Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants
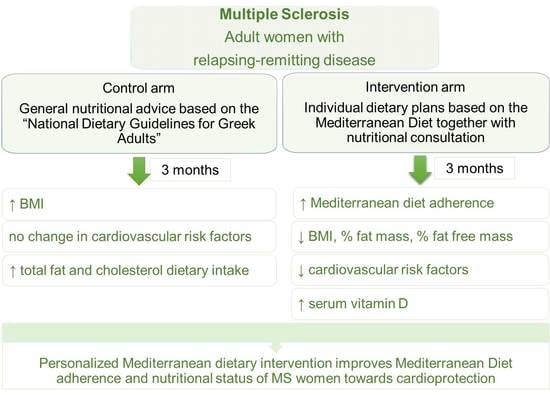
2.3. Study Design
2.4. Study Assessments
2.5. Primary Outcome and Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Dietary Intake and Mediterranean Diet Adherence
3.3. Anthropometrics and Physical Activity
3.4. Blood Markers
3.5. Psychological Distress and Anxiety
3.6. Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Multiple sclerosis: Pathology, Diagnosis and Treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive Multiple Sclerosis: From Pathophysiology to Therapeutic Strategies. Nat. Rev. Drug Discov. 2019, 8, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Axisa, P.P.; Hafler, D.A. Multiple sclerosis: Genetics, Biomarkers, Treatments. Curr. Opin. Neurol. 2016, 29, 345–353. [Google Scholar] [CrossRef]
- Iridoy Zulet, M.; Pulido Fontes, L.; Ayuso Blanco, T.; Lacruz Bescos, F.; Mendioroz Iriarte, M. Epigenetic Changes in Neurology: DNA Methylation in Multiple Sclerosis. Neurologia 2017, 32, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Tzoulaki, I. Environmental Factors and Risk of Multiple Sclerosis: Findings from Meta-Analyses and Mendelian Randomization Studies. Mult. Scler. 2019, 26, 397–404. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Koskinen, J.O.; Laaksonen, M.; Hänninen, A.; Lilius, E.M.; Waris, M. High sensitivity measurement of CRP and disease progression in multiple sclerosis. Neurology 2005, 65, 153–155. [Google Scholar] [CrossRef]
- MS International Federation. Atlas of MS 3D Edition. PART 1: Mapping Multiple Sclerosis around the World Key Epidemiology Findings. Available online: https://www.msif.org/wp-content/uploads/2020/10/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 3 September 2020).
- Wingo, B.C.; Young, H.-J.; Motl, R.W. Body composition differences between adults with multiple sclerosis and BMI-matched controls without MS. Disabil. Health J. 2018, 11, 243–248. [Google Scholar] [CrossRef]
- Gianfrancesco, M.A.; Barcellos, L.F. Obesity and Multiple Sclerosis Susceptibility: A Review. J. Neurol. Neuromed. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Baynard, T.; Hilgenkamp, T.I.M.; Schroeder, E.C.; Motl, R.W.; Fernhall, B. Measures of Adiposity Differentially Correlate with C-reactive Protein Among Persons with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 25, 1–4. [Google Scholar] [CrossRef]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Matveeva, O.; Bogie, J.F.J.; Hendriks, J.J.A.; Linker, R.A.; Haghikia, A.; Kleinewietfeld, M. Western lifestyle and immunopathology of multiple sclerosis. Ann. N. Y. Acad. Sci. 2018, 1417, 71–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.M.; Willett, W.C.; Hernán, M.A.; Olek, M.J.; Ascherio, A. Dietary Fat in Relation to Risk of Multiple Sclerosis among Two Large Cohorts of Women. Am. J. Epidemiol. 2000, 152, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swank, R.L.; Dugan, B.B. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet 1990, 336, 37–39. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Barbero, P.; Caloni, B.; Naldi, P.; Cantello, R.; Dianzani, U.; Comi, C. Vitamin D as a Marker of Impaired Information Processing Speed and Early Disability in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1521. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Bivona, G.; Gambino, C.M.; Iacolino, G.; Ciaccio, M. Vitamin D and the Nervous System. Neurol. Res. 2019, 41, 827–835. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the Mcdonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, P.; Gioxari, A.; Nimee, F.; Skouroliakou, M. Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial. Nutrients 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- National Dietary Guidelines for Adults: Ten steps to healthy eating. Available online: http://www.diatrofikoiodigoi.gr/default.aspx?page=home. (accessed on 13 December 2021).
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Mediterranean Diet and Survival Among Patients with Coronary Heart Disease in Greece. Arch. Intern. Med. 2005, 165, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean Diet Score and its Relation to Clinical and Biological Markers of Cardiovascular Disease Risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability Measures of the Short International Physical Activity Questionnaire (IPAQ) in Greek Young Adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Obesity and Overweight: Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. (accessed on 9 June 2021).
