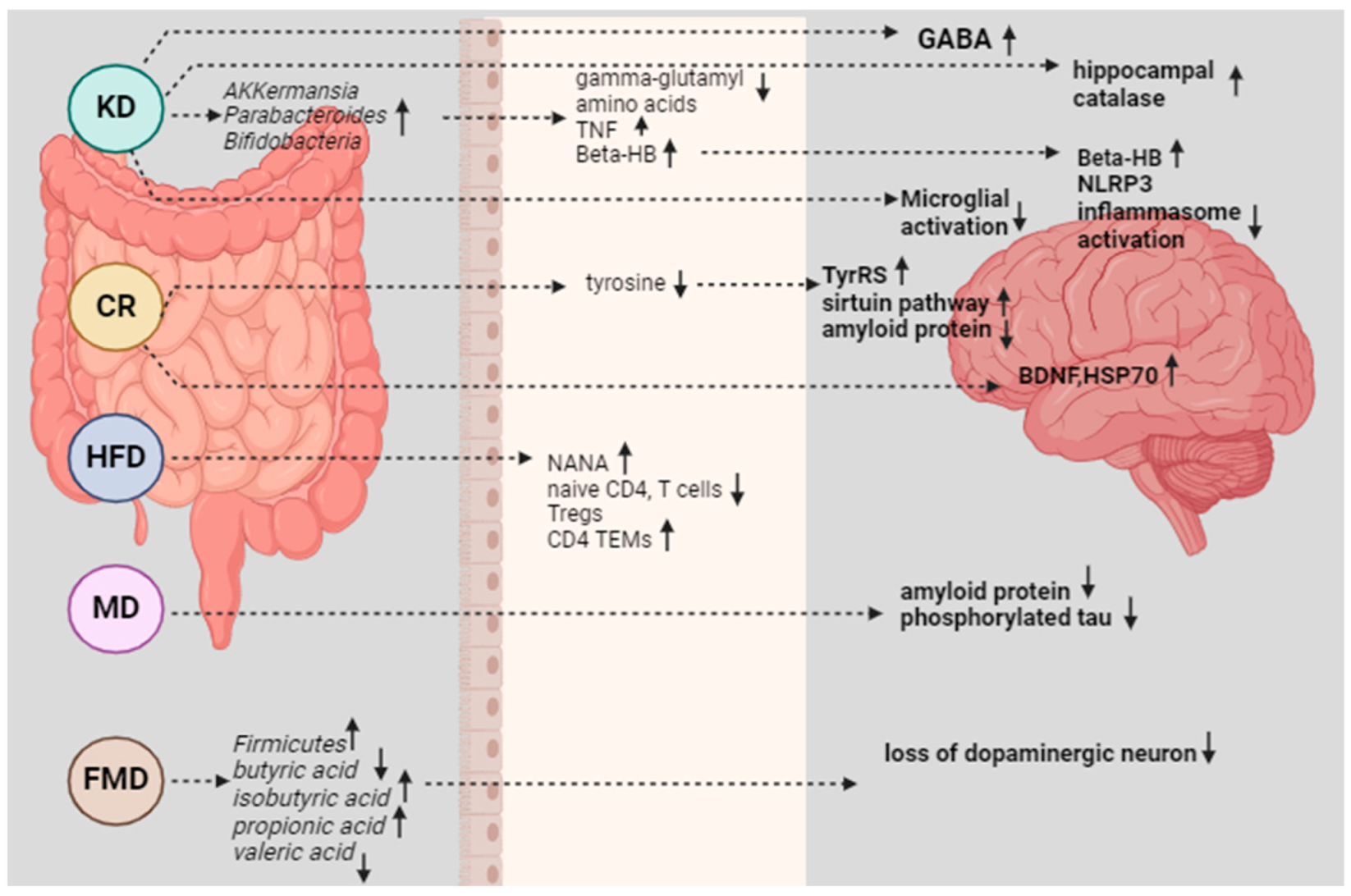
4. Mechanisms of Action
4.1. Inflammation and Oxidative Stress
4.2. Gut-Brain Axis
5. Clinical Studies and Evidence
- Clinical Studies: Numerous clinical trials have investigated the efficacy of the ketogenic diet in managing epilepsy, particularly in drug-resistant cases. For example, a study published in The Lancet Neurology in 2008 conducted a randomized controlled trial involving children with drug-resistant epilepsy, showing that the ketogenic diet led to a significant reduction in seizure frequency compared to controls [6].