- Valentini, A.; Perrone, M.A.; Cianfarani, M.A.; Tarantino, U.; Massoud, R.; Merra, G.; Bernardini, S.; Morris, H.A.; Bertoli, A. Obesity, Vitamin D Status and Physical Activity: 1,25(OH)2D as a Potential Marker of Vitamin D Deficiency in Obese Subjects. Panminerva Med. 2020, 62, 83–92. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Grosomanidis, D.; Massara, P.; Kostara, C.; Papandreou, P.; Ntountaniotis, D.; Xepapadakis, G. Serum Antioxidant Capacity, Biochemical Profile and Body Composition of Breast Cancer Survivors in a Randomized Mediterranean Dietary Intervention Study. Eur. J. Nutr. 2017, 57, 2133–2145. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health Benefits of the Mediterranean Diet. Angiology 2014, 66, 304–318. [Google Scholar] [CrossRef]
- Sedaghat, F.; Jessri, M.; Behrooz, M.; Mirghotbi, M.; Rashidkhani, B. Mediterranean Diet Adherence and Risk of Multiple Sclerosis: A Case-Control Study. Asia Pac. J. Clin. Nutr. 2016, 25, 377–384. [Google Scholar] [CrossRef]
- Black, L.J.; Baker, K.; Ponsonby, A.-L.; van der Mei, I.; Lucas, R.M.; Pereira, G.; Ausimmune Investigator Group. A Higher Mediterranean Diet Score, Including Unprocessed Red Meat, is Associated with Reduced Risk of Central Nervous System Demyelination in a Case-Control Study of Australian Adults. J. Nutr. 2019, 149, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Hadgkiss, E.J.; Jelinek, G.A.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. The Association of Diet with Quality of Life, Disability, and Relapse Rate in an International Sample of People with Multiple Sclerosis. Nutr. Neurosci. 2015, 18, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagur, M.J.; Murcia, M.A.; Jiménez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martínez-Tomé, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, P.; Rossano, R. Nutrition Facts in Multiple Sclerosis. ASN Neuro. 2015, 7, 8185. [Google Scholar] [CrossRef] [Green Version]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Amirinejad, R.; Shirvani-Farsani, Z.; Naghavi Gargari, B.; Sahraian, M.A.; Mohammad Soltani, B.; Behmanesh, M. Vitamin D changes expression of DNA repair genes in the patients with multiple sclerosis. Gene 2021, 781, 145488. [Google Scholar] [CrossRef]
- Esposito, S.; Sparaco, M.; Maniscalco, G.T.; Signoriello, E.; Lanzillo, R.; Russo, C.; Carmisciano, L.; Cepparulo, S.; Lavorgna, L.; Gallo, A.; et al. Lifestyle and Mediterranean Diet Adherence in a Cohort of Southern Italian Patients with Multiple Sclerosis. Mult. Scler. Related Disord. 2021, 47, 102636. [Google Scholar] [CrossRef]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 5, CD004192. [Google Scholar] [CrossRef]
- Munger, K.L.; Chitnis, T.; Ascherio, A. Body Size and Risk of MS in Two Cohorts of US Women. Neurology 2009, 73, 1543–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.D.; Langer-Gould, A.; Gonzales, E.G.; Smith, J.B.; Brennan, V.; Pereira, G.; Lucas, R.M.; Begley, A.; Black, L.J. Obesity, Dieting, and Multiple Sclerosis. Mult. Scler. Rel. Disord. 2019, 39, 101889. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, C.R.; Simpson, S.; Chen, J.; van der Mei, I.; Marck, C.H. Modifiable Factors Associated with Depression and Anxiety in Multiple Sclerosis. Acta Neurol. Scand. 2019, 40, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2019, 24, 248–259. [Google Scholar] [CrossRef]