- Evidence: Meta-analyses and systematic reviews have consistently supported the effectiveness of the ketogenic diet in reducing seizure frequency and improving seizure control in both children and adults with epilepsy. A meta-analysis published in the Cochrane Database of Systematic Reviews in 2020 concluded that the ketogenic diet significantly reduced seizure frequency compared to controls in randomized controlled trials [123].
- Clinical Studies: Clinical trials have investigated the effects of omega-3 fatty acid supplementation on cognitive function and neurodegenerative diseases. For instance, a randomized controlled trial published in J. Alzheimer’s Dis. 2016 examined the effect of omega-3 fatty acids on cognitive decline in older adults and found that supplementation was associated with slower cognitive decline over 4.5 years [124].
- Evidence: Observational studies have also suggested a protective effect of omega-3 fatty acids against cognitive decline and dementia. A systematic review and meta-analysis published in Eur. J. Nutr. 2022 concluded that higher dietary intake or blood levels of omega-3 fatty acids were associated with a lower risk of dementia and Alzheimer’s disease [125].
- Clinical Studies: Clinical trials and observational studies have investigated the impact of the Mediterranean diet on Alzheimer’s disease risk and progression. For example, the PREDIMED-NAVARRA trial, published in JAMA Internal Medicine in 2015, demonstrated that adherence to a Mediterranean diet supplemented with extra-virgin olive oil was associated with improved cognitive function in older adults with high cardiovascular risk [126,127].
- Evidence: Longitudinal cohort studies have provided consistent evidence linking adherence to the Mediterranean diet with a reduced risk of Alzheimer’s disease and slower cognitive decline. A meta-analysis published in the Journal of Alzheimer’s Disease in 2014 found that greater adherence to the Mediterranean diet was associated with a 33% reduction in the risk of Alzheimer’s disease [128].
- Clinical Studies: Clinical trials and observational studies have investigated the role of antioxidants in Parkinson’s disease prevention and management. For example, the DATATOP trial, published in the Archives of Neurology in 1993, found that treatment with the antioxidant vitamin E delayed the progression of disability in early Parkinson’s disease [129].
- Evidence: Epidemiological studies have provided mixed evidence regarding the association between dietary antioxidants and Parkinson’s disease risk. While some studies have suggested a protective effect of antioxidants such as vitamin E and flavonoids, others have found no significant association [130].
6. Methodology
7. Challenges and Limitations
8. Future Perspective and Research Opportunities
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ND | Neurological diseases |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic lateral sclerosis |
| MS | Multiple sclerosis |
| MD | Mediterranean Diet |
| PPARγ2 | Peroxisome proliferator-activated receptor gamma 2 |
| KD | Ketogenic diet |
| MAD | Modified Atkins diet |
| CSF | Cerebrospinal fluid |
| GABA | Gamma-Aminobutyric Acid |
| BDNF | Brain-Derived Neurotrophic Factor |
| BBB | Blood–brain barrier |
| APP | Amyloid-β protein precursor |
| KR | Caloric restriction |
| DASH | Dietary methods to halt hypertension |
| DHA | Docosahexaenoic acid |
| HSP-70 | Heat Shock Protein 70 |
| PUFAs | Polyunsaturated Fatty Acids |
| FMD | Fasting-mimicking diet |
| SCFA | Short chain fatty acids |
| GI | Glycemic index |
| MMKD | Mediterranean-ketogenic diet |
| HSD | High-salt diet |
| mPT | Mitochondrial permeability transition |
| NANA | N-acetylneuraminic acid |
| HFD | High-fat diet |
References
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 15, 2505. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A. Dietary Factors and Cognitive Decline. J. Prev. Alzheimer’s Dis. 2015, 3, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.; Li, H. What Is Brain Health and Why Is It Important? BMJ 2020, 371, m3683. [Google Scholar] [CrossRef]
- Maslowski, K.M.; MacKay, C.R. Diet, Gut Microbiota and Immune Responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea Meira, I.; Romão, T.T.; Do Prado, H.J.P.; Krüger, L.T.; Pires, M.E.P.; Da Conceição, P.O. Ketogenic Diet and Epilepsy: What We Know so Far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The Ketogenic Diet for the Treatment of Childhood Epilepsy: A Randomised Controlled Trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A Ketogenic Diet Modifies Glutamate, Gamma-Aminobutyric Acid and Agmatine Levels in the Hippocampus of Rats: A Microdialysis Study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rudy, L.; Carmen, R.; Daniel, R.; Artemio, R.; Moisés, R.O. Anticonvulsant Mechanisms of the Ketogenic Diet and Caloric Restriction. Epilepsy Res. 2020, 168, 106499. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.; Budney, S.; Deodhar, M.; Matthews, S.A.; Simeone, K.A.; Simeone, T.A. Ketogenic Diet Regulates the Antioxidant Catalase via the Transcription Factor PPARγ2. Epilepsy Res. 2018, 147, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed]
- Yellen, G. Ketone Bodies, Glycolysis, and KATP Channels in the Mechanism of the Ketogenic Diet. Epilepsia 2008, 49 (Suppl. 8), 80–82. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023; pp. 5–25. [Google Scholar]
- Singh-Manoux, A.; Dugravot, A.; Shipley, M.; Brunner, E.J.; Elbaz, A.; Sabia, S.; Kivimaki, M. Obesity Trajectories and Risk of Dementia: 28 Years of Follow-up in the Whitehall II Study. Alzheimer’s Dement. 2018, 14, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Walach, H. Midlife Obesity and Dementia: Meta-Analysis and Adjusted Forecast of Dementia Prevalence in the United States and China. Obesity 2013, 21, E51–E55. [Google Scholar] [CrossRef] [PubMed]
- Cournot, M.; Marquié, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; Ferrières, J.; Ruidavets, J.B. Relation between Body Mass Index and Cognitive Function in Healthy Middle-Aged Men and Women. Neurology 2006, 67, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Gordon, E. Elevated Body Mass Index Is Associated with Executive Dysfunction in Otherwise Healthy Adults. Compr. Psychiatry 2007, 48, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Coppin, G.; Nolan-Poupart, S.; Jones-Gotman, M.; Small, D.M. Working Memory and Reward Association Learning Impairments in Obesity. Neuropsychologia 2014, 65, 146–155. [Google Scholar] [CrossRef]
- WHO. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016; Volume 978, pp. 6–86. [Google Scholar]
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of Metabolic Syndrome on Cognition and Brain: A Selected Review of the Literature. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Lawton, C.L.; Mansfield, M.W.; Dye, L. Impairments in Glucose Tolerance Can Have a Negative Impact on Cognitive Function: A Systematic Research Review. Neurosci. Biobehav. Rev. 2009, 33, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, P.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Helkala, E.L.; Haapala, I.; Nissinen, A.; Rauramaa, R. Metabolic Syndrome and Cognitive Function: A Population-Based Follow-up Study in Elderly Women. Dement. Geriatr. Cogn. Disord. 2006, 23, 29–34. [Google Scholar] [CrossRef]
- CH, R.; Rathor, P. Chapter Nine—Metabolomic of Neurodegenerative Disorder: Alzheimer’s Disease. In Metabolomics in Health and Disease Biology; Ch, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 104, pp. 183–215. [Google Scholar] [CrossRef]