- Paz-Zulueta, M.; Parás-Bravo, P.; Cantarero-Prieto, D.; Blázquez-Fernández, C.; Oterino-Durán, A. A Literature Review of Cost-Of-Illness Studies on the Economic Burden of Multiple Sclerosis. Mult. Scler. Rel. Disord. 2020, 43, 102162. [Google Scholar] [CrossRef]
- Saulle, R.; Semyonov, L.; La Torre, G. Cost and Cost-Effectiveness of the Mediterranean Diet: Results of a Systematic Review. Nutrients 2013, 5, 4566–4586. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The Challenges of Control Groups, Placebos and Blinding in Clinical Trials of Dietary Interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Enrolled Patients (n = 40) | Control Group (n = 20) | Intervention Group (n = 20) | p-Value |
|---|---|---|---|---|
| Females | 40 | 20 | 20 | - |
| Age (years) | 29 ± 6 | 30 ± 8 | 29 ± 4 | NS |
| EDSS | 0.75 ± 1.16 | 1.0±1.3 | 0.75±1.16 | NS |
| Treatment Fingolimod | 40 | 20 | 20 | - |
| BW (kg) | 71.7 ± 17.7 | 72.0 ± 19.4 | 71.4 ± 16.3 | NS |
| BMI (kg/m2) <18.5 18.5–24.9 25–29.9 >30 | 24.4 ± 4.9 0 26 10 4 | 24.71 ± 5.6 0 14 4 2 | 24.0 ± 4.3 0 10 8 2 | NS |
| FM% | 19.2 ± 11.0 | 19.7 ± 11.4 | 18.8 ± 10.8 | NS |
| FFM% | 52.4 ± 10.8 | 52.3 ± 12.1 | 52.6 ± 9.7 | NS |
| Glucose (mg/dL) | 98.5 ± 15.8 | 100.6 ± 6.7 | 96.3 ± 21.6 | NS |
| Total cholesterol (mg/dL) | 193.8 ± 45.6 | 190.2 ± 48.2 | 197.5 ± 43.7 | NS |
| HDL (mg/dL) | 63.0 ± 19.2 | 65.3 ± 16.0 | 60.8 ± 22.2 | NS |
| LDL (mg/dL) | 117.5 ± 38.5 | 118.2 ± 41.7 | 116.7 ± 36.0 | NS |
| TG (mg/dL) | 104.1 ± 62.5 | 103.1 ± 70.6 | 105.2 ± 55.0 | NS |
| CRP (mg/dL) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | NS |
| Vitamin 1,25(OH)2D (ng/L) | 31.5 ± 3.9 | 32.2 ± 3.8 | 30.8 ± 4.0 | NS |
| METs-min/week | 578.1 ± 275.0 | 518.6 ± 288.7 | 637.6 ± 253.8 | NS |
| MedDietScore | 34.2 ± 2.9 | 34.3 ± 3.1 | 34.1 ± 2.7 | NS |
| Total fat (g) | 61.1 ± 8.4 | 59.2 ± 5.8 | 63.0 ± 10.1 | NS |
| Cholesterol dietary (mg) | 177.9 ± 40.6 | 168.7 ± 52.2 | 187.0 ± 22.1 | NS |
| Fiber (g/day) | 19.0 ± 4.3 | 18.0 ± 4.2 | 20.0 ± 4.3 | NS |
| SFAs (g/day) | 17.0 ± 3.3 | 17.7 ± 1.9 | 16.2 ± 4.1 | NS |
| MUFAs (g/day) | 25.8 ± 6.0 | 27.8 ± 4.9 | 23.8 ± 6.4 | NS |
| Anxiety score | 8.8 ± 4.9 | 8.7 ± 4.9 | 8.9 ± 5.1 | NS |
| Depression score | 6.3 ± 3.8 | 6.15 ± 4.1 | 6.5 ± 3.6 | NS |
| Characteristics | Group | Baseline (n= 20) | 3 Months (n = 20) | p-Value | * p-Value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| BW (kg) | Control | 71.4 ± 16.3 | 73.3 ± 16.7 | <0.001 | NS |
| Intervention | 72.0 ± 19.4 | 70.1 ± 18.9 | <0.001 | ||
| BMI (kg/m2) <18.5 18.5–24.99 25–30 >30 | Control | 24.0 ± 4.3 0 12 6 2 | 24.6 ± 4.3 0 10 8 2 | <0.001 | NS |
| Intervention | 24.7 ± 5.6 0 14 4 2 | 24.0 ± 5.3 0 14 4 2 | <0.001 | ||
| FM% | control | 18.8 ± 10.8 | 20.1 ± 11.0 | 0.002 | NS |
| intervention | 19.7 ± 11.4 | 18.5 ± 11.0 | <0.001 | ||
| FFM% | control | 52.6 ± 9.7 | 52.5 ± 10.1 | NS | NS |
| intervention | 52.3 ± 12.1 | 51.5 ± 12.1 | <0.001 | ||
| Glu (mg/dL) | control | 96.3 ± 21.6 | 95.2 ± 20.5 | NS | 0.05 |
| intervention | 100.6 ± 6.7 | 95.3 ± 4.8 | <0.001 | ||
| Total Chol (mg/dL) | control | 197.5 ± 43.7 | 206.8 ± 48.8 | NS | NS |
| intervention | 195.4 ± 33.4 | 193.1 ± 35.8 | NS | ||
| HDL (mg/dL) | control | 60.8 ± 22.2 | 67.5 ± 25.2 | NS | NS |