- Rathor, P.; Ch, R. Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease. Biology 2023, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.; Ying, Z.; Wu, A.; Gomez-Pinilla, F. Coupling Energy Metabolism with a Mechanism to Support Brain-Derived Neurotrophic Factor-Mediated Synaptic Plasticity. Neuroscience 2006, 139, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Martín-Segura, A.; Ahmed, T.; Casadomé-Perales, Á.; Palomares-Perez, I.; Palomer, E.; Kerstens, A.; Munck, S.; Balschun, D.; Dotti, C.G. Age-associated cholesterol reduction triggers brain insulin resistance by facilitating ligand-independent receptor activation and pathway desensitization. Aging Cell 2019, 18, e12932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nawa, H.; Carnahan, J.; Gall, C. BDNF Protein Measured by a Novel Enzyme Immunoassay in Normal Brain and after Seizure: Partial Disagreement with MRNA Levels. Eur. J. Neurosci. 1995, 7, 1527–1535. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF Val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Patel, P.D.; Sant, G.; Meng, C.X.; Teng, K.K.; Hempstead, B.L.; Lee, F.S. Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. J. Neurosci. 2004, 24, 4401–4411. [Google Scholar] [CrossRef] [PubMed]
- Tonra, J.R.; Ono, M.; Liu, X.; Garcia, K.; Jackson, C.; Yancopoulos, G.D.; Wiegand, S.J.; Wong, V. Brain-Derived Neurotrophic Factor Improves Blood Glucose Control and Alleviates Fasting Hyperglycemia in C57BLKS-Lepr(Db)/Lepr(Db) Mice. Diabetes 1999, 48, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.S.H.; Hung, C.C.C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de Novo Mutation Affecting Human TrkB Associated with Severe Obesity and Developmental Delay. Nat. Neurosci. 2004, 7, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The Relationship between Obesity and Cognitive Health and Decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and Cognitive Decline: Role of Inflammation and Vascular Changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.T. B6-Responsive Disorders: A Model of Vitamin Dependency. J. Inherit. Metab. Dis. 2006, 29, 317–326. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Essential Nutrition; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Trapl, E.S.; Smith, S.; Joshi, K.; Osborne, A.; Matos, A.T.; Bolen, S. Dietary Impact of Produce Prescriptions for Patients with Hypertension. Prev. Chronic Dis. 2018, 15, E138. [Google Scholar] [CrossRef] [PubMed]
- Seligman, H.K.; Lyles, C.; Marshall, M.B.; Prendergast, K.; Smith, M.C.; Headings, A.; Bradshaw, G.; Rosenmoss, S.; Waxman, E. A Pilot Food Bank Intervention Featuring Diabetes-Appropriate Food Improved Glycemic Control among Clients in Three States. Health Aff. 2015, 34, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary Fats and Membrane Function: Implications for Metabolism and Disease. Biol. Rev. Camb. Philos. Soc. 2005, 80, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Transport of nutrients and hormones through the blood-brain barrier. Fed. Proc. 1984, 43, 201–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M. Intestinal Absorption of Water-Soluble Vitamins in Health and Disease. Biochem. J. 2011, 437, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Mehta, S.; Gupta, D.; Sheikh, S.; Pallagatti, S.; Singh, R.; Singla, I. Clinical & Immunological Erythematosus Patients Characteristics in Systemic Lupus Maryam. J. Dent. Educ. 2012, 76, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-Soluble Vitamins: Updated Review of Their Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Georgieff, M.K. Preterm Nutrition and the Brain. World Rev. Nutr. Diet. 2014, 110, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.; Fujima, L.S.; Hovda, D.A. Age-Dependent Reduction of Cortical Contusion Volume by Ketones after Traumatic Brain Injury. J. Neurosci. Res. 2005, 82, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Chytrova, G.; Ying, Z.; Gomez-Pinilla, F. Exercise Contributes to the Effects of DHA Dietary Supplementation by Acting on Membrane-Related Synaptic Systems. Brain Res. 2010, 1341, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (-)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Gasior, M.; Vining, E.P.G.; Rogawski, M.A. The Neuropharmacology of the Ketogenic Diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.M.; Degnan, A.J. GABA-Based Evaluation of Neurologic Conditions: MR Spectroscopy. Am. J. Neuroradiol. 2013, 34, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, M.; Elfving, Å.; Ungerstedt, U.; Åmark, P. The Ketogenic Diet Influences the Levels of Excitatory and Inhibitory Amino Acids in the CSF in Children with Refractory Epilepsy. Epilepsy Res. 2005, 64, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; O’Leary, E.I.; Tanner, G.R. The Ketogenic Diet Metabolite Beta-Hydroxybutyrate (β-HB) Reduces Incidence of Seizure-like Activity (SLA) in a K Atp- and GABA b-Dependent Manner in a Whole-Animal Drosophila Melanogaster Model. Epilepsy Res. 2017, 133, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic Control of Vesicular Glutamate Transport and Release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Hrynevich, S.V.; Waseem, T.V.; Hébert, A.; Pellerin, L.; Fedorovich, S.V. β-Hydroxybutyrate Supports Synaptic Vesicle Cycling but Reduces Endocytosis and Exocytosis in Rat Brain Synaptosomes. Neurochem. Int. 2016, 93, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Martínez, I.; Walker, A.; Kunkel, B.; Schmitt-Kopplin, P.; Walter, J.; Krishnamoorthy, G. Dietary Non-Fermentable Fiber Prevents Autoimmune Neurological Disease by Changing Gut Metabolic and Immune Status. Sci. Rep. 2018, 8, 10431. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Lee, H.; Camins, A.; Pallàs, M.; Casadesus, G.; Smith, M.A.; Zhu, X. The Sirtuin Pathway in Ageing and Alzheimer Disease: Mechanistic and Therapeutic Considerations. Lancet Neurol. 2011, 10, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate Inhibits Inflammasome Activation to Attenuate Alzheimer’s Disease Pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Marder, K.; Gu, Y.; Eberly, S.; Tanner, C.M.; Scarmeas, N.; Oakes, D.; Shoulson, I. Relationship of Mediterranean Diet and Caloric Intake to Phenoconversion in Huntington Disease. JAMA Neurol. 2013, 70, 1382–1388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bushara, K.O.; Nance, M.; Gomez, C.M. Antigliadin Antibodies in Huntington’s Disease. Neurology 2004, 62, 132–133. [Google Scholar] [CrossRef] [PubMed]
- D’amico, E.; Grosso, G.; Nieves, J.W.; Zanghì, A.; Factor-Litvak, P.; Mitsumoto, H. Metabolic Abnormalities, Dietary Risk Factors and Nutritional Management in Amyotrophic Lateral Sclerosis. Nutrients 2021, 13, 2273. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.C.; Dorst, J.; Dreyhaupt, J.; Weishaupt, J.H.; Kassubek, J.; Weiland, U.; Meyer, T.; Petri, S.; Hermann, A.; Emmer, A.; et al. Effect of High-Caloric Nutrition on Survival in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2020, 87, 206–216. [Google Scholar] [CrossRef]
- Provensi, G.; Schmidt, S.D.; Boehme, M.; Bastiaanssen, T.F.S.; Rani, B.; Costa, A.; Busca, K.; Fouhy, F.; Strain, C.; Stanton, C.; et al. Preventing Adolescent Stress-Induced Cognitive and Microbiome Changes by Diet. Proc. Natl. Acad. Sci. USA 2019, 116, 9644–9651. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.D.; Chen, H.; Bertoni, A.G.; Rapp, S.R.; Fitzpatrick, A.L.; Luchsinger, J.A.; Wood, A.C.; Hughes, T.M.; Burke, G.L.; Hayden, K.M. DASH Diet Adherence and Cognitive Function: Multi-Ethnic Study of Atherosclerosis. Clin. Nutr. ESPEN 2021, 46, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Li, H.; Wang, Y.; Barnes, L.; Schneider, J.A.; Bennett, D.A.; Morris, M.C. Relation of DASH- and Mediterranean-like Dietary Patterns to Cognitive Decline in Older Persons. Neurology 2014, 83, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.M.; Pipingas, A.; Scholey, A.B. Investigation of the Effects of Solid Lipid Curcumin on Cognition and Mood in a Healthy Older Population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The Emerging Role of Nutrition in Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in Neuropsychiatric Disorders: Friend or Foe? Front. Cell. Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Shinkai, S.; Shiroma, H.; Taniguchi, Y.; Tsuchida, S.; Kariya, T.; Kawahara, T.; Kobayashi, Y.; Kohda, N.; Ushida, K.; et al. Identification of Faecalibacterium Prausnitzii Strains for Gut Microbiome-Based Intervention in Alzheimer’s-Type Dementia. Cell Rep. Med. 2021, 2, 100398. [Google Scholar] [CrossRef]