| intervention | 65.3 ± 16.0 | 63.4 ± 17.5 | NS | ||
| LDL (mg/dL) | control | 116.7 ± 36.0 | 119.8 ± 42.3 | NS | NS |
| intervention | 118.2 ± 41.7 | 115.0 ± 47.3 | NS | ||
| TG (mg/dL) | control | 105.2 ± 55.0 | 114.9 ± 6.0 | NS | NS |
| intervention | 103.1 ± 70.6 | 90.7 ± 51.4 | NS | ||
| CRP (mg/dL) | control | 0.7 ± 0.1 | 0.7 ± 0.1 | NS | NS |
| intervention | 0.7 ± 0.1 | 0.7 ± 0.1 | NS | ||
| Vitamin 1,25(OH)2D (ng/L) | control | 30.8 ± 4.0 | 38.9 ± 5.9 | <0.001 | <0.001 |
| intervention | 32.2 ± 3.8 | 49.1 ± 11.3 | <0.001 | ||
| METs-min/week | control | 637.6 ± 253.8 | 611.5 ± 224.2 | NS | NS |
| intervention | 518.6 ± 288.7 | 550.6 ± 282.1 | 0.013 | ||
| MedDietScore | control | 34.1 ± 2.7 | 34.3 ± 2.5 | NS | <0.001 |
| intervention | 34.3 ± 3.1 | 37.6 ± 2.2 | <0.001 | ||
| Total fat (g) | Control | 63.0 ± 10.1 | 65.6 ± 10.3 | <0.001 | <0.001 |
| intervention | 59.2 ± 5.8 | 53.6 ± 5.6 | <0.001 | ||
| Cholesterol dietary (mg) | Control | 187.0 ± 22.1 | 200.5 ± 25.3 | <0.001 | <0.001 |
| intervention | 168.7 ± 52.2 | 151.1 ± 44.9 | <0.001 | ||
| Fiber (g/d) | control | 20.0 ± 4.3 | 21.2 ± 3.8 | 0.068 | NS |
| intervention | 18.0 ± 4.2 | 23.3 ± 4.5 | <0.001 | ||
| SFAs (g/d) | control | 16.2 ± 4.1 | 16.5 ± 4.3 | NS | NS |
| intervention | 17.7 ± 1.9 | 14.9 ± 2.8 | 0.004 | ||
| MUFAs (g/d) | control | 23.8 ± 6.4 | 23.3 ± 6.4 | NS | <0.001 |
| intervention | 27.8 ± 4.9 | 33.0 ± 5.2 | <0.001 | ||
| Anxiety score | control | 8.9 ± 5.1 | 7.1 ± 3.6 | 0.050 | 0.014 |
| intervention | 8.6 ± 4.9 | 4.2 ± 3.6 | <0.001 | ||
| Depression score | control | 6.5 ± 3.6 | 6.4 ± 4.2 | NS | 0.020 |
| intervention | 6.2 ± 4.1 | 3.6 ± 3.0 | <0.001 |
| Independent Variables | B | S.E. | Wald | Sig. | Exp(B) | 95% C.I. for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.432 | 0.344 | 1.581 | 0.209 | 1.540 | 0.786 | 3.021 |
| BMI | 0.112 | 0.333 | 0.114 | 0.736 | 1.119 | 0.582 | 2.151 |
| FM | 0.039 | 0.151 | −0.068 | 0.794 | 1.040 | 0.774 | 1.397 |
| HDL | −0.035 | 0.044 | 0.649 | 0.421 | 0.966 | 0.887 | 1.052 |
| TG | −0.019 | 0.012 | 2.576 | 0.108 | 0.982 | 0.960 | 1.004 |
| CRP | 1.748 | 8.902 | 0.039 | 0.421 | 0.966 | 0.887 | 1.052 |
| MedDietScore | 1.142 | 0.562 | 4.136 | 0.042 | 3.134 | 1.042 | 9.424 |
| Cholesterol intake | −0.060 | 0.025 | 5.489 | 0.019 | 0.942 | 0.896 | 0.990 |
| SFAs | −0.340 | 0.266 | 1.626 | 0.202 | 0.712 | 0.422 | 1.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, P.; Gioxari, A.; Daskalou, E.; Vasilopoulou, A.; Skouroliakou, M. Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study. Dietetics 2022, 1, 25-38. https://doi.org/10.3390/dietetics1010004
Papandreou P, Gioxari A, Daskalou E, Vasilopoulou A, Skouroliakou M. Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study. Dietetics. 2022; 1(1):25-38. https://doi.org/10.3390/dietetics1010004
Chicago/Turabian StylePapandreou, Panos, Aristea Gioxari, Efstratia Daskalou, Archontia Vasilopoulou, and Maria Skouroliakou. 2022. "Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study" Dietetics 1, no. 1: 25-38. https://doi.org/10.3390/dietetics1010004
APA StylePapandreou, P., Gioxari, A., Daskalou, E., Vasilopoulou, A., & Skouroliakou, M. (2022). Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study. Dietetics, 1(1), 25-38. https://doi.org/10.3390/dietetics1010004