- Dei Cas, M.; Paroni, R.; Signorelli, P.; Mirarchi, A.; Cerquiglini, L.; Troiani, S.; Cataldi, S.; Codini, M.; Beccari, T.; Ghidoni, R.; et al. Human Breast Milk as Source of Sphingolipids for Newborns: Comparison with Infant Formulas and Commercial Cow’s Milk. J. Transl. Med. 2020, 18, 481. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T.; et al. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimer’s Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E.; Laliena, A.; Vallejo, D.; Tuñón, M.J.; Rodríguez-López, J.M.; Rodríguez-Pérez, R.; García-Fernández, M.C. Effects of a Low-Fat Diet with Antioxidant Supplementation on Biochemical Markers of Multiple Sclerosis Long-Term Care Residents. Nutr. Hosp. 2013, 28, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Baleztena, J.; Ruiz-Canela, M.; Sayon-Orea, C.; Pardo, M.; Añorbe, T.; Gost, J.I.; Gomez, C.; Ilarregui, B.; Bes-Rastrollo, M. Association between Cognitive Function and Supplementation with Omega-3 PUFAs and Other Nutrients in ≥ 75 Years Old Patients: A Randomized Multicenter Study. PLoS ONE 2018, 13, e0193568. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-Chain Amino Acids for People with Hepatic Encephalopathy. Am. Fam. Physician 2017, 5, CD001939. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura, M.; Boison, D.; Masino, S.A. Ketogenic Diet Improves Core Symptoms of Autism in BTBR Mice. PLoS ONE 2013, 8, e65021. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Pauls, D.L.; Santangelo, S.L.; Spiegelman, D.; Ascherio, A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. J. Autism Dev. Disord. 2011, 41, 618–627, Erratum in J. Autism Dev Disord. 2011, 41, 628.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lu, H.; Lewis, T.L.; Li, L. Intake of Sucrose-Sweetened Water Induces Insulin Resistance and Exacerbates Memory Deficits and Amyloidosis in a Transgenic Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2007, 282, 36275–36282. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Petramfar, P.; Hajari, F.; Yousefi, G.; Azadi, S.; Hamedi, A. Efficacy of Oral Administration of Licorice as an Adjunct Therapy on Improving the Symptoms of Patients with Parkinson’s Disease, A Randomized Double Blinded Clinical Trial. J. Ethnopharmacol. 2020, 247, 112226. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.; Panjwani, S.; Mohan, V.; Joshi, V.; Thakurdesai, P.A. Efficacy and Safety of Standardized Extract of Trigonella Foenum-Graecum l Seeds as an Adjuvant to L-Dopa in the Management of Patients with Parkinson’s Disease. Phytother. Res. 2014, 28, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Dioguardi, F.; Bianchi, G.; Zoli, M.; Bellati, G.; Roffi, L.; Martines, D.; Abbiati, R. Long-Term Oral Branched-Chain Amino Acid Treatment in Chronic Hepatic Encephalopathy. J. Hepatol. 1990, 11, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Park, T.; Barnes, J.; Kevala, K.; Chen, H.; Kim, H.Y. Reduced Acute Neuroinflammation and Improved Functional Recovery after Traumatic Brain Injury by α-Linolenic Acid Supplementation in Mice. J. Neuroinflamm. 2016, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhao, J.; Wu, J.; Xu, D.; Meng, X.; Jiang, P.; Shi, H.; Ge, X.; Yang, X.; Hu, M.; et al. Dimethyl Itaconate Ameliorates Cognitive Impairment Induced by a High-Fat Diet via the Gut-Brain Axis in Mice. Microbiome 2023, 11, 30. [Google Scholar] [CrossRef]
- Suzzi, S.; Croese, T.; Ravid, A.; Gold, O.; Clark, A.R.; Medina, S.; Kitsberg, D.; Adam, M.; Vernon, K.A.; Kohnert, E.; et al. N-Acetylneuraminic Acid Links Immune Exhaustion and Accelerated Memory Deficit in Diet-Induced Obese Alzheimer’s Disease Mouse Model. Nat. Commun. 2023, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized Crossover Trial of a Modified Ketogenic Diet in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- CH, R.; Tiwari, A.; Verma, T. Chapter Six—Metabolomics Applications in Type 2 Diabetes Mellitus. In Metabolomics in Health and Disease Biology; Ch, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 104, pp. 109–128. [Google Scholar] [CrossRef]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and Metabolic Syndrome: A Narrative Review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Redman, L.M. Impact of Calorie Restriction on Energy Metabolism in Humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.N.; Burhans, M.S.; Clark, J.P.; Howell, P.R.; Polewski, M.A.; DeMuth, T.M.; Eliceiri, K.W.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Aging and Caloric Restriction Impact Adipose Tissue, Adiponectin, and Circulating Lipids. Aging Cell 2017, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Trepiana, J.; Gómez-Zorita, S.; Fernández-Quintela, A.; González, M.; Portillo, M.P. Effects of Resveratrol and Its Analogue Pterostilbene, on NOV/CCN3 Adipokine in Adipose Tissue from Rats Fed a High-Fat High-Sucrose Diet. J. Physiol. Biochem. 2019, 75, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.T.; Pan, W.H.; Lin, M.N.; Lin, C.L. Vegetarian Diet, Change in Dietary Patterns, and Diabetes Risk: A Prospective Study. Nutr. Diabetes 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Al-qawasmeh, R.H.; Tayyem, R.F. Current Research in Nutrition and Food Science Dietary and Lifestyle Risk Factors and Metabolic Syndrome: Literature Review. Curr. Res. Nutr. Food Sci. J. 2018, 6, 594–608. [Google Scholar] [CrossRef]
- Baudrand, R.; Campino, C.; Carvajal, C.A.; Olivieri, O.; Guidi, G.; Faccini, G.; Vöhringer, P.; Cerda, J.; Owen, G.; Kalergis, A.; et al. High Sodium Intake Is Associated with Increased Glucocorticoid Production, Insulin Resistance and Metabolic Syndrome. Clin. Endocrinol. 2014, 80, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Rathor, P.; Trivedi, P.K.; Ch, R. Multi-omics reveal interplay between circadian dysfunction and type2 diabetes. Biology 2023, 12, 301. [Google Scholar] [CrossRef]
- Guzmán, M.; Geelen, M.J.H. Regulation of Fatty Acid Oxidation in Mammalian Liver. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1993, 1167, 227–241. [Google Scholar] [CrossRef]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic Diets, Mitochondria, and Neurological Diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Casteilla, L.; Carrière, A.; Galinier, A.; Benani, A.; Carneiro, L.; Pénicaud, L. Balancing Mitochondrial Redox Signaling: A Key Point in Metabolic Regulation. Antioxid. Redox Signal. 2011, 14, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Izzo, V.; Bravo-San Pedro, J.M.; Sica, V.; Kroemer, G.; Galluzzi, L. Mitochondrial Permeability Transition: New Findings and Persisting Uncertainties. Trends Cell Biol. 2016, 26, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Simeone, K.A.; Simeone, T.A.; Pandya, J.D.; Wilke, J.C.; Ahn, Y.; Geddes, J.W.; Sullivan, P.G.; Rho, J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015, 78, 77–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sander, D.; Kearney, M.T. Reducing the Risk of Stroke in Type 2 Diabetes: Pathophysiological and Therapeutic Perspectives. J. Neurol. 2009, 256, 1603–1619. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.J.; Rebec, G.V. Neurobiology of Vitamin C: Expanding the Focus from Antioxidant to Endogenous Neuromodulator. Pharmacol. Res. 2019, 146, 104321. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Browning, K.N.; Camilleri, M. Parkinson Disease and the Gut: New Insights into Pathogenesis and Clinical Relevance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered Gut Microbiota and Inflammatory Cytokine Responses in Patients with Parkinson’s Disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Yaffe, K.; Orwoll, E.S.; Ix, J.H.; You, Z.; Barrett-Connor, E.; Hoffman, A.R.; Chonchol, M. Serum Sodium and Cognition in Older Community-Dwelling Men. Clin. J. Am. Soc. Nephrol. 2018, 13, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.C.; Demetriou, C.A.; Zamba-Papanicolaou, E. Dietary Intake, Mediterranean Diet Adherence and Caloric Intake in Huntington’s Disease: A Review. Nutrients 2020, 12, 2946. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Jia, X.B.; Sun, M.F.; Zhu, Y.L.; Qiao, C.M.; Zhang, B.P.; Zhao, L.P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. eBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.C.; Morrison, D.J.; Edwards, C.A. Impact of the Source of Fermentable Carbohydrate on SCFA Production by Human Gut Microbiota In Vitro—A Systematic Scoping Review and Secondary Analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic Diet for Epilepsy: An Overview of Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2022, 76, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; De Jager, C.A. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and Supplemental Long-Chain Omega-3 Fatty Acids as Moderators of Cognitive Impairment and Alzheimer’s Disease. Eur. J. Nutr. 2022, 61, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean Diet Improves Cognition: The PREDIMED-NAVARRA Randomised Trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean Diet with Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shoulson, I. DATATOP: A Decade of Neuroprotective Inquiry. Parkinson Study Group. Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism. Ann. Neurol. 1998, 44 (Suppl. 1), S160–S166. [Google Scholar] [PubMed]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary Antioxidants and Risk of Parkinson’s Disease: A Systematic Review and Dose–Response Meta-Analysis of Observational Studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.M. Organizing Knowledge Syntheses: A Taxonomy of Literature Reviews. Knowl. Soc. 1988, 1, 104. [Google Scholar] [CrossRef]
- Levy, Y.; Ellis, T.J. A Systems Approach to Conduct an Effective Literature Review in Support of Information Systems Research. Informing Sci. 2006, 9, 181–211. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Harden, A. Methods for the Thematic Synthesis of Qualitative Research in Systematic Reviews. BMC Med. Res. Methodol. 2008, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.M.; Valentine, J.C.; Hedges, L.V. Handbook of Research Synthesis and Meta-Analysis, 2nd ed.Russell Sage Foundation: New York, NY, USA, 2014; Volume 389. [Google Scholar]
- Okoli, C.; Schabram, K. A Guide to Conducting a Systematic Literature Review of Information Systems Research. Available online: https://ssrn.com/abstract=1954824 (accessed on 5 May 2010).
- Strech, D.; Sofaer, N. How to Write a Systematic Review of Reasons. J. Med. Ethics 2012, 38, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.A.; Urbanowicz, R.J.; Naj, A.C.; Moore, J.H. Genetic Heterogeneity: Challenges, Impacts, and Methods through an Associative Lens. Genet. Epidemiol. 2022, 46, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.G.; Sanz-Lorente, M.; Sanz-Valero, J.; López-Pintor, E. Compliance and Adherence to Enteral Nutrition Treatment in Adults: A Systematic Review. Nutrients 2019, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient Synergy: Definition, Evidence, and Future Directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites, and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lobo, F.; Haase, J.; Brandhorst, S. The Effects of Dietary Interventions on Brain Aging and Neurological Diseases. Nutrients 2022, 14, 5086. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathor, P.; Ch, R. The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review. Dietetics 2024, 3, 289-307. https://doi.org/10.3390/dietetics3030023
Rathor P, Ch R. The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review. Dietetics. 2024; 3(3):289-307. https://doi.org/10.3390/dietetics3030023
Chicago/Turabian StyleRathor, Priya, and Ratnasekhar Ch. 2024. "The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review" Dietetics 3, no. 3: 289-307. https://doi.org/10.3390/dietetics3030023
APA StyleRathor, P., & Ch, R. (2024). The Impacts of Dietary Intervention on Brain Metabolism and Neurological Disorders: A Narrative Review. Dietetics, 3(3), 289-307. https://doi.org/10.3390/dietetics3030023






